Synthesis and Electrochemical Properties of Ti-Si Alloys Prepared by Mechanical Alloying and Heat Treatment
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
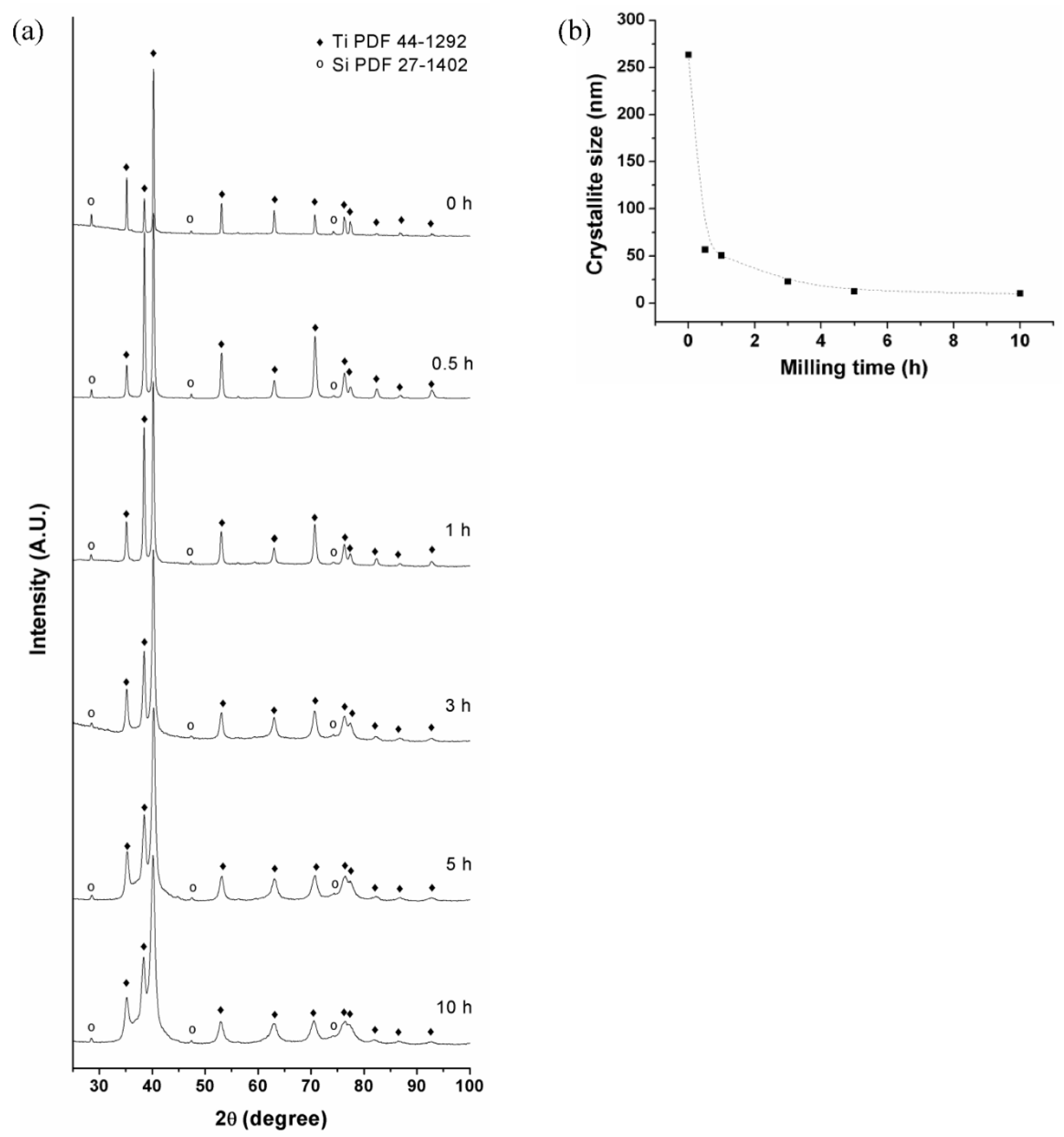
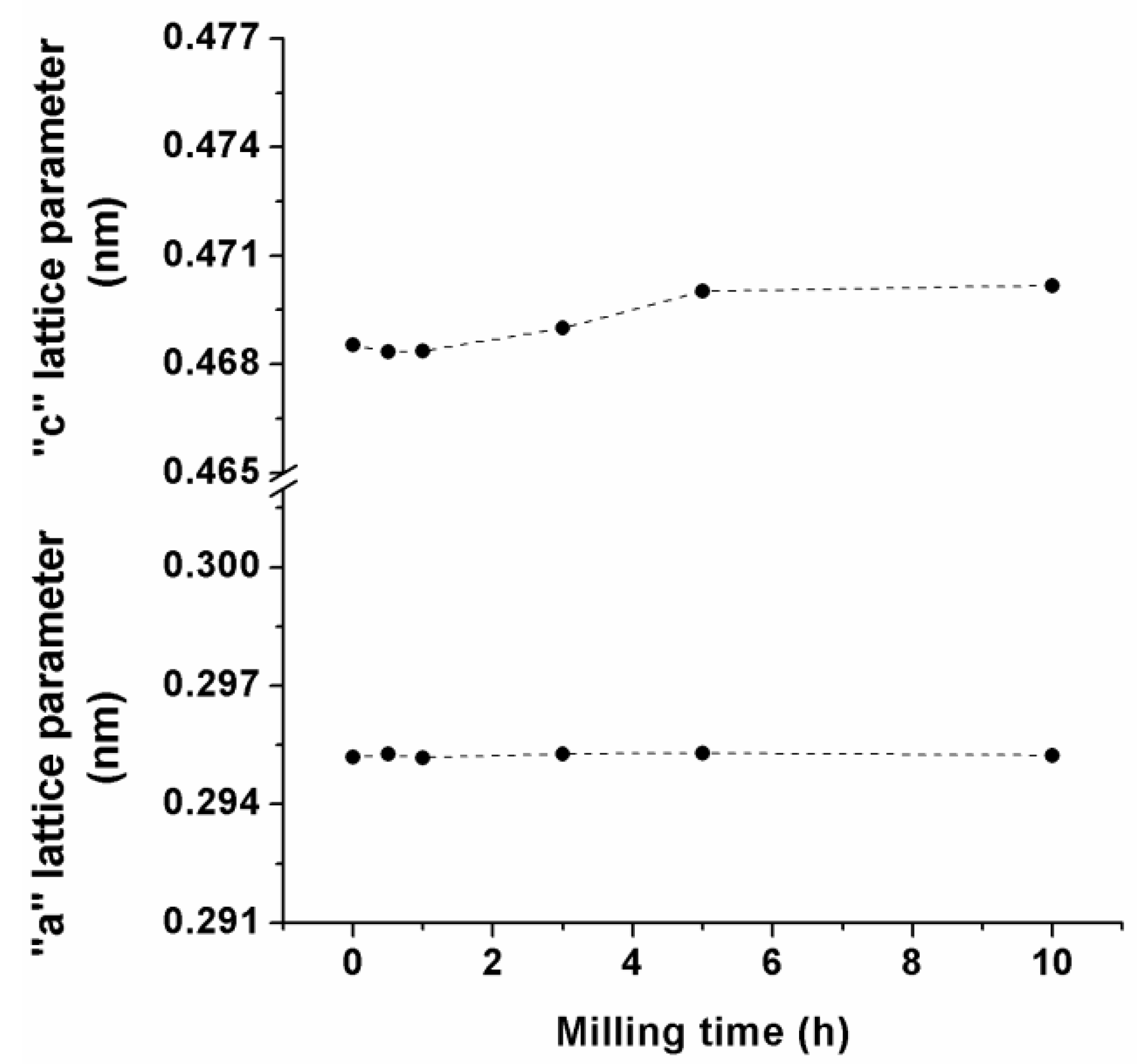
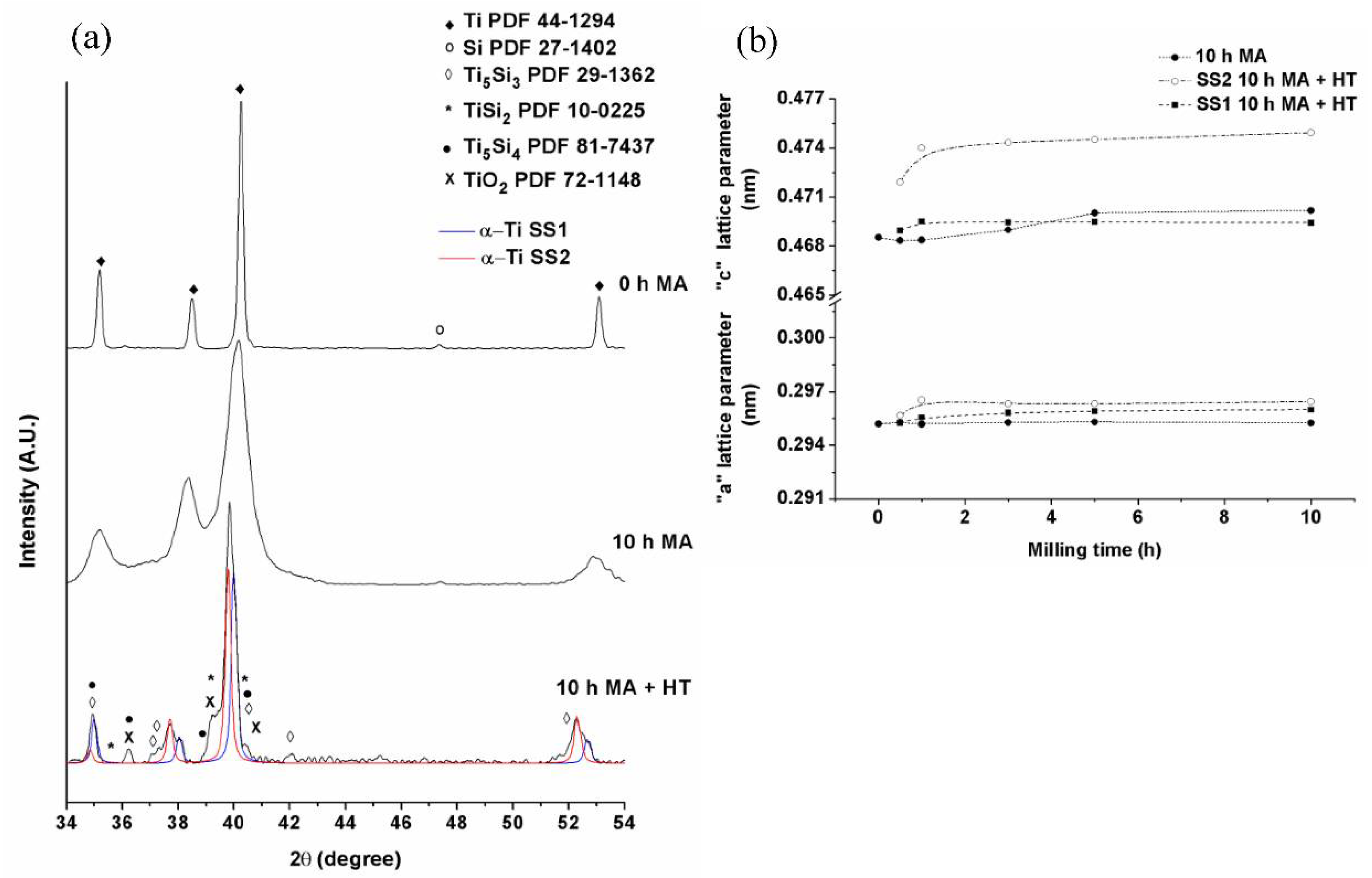
3.1. Mechanical Alloying
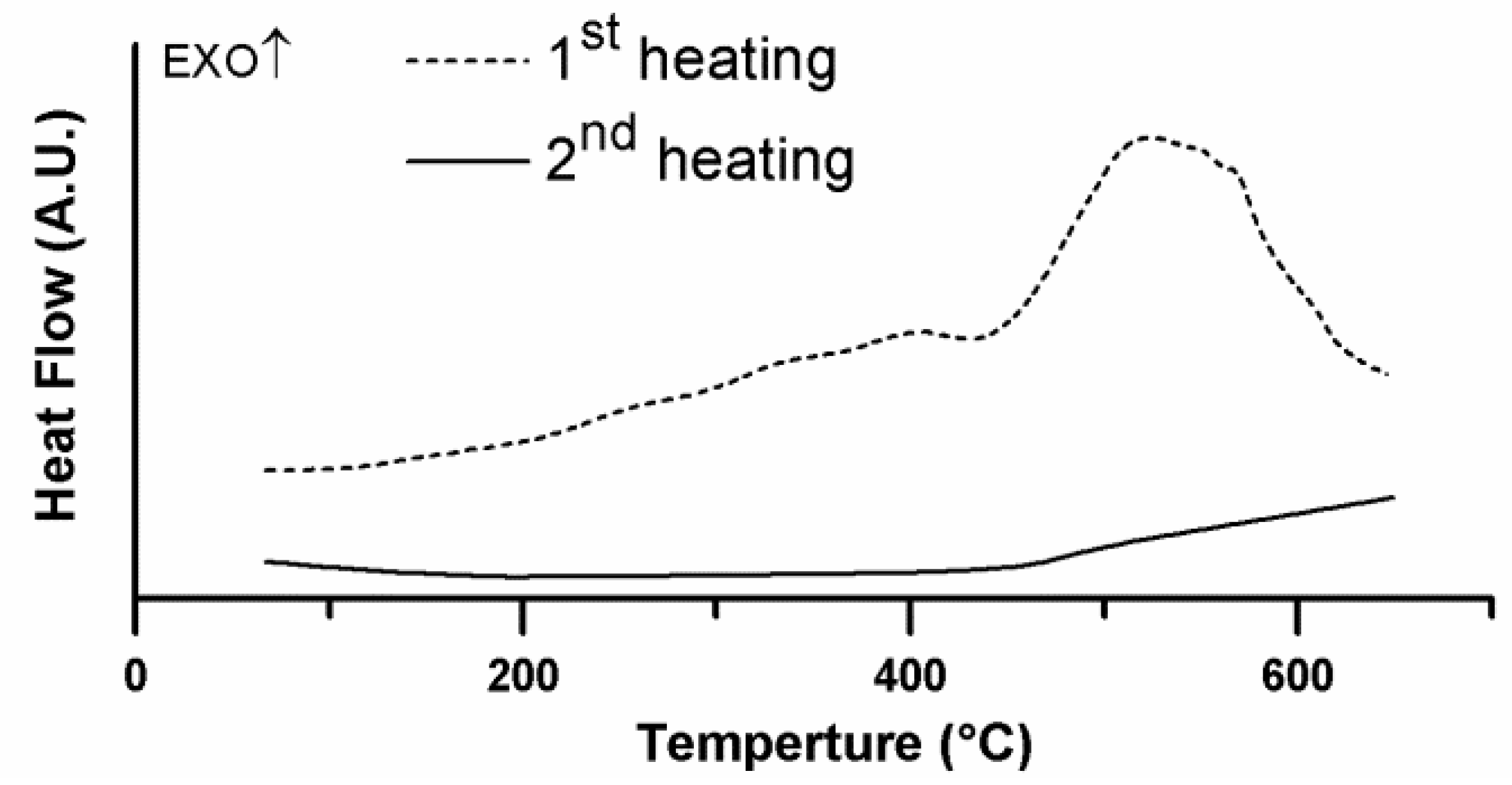
3.2. Thermal Stability
3.3. Open Circuit Potential Measurements
3.4. Linear Voltammetry Measurements
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Felder, A.; Prengaman, D. Lead alloys for permanent anodes in the nonferrous metals industry. JOM J. Miner. Met. Mater. Soc. 2006, 58, 28–31. [Google Scholar] [CrossRef]
- Camurri, C.; Araneda, E.; Pagliero, A.; Dile, J. Optimal operational conditions during production of lead calcium-tin anodes for improve their mechanical properties. Mater. Sci. Forum 2005, 475–479, 2631–2634. [Google Scholar] [CrossRef]
- Beer, H. Electrode and Coating Therefor. U.S. Patent 3,632,498 A, 4 January 1972. [Google Scholar]
- Lodi, G.; Sivieri, E.; de Battisti, A.; Trasatti, S. Ruthenium dioxide-based film electrodes-effect of chemical composition and surface morphology on oxygen evolution in acid solutions. J. Appl. Electrochem. 1978, 8, 135–143. [Google Scholar] [CrossRef]
