1. Introduction
Copper is a metal with myriad applications in diverse fields, such as electronics, construction, transportation industry, biology, catalytic processes and sensors [
1]. Nevertheless, in some applications, such as structures for nautical applications and heat exchangers, the metal does not present appropriate resistance to corrosion. The combustion chamber of a water heater is a gas-liquid heat exchanger. Hot combustion gases flow through the interior of the so-called skirt, where they intersect the pipes within, where the water is transported to the outer tubes, designated serpentines. These work conditions can induce morphological and microstructural changes of the material, with significant implications, including mechanical fatigue and corrosion. In fact, the corrosion resistance of copper is evident when used in fresh and saline cooling waters [
2], but not when subjected to corrosive environments at high temperatures. Nevertheless, there is not much work describing the implications of the evolution of the copper’s properties in terms of performance during a complete life cycle as a heat exchanger in a combustion chamber.
The thermal stresses arise when a heated body is prevented from expanding. Thermal and physical stresses are identical: the thermal stress induced in the material by preventing it from expanding is equivalent to the compression stress that would have to be applied to return it to its original shape if the material had been allowed to freely expand [
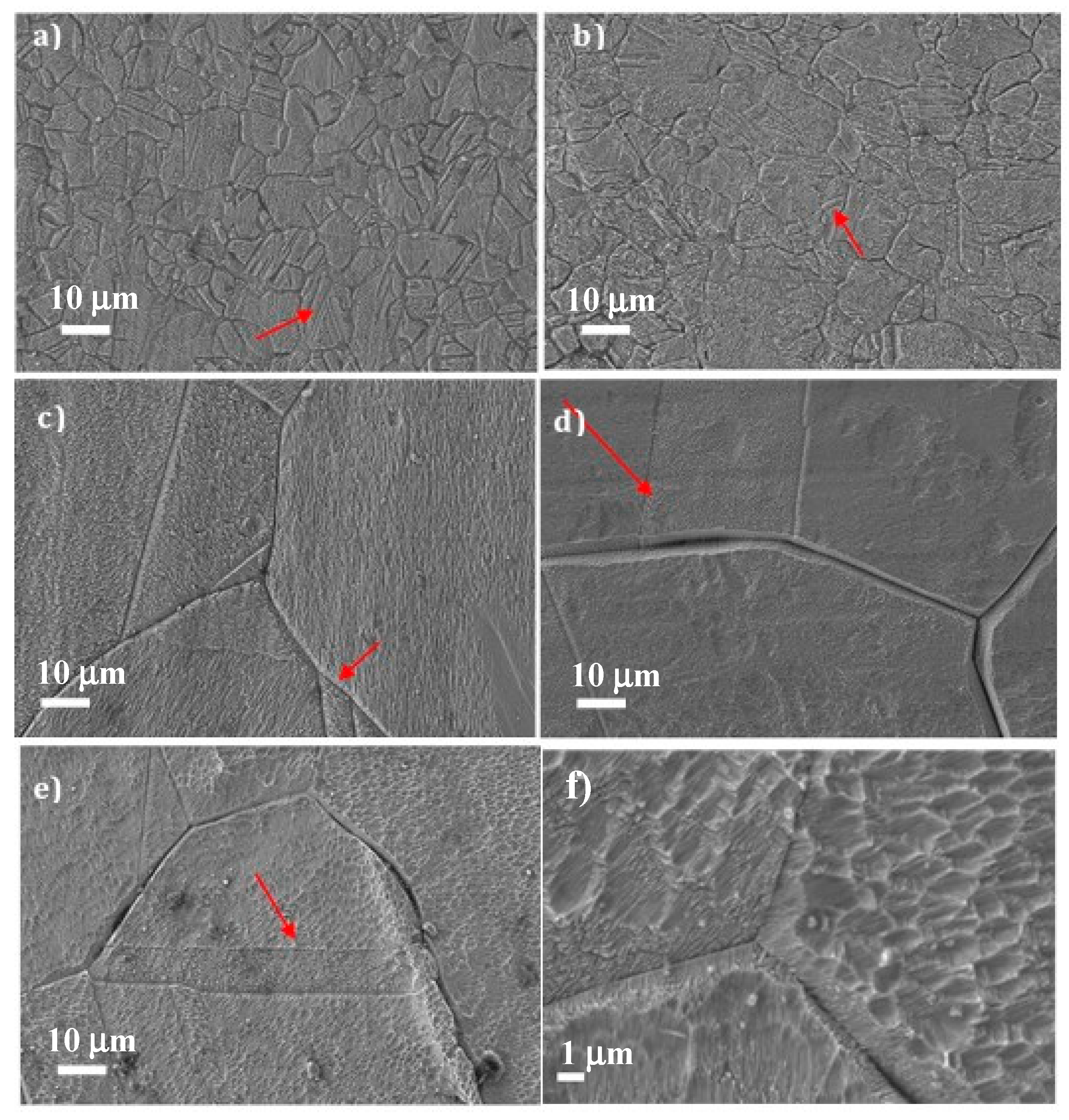
3]. A high number of thermal cycles induce low-magnitude cyclic stresses that locally trigger dislocations, causing microplasticity. Thus, thermal fatigue can play an important role in surface topography, as in the long term, these dislocations produce internal stresses that develop intrusions and extrusions that arise on the surface of the material [
