Experimental Study of Surface Characteristic in Electrochemical Machining of 35CrMo Steel
Abstract
:1. Introduction
2. Experimental
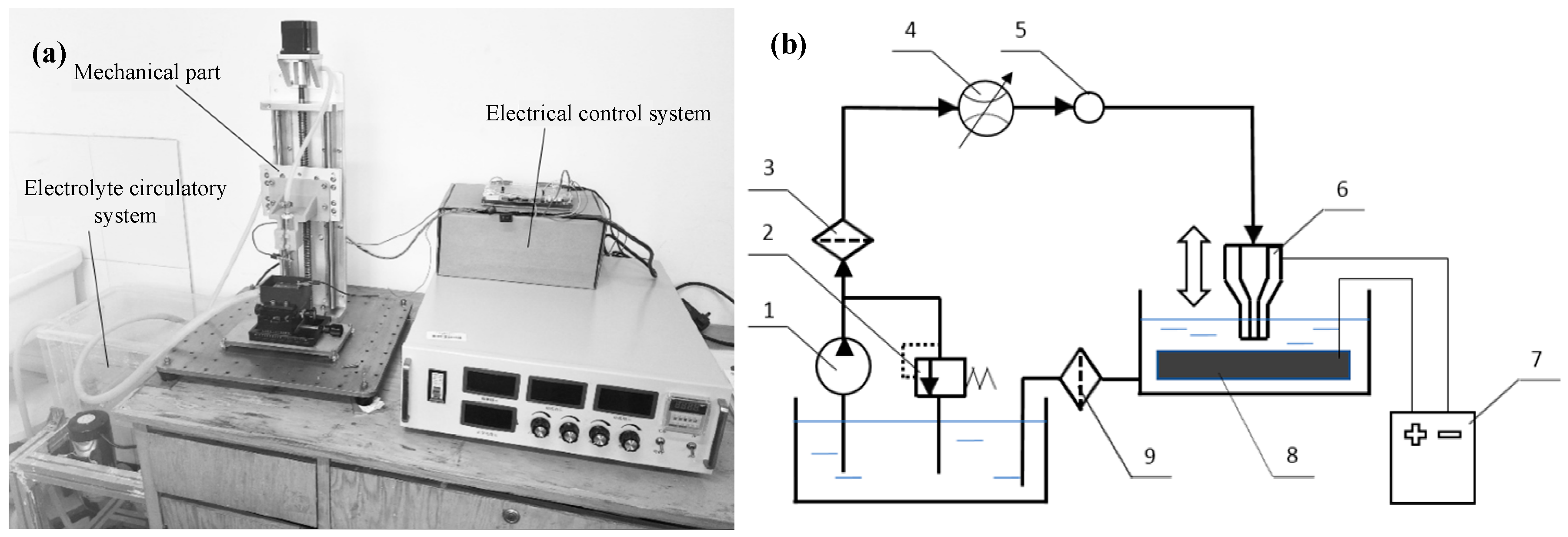
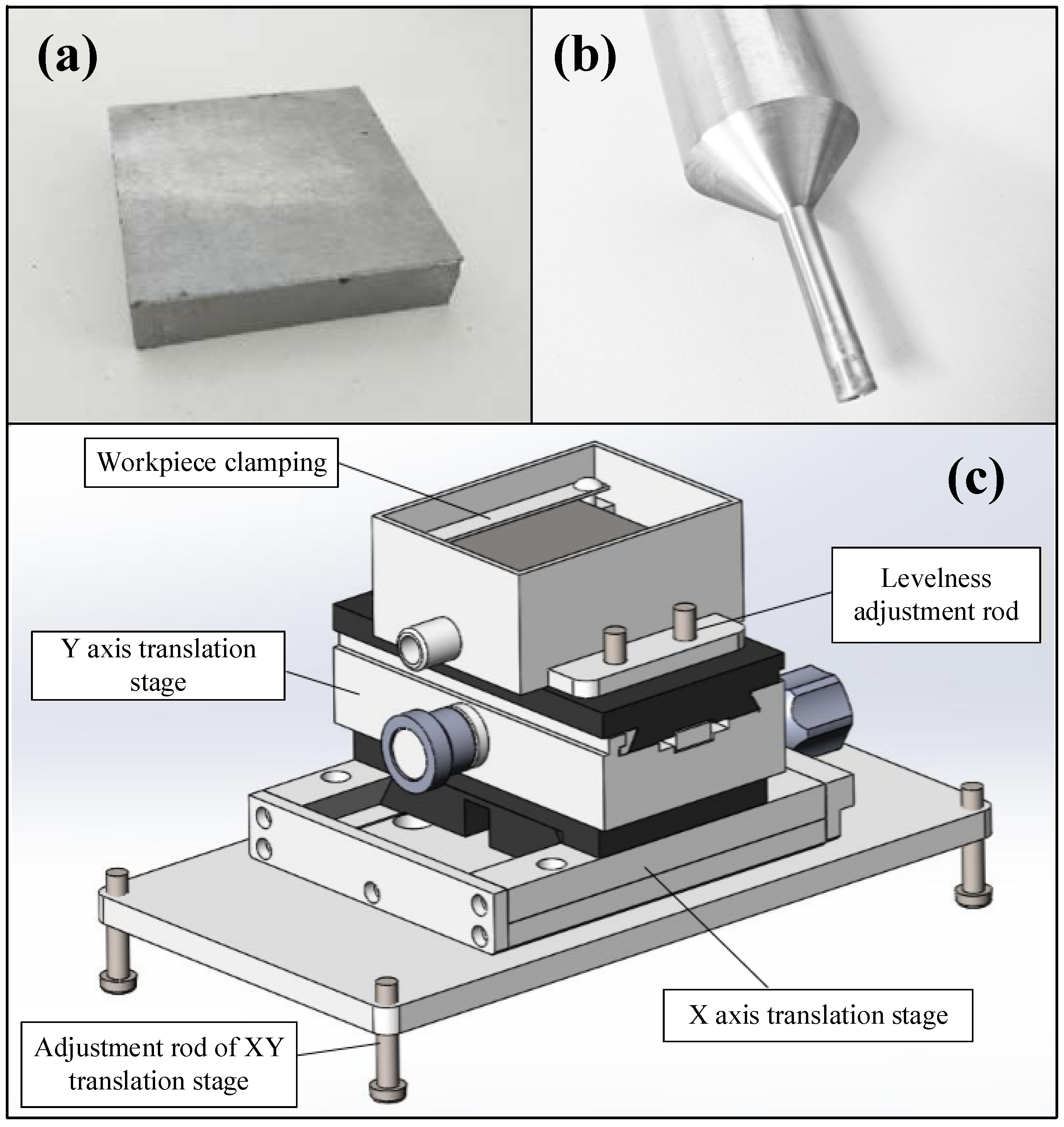
2.1. Experimental System and Materials
2.2. Machining Parameters
2.3. Experimental Processes
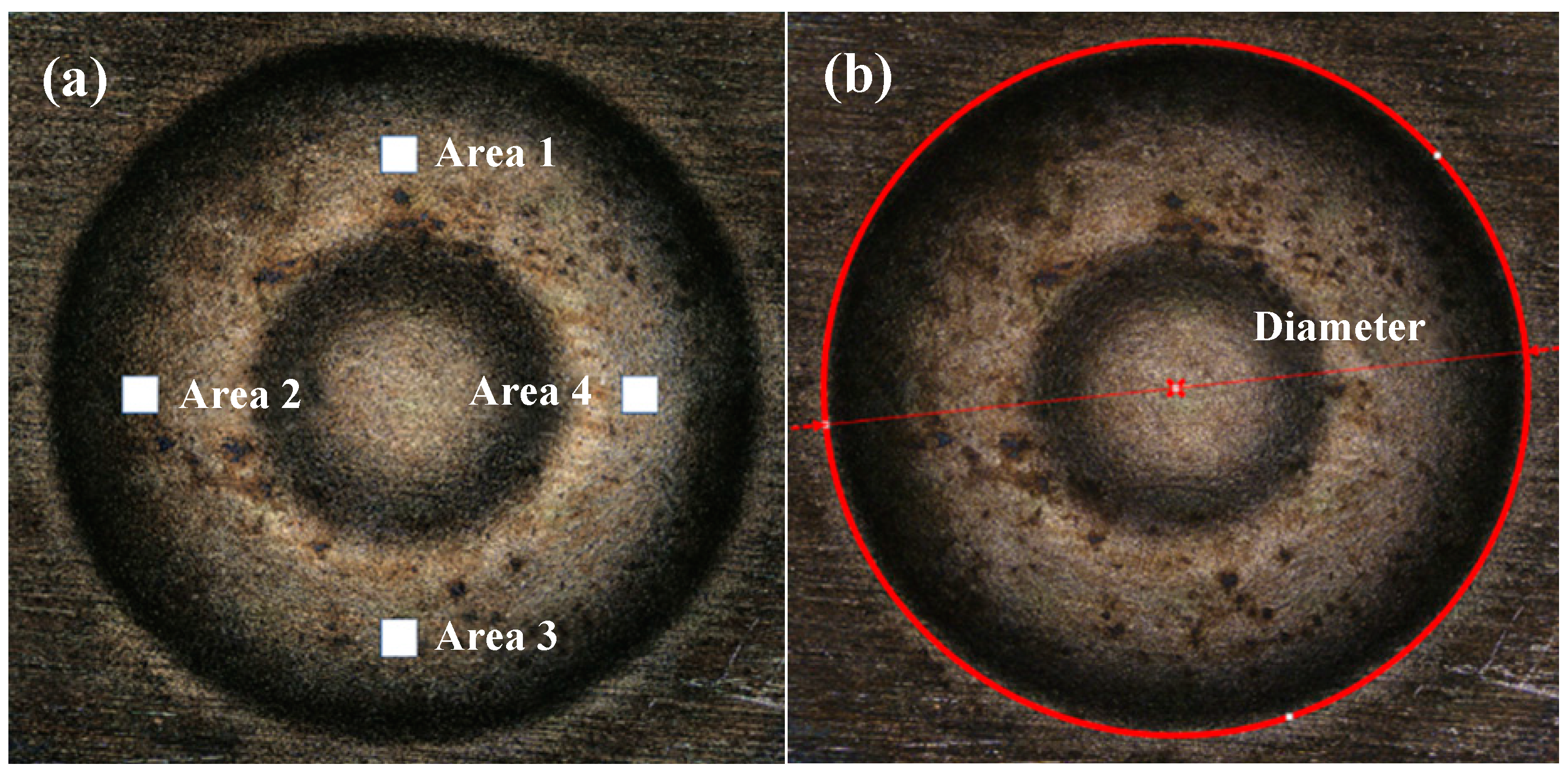
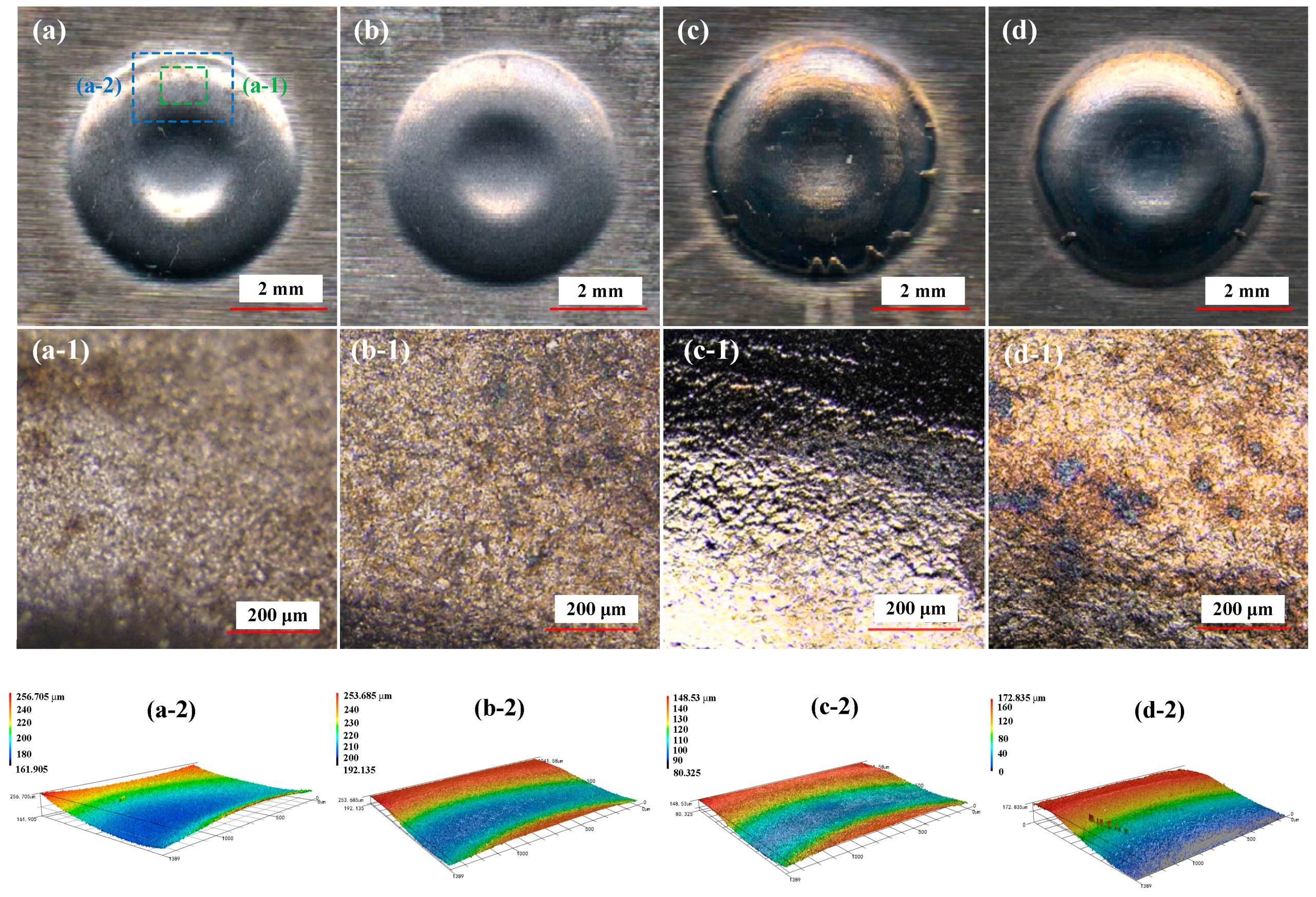
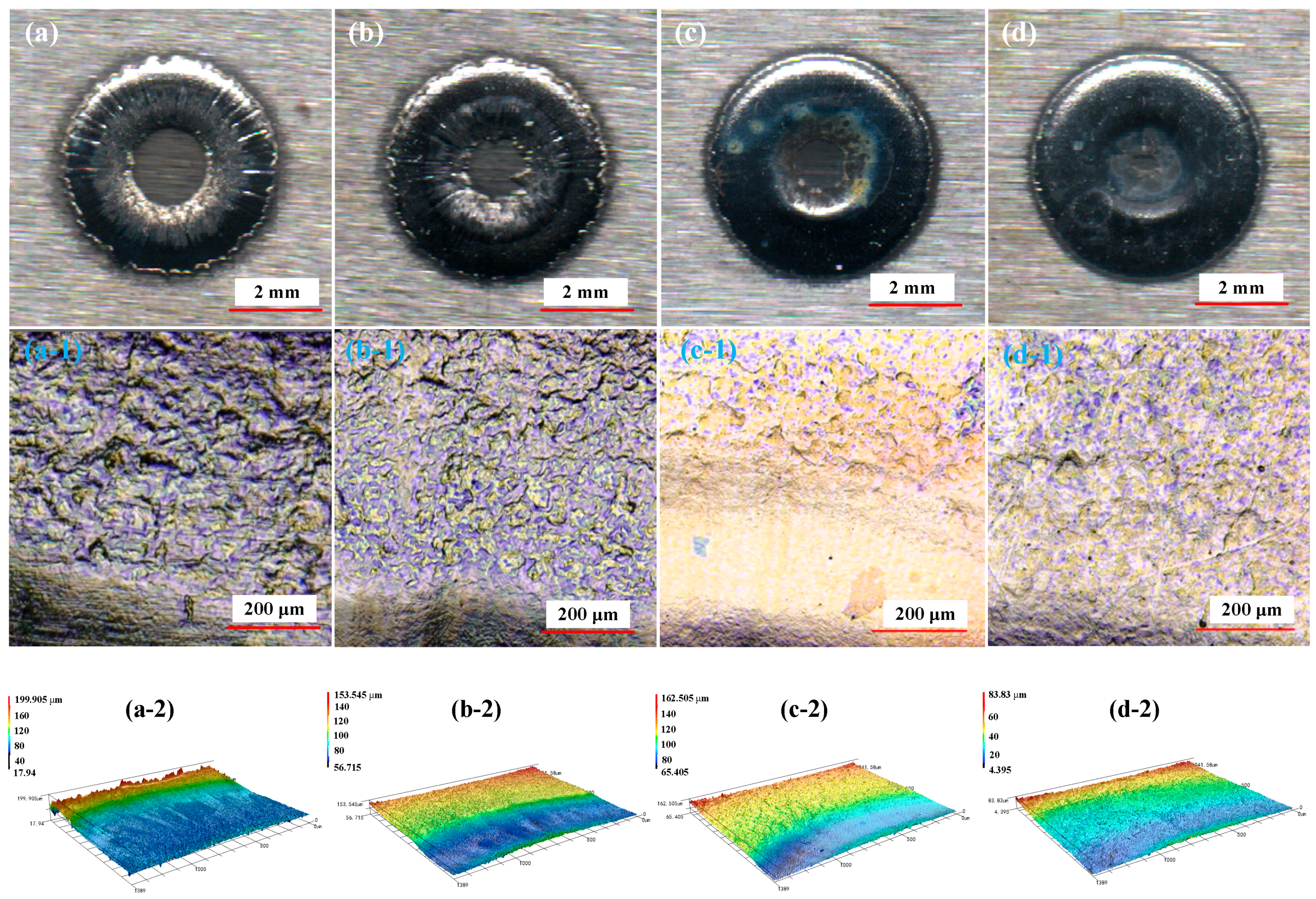
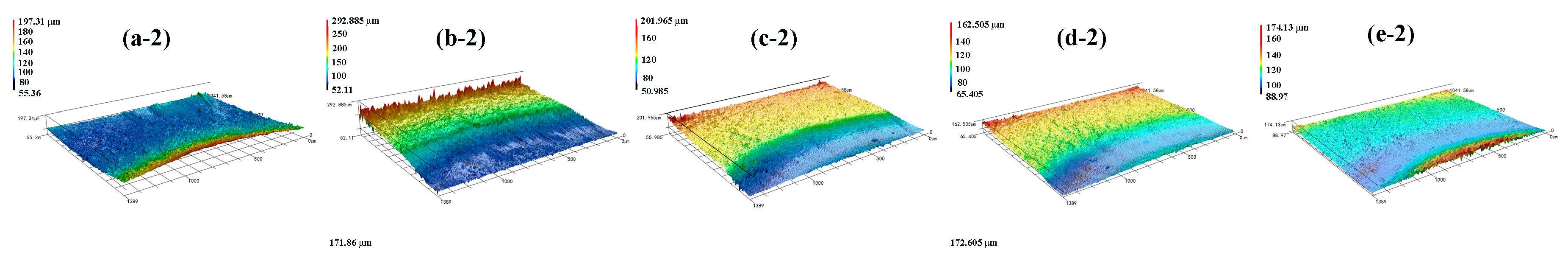
2.4. Result Characterization
3. Electrochemical Machining Experiment and Analysis in NaCl Electrolyte
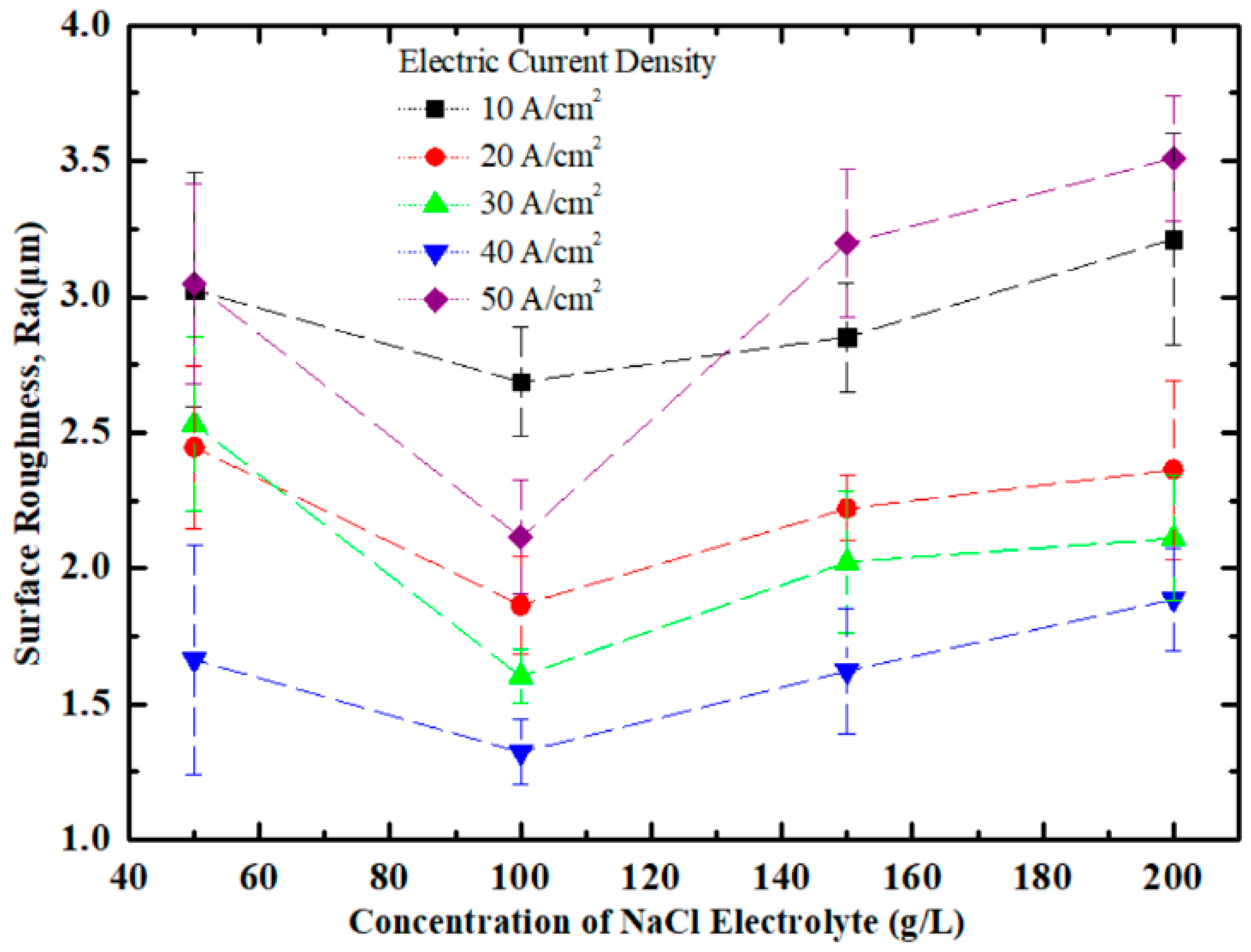
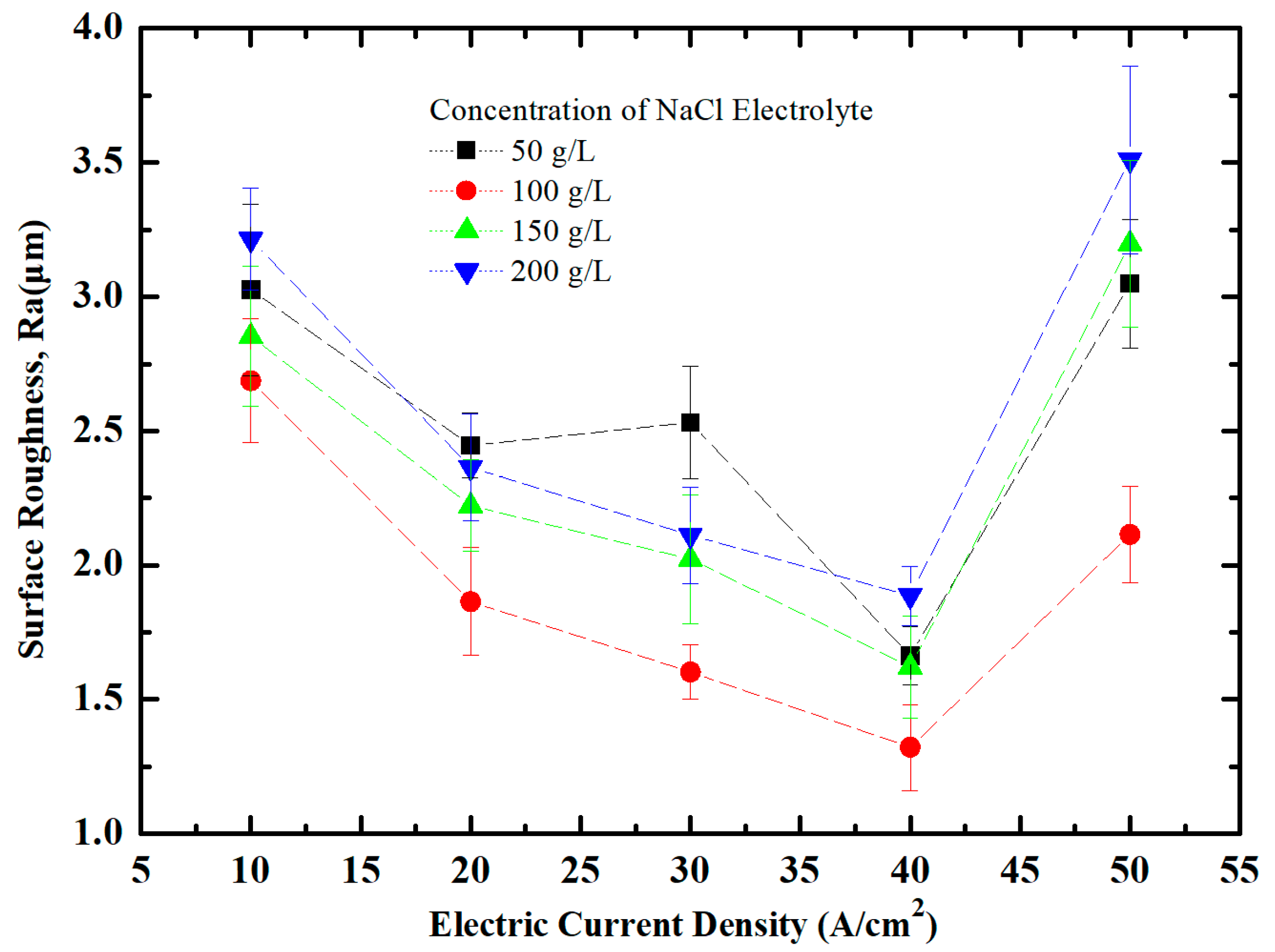
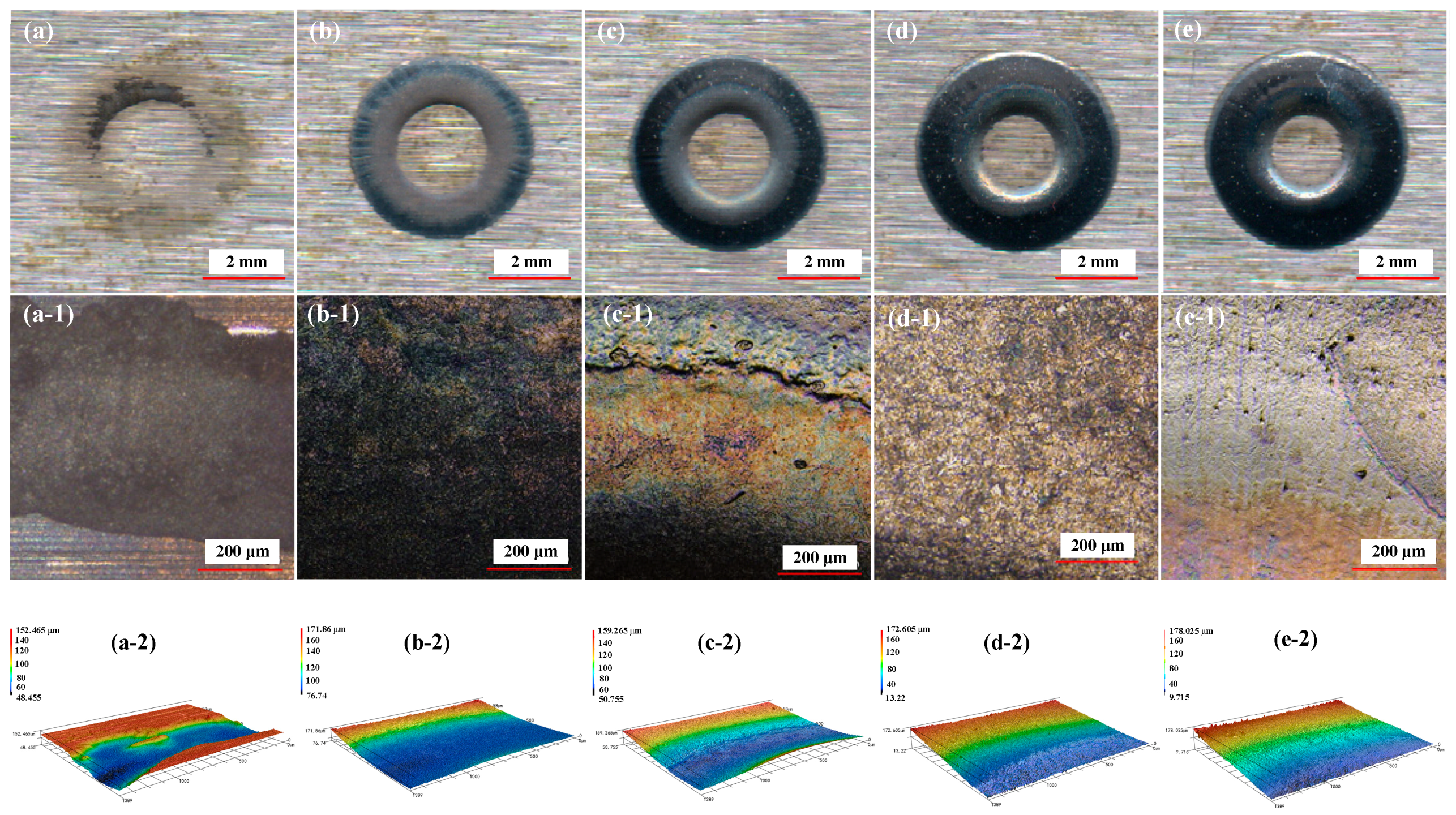
3.1. Effect of NaCl Electrolyte Concentration on Surface Quality of Workpiece
3.2. Effect of Current Density on Surface Quality of Workpiece
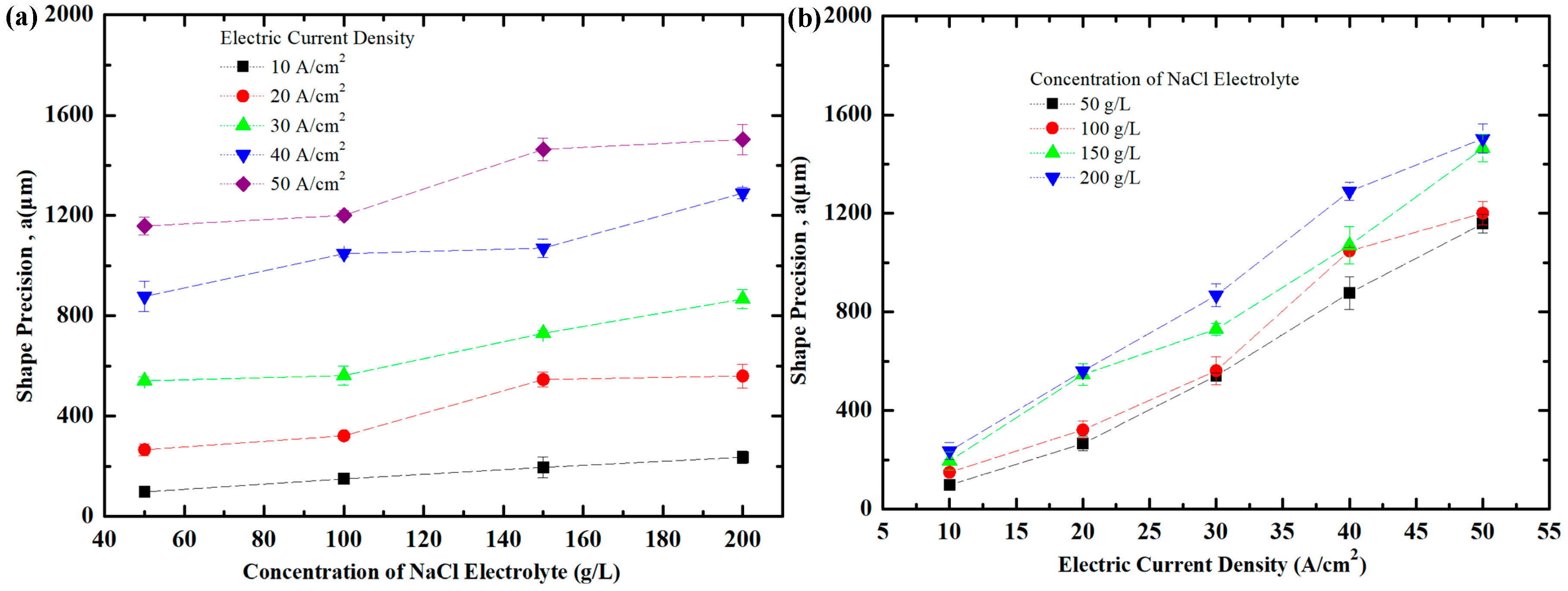
3.3. Effect of Current Density and NaCl Electrolyte Concentration on Shape Precision of Workpiece
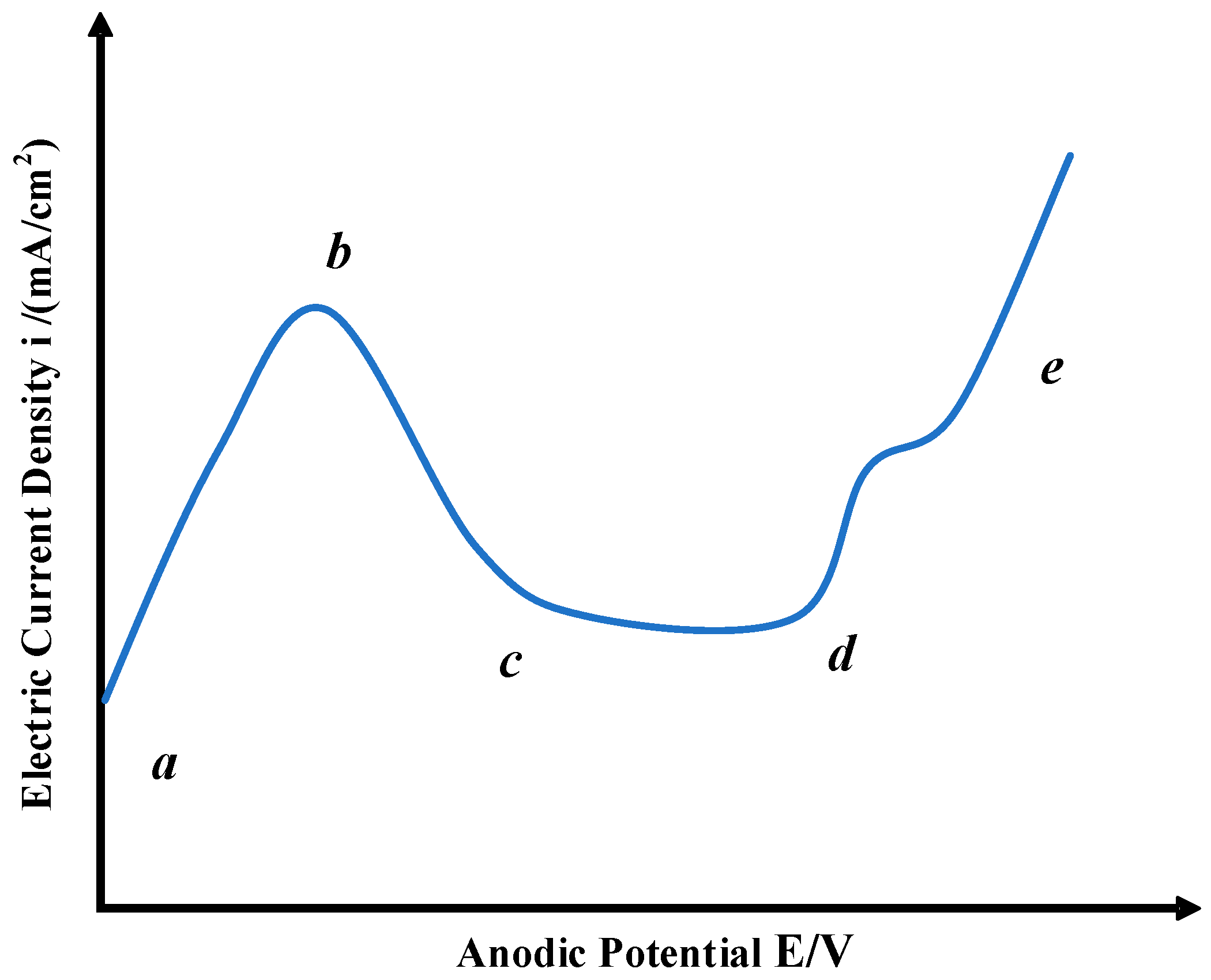
3.4. Electrolytic Reaction Principle of 35CrMo Steel in NaCl Electrolyte
4. Electrochemical Machining Experiment and Analysis of NaClO3 Electrolyte
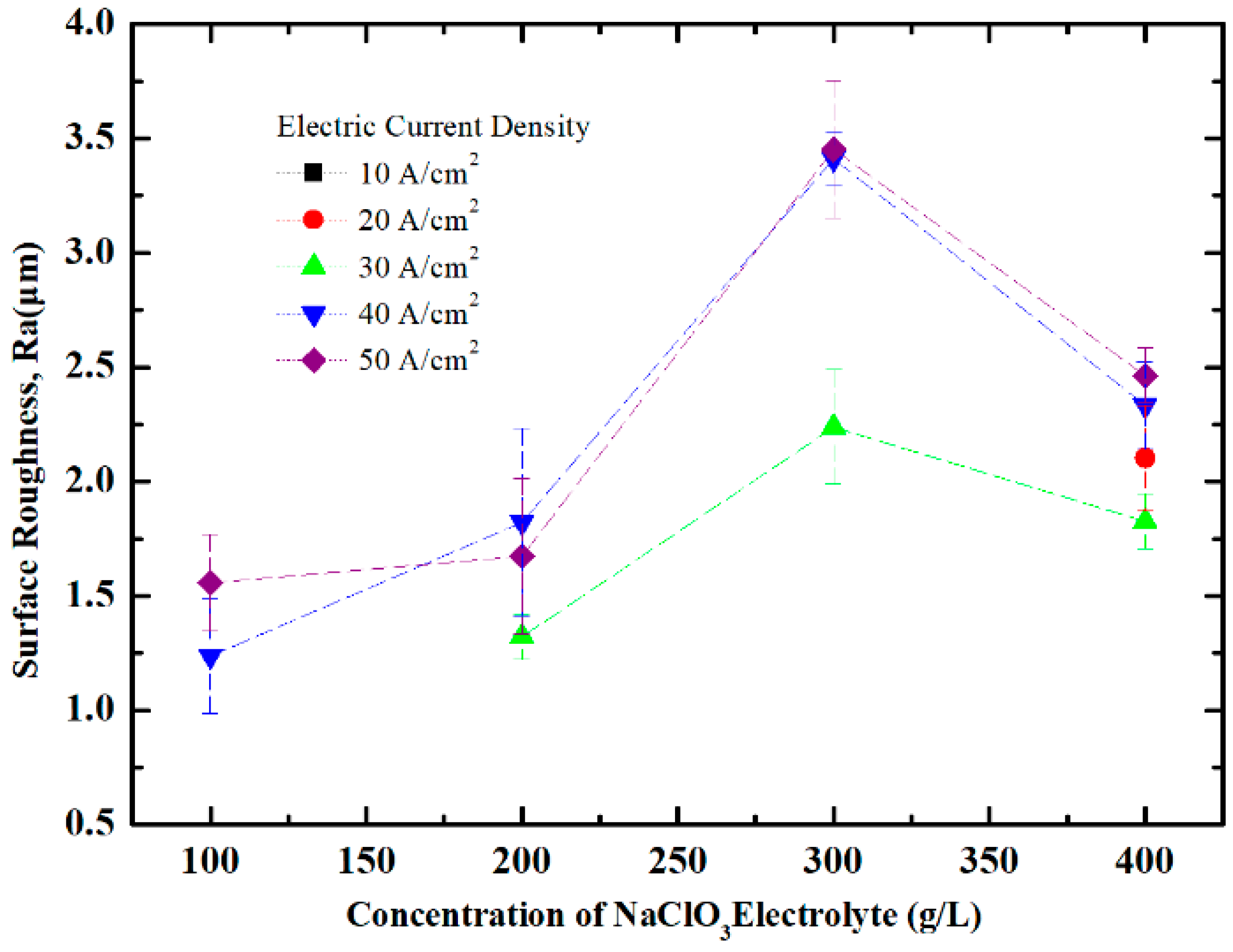