- Otogawa, R.; Morimitsu, M.; Matsunaga, M. Effects of microstructure of IrO2-based anodes on electrocatalytic properties. Electrochim. Acta 1998, 44, 1509–1513. [Google Scholar] [CrossRef]
- Vazquez-Gomez, L.; Ferro, S.; De Battisti, A. Preparation and characterization of RuO ternary mixtures for advanced electrochemical technology. Appl. Catal. B 2006, 67, 34–40. [Google Scholar] [CrossRef]
- Comninellis, C.; Vercesi, G.P. Characterization of DSA®-type oxygen evolving electrodes: Choice of a coating. J. Appl. Electrochem. 1991, 21, 335–345. [Google Scholar] [CrossRef]
- Rolewicz, J.; Comninellis, C.; Hinden, J. Charactérisation des électrodes de type DSA pour le dégagement de O2-I. L’électrode Ti/IrO2-Ta2O5. Electrochim. Acta 1988, 33, 573–580. [Google Scholar] [CrossRef]
- Bagri, P. Evaluation of Coated Titanium Anodes. Master’s Thesis, Utah University, Salt Lake City, UT, USA, 2012. [Google Scholar]
- Moats, M. Will lead-based anodes ever be replaced in aqueous electrowinning? JOM J. Miner. Met. Mater. Soc. 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Hu, J.M.; Meng, H.M.; Zhang, J.Q.; Cao, C.N. Degradation mechanism of long service life Ti/IrO2-Ta2O5 oxide anodes in sulphuric acid. Corros. Sci. 2002, 44, 1655–1668. [Google Scholar] [CrossRef]
- Jiang, Z.; Dai, X.; Middeton, H. Effect of silicon on corrosion resistance of Ti–Si alloys. Mater. Sci. Eng. B Adv. 2011, 176, 79–86. [Google Scholar] [CrossRef]