4]. A relationship has been found between grain orientation and surface degradation induced by thermal fatigue in polycrystalline copper, as well as a proportional correspondence between the increased roughness of polycrystalline copper subjected to thermal fatigue and the increase in hardness [
4,
5].
In components subjected to high fatigue cycles, the crack nucleation stage is the most relevant, as it corresponds to about 90% of the life of the material [
6]. For metals with a face-centered cubic (FCC) structure (such as copper), crack nucleation occurs preferentially along grain boundaries, along with the persisting sliding bands, or as a combination of both. Cracks nucleate along grain boundaries as a result of incompatibility of deformation of this zone with the rest of the material, whereas the inclusions and exclusions caused by persistent sliding bands are the main reason why crack nucleation arises along them.
The fatigue process is particularly sensitive to the surface state; in homogeneous materials without significant internal defects, the microcracks always start on the free surfaces [
7,
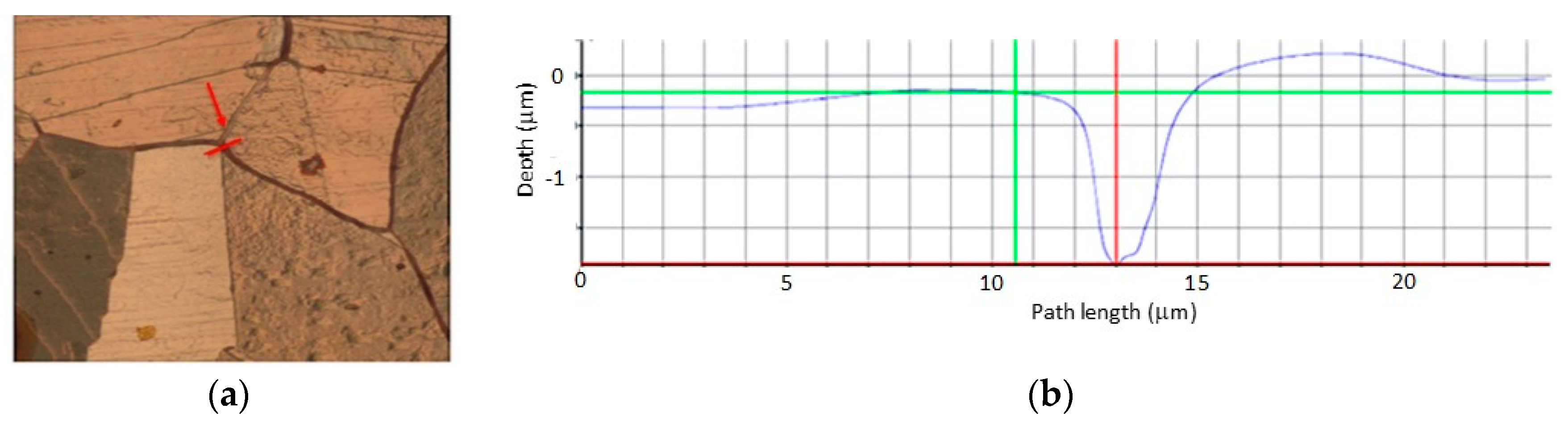
8]. The cracks are nucleated at the sites where cyclic plastic deformation has higher values as a result of higher stress concentrations. Although there are always microscopic stress concentrators that significantly affect the material, the surface tension level is very susceptible to surface topography; for example, roughness with a depth of 1 μm can cause a stress increase of about 10% [
9]. Crack propagation depends mainly on the direction of the applied force [
10,
11]. However, the velocity of propagation is greatly increased by corrosion, which accelerates the fatigue cracking process.
