316L Stainless Steel Manufactured by Selective Laser Melting and Its Biocompatibility with or without Hydroxyapatite Coating
Abstract
1. Introduction
2. Experimental Section
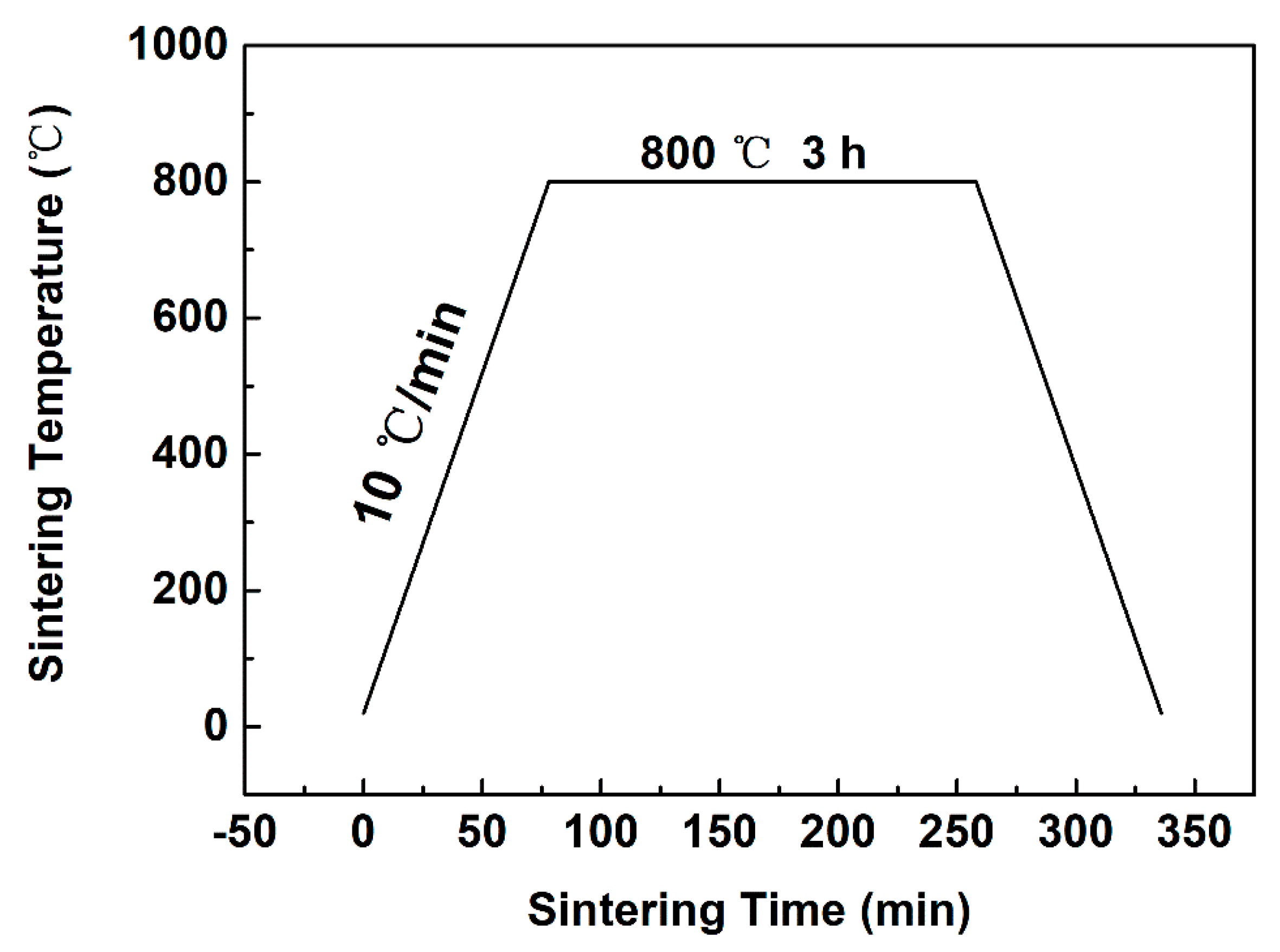
2.1. Material Preparation and Characterization
2.2. Cell Culture and Proliferation Study
2.3. Cell Morphology Study
2.4. Statistical Comparisons for the Biocompatibility Study
3. Results
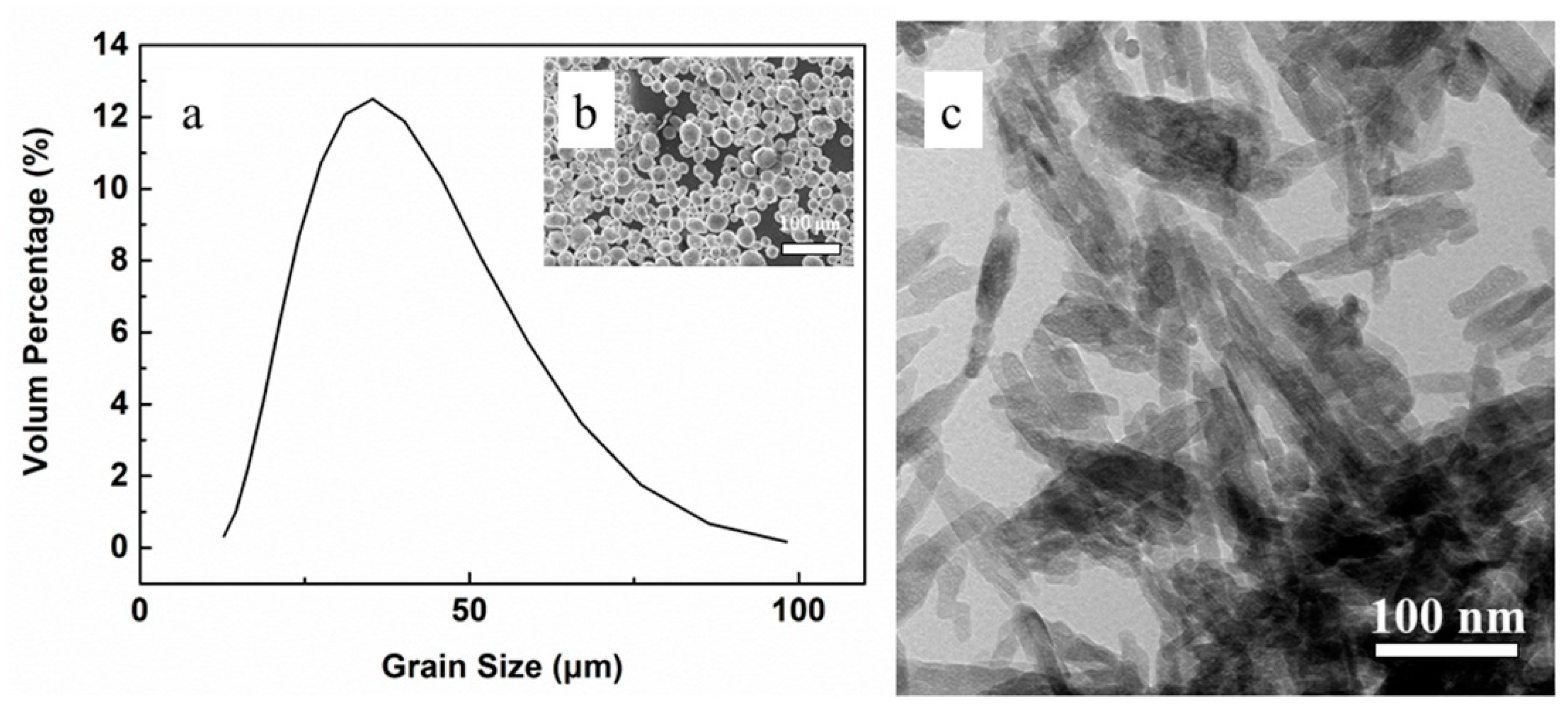
3.1. Powder Characterization
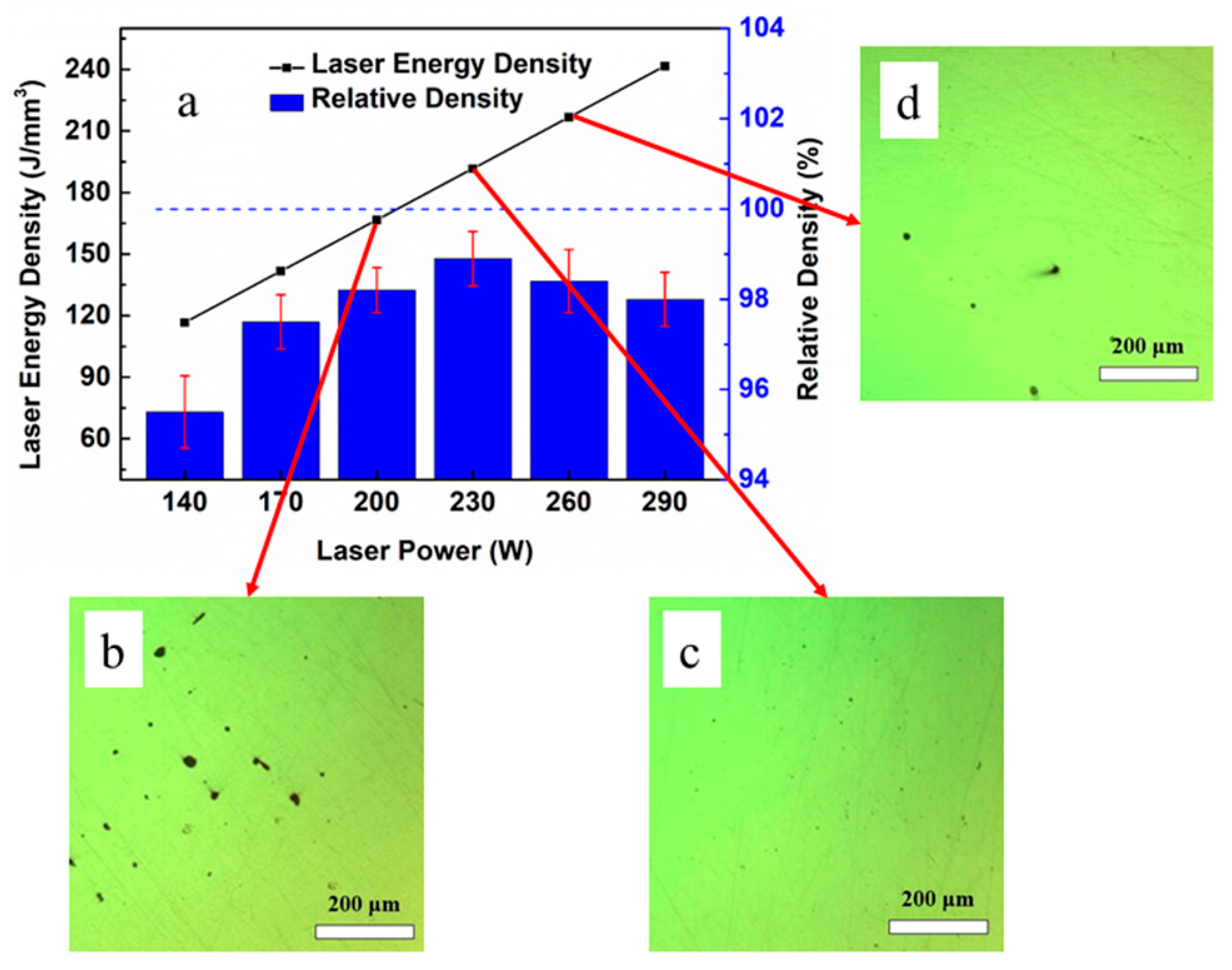
3.2. Density of the As-Printed 316L Steel
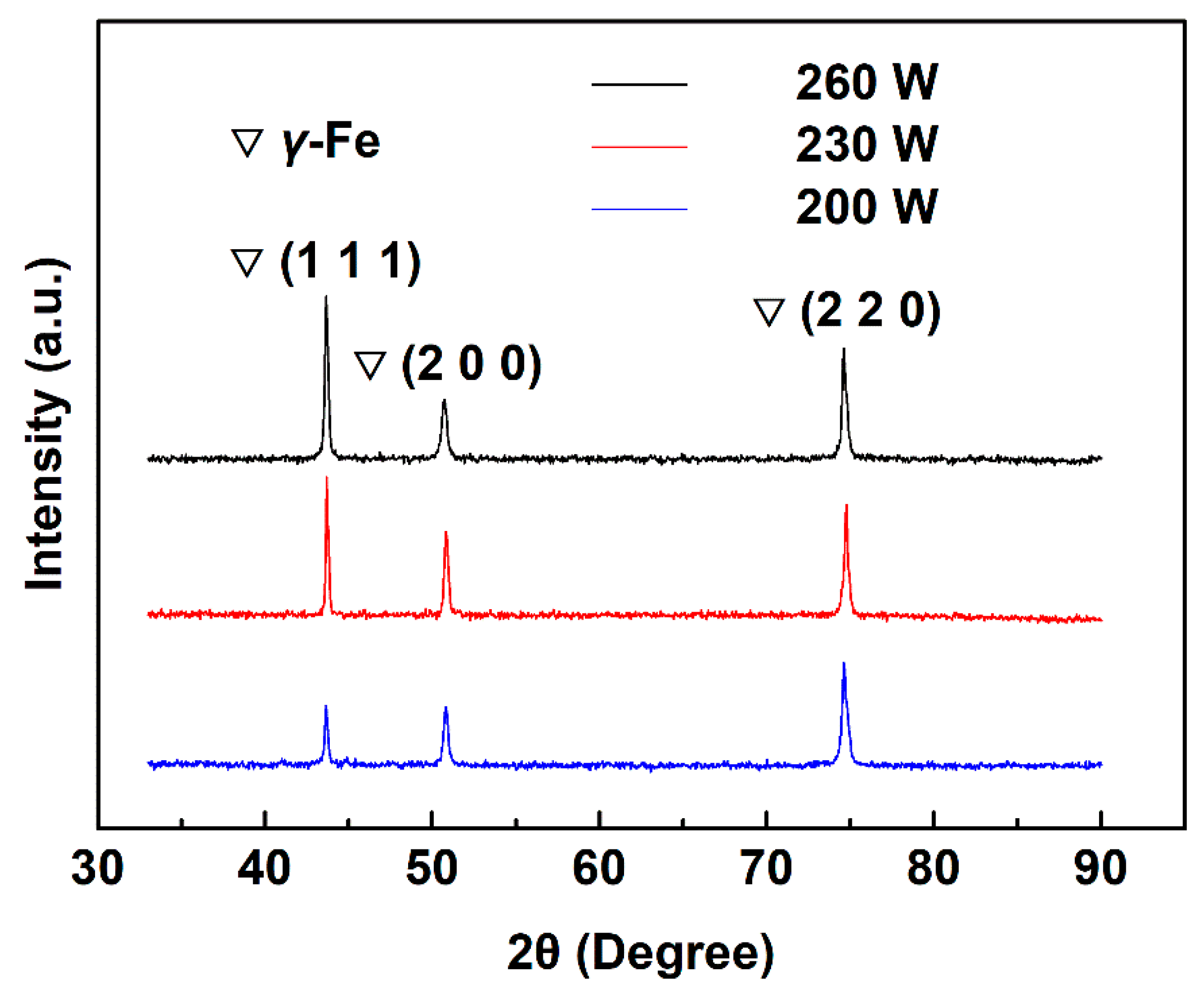
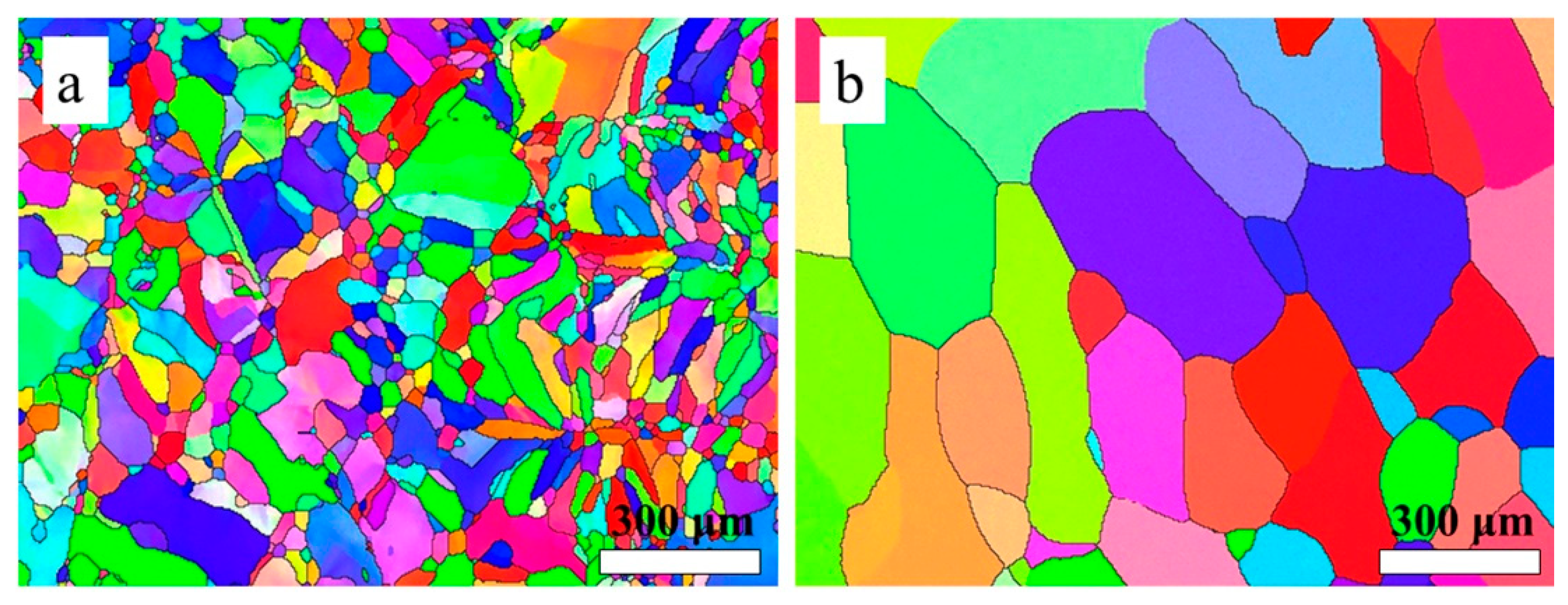
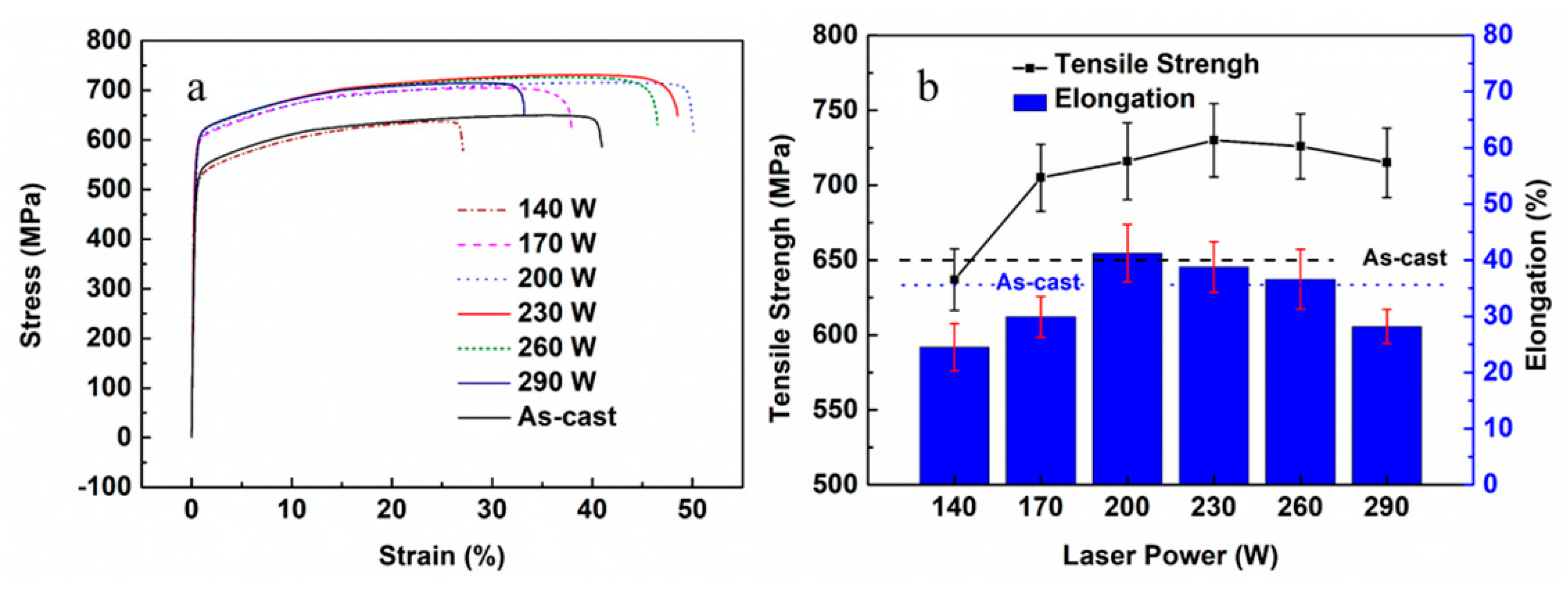
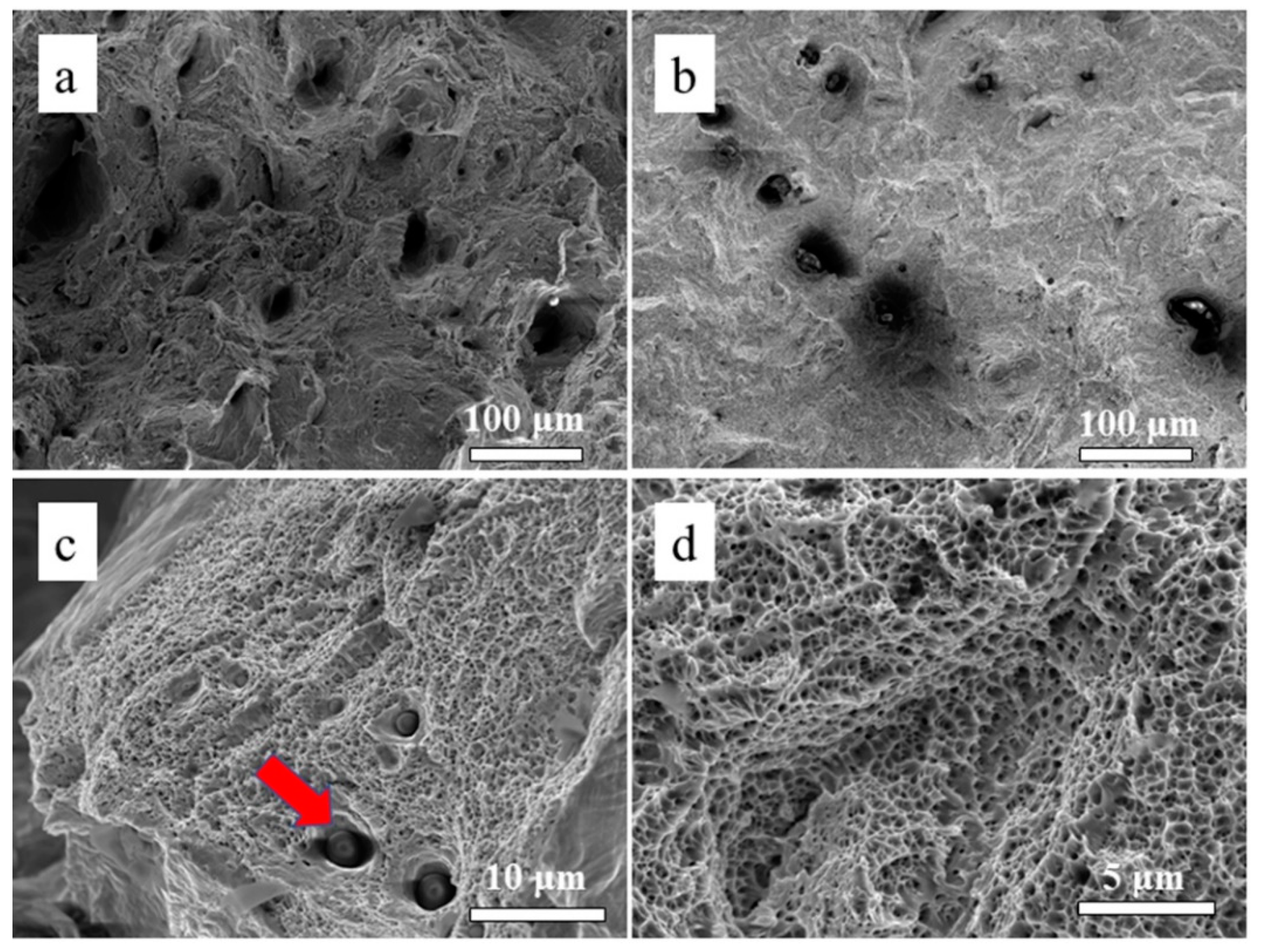
3.3. Microstructure and Mechanical Properties of the As-Printed 316L Stainless Steel
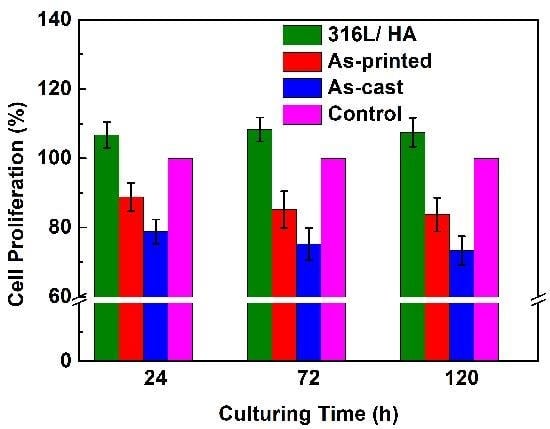
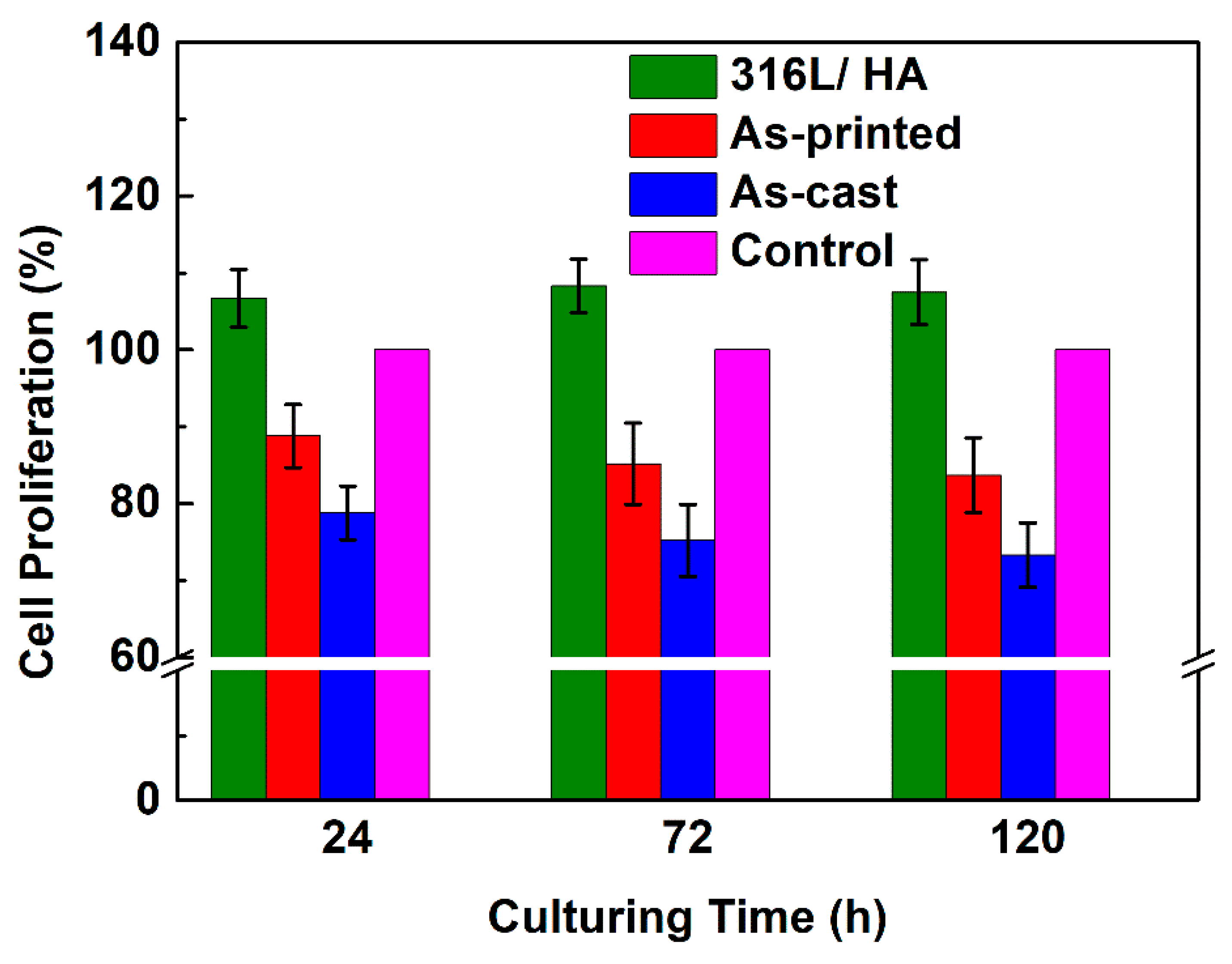
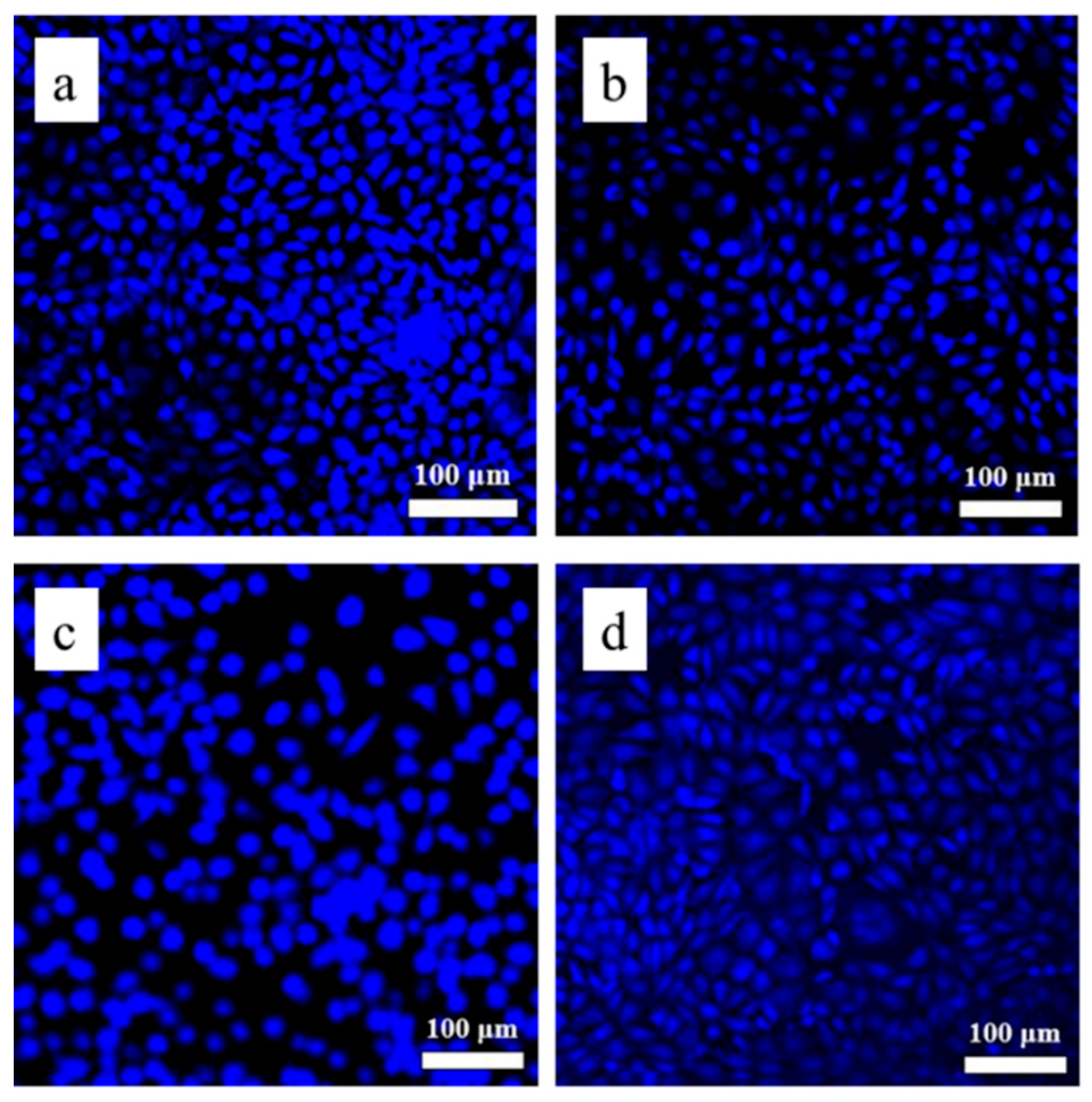
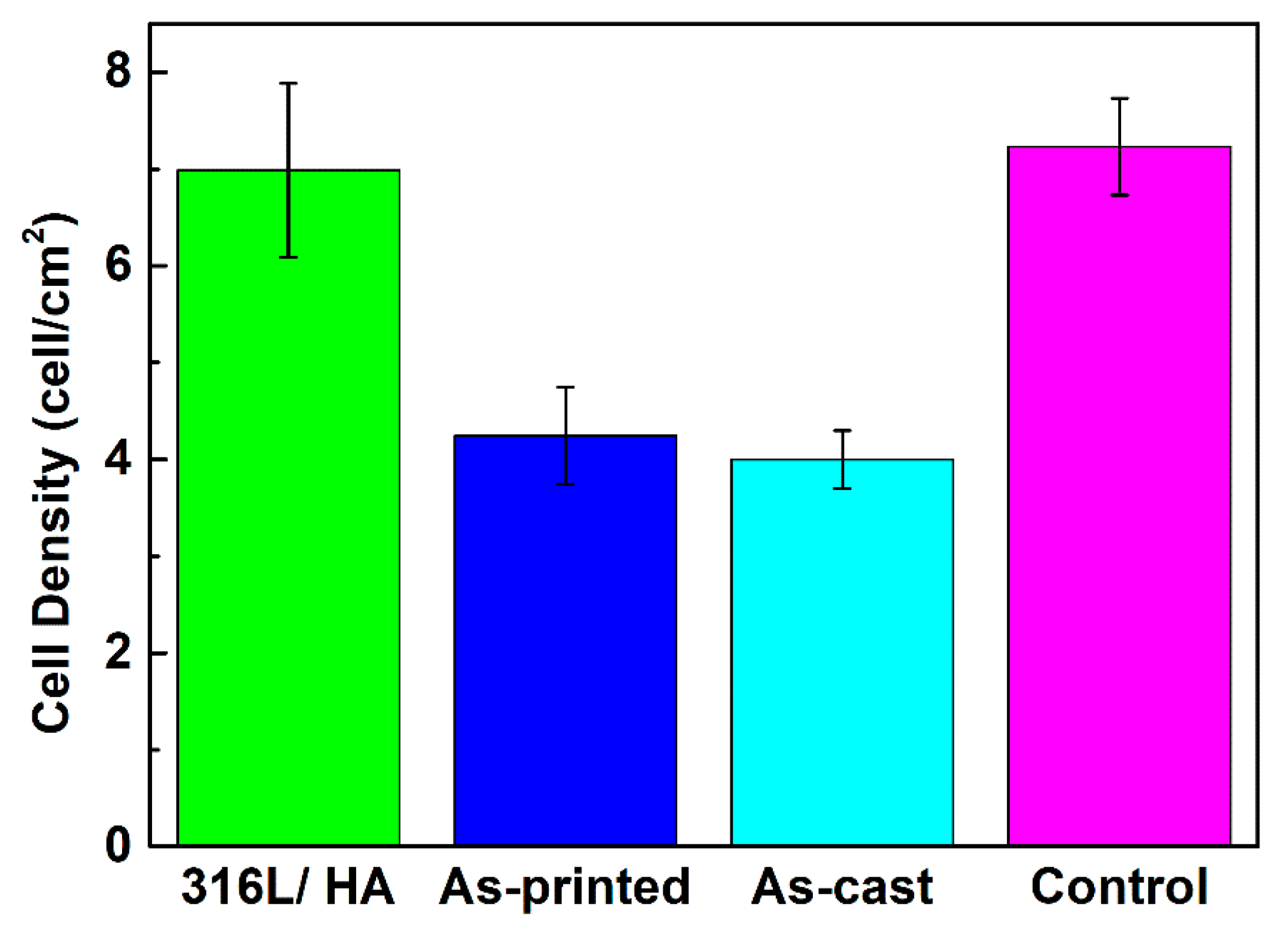
3.4. Biocompatibility of the As-Printed 316L Stainless Steel
4. Discussion
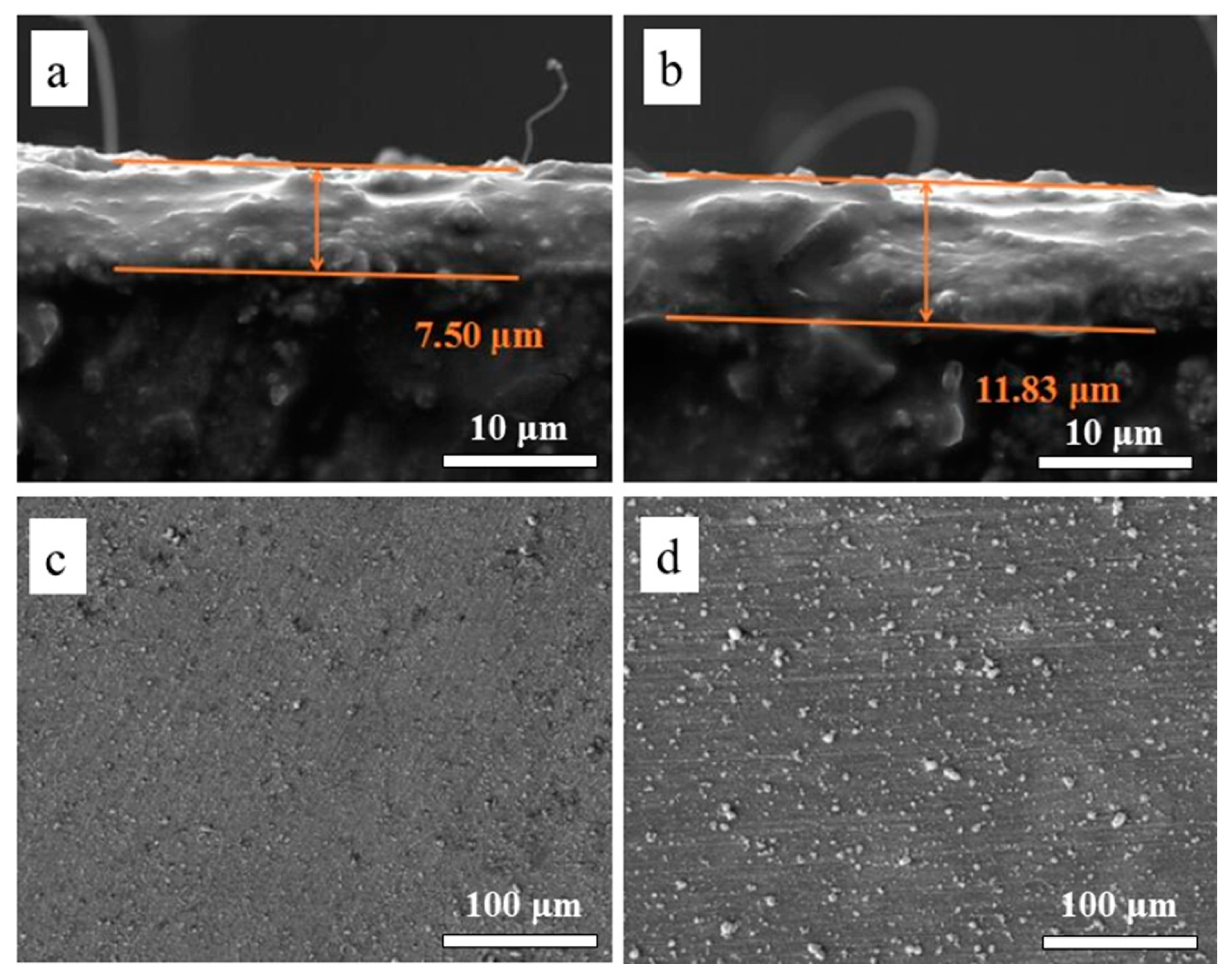
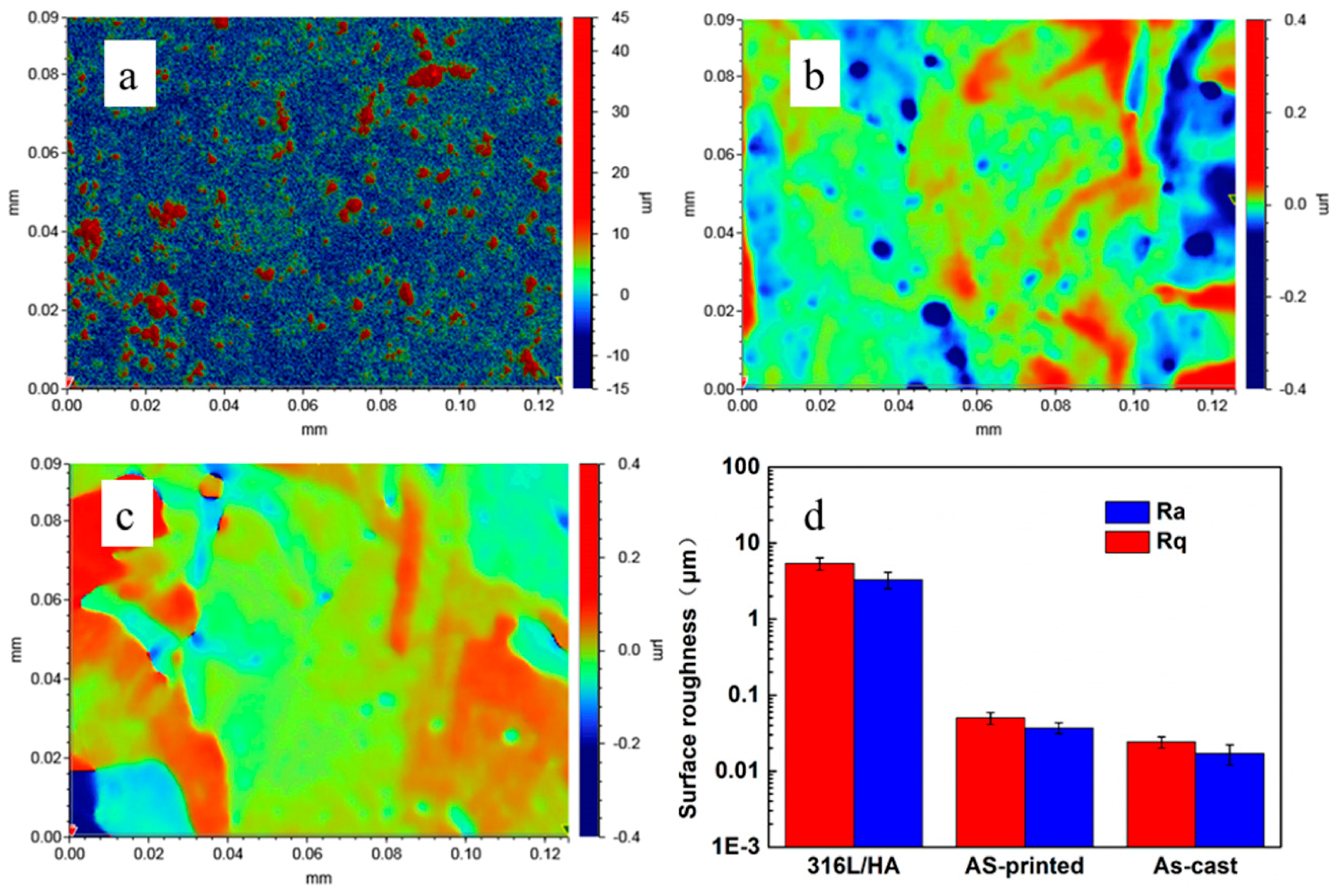
4.1. Discussion on the Surface Roughness and Cellular Activities
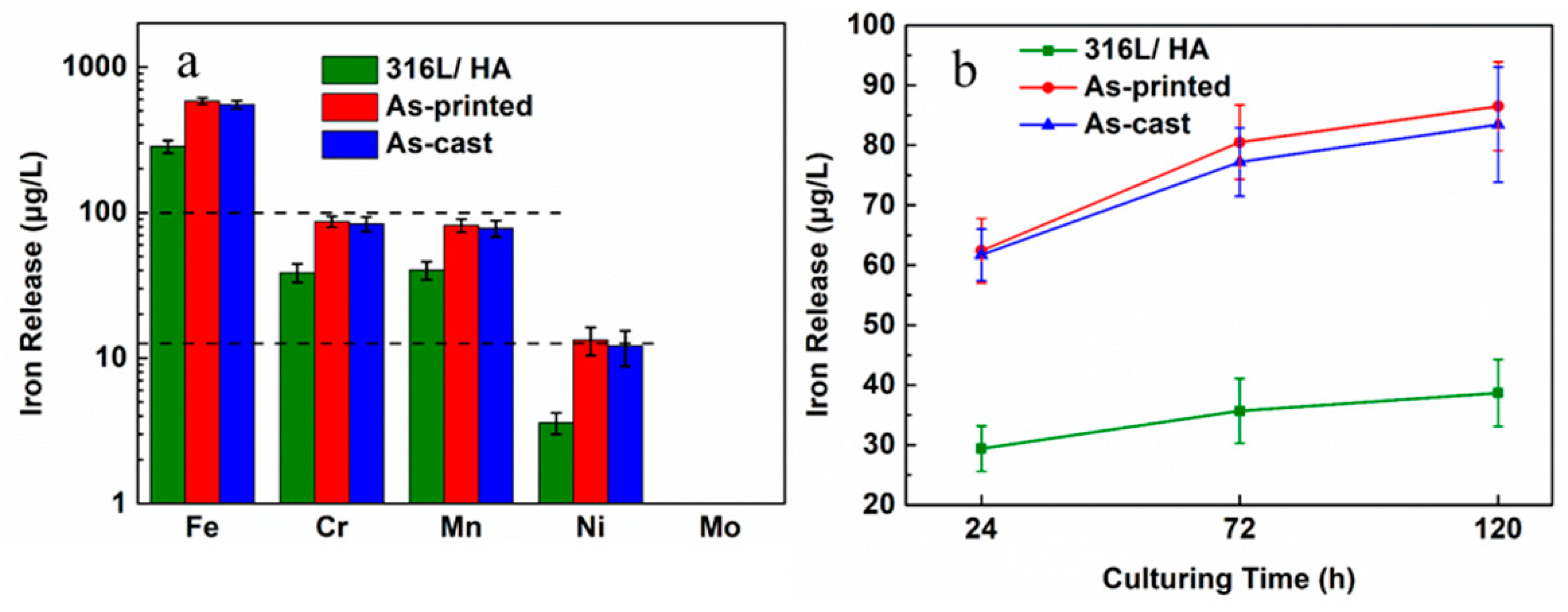
4.2. Discussion on the Biocompatibility of the 316L+ HA
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ehtemam-Haghighi, S.; Prashanth, K.G.; Attar, H.; Chaubey, A.K.; Cao, G.H.; Zhang, L.C. Evaluation of mechanical and wear properties of Ti-xNb-7Fe alloys designed for biomedical applications. Mater. Des. 2016, 111, 592–599. [Google Scholar] [CrossRef]
- Baddoo, N.R. Stainless steel in construction: A review of research, applications, challenges and opportunities. J. Constr. Steel Res. 2008, 64, 1199–1206. [Google Scholar] [CrossRef]