- ASM International. Handbook Committee, ASM Handbook Volume 3: Alloy Phase Diagrams; ASM International: Novelty, OH, USA, 1992; p. 1741. ISBN 0-87170-381-5. [Google Scholar]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Benjamin, J.S. Dispersion strengthened superalloys by mechanical alloying. Metall. Trans. 1970, 1, 2943–2951. [Google Scholar] [CrossRef]
- Guzmán, D.; Ordoñez, S.; Serafini, D.; Rojas, P.; Bustos, O. Effect of the milling energy on the production and thermal stability of amorphous Mg50Ni50. J. Alloy. Compd. 2009, 471, 435–441. [Google Scholar] [CrossRef]
- Lee, P.; Cheng, Y.; Chen, J.; Hu, C. Formation and corrosion behavior of mechanically-alloyed Cu–Zr–Ti bulk metallic glasses. Metals 2017, 7, 148. [Google Scholar] [CrossRef]
- Guzmán, D.; Rivera, O.; Aguilar, C.; Ordoñez, S.; Martínez, C.; Serafini, D.; Rojas, P. Mechanical alloying and subsequent heat treatment of Ag–Zn powders. Trans. Nonferr. Met. Soc. 2013, 23, 2071–2078. [Google Scholar] [CrossRef]
- Dayani, D.; Shokuhfar, A.; Vaezi, M.; Rezaei, S.; Hosseinpour, S. Structural and mechanical evaluation of a nanocrystalline Al–5 wt %Si alloy produced by mechanical alloying. Metals 2017, 7, 232. [Google Scholar] [CrossRef]