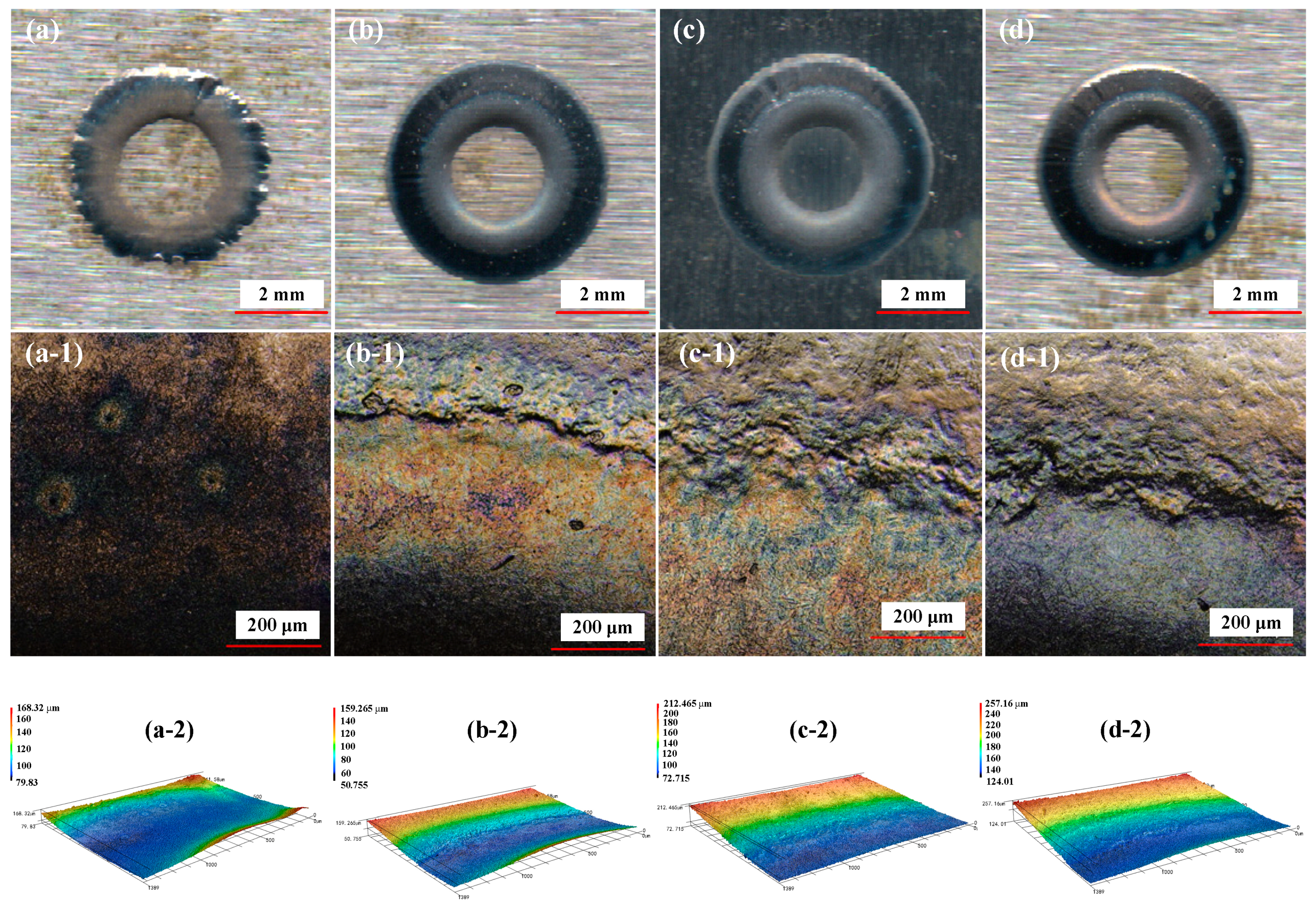
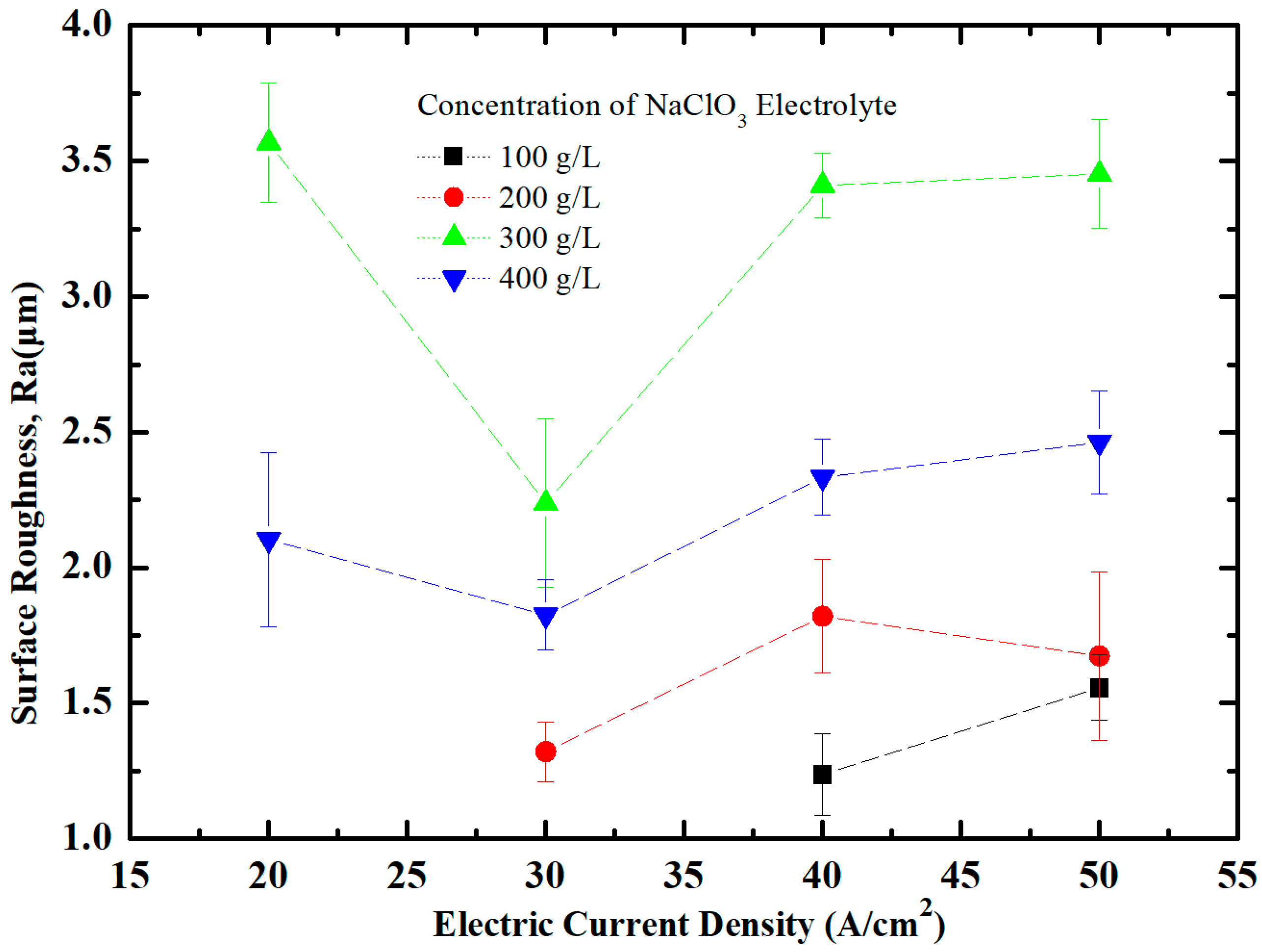
4.1. Effect of NaClO3 Electrolyte Concentration on Surface Quality of Workpiece
4.2. Effect of Current Density on Surface Quality of Workpiece
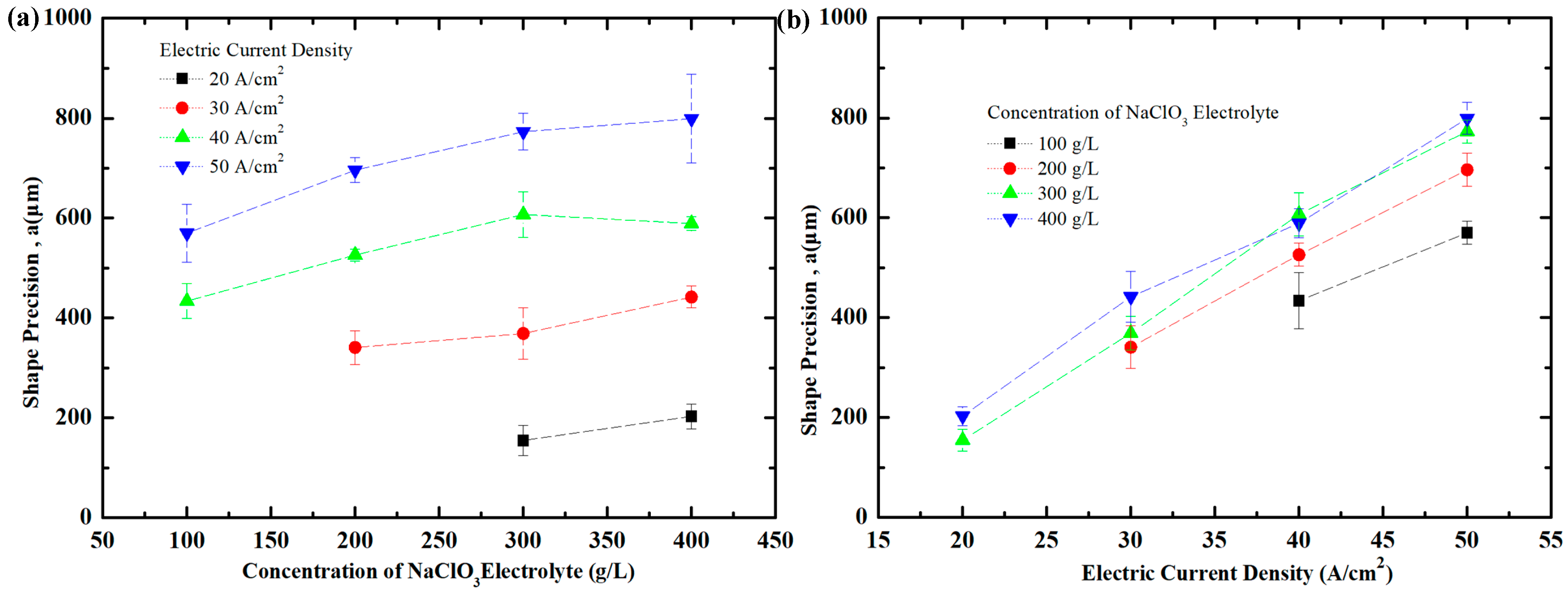
4.3. Effect of Current Density and NaClO3 Electrolyte Concentration on Shape Precision of Workpiece
4.4. Electrolytic Reaction Principle of 35CrMo Steel in NaClO3 Electrolyte
5. Electrochemical Machining Experiment and analysis Of NaNO3 Electrolyte
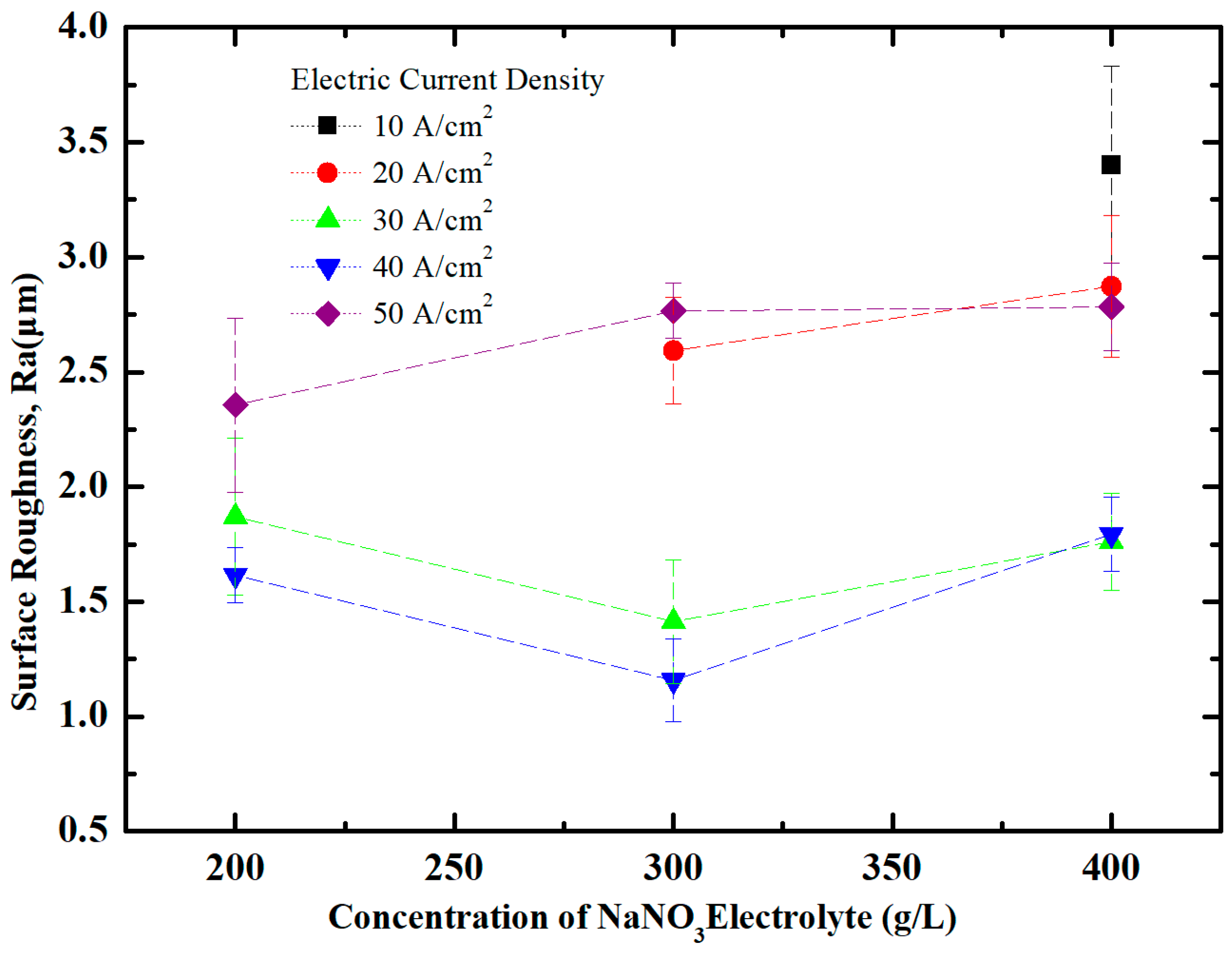
5.1. Effect of NaNO3 Electrolyte Concentration on Surface Quality of Workpiece
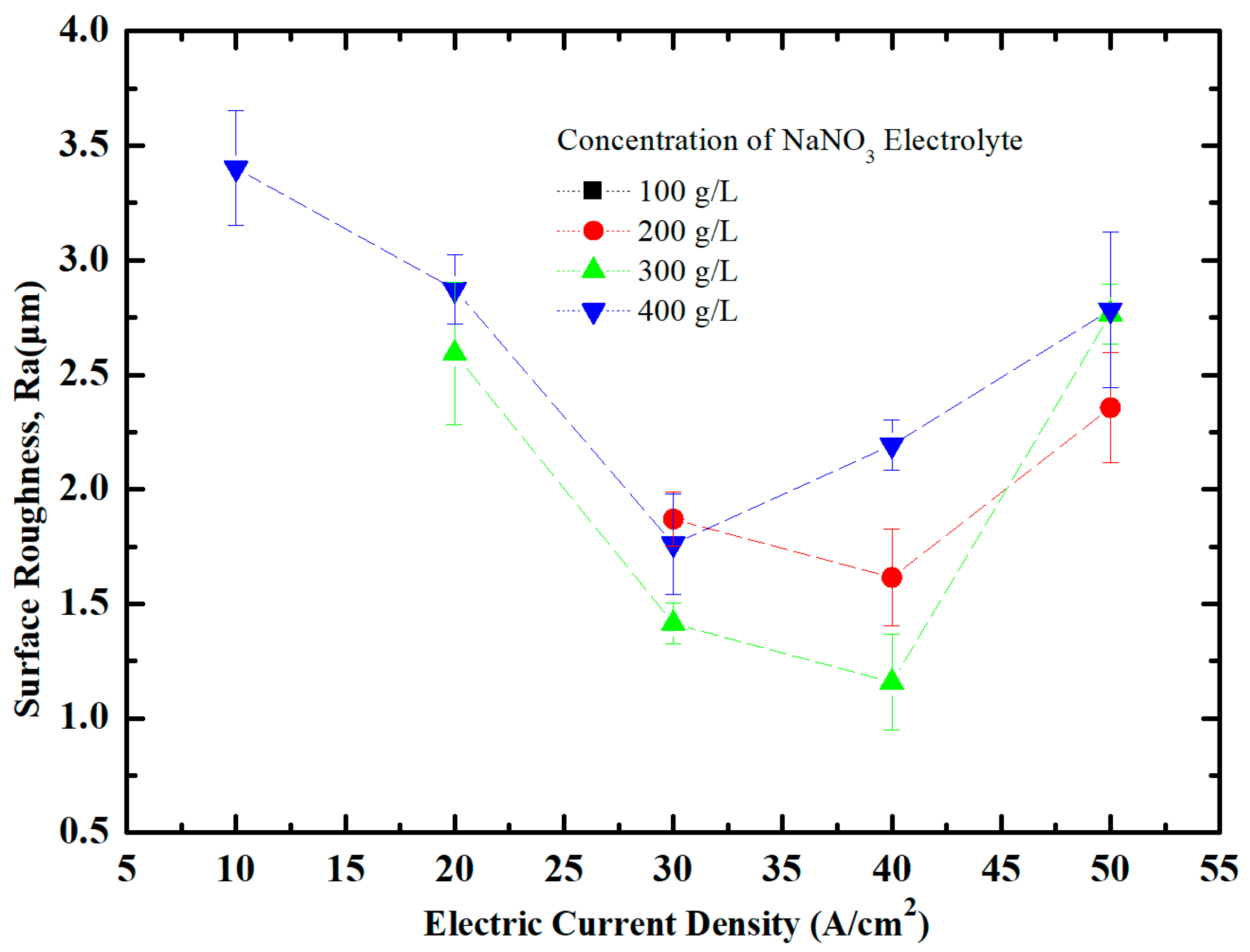
5.2. Effect of Current Density on Surface Quality of Workpiece
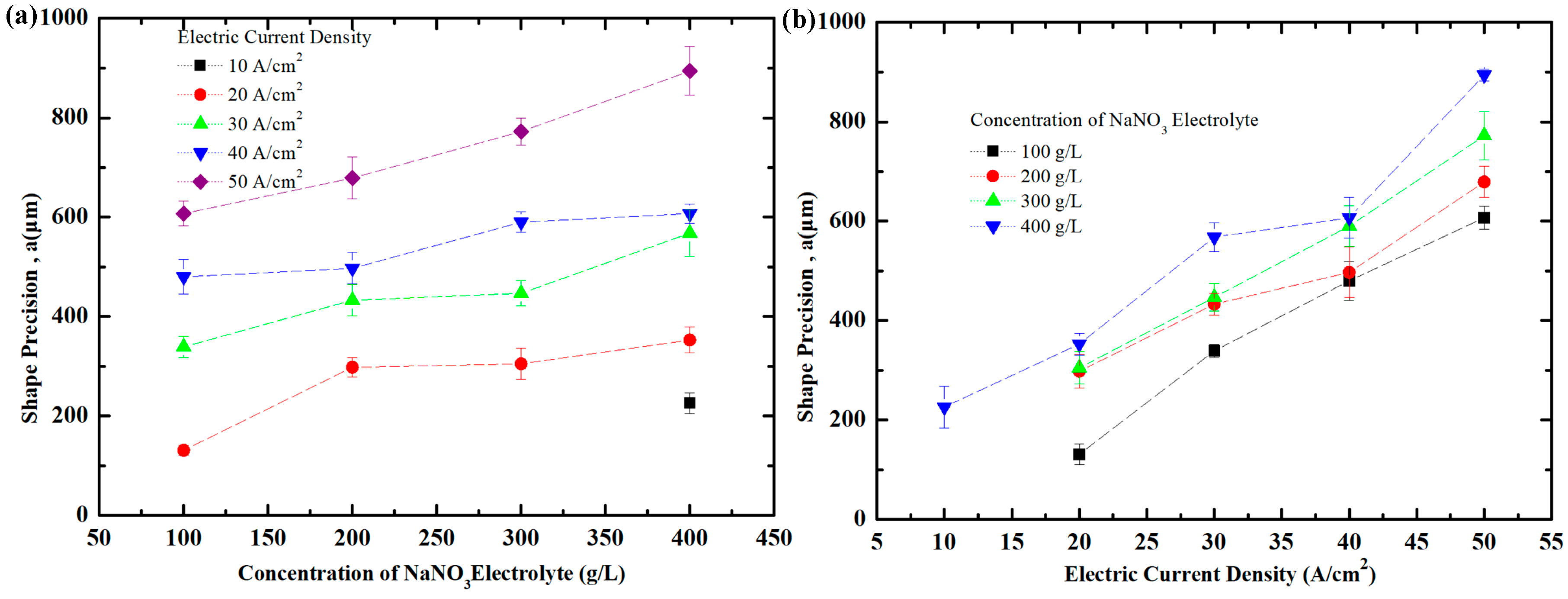
5.3. Effect of Current Density and NaNO3 Electrolyte Concentration on Shape Precision of Workpiece
5.4. Electrolytic Reaction Principle of 35CrMo Steel in NaNO3 Electrolyte
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, W.; Zhou, H.; Liu, W.; Chao, H.; Gu, Y.X. Drilling difficulties and countermeasures for deep and ultra-deep wells in Kara Jeddah block, Fergana basin. Drill. Product. Technol. 2017, 1, 18–20. [Google Scholar]