- Ziewiec, A.; Tasak, E.; Witkowska, M.; Ziewiec, K. Microstructure and properties of welds of semi-austenitic precipitation hardening stainless steel after heat treatment. Arch. Metall. Mater. 2013, 58, 613–617. [Google Scholar] [CrossRef]
- Ehtemam-Haghighi, S.; Lu, H.B.; Jian, G.Y.; Cao, G.H.; Habibi, D.; Zhang, L.C. Effect of α″ martensite on the microstructure and mechanical properties of beta-type Ti–Fe–Ta alloys. Mater. Des. 2015, 76, 47–54. [Google Scholar] [CrossRef]
- Barnes, D.; Johnson, S.; Snell, R.; Best, S. Using scratch testing to measure the adhesion strength of calcium phosphate coatings applied to poly (carbonate urethane) substrates. J. Mech. Behav. Biomed. Mater. 2012, 6, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Okulov, I.V.; Volegov, A.S.; Attar, H.; Bönisch, M.; Ehtemam-Haghighi, S.; Calin, M.; Eckert, J. Composition optimization of low modulus and high-strength TiNb-based alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2017, 65, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Frazier, W.E. Metal Additive Manufacturing: A Review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Yarlagadda, P.K.; Chandrasekharan, M.; Shyan, J.Y.M. Recent advances and current developments in tissue scaffolding. Bio-Med. Mater. Eng. 2005, 15, 159–177. [Google Scholar]
- Miranda, G.; Faria, S.; Bartolomeu, F.; Pinto, E.; Madeira, S.; Mateus, A.; Carvalho, O. Predictive models for physical and mechanical properties of 316L stainless steel produced by selective laser melting. Mater. Sci. Eng. A 2016, 657, 43–56. [Google Scholar] [CrossRef]
- Wang, D.; Song, C.; Yang, Y.; Bai, Y. Investigation of crystal growth mechanism during selective laser melting and mechanical property characterization of 316L stainless steel parts. Mater. Des. 2016, 100, 291–299. [Google Scholar] [CrossRef]
- Sumita, M.; Hanawa, T.; Teoh, S.H. Development of nitrogen-containing nickel-free austenitic stainless steels for metallic biomaterials. Mater. Sci. Eng. A 2004, 24, 753–760. [Google Scholar] [CrossRef]