- Liu, E.; Jia, J.; Bai, Y.; Wang, W.; Gao, Y. Study on preparation and mechanical property of nanocrystalline NiAl intermetallic. Mater. Des. 2014, 53, 596–601. [Google Scholar] [CrossRef]
- Nobuki, T.; Moriya, T.; Hatate, M.; Crivello, J.; Cuevas, F.; Joubert, J. Synthesis of TiFe hydrogen absorbing alloys prepared by mechanical alloying and SPS treatment. Metals 2018, 8, 264. [Google Scholar] [CrossRef]
- Aguilar, C.; Castro, F.; Martínez, V.; Guzmán, D.; de las Cuevas, F.; Lozada, L.; Vielma, N. Structural study of nanocrystalline solid solution of Cu–Mo obtained by mechanical alloying. Mater. Sci. Eng. A Struct. 2012, 548, 189–194. [Google Scholar] [CrossRef]
- Ahn, A.H.; Chung, H.S.; Watanabe, R.; Park, Y.H. Microstrutural refinemet amorphization in Ti-Al, Ti-Si and Si-W system by mechanical alloying. Mater. Sci. Forum. 1992, 88–90, 347–354. [Google Scholar] [CrossRef]
- Oleszak, D.; Burzynska-Szyszko, M.; Matyja, H. Structural changes during mechanical alloying of elemental Al-Ti, Al-Nb and Ti-Si powders. J. Mater. Sci. Lett. 1993, 12, 3–5. [Google Scholar] [CrossRef]
- Oehring, M.; Bormann, R. Nanocrystalline alloys prepared by mechanical allotting and ball milling. Mater. Sci. Eng. A Struct. 1991, 134, 1330–1333. [Google Scholar] [CrossRef]
