Glow Discharge Optical Emission Spectrometer Calibration Using Hydrogenated Zr-2.5Nb Alloy Standard Samples
Abstract
:1. Introduction
2. Materials and Research Methods
2.1. Standard Samples Production
2.2. Standart Samples Characterization
2.3. Optimization and Calibration of GD-OES
3. Results and Discussion
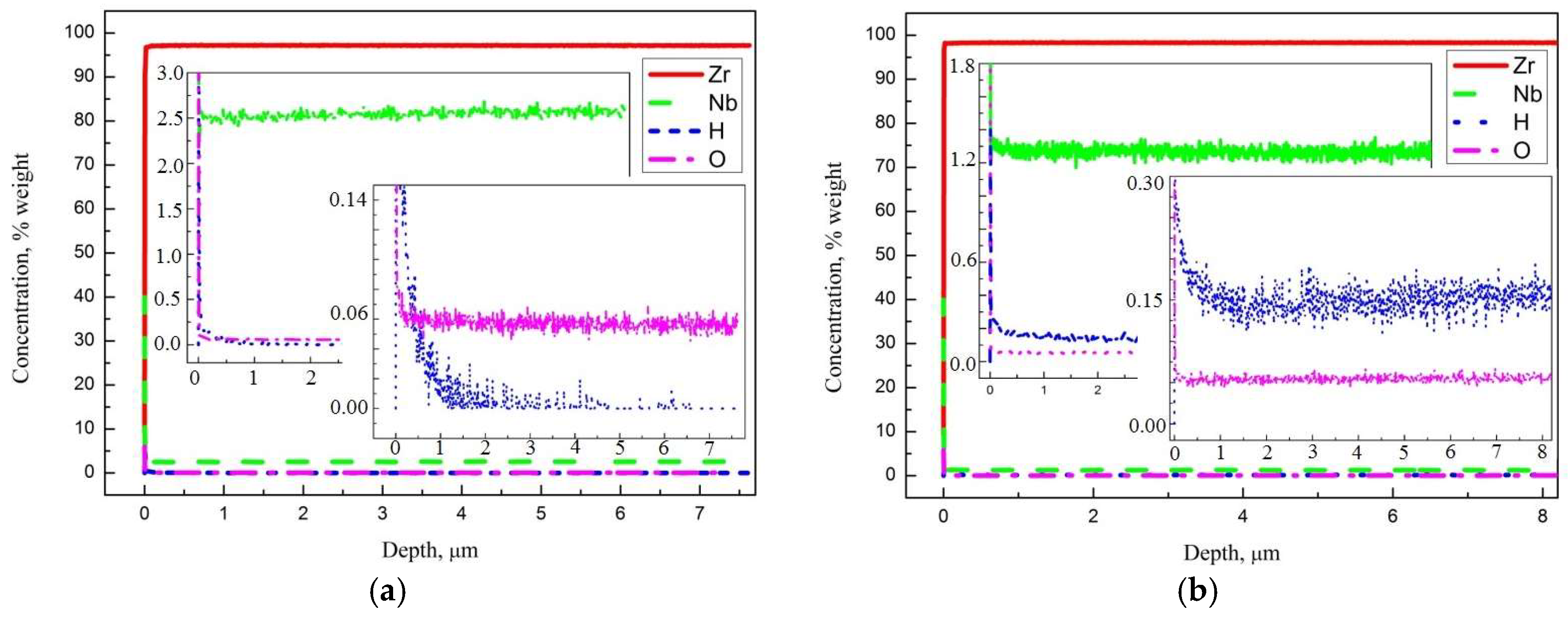
3.1. Sample Characterization
3.2. Optimization of GD-OES
3.3. Calibration of GD-OES with the Use of Zr-2.5Nb Samples
3.4. Measurements of the Other Samples in the Calibration
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Azevedo, C.R.F. Selection of fuel cladding material for nuclear fission reactors. Eng. Fail. Anal. 2011, 18, 1943–1962. [Google Scholar] [CrossRef]
- Hallstadius, L.; Johnson, S.; Lahoda, E. Cladding for performance fuel. Prog. Nucl. Energy 2012, 57, 71–76. [Google Scholar] [CrossRef]
- Fukai, Y. The Metal-Hydrogen System; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2005; Volume 21. [Google Scholar]
- Puls Manfred, P. The Effect of Hydrogen and Hydrides on the Integrity of Zirconium Alloy Components: Delayed Hydride Cracking; MPP Consulting: Oakville, ON, Canada; Springer: London, UK, 2012; 451p. [Google Scholar]
- Sawatzky, A.; Ells, C.E. Understanding hydrogen in zirconium. In Zirconium in the Nuclear Industry: Twelfth International Symposium; ASTM STP 1354; ASTM International: West Conshohocken, PA, USA, 2000; pp. 32–48. [Google Scholar]
- Zielinski, A.; Sobieszczyk, S. Hydrogen-enhanced degradation and oxide effects in zirconium alloys for nuclear applications. Int. J. Hydrog. Energy 2011, 36, 8619–8629. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Nikitenkov, N.N.; Sutygina, A.N.; Syrtanov, M.S.; Vilkhivskaya, O.V.; Pryamushko, T.S.; Kudiiarov, V.N.; Volesky, L. Effect of titanium ion implantation and deposition on hydrogenation behavior of Zr-1Nb alloy. Surf. Coat. Technol. 2016, 308, 2–9. [Google Scholar] [CrossRef]