- Savalani, M.M.; Hao, L.; Dickens, P.M.; Zhang, Y.; Tanner, K.E.; Harris, R.A. The effects and interactions of fabrication parameters on the properties of selective laser sintered hydroxyapatite polyamide composite biomaterials. Rapid Prototyp. J. 2012, 18, 16–27. [Google Scholar] [CrossRef]
- Geiger, F.; Kunze, K.; Etter, T. Tailoring the texture of IN738LC processed by selective laser melting (SLM) by specific scanning strategies. Mater. Sci. Eng. A 2016, 661, 240–246. [Google Scholar] [CrossRef]
- Carter, L.N.; Martin, C.; Withers, P.J.; Attallah, M.M. The influence of the laser scan strategy on grain structure and cracking behaviour in SLM powder-bed fabricated nickel superalloy. J. Alloys Compd. 2014, 615, 338–347. [Google Scholar] [CrossRef]
- Duraccio, D.; Mussano, F.; Faga, M.G. Biomaterials for dental implants: Current and future trends. J. Mater. Sci. 2015, 50, 4779–4812. [Google Scholar] [CrossRef]
- Xin, X.Z.; Xiang, N.; Chen, J.; Wei, B. In vitro biocompatibility of Co–Cr alloy fabricated by selective laser melting or traditional casting techniques. Mater. Lett. 2012, 88, 101–103. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Lin, S.F.; Hou, Y.H.; Wang, D.W.; Zhou, P.; Han, P.L.; Yan, M. Layered surface structure of gas-atomized high Nb-containing TiAl powder and its impact on laser energy absorption for selective laser melting. Appl. Surf. Sci. 2018, 441, 210–217. [Google Scholar] [CrossRef]
- Yan, J.J.; Zheng, D.L.; Li, H.X.; Jia, X.; Sun, J.F.; Li, Y.L.; Yan, M. Selective laser melting of H13: Microstructure and residual stress. J. Mater. Sci. 2017, 52, 12476–12485. [Google Scholar] [CrossRef]
- Attar, H.; Ehtemam-Haghighi, S.; Kent, D.; Wu, X.; Dargusch, M.S. Comparative study of commercially pure titanium produced by laser engineered net shaping, selective laser melting and casting processes. Mater. Sci. Eng. A 2017, 705, 385–393. [Google Scholar] [CrossRef]
