Effect of Nitric and Oxalic Acid Addition on Hard Anodizing of AlCu4Mg1 in Sulphuric Acid
Abstract
:1. Introduction
2. Materials and Methods
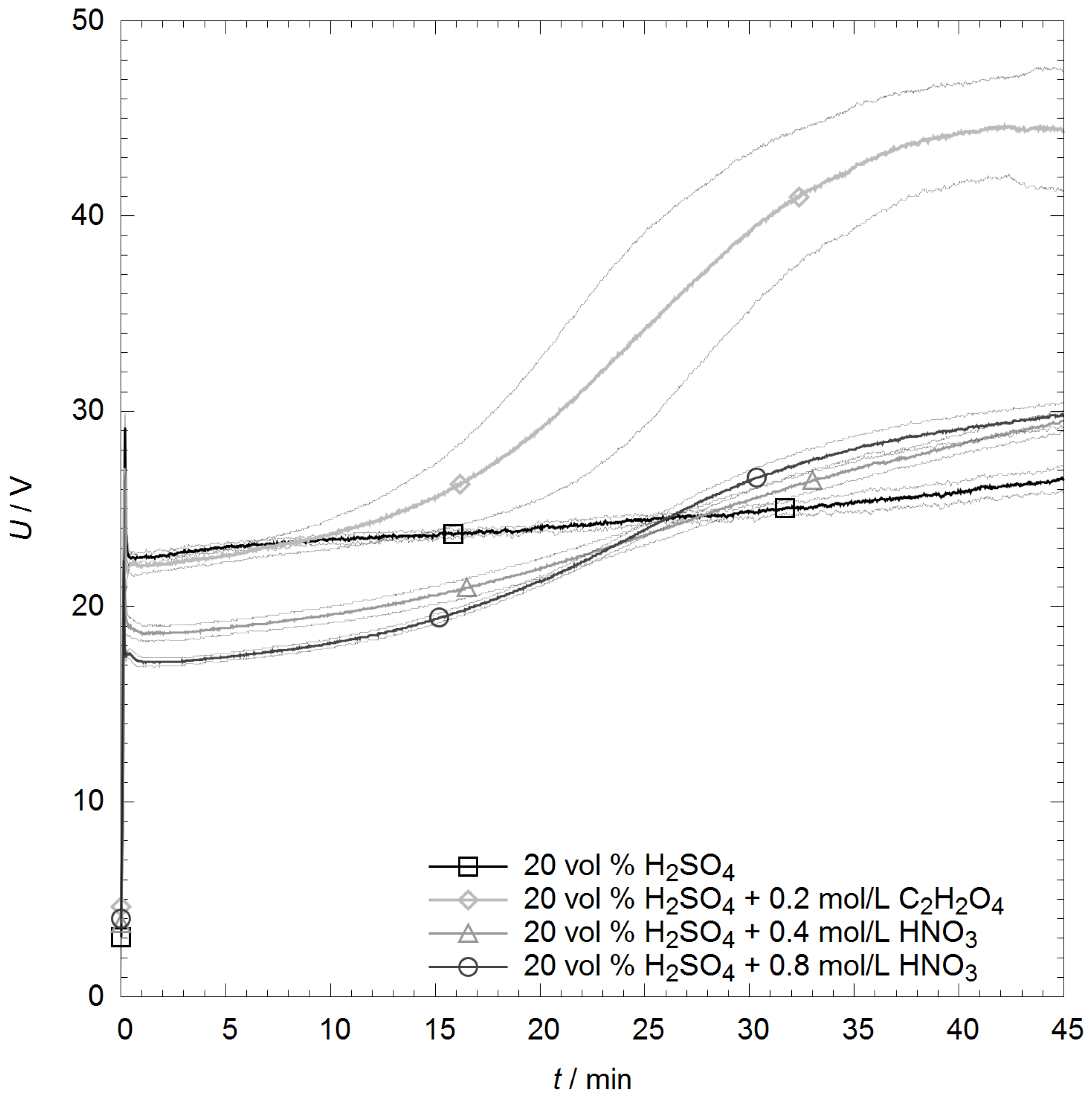
2.1. Anodizing Process
2.2. Coating and Process Characterization
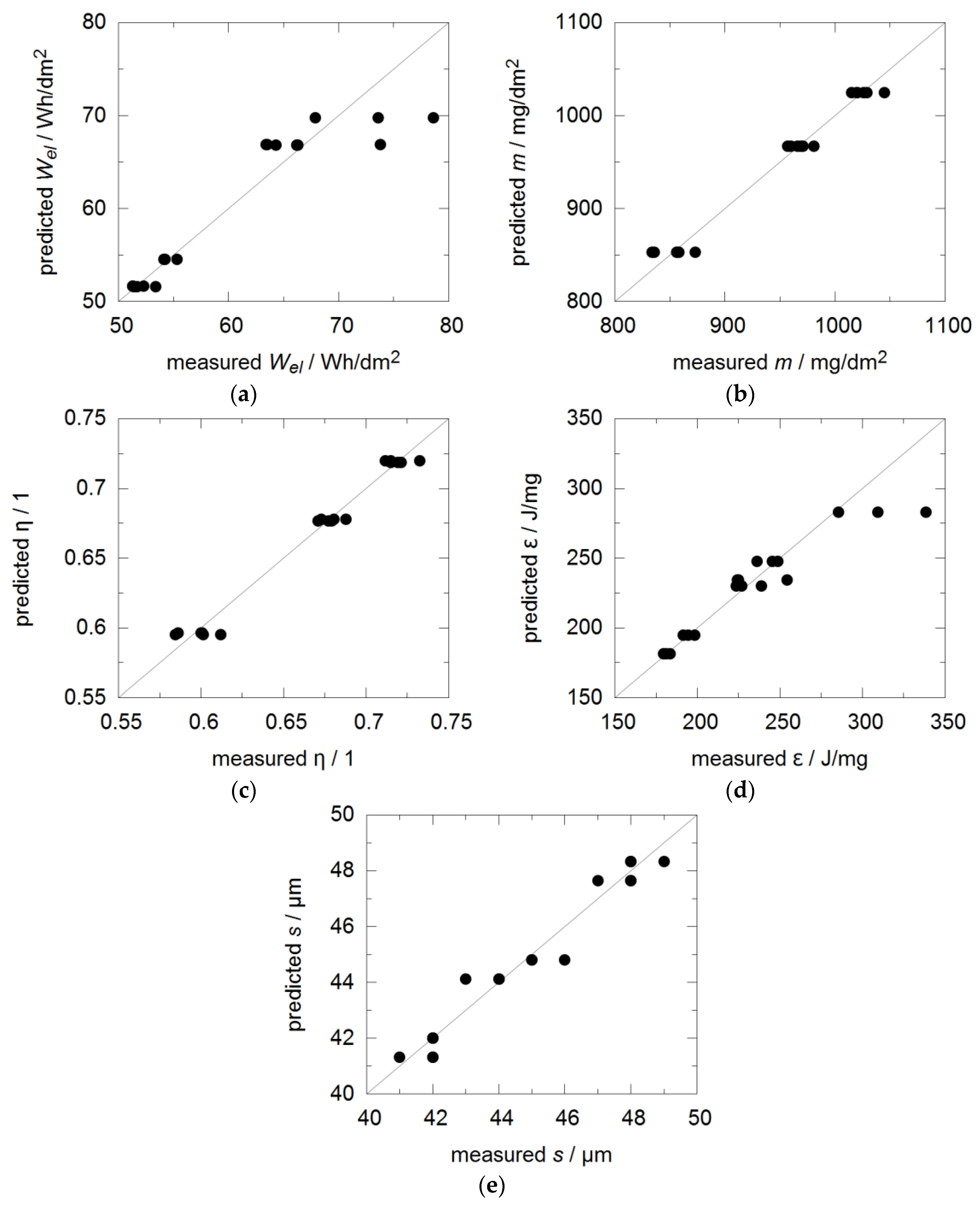
2.3. DOE-Set Up and Exploitation
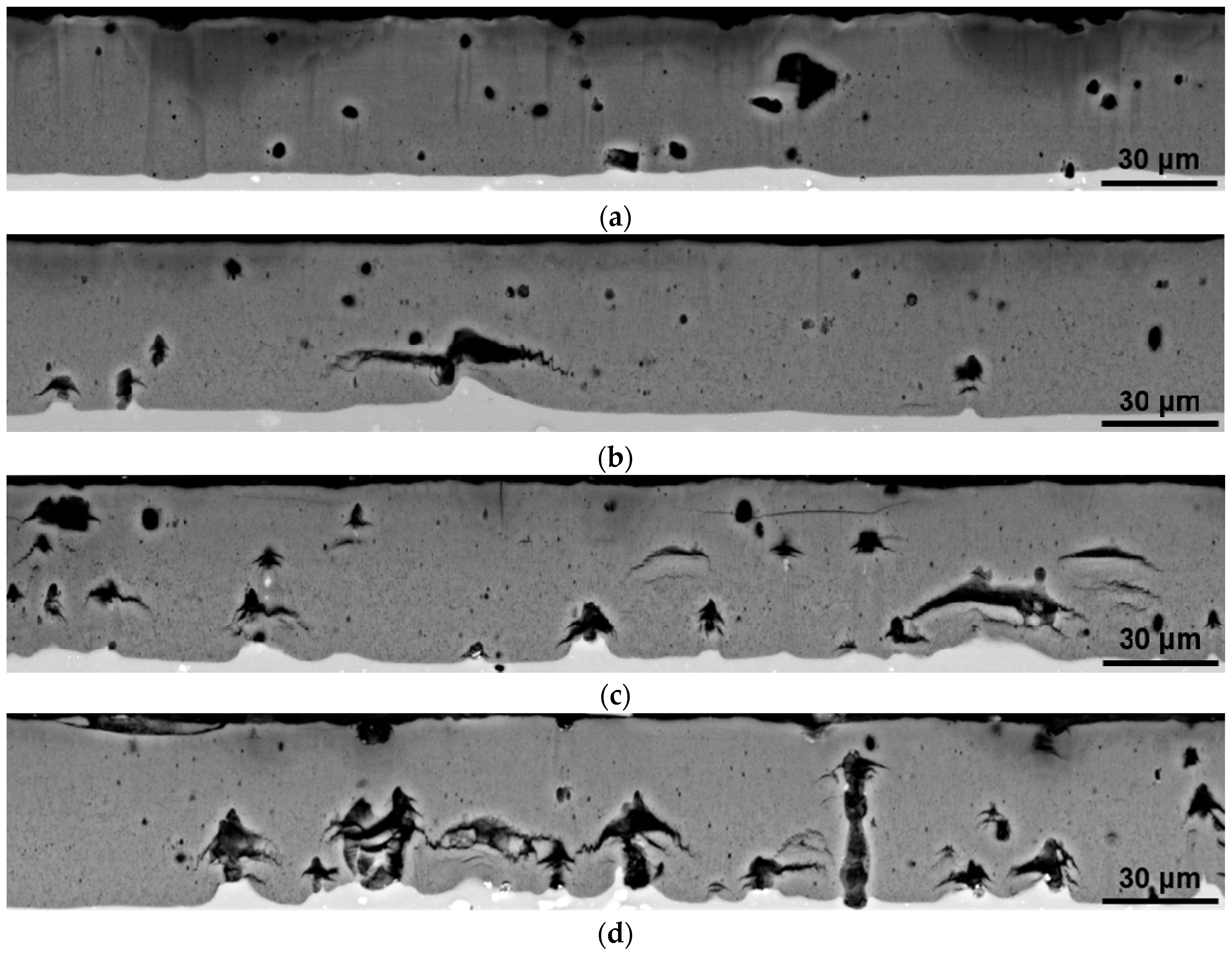
2.4. Microstructure Characterisation
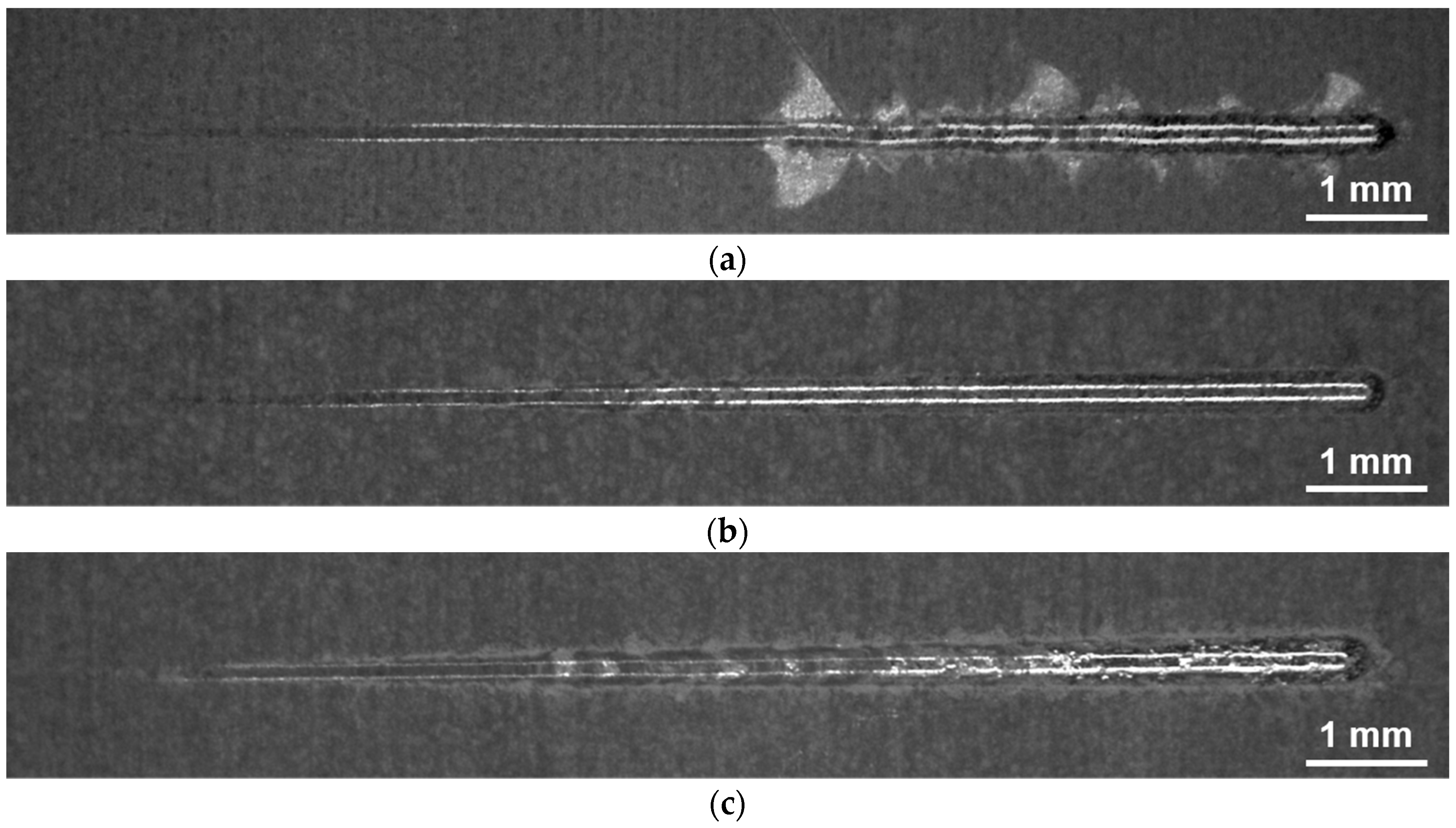
2.5. Scratch Testing and Profilometry
3. Results
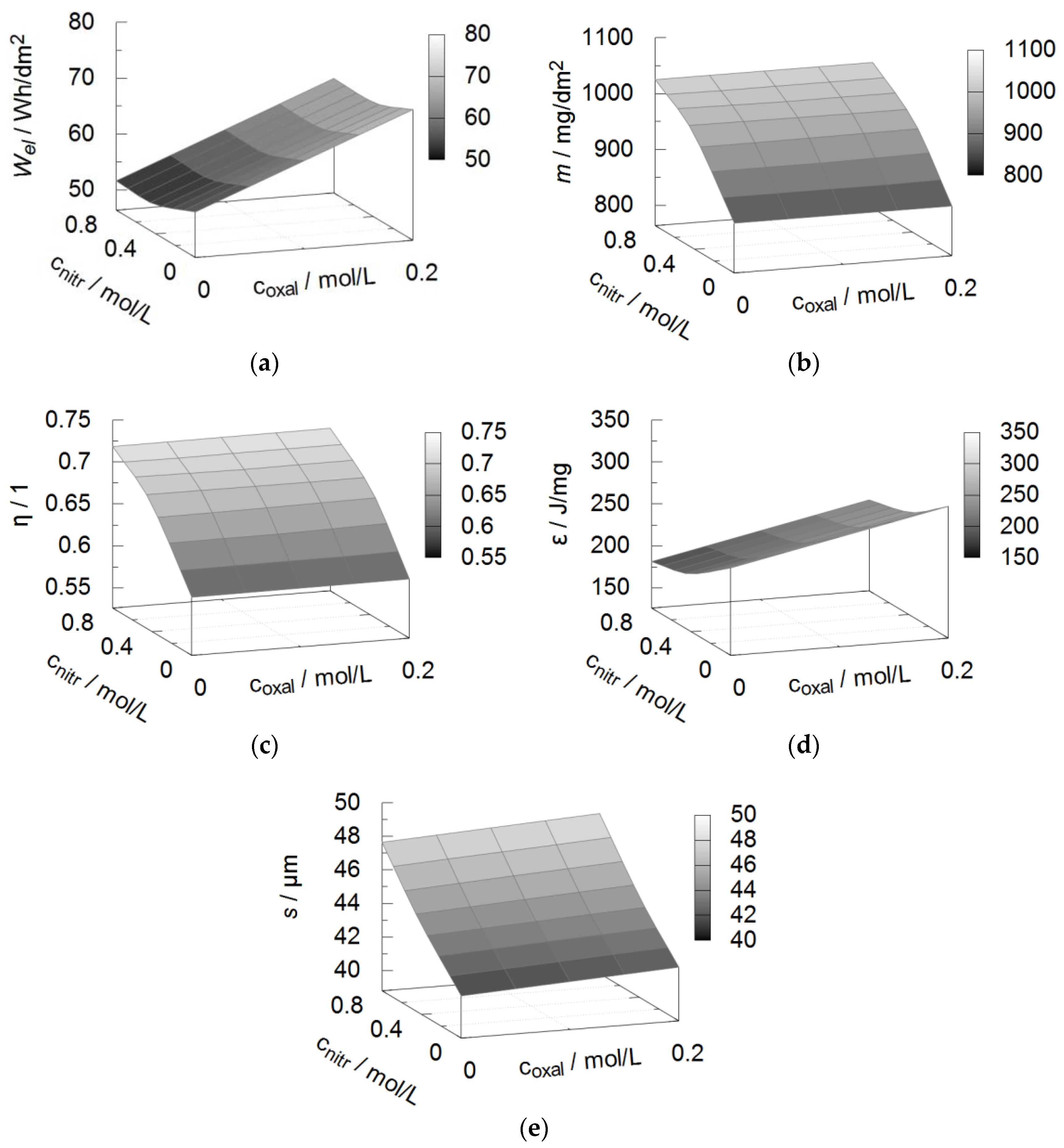
3.1. Energy Efficiency
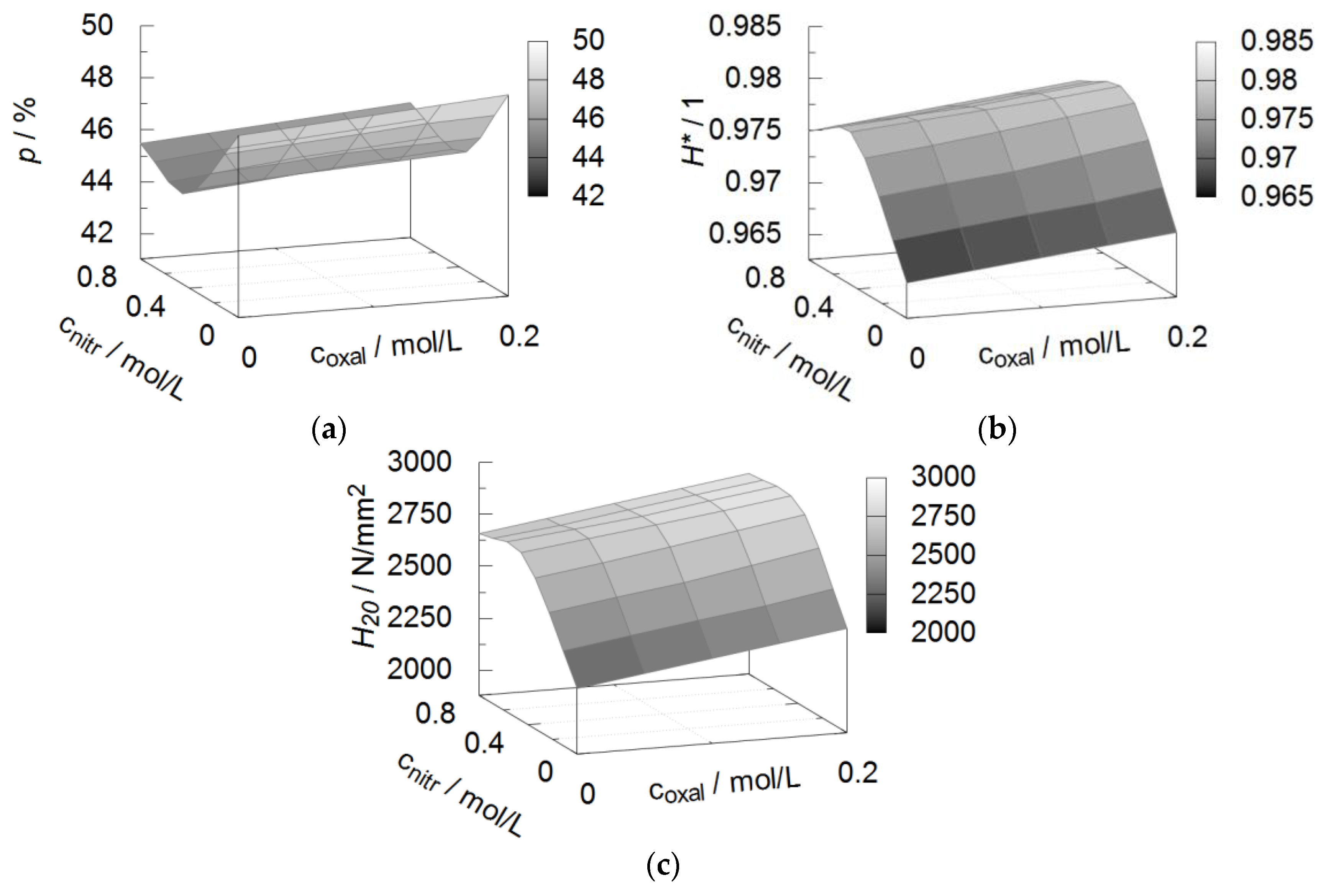
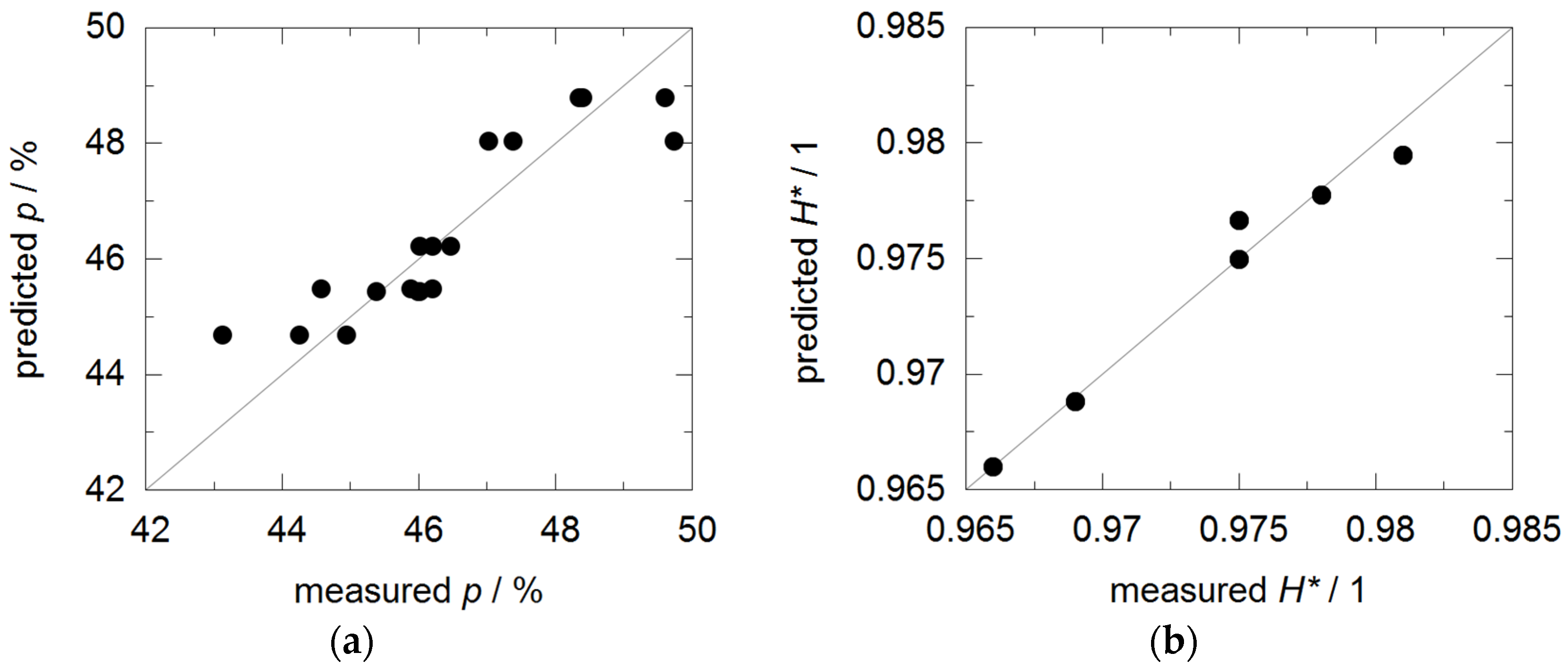
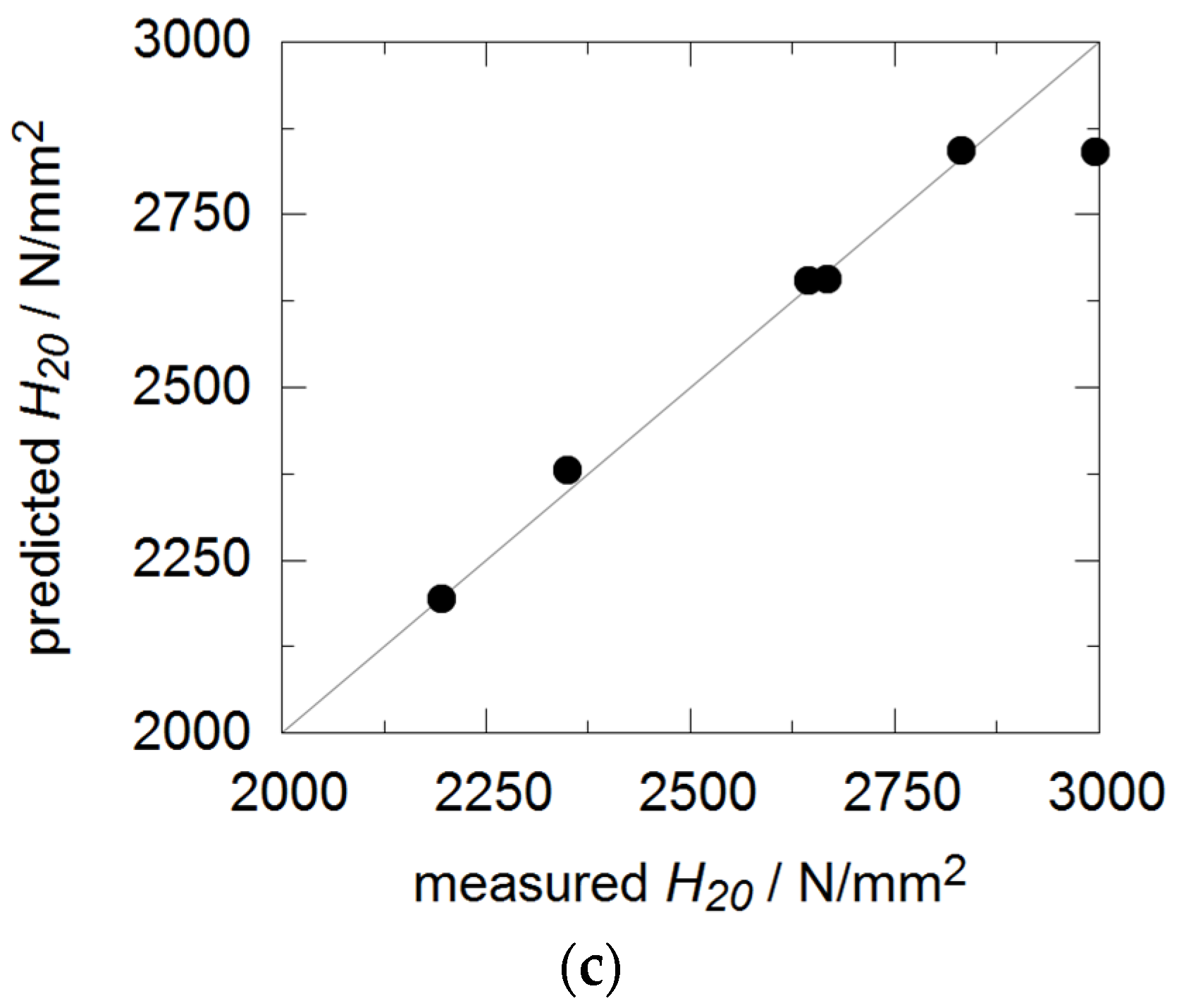
3.2. Coating Porosity and Hardness
3.3. Coating Adhesion and Abrasion Resistance