Copper is not found in nature in its “pure” form, but is extracted from minerals that contain it [
12]. In fact, metals have higher surface energy and therefore present a tendency to return to a state of lower energy, such as an oxide, which is chemically more stable. Therefore, when copper is subjected to the right chemical environment with the appropriate energy input, corrosion reaction will be triggered. When metals are used in applications at high temperatures, they are particularly susceptible to oxidation/corrosion, as the rate of reaction increases exponentially with temperature for both general [
13] and pitting corrosion [
14].
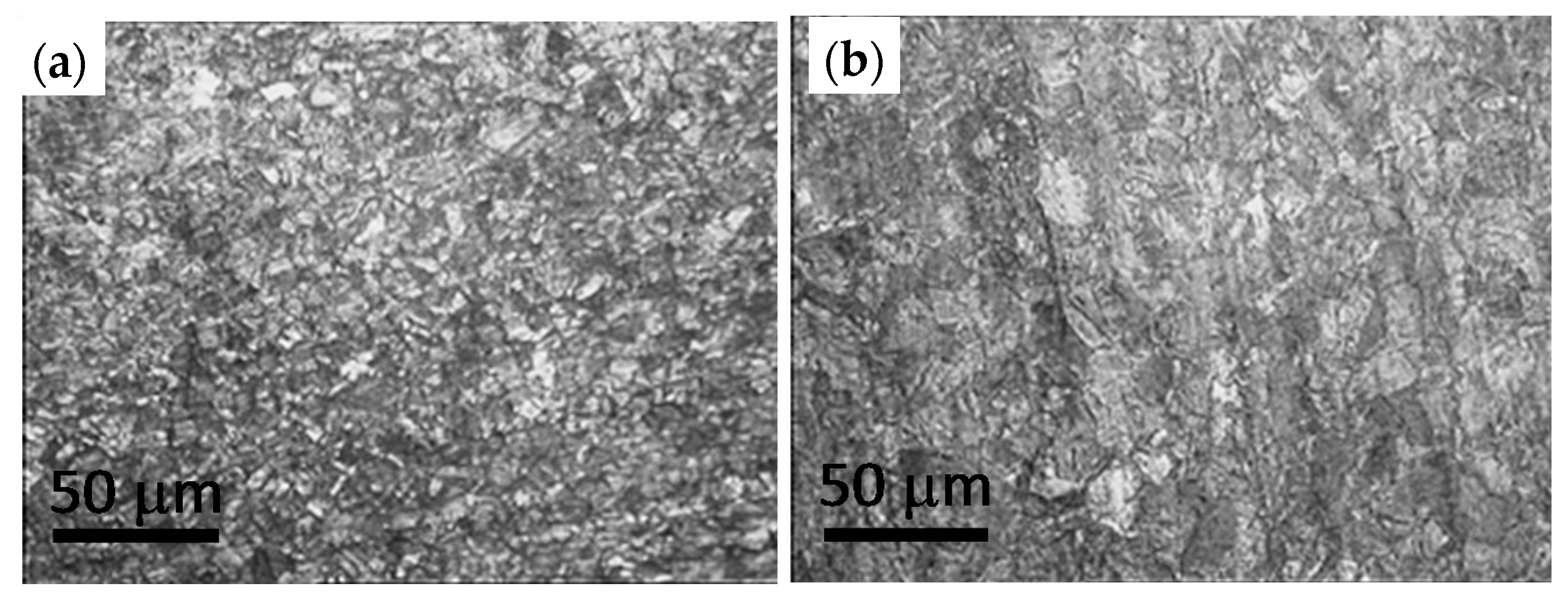
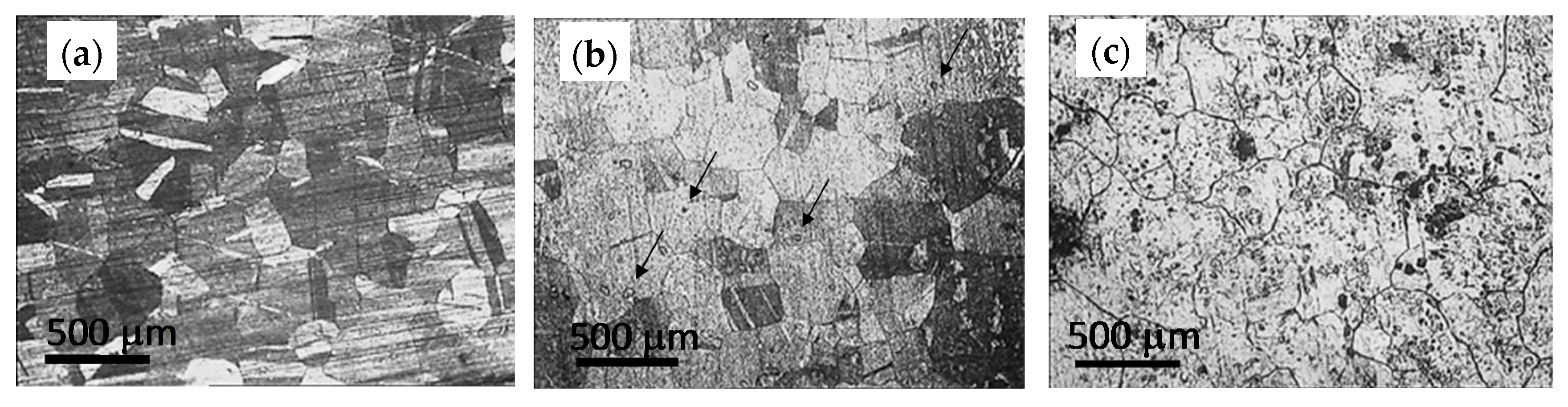