- Zheng, X.T.; Wu, K.W.; Wang, W.; Yu, J.Y.; Xu, J.M.; Ma, L.W. Low cycle fatigue and ratcheting behavior of 35CrMo structural steel at elevated temperature. Nucl. Eng. Des. 2017, 314, 285–292. [Google Scholar] [CrossRef]
- Hu, Z.; Fan, J.; Wu, S.; Dai, H.; Liu, S. Characteristics of metal magnetic memory testing of 35CrMo steel during fatigue loading. Metals 2018, 8, 119. [Google Scholar] [CrossRef]
- Liang, G.; Chen, S.H.; Zhou, Y.J.; Mao, D.H. Numerical simulation and experimental study of an ultrasonic waveguide for ultrasonic casting of 35CrMo steel. J. Iron Steel Res. Int. 2016, 23, 772–777. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, X.; Yu, J.; Lin, W.; Wang, C.; Xu, J. Time-dependent ratcheting of 35CrMo structural steel at elevated temperature considering stress rates. Mater. High Temp. 2017, 34, 172–178. [Google Scholar] [CrossRef]
- Xiao, Z.; Huang, Y.; Liu, Y. Plastic deformation behavior and processing maps of 35CrMo steel. J. Mater. Eng. Perform. 2016, 25, 1219–1227. [Google Scholar] [CrossRef]