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Giovanardi, R.; Fontanesi, C.; Dallabarba, W. Adsorption of organic compounds at the aluminium oxide/aqueous solution interface during the aluminium anodizing process. Electrochim. Acta 2011, 56, 3128–3138. [Google Scholar] [CrossRef]
- Bensalah, W.; Elleuch, K.; Feki, M.; Wery, M.; Ayedi, H.F. Mechanical and Abrasive Wear Properties of Anodic Oxide Layers Formed on Aluminium. J. Mater. Sci. Technol. 2009, 25, 508–512. [Google Scholar]
- Sieber, M.; Morgenstern, R.; Lampke, T. Anodic oxidation of the AlCu4Mg1 aluminium alloy with dynamic current control. Surf. Coat. Technol. 2016, 302, 515–522. [Google Scholar] [CrossRef]
- Thompson, G.E.; Habazaki, H.; Shimizu, K.; Sakairi, M.; Skeldon, P.; Zhou, X.; Wood, G.C. Anodizing of aluminium alloys. Aircr. Eng. Aerosp. Technol. 1999, 71, 228–238. [Google Scholar] [CrossRef]
- Hashimoto, T.; Zhou, X.; Skeldon, P.; Thompson, G.E. Structure of the Copper–Enriched Layer Introduced by Anodic Oxidation of Copper-Containing Aluminium Alloy. Electrochim. Acta 2015, 179, 394–401. [Google Scholar] [CrossRef]
- Curioni, M.; Roeth, F.; Garcia-Vergara, S.J.; Hashimoto, T.; Skeldon, P.; Thompson, G.E.; Ferguson, J. Enrichment, incorporation and oxidation of copper during anodizing of aluminium-copper alloys. Surf. Interface Anal. 2010, 42, 234–240. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Thompson, G.E.; Curioni, M.; Zhong, X.; Koroleva, E.; Skeldon, P.; Thomson, P.; Fowles, M. Discontinuities in the porous anodic film formed on AA2099-T8 aluminium alloy. Corros. Sci. 2011, 53, 4141–4151. [Google Scholar] [CrossRef]