- Attar, H.; Ehtemam-Haghighi, S.; Kent, D.; Okulov, I.V.; Wendrock, H.; Bӧnisch, M.; Volegov, A.S.; Calin, M.; Eckert, J.; Dargusch, M.S. Nanoindentation and wear properties of Ti and Ti-Tib composite materials produced by selective laser melting. Mater. Sci. Eng. A 2017, 688, 20–26. [Google Scholar] [CrossRef]
- Kong, D.; Ni, X.; Dong, C.; Lei, X.; Zhang, L.; Man, C.; Li, X. Bio-functional and anti-corrosive 3D printing 316L stainless steel fabricated by selective laser melting. Mater. Des. 2018, 152, 88–101. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Buciumeanu, M.; Pinto, E.; Alves, N.; Carvalho, O.; Silva, F.S.; Miranda, G. 316L stainless steel mechanical and tribological behavior—a comparison between selective laser melting, hot pressing and conventional casting. Addit. Manuf. 2017, 16, 81–89. [Google Scholar] [CrossRef]
- Čapek, J.; Machova, M.; Fousova, M.; Kubásek, J.; Vojtěch, D.; Fojt, J.; Ruml, T. Highly porous, low elastic modulus 316L stainless steel scaffold prepared by selective laser melting. Mater. Sci. Eng. C 2016, 69, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Biemond, J.E.; Hannink, G.; Verdonschot, N.; Buma, P. Bone ingrowth potential of electron beam and selective laser melting produced trabecular-like implant surfaces with and without a biomimetic coating. J. Mater. Sci. Mater. Med. 2013, 24, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Bagherifard, S.; Hickey, D.J.; De-Luca, A.C.; Malheiro, V.N.; Markaki, A.E.; Guagliano, M.; Webster, T.J. The influence of nanostructured features on bacterial adhesion and bone cell functions on severely shot peened 316L stainless steel. Biomaterials 2015, 73, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Yuan, Y.; Li, D.; Li, Y.; Chen, J. Effects of scanning speed on in vitro biocompatibility of 316L stainless steel parts elaborated by selective laser melting. Int. J. Adv. Manuf. Technol. 2017, 92, 4379–4385. [Google Scholar] [CrossRef]