- Xiao, Z.; Huang, Y.; Liu, H.; Wang, S. Hot tensile and fracture behavior of 35CrMo steel at elevated temperature and strain rate. Metals 2016, 6, 210. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, S.; Xiao, Z.; Liu, H. Critical condition of dynamic recrystallization in 35CrMo steel. Metals 2017, 7, 161. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Y.; Xiao, Z.; Liu, Y.; Liu, H. A modified Johnson-Cook model for hot deformation behavior of 35CrMo steel. Metals 2017, 7, 337. [Google Scholar] [CrossRef]
- Lohrengel, M.M.; Rataj, K.P.; Münninghoff, T. Electrochemical machining—Mechanisms of anodic dissolution. Electrochim. Acta 2016, 201, 348–353. [Google Scholar] [CrossRef]
- Rama Rao, S.; Padmanabhan, G. Linear modelling of the electrochemical machining process using full factorial design of experiments. J. Adv. Mech. Eng. 2013, 1, 13–23. [Google Scholar]
- Florence, T.; Dharmalingam, S.; Ragupathy, V.; Satishkumar, P. Machinablity study on electrochemical machining—A review. Int. J. ChemTech Res. 2015, 7, 2596–2600. [Google Scholar]
- Wang, G.Q.; Li, H.S.; Qu, N.S.; Zhu, D. Investigation of the hole-formation process during double-sided through-mask electrochemical machining. J. Mater. Process. Technol. 2016, 234, 95–101. [Google Scholar] [CrossRef]