- Park, Y.; Hashimoto, H.; Wtanabe, R. Phase evolution and formation process of compound during ball milling of Ti-Si powder mixtures. Mater. Sci. Eng. A Struct. 1994, 181–182, 1212–1216. [Google Scholar] [CrossRef]
- Yang, J.; Wu, J.; Hua, W. Study on mechanical alloying and subsequent heat treatment of the Ti–Si system. Phys. B Condens. Matter 2000, 279, 241–245. [Google Scholar] [CrossRef]
- Gu, Y.W.; Goi, L.S.; Jarfors, A.E.W.; Butler, D.L.; Lim, C.S. Structural evolution in Ti–Si alloy synthesized by mechanical alloying. Phys. B Condens. Matter 2004, 32, 299–304. [Google Scholar] [CrossRef]
- Rietveld, H. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Lutterotti, L. MAUD—Materials Analysis Using Diffraction. Available online: http://maud.radiographema.eu/ (accessed on 16 April 2018).
- Chaubey, A.; Scudino, S.; Khoshkhoo, M.; Prashanth, K.; Mukhopadhyay, N.; Mishra, B.; Eckert, J. Synthesis and Characterization of Nanocrystalline Mg-7.4%Al Powders Produced by Mechanical Alloying. Metals 2013, 3, 58–68. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling; Marcel Dekker: New York, NY, USA, 2004; ISBN 0-8247-4103-X. [Google Scholar]
- Lee, P.; Yang, J.; Lin, H. Amorphization behaviour in mechanically alloyed Ni—Ta powders. J. Mater. Sci. 1998, 33, 235–239. [Google Scholar] [CrossRef]