- Bayraktaroglu, E.; Gulsoy, H.O.; Gulsoy, N.; Er, O.; Kilic, H. Effect of boron addition on injection molded 316L stainless steel: Mechanical, corrosion properties and in vitro bioactivity. Bio-Med. Mater. Eng. 2012, 22, 333–349. [Google Scholar]
- Köse, C.; Kacar, R. In vitro bioactivity and corrosion properties of laser beam welded medical grade AISI 316L stainless steel in simulated body fluid. Int. J. Electrochem. Sci. 2016, 11, 2762–2777. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite—past, present, and future in bone regeneration. Bone Tissue Regener Insights 2016, 6, 9. [Google Scholar] [CrossRef]
- Ramires, P.A.; Wennerberg, A.; Johansson, C.B.; Cosentino, F.; Tundo, S.; Milella, E. Biological behavior of sol-gel coated dental implants. J. Mater. Sci. Mater. Med. 2003, 14, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Paital, S.R.; Dahotre, N.B. Calcium phosphate coatings for bio-implant applications: Materials, performance factors, and methodologies. Mater. Sci. Eng. R Rep. 2009, 66, 1–70. [Google Scholar] [CrossRef]
- Ergün, Y.; Başpınar, M.S. Effect of acid passivation and H2 sputtering pretreatments on the adhesive strength of sol–gel derived Hydroxyapatite coating on titanium surface. Int. J. Hydrog. Energy 2017, 42, 20420–20429. [Google Scholar] [CrossRef]
- Metikoš-Huković, M.; Tkalčec, E.; Kwokal, A.; Piljac, J. An in vitro study of Ti and Ti-alloys coated with sol–gel derived hydroxyapatite coatings. Surf. Coat. Tech. 2003, 165, 40–50. [Google Scholar] [CrossRef]
- Li, X.P.; O’Donnell, K.M.; Sercombe, T.B. Selective laser melting of Al-12Si alloy: Enhanced densification via powder drying. Addit. Manuf. 2016, 10, 10–14. [Google Scholar] [CrossRef]