- Ayyappan, S.; Sivakumar, K. Investigation of electrochemical machining characteristics of 20MnCr5 alloy steel using potassium dichromate mixed aqueous NaCl electrolyte and optimization of process parameters. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2015, 229, 1984–1996. [Google Scholar] [CrossRef]
- Ayyappan, S.; Sivakumar, K. Enhancing the performance of electrochemical machining of 20MnCr5 alloy steel and optimization of process parameters by PSO-DF optimizer. Int. J. Adv. Manuf. Technol. 2016, 82, 2053–2064. [Google Scholar] [CrossRef]
- Ayyappan, S.; Sivakumar, K. Experimental investigation on the performance improvement of electrochemical machining process using oxygen-enriched electrolyte. Int. J. Adv. Manuf. Technol. 2014, 75, 479–487. [Google Scholar] [CrossRef]
- Britto, P.A.; Lenin, N. Optimization of process parameters of ECM by RSM on AISI 202 steel. Carbon Sci. Technol. 2015, 7, 28–33. [Google Scholar]
- Kirchner, V.; Cagnon, L.; Schuster, R.; Ertl, G. Electrochemical machining of stainless steel microelements with ultrashort voltage pulses. Appl. Phys. Lett. 2001, 79, 1721–1723. [Google Scholar] [CrossRef]
- Das, M.K.; Kumar, K.; Barman, T.K.; Sahoo, P. Optimization of surface roughness and MRR in electrochemical machining of EN31 tool steel using grey-Taguchi approach. Procedia Mater. Sci. 2014, 6, 729–740. [Google Scholar] [CrossRef]