- Ecker, J.; Holzer, J.C.; Krill, C.E.; Johnson, W.L. Structural and thermodynamic properties of nanocrystalline FCC metals prepared by mechanical attrition. J. Mater. Res. 1992, 7, 1751–1761. [Google Scholar] [CrossRef]
- Ungár, T. Microstructural parameters from X-ray diffraction peak broadening. Scr. Mater. 2004, 51, 777–781. [Google Scholar] [CrossRef]
- Froes, F.H. Titanium: Physical Metallurgy, Processing and Applications; ASM International: Novelty, OH, USA, 2015; pp. 98–99. ISBN 978-1-62708-079-8. [Google Scholar]
- Simổes, F.; Trindade, B.; Santos, J.A.; Froes, F.H. Ti-Al and Ti-Si Intermetallics Powders Coated with Aluminium. Mater. Technol. Adv. Perform. Mater. 2003, 18, 98–101. [Google Scholar] [CrossRef]
- Andersson, S.; Collen, B.; Kuyenstierna, U. Phase Analysis Studies on the Titanium–Oxygen System. Acta Chem. Scand. 1957, 11, 1641–1652. [Google Scholar] [CrossRef]
- Montanari, R.; Costanza, G.; Tata, M.E.; Testani, C. Lattice expansion of Ti-6Al-4V by nitrogen and oxygen absorption. Mater. Charact. 2008, 59, 334–337. [Google Scholar] [CrossRef]
- Blanter, M.S.; Granovskiy, E.B.; Magalas, L.B. Interaction of dissolved atoms and relaxation due to interstitial atoms in hcp metals. Mater. Sci. Eng. A Struct. 2004, 370, 88–92. [Google Scholar] [CrossRef]
- Contieri, R.; Zanotello, M.; Caram, R. Recrystallization and grain growth in highly cold worked CP-Titanium. Mater. Sci. Eng. A Struct. 2010, 527, 3994–4000. [Google Scholar] [CrossRef]