- Vaithilingam, J.; Goodridge, R.D.; Hague, R.J.; Christie, S.D.; Edmondson, S. The effect of laser remelting on the surface chemistry of Ti6al4V components fabricated by selective laser melting. J. Mater. Process. Technol. 2016, 232, 1–8. [Google Scholar] [CrossRef]
- Suyalatu, X.; Takayoshi, N.; Norio, H.; Hitoshi, S. Microstructure and mechanical properties of ti-x alloys fabricated by selective laser melting process for new biomaterial devices. Front. Bioeng. Biotechnol. 2016, 4. [Google Scholar] [CrossRef]
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A 2002, 33A, 477–486. [Google Scholar] [CrossRef]
- Wierzbicki, A.; Cheung, H.S. Molecular modeling of inhibition of hydroxyapatite by phosphocitrate. THEOCHEM 2000, 529, 73–82. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, G.; Lian, J. A chemical conversion hydroxyapatite coating on AZ60 magnesium alloy and its electrochemical corrosion behaviour. Int. J. Electrochem. Sci. 2012, 7, 11497–11511. [Google Scholar]
- Evans, S.L.; Gregson, P.J. Composite technology in load-bearing orthopaedic implants. Biomaterials 1998, 19, 1329–1342. [Google Scholar] [CrossRef]
- Pellino, G.; Sciaudone, G.; Caserta, V.; Candilio, G.; Gilda Serena, D.F.; Gagliardi, S.; Landino, I.; Patturelli, M.; Riegler, G.; Di Caprio, E.L.; et al. Fatigue in inflammatory bowel diseases: Relationship with age and disease activity. Int. J. Surg. 2014, 12, S60–S63. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Casagrande, T.; Zhitomirsky, I. Electrophoretic deposition of hydroxyapatite-CaSiO3-chitosan composite coatings. J. Colloid Interface Sci. 2008, 330, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.F.; Liu, R.F.; Tang, X.L. Electrophoretic deposition of silicon substituted hydroxyapatite coatings from n-butanol-chloroform mixture. J. Mater. Sci. Mater. Med. 2008, 19, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Hinds, G.; Wickström, L.; Mingard, K.; Turnbull, A. Impact of surface condition on sulphide stress corrosion cracking of 316L stainless steel. Corros. Sci. 2013, 71, 43–52. [Google Scholar] [CrossRef]