- Nagase, F. Hydride behavior in Zircaloy cladding tube during high-temperature transients. J. Nucl. Mater. 2011, 415, 117–122. [Google Scholar] [CrossRef]
- Motta, A.T.; Chen, L.Q. Hydride formation in zirconium alloys. J. Miner. Met. Mater. Soc. 2012, 64, 1403–1408. [Google Scholar] [CrossRef]
- Laptev, R.S.; Lider, A.M.; Bordulev, Y.S.; Kudiiarov, V.N.; Gvozdyakov, D.V. The evolution of defects in zirconium in the process of hydrogen sorption and desorption. Key Eng. Mater. 2016, 683, 256. [Google Scholar] [CrossRef]
- Huang, J.-H.; Yeh, M.-S. Gaseous hydrogen embrittlement of a hydrided zirconium alloy. Metall. Mater. Trans. A 1998, 29, 1047–1056. [Google Scholar] [CrossRef]
- Kim, Y.S.; Matvienko, Y.G.; Cheong, Y.M.; Kim, S.S.; Kwon, S.C. A model of the threshold stress intensity factor КiH for delayed hydride cracking of Zr-2,5Nb alloy. J. Nucl. Mater. 2000, 278, 251–257. [Google Scholar] [CrossRef]
- Nagase, F.; Fuketa, T. Investigation of hydride rim effect on failure of Zircaloy-4 cladding with tube burst test. J. Nucl. Sci. Technol. 2005, 42, 58–65. [Google Scholar] [CrossRef]
- Hanson, B.; Shimskey, R.; Lavender, C.; MacFarlan, P.; Eslinger, P. Hydride Rim Formation in Unirradiated Zircaloy. Available online: https://www.energy.gov/sites/prod/files/2013/08/f2/HydrideRimFormationZircaloy.pdf (accessed on 22 March 2018).
- Shimskey, R.; Hanson, B.; MacFarlan, P. Optimization of Hydride Rim Formation in Unirradiated Zr-4 Cladding. Available online: https://www.pnnl.gov/main/publications/external/technical_reports/PNNL-22835.pdf (accessed on 22 March 2018).