- Morgenstern, R.; Dietrich, D.; Sieber, M.; Lampke, T. Influence of the heat treatment condition of alloy AlCu4Mg1 on the microstructure and properties of anodic oxide layers. IOP Conf. Ser. Mater. Sci. 2017, 181, 012043. [Google Scholar] [CrossRef]
- Morgenstern, R.; Nickel, D.; Dietrich, D.; Scharf, I.; Lampke, T. Anodic Oxidation of AMCs: Influence of Process Parameters on Coating Formation. Mater. Sci. Forum 2015, 825–826, 636–644. [Google Scholar] [CrossRef]
- Shih, H.-H.; Tzou, S.-L. Study of anodic oxidation of aluminum in mixed acid using a pulsed current. Surf. Coat. Technol. 2000, 124, 278–285. [Google Scholar] [CrossRef]
| Element | Al | Cu | Mg | Mn | Si | Fe | Cr | Zn | Ti |
|---|---|---|---|---|---|---|---|---|---|
| wt % | balance | 3.9–4.9 | 1.2–1.8 | 0.3–0.9 | ≤0.5 | ≤0.5 | ≤0.1 | ≤0.25 | ≤0.15 |
| Property | Symbol | Unit | Oxalic/Nitric Acid Addition in mol/L | |||||
|---|---|---|---|---|---|---|---|---|
| 0/0 | 0/0.4 | 0/0.8 | 0.2/0 | 0.2/0.4 | 0.2/0.8 | |||
| Coating thickness | s | µm | 42 ± 5 | 44 ± 2 | 48 ± 2 | 42 ± 3 | 45 ± 3 | 48 ± 3 |
| Specific mass | m | mg/dm² | 855 ± 16 | 964 ± 5 | 1025 ± 4 | 850 ± 10 | 971 ± 9 | 1026 ± 14 |
| El. energy Consumption | Wel | Wh/dm² | 54.5 ± 0.6 | 52.2 ± 0.9 | 51.6 ± 0.5 | 73 ± 5 | 65.6 ± 1.0 | 67 ± 5 |
| Current eff. | η | % | 59.9 ± 1.2 | 67.6 ± 0.4 | 71.9 ± 2.7 | 59.6 ± 0.7 | 68.0 ± 0.6 | 72.0 ± 1.0 |
| Energy eff. | ε | J/mg | 230 ± 7 | 195 ± 3 | 181 ± 2 | 311 ± 22 | 243 ± 6 | 234 ± 14 |
| Property | Symbol | Unit | a1 | a2 | a3 | a4 | R2 |
|---|---|---|---|---|---|---|---|
| Coating thickness | s | µm | 40 | 3 | 2.3 | 6 | 0.96 |