- Vashista, M.; Paul, S. Correlation between full width at half maximum (FWHM) of XRD peak with residual stress on ground surfaces. Philos. Mag. 2012, 92, 4194–4204. [Google Scholar] [CrossRef]
- Simonovski, I.; Nilsson, K.F.; Cizelj, L. Crack tip displacements of microstructurally small cracks in 316L steel and their dependence on crystallographic orientations of grains. Fatigue Fract. Eng. Mater. Struct. 2007, 30, 463–478. [Google Scholar] [CrossRef]
- Martínez, S.; Lamikiz, A.; Ukar, E.; Calleja, A.; Arrizubieta, J.A.; Lacalle Lopez de, L.N. Analysis of the regimes in the scanner-based laser hardening process. Opt. Lasers Eng. 2017, 90, 72–80. [Google Scholar] [CrossRef]
- Martínez Krahmer, D.; Polvorosa, R.; López de Lacalle, L.N.; Alonso-Pinillos, U.; Abate, G.; Riu, F. Alternatives for Specimen manufacturing in Tensile Testing of Steel Plates. Exp. Tech. 2016, 40, 1555–1565. [Google Scholar] [CrossRef]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, L.; Wikman, S.; Cui, D.; Shen, Z. Intragranular cellular segregation network structure strengthening 316L stainless steel prepared by selective laser melting. J. Nucl. Mater. 2016, 470, 170–178. [Google Scholar] [CrossRef]
- Hao, L.; Dadbakhsh, S.; Seaman, O.; Felstead, M. Selective laser melting of a stainless steel and hydroxyapatite composite for load-bearing implant development. J. Mater. Process. Technol. 2009, 209, 5793–5801. [Google Scholar] [CrossRef]
- Yu, S.; Wang, B.; Pan, Y.; Chen, Z.; Meng, F.; Duan, S.; Ma, W. Cleaner production of spherical nanostructure chromium oxide (Cr2O3) via a facile combination membrane and hydrothermal approach. J. Cleaner Prod. 2018, 176, 636–644. [Google Scholar] [CrossRef]