- Steinbruck, M. Hydrogen absorption by zirconium alloys at high temperatures. J. Nucl. Mater. 2004, 334, 58–64. [Google Scholar] [CrossRef]
- Pundt, A.; Kirchheim, R. Hydrogen in metals: Microstructural aspects. Annu. Rev. Mater. Res. 2006, 36, 555–608. [Google Scholar] [CrossRef]
- Terrani, K.A.; Balooch, M.; Wongsawaeng, D.; Jaiyen, S.; Olander, D.R. The kinetics of hydrogen desorption from and adsorption on zirconium hydride. J. Nucl. Mater. 2010, 397, 61–68. [Google Scholar] [CrossRef]
- Kammenzind, B.K.; Berquist, B.M.; Bajaj, R. The long-range migration of hydrogen through ziicaloy in response to tensile and compressive stress gradients. In Zirconium in the Nuclear Industry: Twelfth International Symposium; ASTM STP 1354; ASTM International: West Conshohocken, PA, USA, 2000; pp. 196–233. [Google Scholar]
- Shmakov, A.A.; Smirnov, E.A.; Bruchertseifer, H. Hydrogen diffusion and distribution in oxidized zirconium alloys by thermo-release method. Metallofiz. Noveishie Technol. 1999, 21, 35–39. (In Russian) [Google Scholar]
- Shi, S.Q. Diffusion-controlled hydride growth near crack tip in zirconium under temperature transients. J. Nucl. Mater. 1999, 275, 318–323. [Google Scholar] [CrossRef]
- Takahara, H.; Ishigami, R.; Kodama, K.; Kojyo, A.; Nakamurac, T.; Oka, Y. Hydrogen analysis in diamond-like carbon by glow discharge optical emission spectroscopy. J. Anal. At. Spectrom. 2016, 31, 940–947. [Google Scholar] [CrossRef]
- Nelis, T.; Payling, R. Glow Discharge Optical Emission Spectroscopy: A Practical Guide; RSC Analytical Spectroscopy Monographs; The Royal Society for Chemistry: Cambridge, UK, 2003. [Google Scholar]
- Mikhaylov, A.A.; Priamushko, T.S.; Babikhina, M.N.; Kudiiarov, V.N.; Heller, R.; Laptev, R.S.; Lider, A.M. Hydrogen calibration of GD-spectrometer using Zr-1Nb alloy. Appl. Surf. Sci. 2017, 432, 85–89. [Google Scholar] [CrossRef]
- Marcus, R.K.; Broekaert, J.A.C. Glow Discharge Plasmas in Analytical Spectroscopy; J. Wiley: New York, NY, USA, 2003. [Google Scholar]
- Takahara, H.; Shikano, M.; Kobayashi, H. Quantification of lithium in LIB electrodes with glow discharge optical emission spectroscopy (GD-OES). J. Power Sources 2013, 244, 252–258. [Google Scholar] [CrossRef]
- Heikkila, I.; Eggertson, C.; Randelius, M.; Caddeo-Johansson, S.; Chasoglou, D. First experiences on characterization of surface oxide films in powder particles by Glow Discharge Optical Emission Spectroscopy (GD-OES). Met. Powder Rep. 2016, 71, 261–264. [Google Scholar] [CrossRef]
- Weiss, Z. Calibration methods in glow discharge optical emission spectroscopy: A tutorial review. J. Anal. At. Spectrom. 2015, 30, 1038–1049. [Google Scholar] [CrossRef]
- Winchester, M.R.; Payling, R. Radio-frequency glow discharge spectrometry: A critical review. Spectrochim Acta A 2004, 59, 607–666. [Google Scholar] [CrossRef]
- Voskuilen, T.; Zheng, Y.; Pourpoint, T. Development of a Sievert apparatus for characterization of high pressure hydrogen sorption materials. Int. J. Hydrog. Energy 2010, 35, 10387–10395. [Google Scholar] [CrossRef]
- Cheng, H.H.; Deng, X.X.; Li, S.L.; Chen, W.; Chen, D.M.; Yang, K. Design of PC based high pressure hydrogen absorption/desorption apparatus. Int. J. Hydrog. Energy 2007, 32, 3046–3053. [Google Scholar] [CrossRef]
- Gas Reaction Controller, Operation Manual. Advanced Materials Corporation. Available online: http://www.advanced-material.com (accessed on 22 May 2018).