| Specific mass | m | mg/dm² | 900 | −0.7 | −180 | 400 | 0.98 |
| El. energy consumption | Wel | Wh/dm² | 50 | 80 | 9 | −11 | 0.87 |
| Current efficiency | η | % | 0.6 | 0.006 | −0.12 | 0.25 | 0.98 |
| Energy efficiency | ε | J/mg | 230 | 270 | 70 | −120 | 0.86 |
| Property | Symbol | Unit | Oxalic/Nitric Acid Addition in mol/L | |||||
|---|---|---|---|---|---|---|---|---|
| 0/0 | 0/0.4 | 0/0.8 | 0.2/0 | 0.2/0.4 | 0.2/0.8 | |||
| Porosity | p | % | 48.0 ± 1.2 | 44.1 ± 0.8 | 45.6 ± 0.7 | 48.8 ± 0.6 | 45.8 ± 0.3 | 46.2 ± 0.2 |
| Hardness | H20 | N/mm² | 2200 | 2600 | 2700 | 2300 | 3000 | 2800 |
| Hardness decline | H* | 0.966 | 0.975 | 0.975 | 0.969 | 0.981 | 0.978 | |
| Property | Symbol | Unit | a1 | a2 | a3 | a4 | R2 |
|---|---|---|---|---|---|---|---|
| Porosity | p | % | 50 | 4 | 13 | −14 | 0.81 |