- Ramos, A.S.; Nunes, C.A.; Coelho, G.C. On the peritectoid Ti3Si formation in Ti–Si alloys. Mater. Charact. 2006, 56, 107–111. [Google Scholar] [CrossRef]
- Hsu, H.C.; Wu, S.C.; Hsu, S.K.; Li, Y.C.; Ho, W.F. Structure and mechanical properties of as-cast Ti-Si alloys. Intermetallics 2014, 47, 11–16. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed.; NACE International: Houston, TX, USA, 1974; p. 219. ISBN 0915567989. [Google Scholar]
- Muñoz-Portero, M.J.; García-Antón, J.; Guiñón, J.L.; Leiva-García, R. Pourbaix diagrams for titanium in concentrated aqueous lithium bromide solutions at 25 °C. Corros. Sci. 2011, 53, 1440–1450. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.; Lim, S. Microstructure and elastic modulus of Ti–Nb–Si ternary alloys for biomedical applications. Scr. Mater. 2006, 54, 887–891. [Google Scholar] [CrossRef]
- Hsu, H.; Wu, S.; Hsu, S.; Liao, Y.; Ho, W. Effect of different post-treatments on the bioactivity of alkali-treated Ti–5Si alloy. BioMed. Mater. Eng. 2017, 28, 503–514. [Google Scholar] [CrossRef] [PubMed]




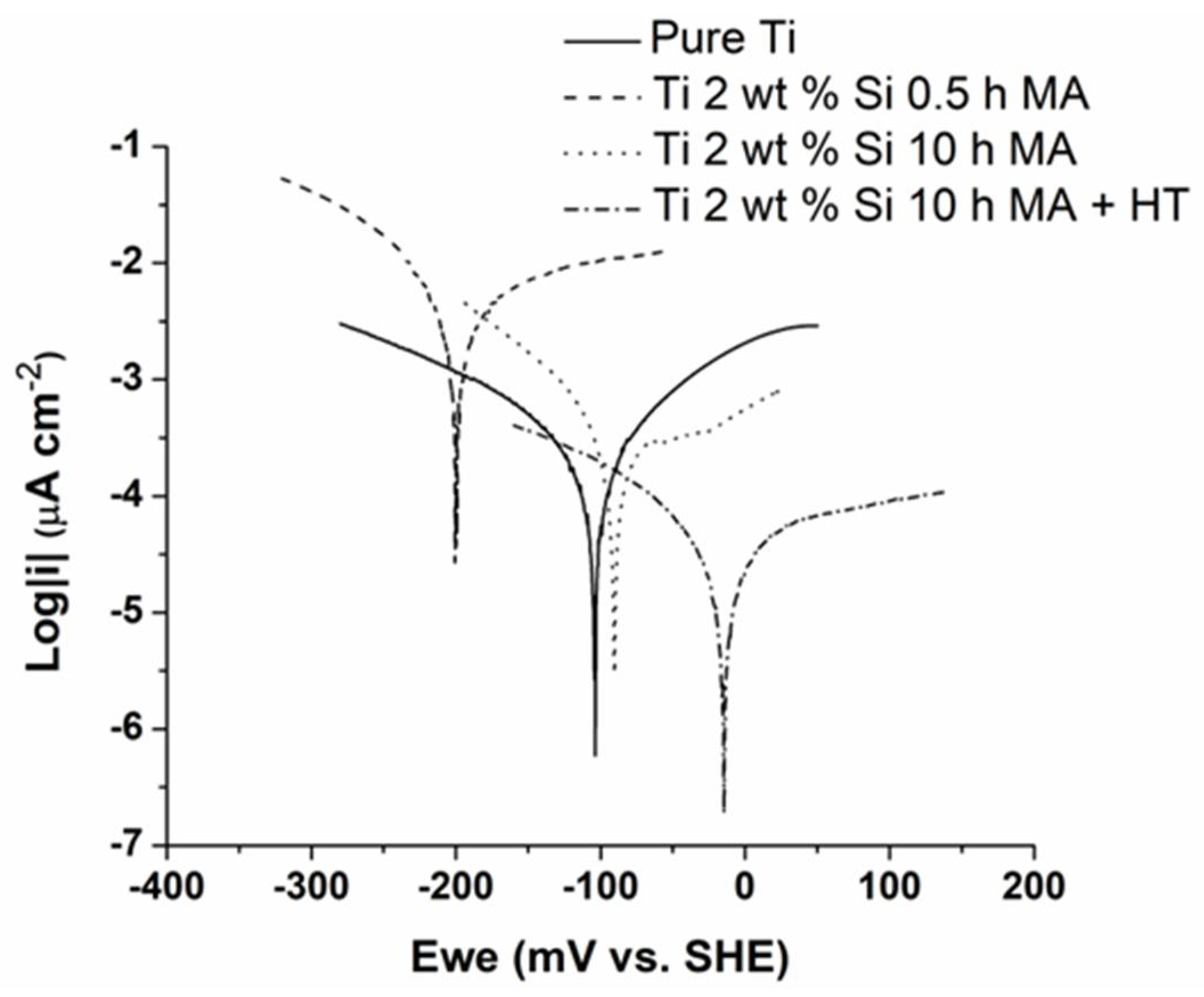

| Corrosion Parameters | Pure Ti | Ti 2 wt % Si | ||
|---|---|---|---|---|
| 0.5 h | 10 h | 10 h + HT | ||