- Schubert, C.; Hoffmann, V.; Kummel, A.; Sinn, J.; Hartel, M.; Reuther, A.; Thomalla, M.; Gemming, T.; Eckertcd, J.; Leyense, C. Compositional depth profiling of diamond-like carbon layers by glow discharge optical emission spectroscopy. J. Anal. At. Spectrom. 2016, 31, 2207–2212. [Google Scholar] [CrossRef]
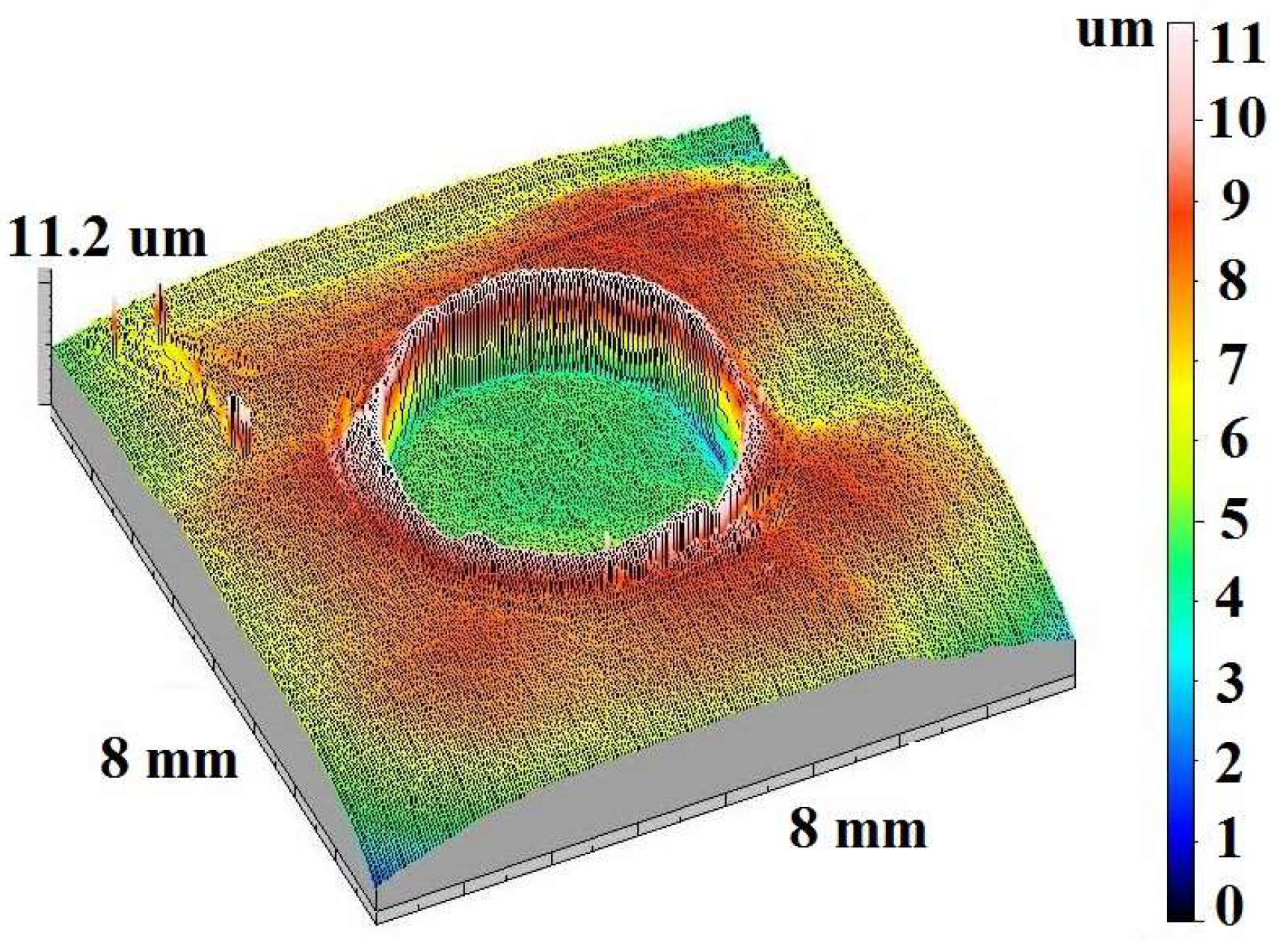
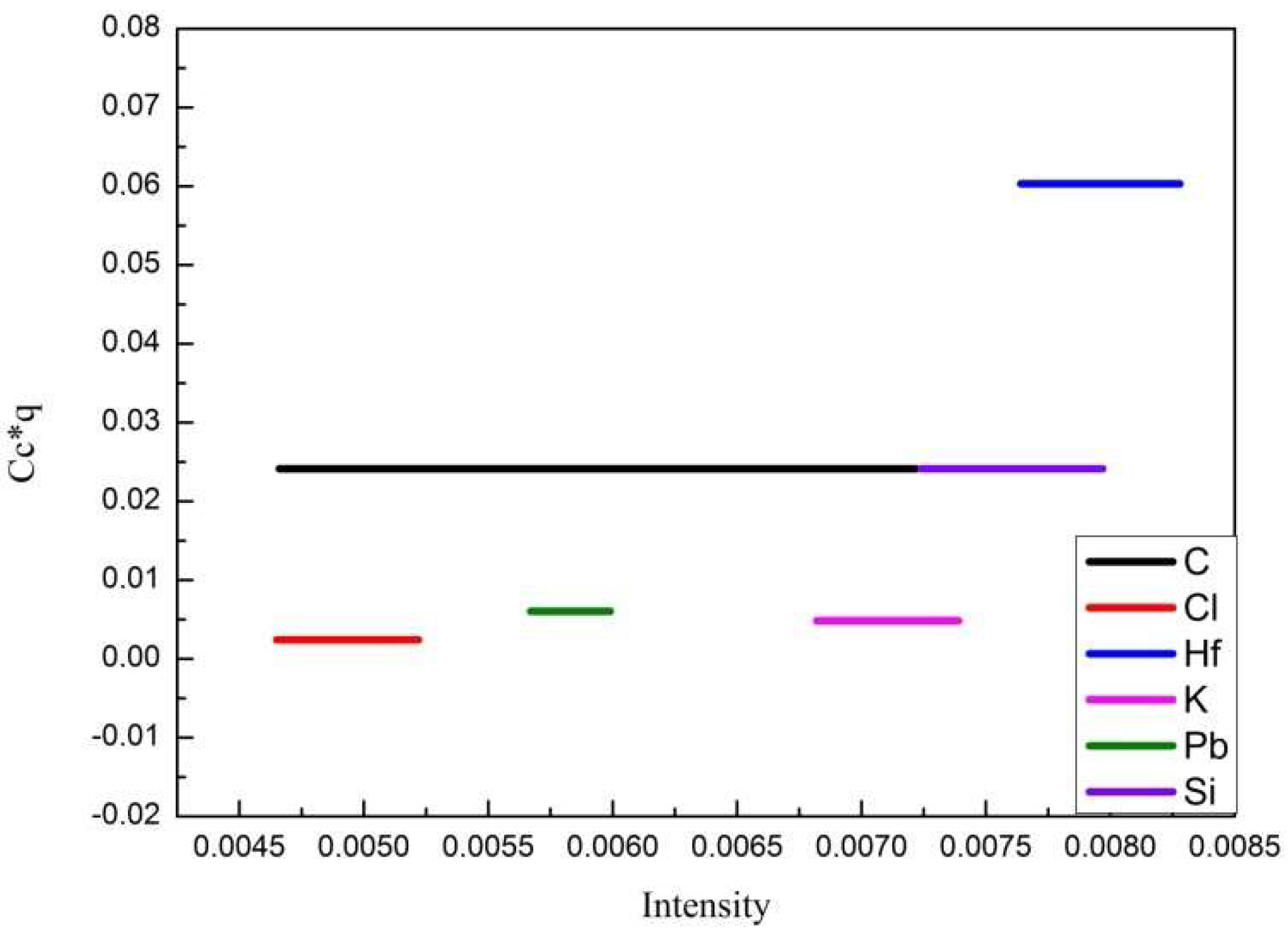
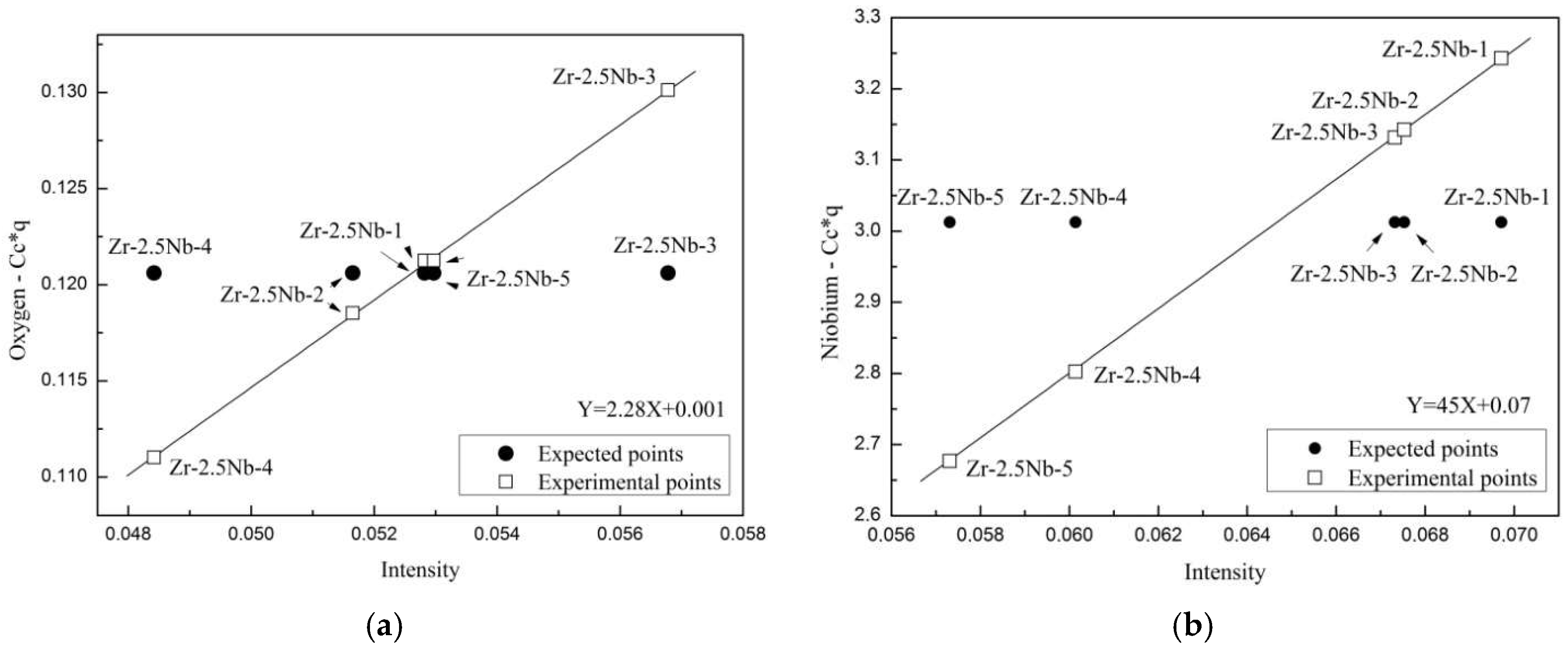
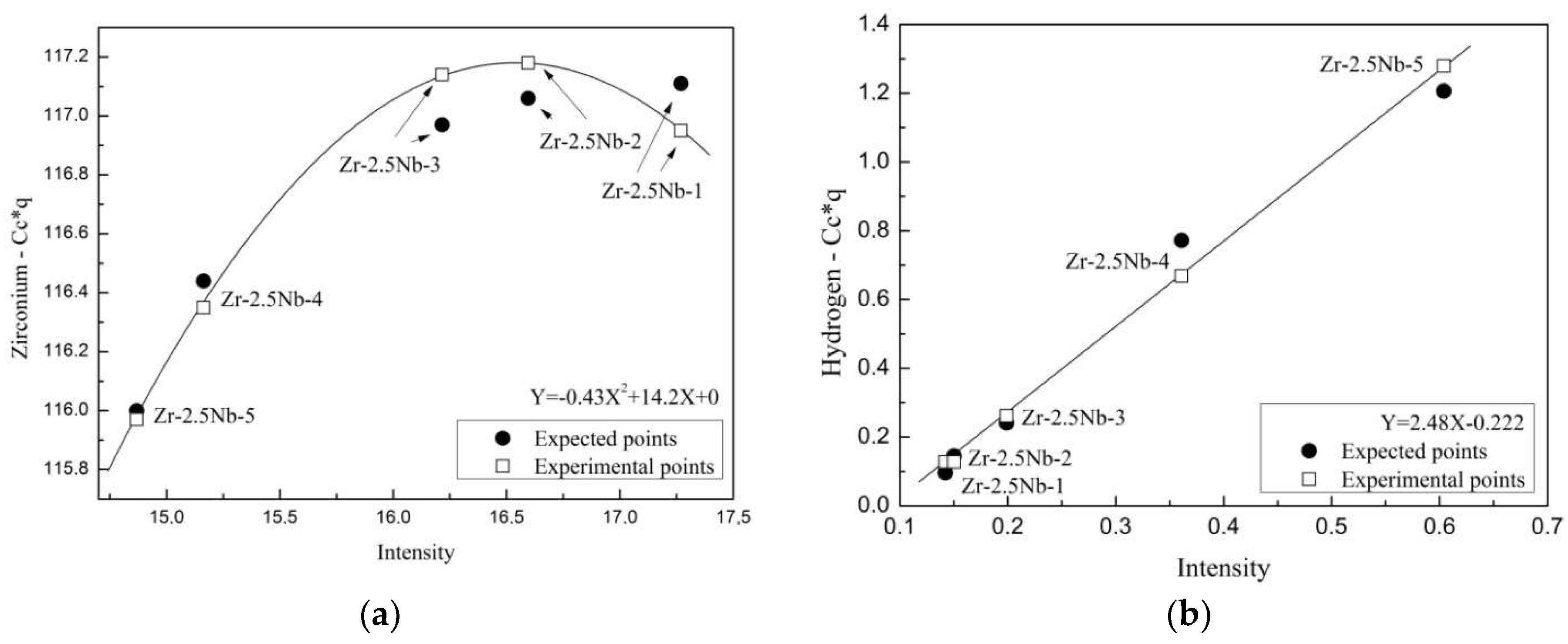
| Element | Content, Weight % | Element | Content, Weight % | Element | Content, Weight % |
|---|---|---|---|---|---|
| Zr | Balance | Cr | 0.02 | Mo | 0.005 |
| Nb(for Zr1Nb) | 0.9–1.1 | C | 0.02 | Pb | 0.005 |