- Klocke, F.; Harst, S.; Zeis, M.; Klink, A. Modeling and simulation of the microstructure evolution of 42CrMo4 steel during electrochemical machining. Procedia CIRP 2018, 68, 505–510. [Google Scholar] [CrossRef]
- Singh, R.; Gandotra, N.; Solaiyappan, A. Study of different work materials effect on surface roughness in electrochemical machining process. Int. J. Res. Eng. Technol. 2015, 4, 505–513. [Google Scholar]
- Asokan, P.; Ravikumar, R.; Jeyapaul, R.; Santhi, M. Development of multi-objective optimization models for electrochemical machining process. Int. J. Adv. Manuf. Technol. 2008, 39, 55–63. [Google Scholar] [CrossRef]
- Senthilkumar, C.; Ganesan, G.; Karthikeyan, R. Study of electrochemical machining characteristics of Al/SiCp composites. Int. J. Adv. Manuf. Technol. 2009, 43, 256–263. [Google Scholar] [CrossRef]
- Rao, S.R.; Padmanabhan, G. Effect of process variables on metal removal rate in electrochemical machining of Al-B4C composites. Arch. Appl. Sci. Res. 2012, 4, 1844–1849. [Google Scholar]
- Kalaimathi, M.; Venkatachalam, G.; Makhijani, N.; Agrawal, A.; Sivakumar, M. Investigations on machining of Monel 400 alloys using electrochemical machining with sodium nitrate as electrolyte. Appl. Mech. Mater. 2014, 592–594, 467–472. [Google Scholar] [CrossRef]
- Kalaimathi, M.; Venkatachalam, G.; Sivakumar, M. Experimental investigations on the electrochemical machining characteristics of Monel 400 alloys and optimization of process parameters. Jordan J. Mech. Ind. Eng. 2014, 8, 143–151. [Google Scholar]
- Martin, A.; Eckart, C.; Lehnert, N.; Hackert-Oschätzchen, M.; Schubert, A. Generation of defined surface waviness on tungsten carbide by jet electrochemical machining with pulsed current. Procedia CIRP 2016, 45, 231–234. [Google Scholar] [CrossRef]
- Kapil, G. Advanced Manufacturing Technologies: Modern Machining, Advanced Joining, Sustainable Manufacturing (Materials Forming, Machining and Tribology), 1st ed.; Springer: Berlin, Germany, 2017. [Google Scholar]
- Zhang, C.; Zhang, Y.; Chen, X.; Li, W.; Liu, G. Investigation of the electrochemical dissolution behavior of tungsten during electrochemical machining. Int. J. Adv. Manuf. Technol. 2018, 49, 1–8. [Google Scholar] [CrossRef]
- Sekar, T. Experimental Investigation on Electrochemical Machining; LAP LAMBERT Academic Publishing: Washington, DC, USA, 2017. [Google Scholar]