| Eoc, mV/SHE | 138 | 146 | 147 | 218 |
| Ecorr, mV/SHE | −104 | −204 | −90 | −13 |
| icorr, (µA cm−2) | 4.70 | 81.63 | 3.00 | 0.45 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán, D.; García, C.; Soliz, Á.; Sepúlveda, R.; Aguilar, C.; Rojas, P.; Iturriza, I.; Luno-Bilbao, C. Synthesis and Electrochemical Properties of Ti-Si Alloys Prepared by Mechanical Alloying and Heat Treatment. Metals 2018, 8, 417. https://doi.org/10.3390/met8060417
Guzmán D, García C, Soliz Á, Sepúlveda R, Aguilar C, Rojas P, Iturriza I, Luno-Bilbao C. Synthesis and Electrochemical Properties of Ti-Si Alloys Prepared by Mechanical Alloying and Heat Treatment. Metals. 2018; 8(6):417. https://doi.org/10.3390/met8060417
Chicago/Turabian StyleGuzmán, Danny, Carolina García, Álvaro Soliz, Rossana Sepúlveda, Claudio Aguilar, Paula Rojas, Iñigo Iturriza, and Carmen Luno-Bilbao. 2018. "Synthesis and Electrochemical Properties of Ti-Si Alloys Prepared by Mechanical Alloying and Heat Treatment" Metals 8, no. 6: 417. https://doi.org/10.3390/met8060417
APA StyleGuzmán, D., García, C., Soliz, Á., Sepúlveda, R., Aguilar, C., Rojas, P., Iturriza, I., & Luno-Bilbao, C. (2018). Synthesis and Electrochemical Properties of Ti-Si Alloys Prepared by Mechanical Alloying and Heat Treatment. Metals, 8(6), 417. https://doi.org/10.3390/met8060417