| Hardness | H20 | N/mm² | 2200 | 900 | −1400 | 1700 | 0.94 |
| Hardness decline | H* | 1 | 0.014 | −0.04 | 0.04 | 0.97 |
| Property | Sym-Bol | Unit | Oxalic/Nitric Acid Addition in mol/L | |||||
|---|---|---|---|---|---|---|---|---|
| 0/0 | 0/0.4 | 0/0.8 | 0.2/0 | 0.2/0.4 | 0.2/0.8 | |||
| Critical normal force | Fc | N | 48.7 ± 2.9 | 45.0 ± 4 | - | 33.3 ± 2.0 | 42.0 ± 5 | - |
| Scratch energy density | WR | J/mm³ | 1.6 ± 0.2 | 1.7 ± 0.2 | 0.9 ± 0.4 | 1.3 ± 0.1 | 1.6 ± 0.2 | 1.0 ± 0.3 |
| Tangential force | Ft | N | 0.4 ± 0.0 | 0.5 ± 0.0 | 1.4 ± 0.2 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.9 ± 0.1 |
| Cross-section area | A | μm² | 270 ± 40 | 309 ± 24 | 1800 ± 700 | 347 ± 24 | 350 ± 50 | 1100 ± 500 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sieber, M.; Morgenstern, R.; Scharf, I.; Lampke, T. Effect of Nitric and Oxalic Acid Addition on Hard Anodizing of AlCu4Mg1 in Sulphuric Acid. Metals 2018, 8, 139. https://doi.org/10.3390/met8020139
Sieber M, Morgenstern R, Scharf I, Lampke T. Effect of Nitric and Oxalic Acid Addition on Hard Anodizing of AlCu4Mg1 in Sulphuric Acid. Metals. 2018; 8(2):139. https://doi.org/10.3390/met8020139
Chicago/Turabian StyleSieber, Maximilian, Roy Morgenstern, Ingolf Scharf, and Thomas Lampke. 2018. "Effect of Nitric and Oxalic Acid Addition on Hard Anodizing of AlCu4Mg1 in Sulphuric Acid" Metals 8, no. 2: 139. https://doi.org/10.3390/met8020139
APA StyleSieber, M., Morgenstern, R., Scharf, I., & Lampke, T. (2018). Effect of Nitric and Oxalic Acid Addition on Hard Anodizing of AlCu4Mg1 in Sulphuric Acid. Metals, 8(2), 139. https://doi.org/10.3390/met8020139