| Element | C | Si | Mn | Cr | Mo | Ni | Cu | P/S |
|---|---|---|---|---|---|---|---|---|
| Composition | 0.32~0.40 | 0.17~0.37 | 0.40~0.70 | 0.80~1.10 | 0.15~0.25 | ≤0.30 | ≤0.30 | ≤0.035 |
| Element | |||||
| Potential | −0.740 | −0.440 | −0.220 | +0.401 | +1.36 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Liu, S.; Yue, W.; Xiao, H.; Zhang, P. Experimental Study of Surface Characteristic in Electrochemical Machining of 35CrMo Steel. Metals 2018, 8, 509. https://doi.org/10.3390/met8070509
Zhang H, Liu S, Yue W, Xiao H, Zhang P. Experimental Study of Surface Characteristic in Electrochemical Machining of 35CrMo Steel. Metals. 2018; 8(7):509. https://doi.org/10.3390/met8070509
Chicago/Turabian StyleZhang, Hang, Shuhai Liu, Weichao Yue, Huaping Xiao, and Peng Zhang. 2018. "Experimental Study of Surface Characteristic in Electrochemical Machining of 35CrMo Steel" Metals 8, no. 7: 509. https://doi.org/10.3390/met8070509
APA StyleZhang, H., Liu, S., Yue, W., Xiao, H., & Zhang, P. (2018). Experimental Study of Surface Characteristic in Electrochemical Machining of 35CrMo Steel. Metals, 8(7), 509. https://doi.org/10.3390/met8070509