| Nb(for Zr2.5Nb) | 2.4–2.7 | Ca | 0.01 | Ti | 0.005 |
| O | 0.1 (max) | Al | 0.008 | K | 0.004 |
| Fe | 0.05 | Ni | 0.007 | Cl | 0.002 |
| Hf | 0.05 | N | 0.006 | Mn | 0.002 |
| Si | 0.02 | Cu | 0.005 | Li | 0.0008 |
| Element | Wavelength, nm | Element | Wavelength, nm | Element | Wavelength, nm |
|---|---|---|---|---|---|
| H | 121.574 | Fe | 271.445 | Ti | 365.355 |
| O | 130.492 | Hf | 286.641 | Mo | 386.416 |
| Cl | 134.730 | Si | 288.162 | Al | 396.157 |
| N | 149.268 | Nb | 316.345 | Ca | 422.679 |
| C | 156.149 | Cu | 324.759 | Cr | 425.439 |
| Pb | 220.357 | Zr | 339.203 | Li | 670.800 |
| Mn | 257.614 | Ni | 341.482 | K | 766.500 |
| Sample | Hydrogenation Time, min | Volumetrically, Weight % | Changing of Weight, Weight % |
|---|---|---|---|
| Zr-2.5Nb-1 | 10 | 0.07 ± 0.01 | 0.08 ± 0.01 |
| Zr-2.5Nb-2 | 13 | 0.10 ± 0.01 | 0.12 ± 0.01 |
| Zr-2.5Nb-3 | 17 | 0.19 ± 0.02 | 0.20 ± 0.03 |
| Zr-2.5Nb-4 | 43 | 0.63 ± 0.03 | 0.64 ± 0.05 |
| Zr-2.5Nb-5 | 57 | 0.90 ± 0.07 | 1.00 ± 0.09 |
| Sample | Concentration | Zr | Nb | O | H |
|---|---|---|---|---|---|
| Zr-2.5Nb-1 | C1, weight % | 97.100 | 2.500 | 0.100 | 0.080 |
| C2, weight % | 96.970 | 2.689 | 0.100 | 0.106 | |
| Zr-2.5Nb-2 | C1, weight % | 97.060 | 2.500 | 0.100 | 0.120 |
| C2, weight % | 97.160 | 2.606 | 0.098 | 0.106 | |
| Zr-2.5Nb-3 | C1, weight % | 96.990 | 2.500 | 0.100 | 0.200 |