- Yang, D.; Lü, X.; Hong, Y.; Xi, T.; Zhang, D. The molecular mechanism for effects of TiN coating on NiTi alloy on endothelial cell function. Biomaterials 2014, 35, 6195–6205. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Lü, X.; Hong, Y.; Xi, T.; Zhang, D. The molecular mechanism of mediation of adsorbed serum proteins to endothelial cells adhesion and growth on biomaterials. Biomaterials 2013, 34, 5747–5758. [Google Scholar] [CrossRef] [PubMed]



| Cr | 16–18 | Ni | 12–14 | Mo | 2–3 |
|---|---|---|---|---|---|
| Mn | ≤2 | Si | ≤0.75 | C | ≤0.1 |
| P | ≤0.1 | S | ≤0.1 | Fe | remainder |
| Sample | 2θ | Intensity | FWHM |
|---|---|---|---|
| Standard | 43.582 | ||
| 140 W | 43.695 | 2369 | 0.201 |
| 170 W | 43.55 | 2357 | 0.241 |
| 200 W | 43.568 | 2437 | 0.234 |
| 230 W | 43.696 | 3956 | 0.203 |
| 260 W | 43.657 | 4548 | 0.199 |
| 290 W | 43.64 | 5058 | 0.180 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Jia, X.; Gu, R.; Zhou, P.; Huang, Y.; Sun, J.; Yan, M. 316L Stainless Steel Manufactured by Selective Laser Melting and Its Biocompatibility with or without Hydroxyapatite Coating. Metals 2018, 8, 548. https://doi.org/10.3390/met8070548
Luo J, Jia X, Gu R, Zhou P, Huang Y, Sun J, Yan M. 316L Stainless Steel Manufactured by Selective Laser Melting and Its Biocompatibility with or without Hydroxyapatite Coating. Metals. 2018; 8(7):548. https://doi.org/10.3390/met8070548
Chicago/Turabian StyleLuo, Jiapeng, Xiao Jia, Ruinan Gu, Peng Zhou, Yongjiang Huang, Jianfei Sun, and Ming Yan. 2018. "316L Stainless Steel Manufactured by Selective Laser Melting and Its Biocompatibility with or without Hydroxyapatite Coating" Metals 8, no. 7: 548. https://doi.org/10.3390/met8070548
APA StyleLuo, J., Jia, X., Gu, R., Zhou, P., Huang, Y., Sun, J., & Yan, M. (2018). 316L Stainless Steel Manufactured by Selective Laser Melting and Its Biocompatibility with or without Hydroxyapatite Coating. Metals, 8(7), 548. https://doi.org/10.3390/met8070548