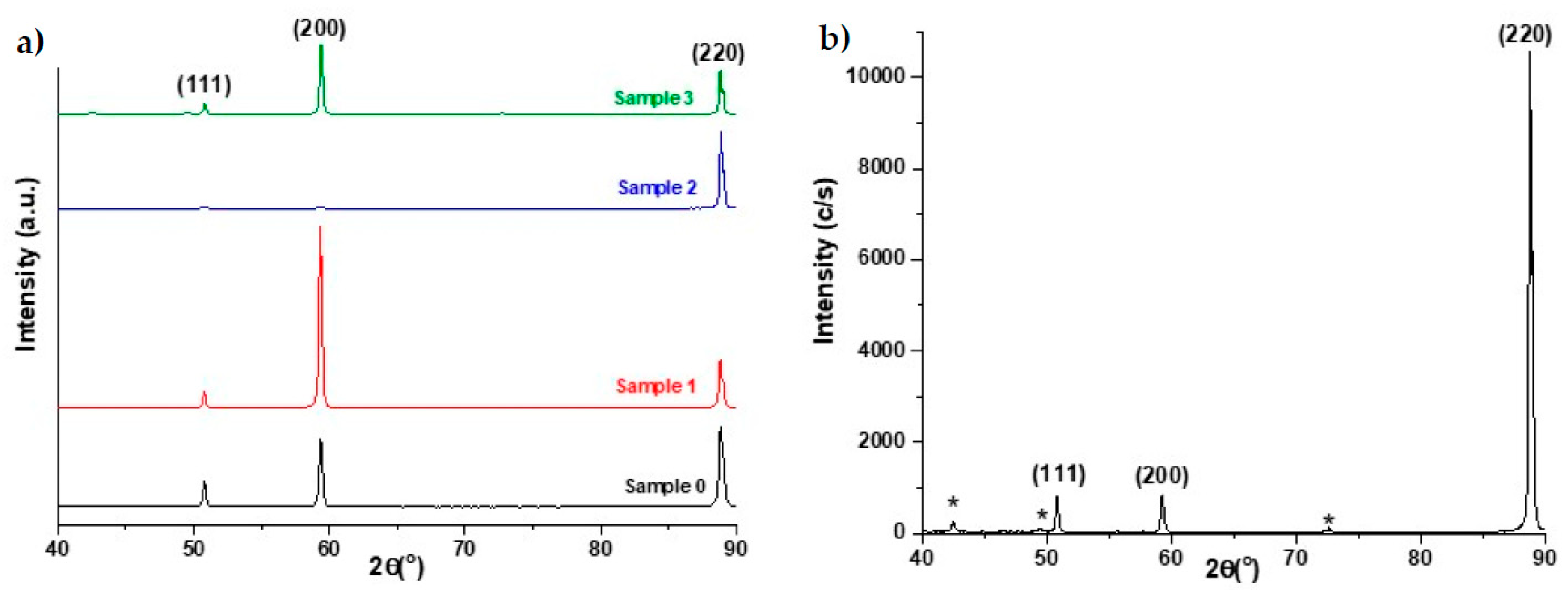
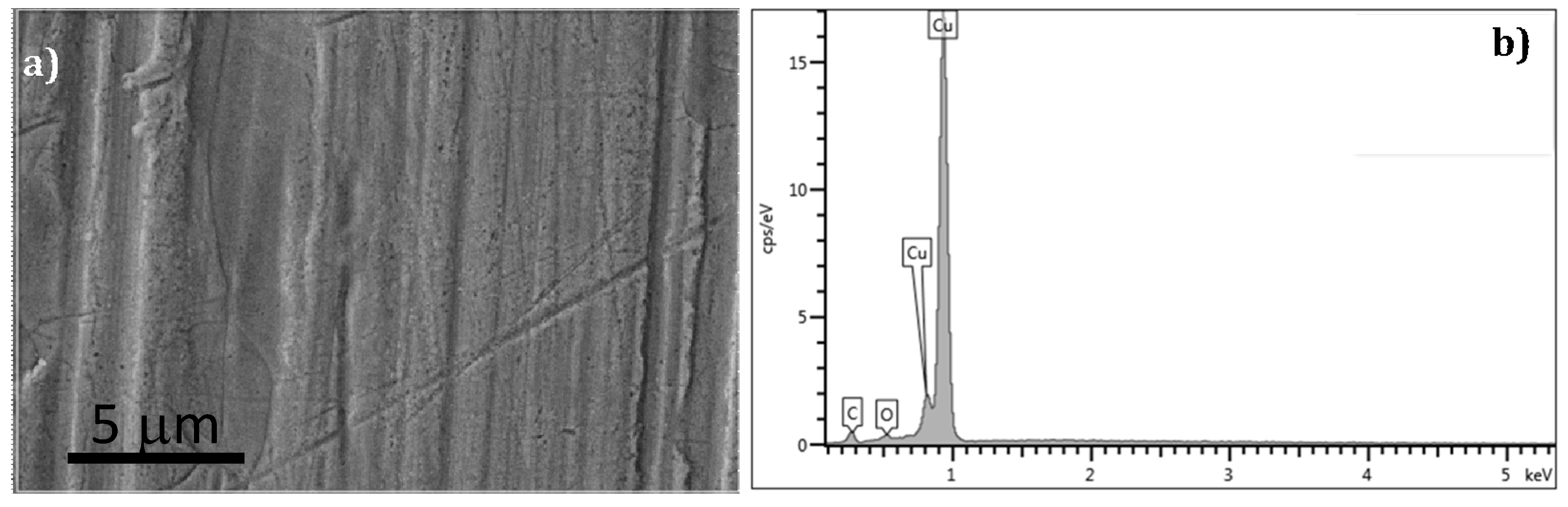
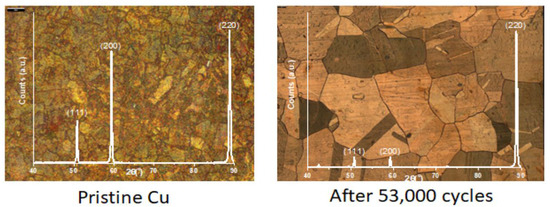
In this work, we describe the results from the characterization of the evolution of structural, surface and mechanical properties of copper used as a combustion chamber during its life cycle; from the copper sheet to the used combustion chamber after 53,000 cycles.