| C2, weight % | 97.130 | 2.597 | 0.108 | 0.217 | |
| Zr-2.5Nb-4 | C1, weight % | 96.550 | 2.500 | 0.100 | 0.640 |
| C2, weight % | 96.480 | 2.324 | 0.092 | 0.554 | |
| Zr-2.5Nb-5 | C1, weight % | 96.400 | 2.500 | 0.100 | 1.000 |
| C2, weight % | 96.817 | 2.124 | 0.103 | 0.956 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priamushko, T.S.; Mikhaylov, A.A.; Babikhina, M.N.; Kudiiarov, V.N.; Laptev, R.S. Glow Discharge Optical Emission Spectrometer Calibration Using Hydrogenated Zr-2.5Nb Alloy Standard Samples. Metals 2018, 8, 372. https://doi.org/10.3390/met8050372
Priamushko TS, Mikhaylov AA, Babikhina MN, Kudiiarov VN, Laptev RS. Glow Discharge Optical Emission Spectrometer Calibration Using Hydrogenated Zr-2.5Nb Alloy Standard Samples. Metals. 2018; 8(5):372. https://doi.org/10.3390/met8050372
Chicago/Turabian StylePriamushko, Tatyana S., Andrey A. Mikhaylov, Maria N. Babikhina, Viktor N. Kudiiarov, and Roman S. Laptev. 2018. "Glow Discharge Optical Emission Spectrometer Calibration Using Hydrogenated Zr-2.5Nb Alloy Standard Samples" Metals 8, no. 5: 372. https://doi.org/10.3390/met8050372
APA StylePriamushko, T. S., Mikhaylov, A. A., Babikhina, M. N., Kudiiarov, V. N., & Laptev, R. S. (2018). Glow Discharge Optical Emission Spectrometer Calibration Using Hydrogenated Zr-2.5Nb Alloy Standard Samples. Metals, 8(5), 372. https://doi.org/10.3390/met8050372







