Hydrophobic and Corrosion Behavior of Sol-Gel Hybrid Coatings Based on the Combination of TiO2 NPs and Fluorinated Chains for Aluminum Alloys Protection
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
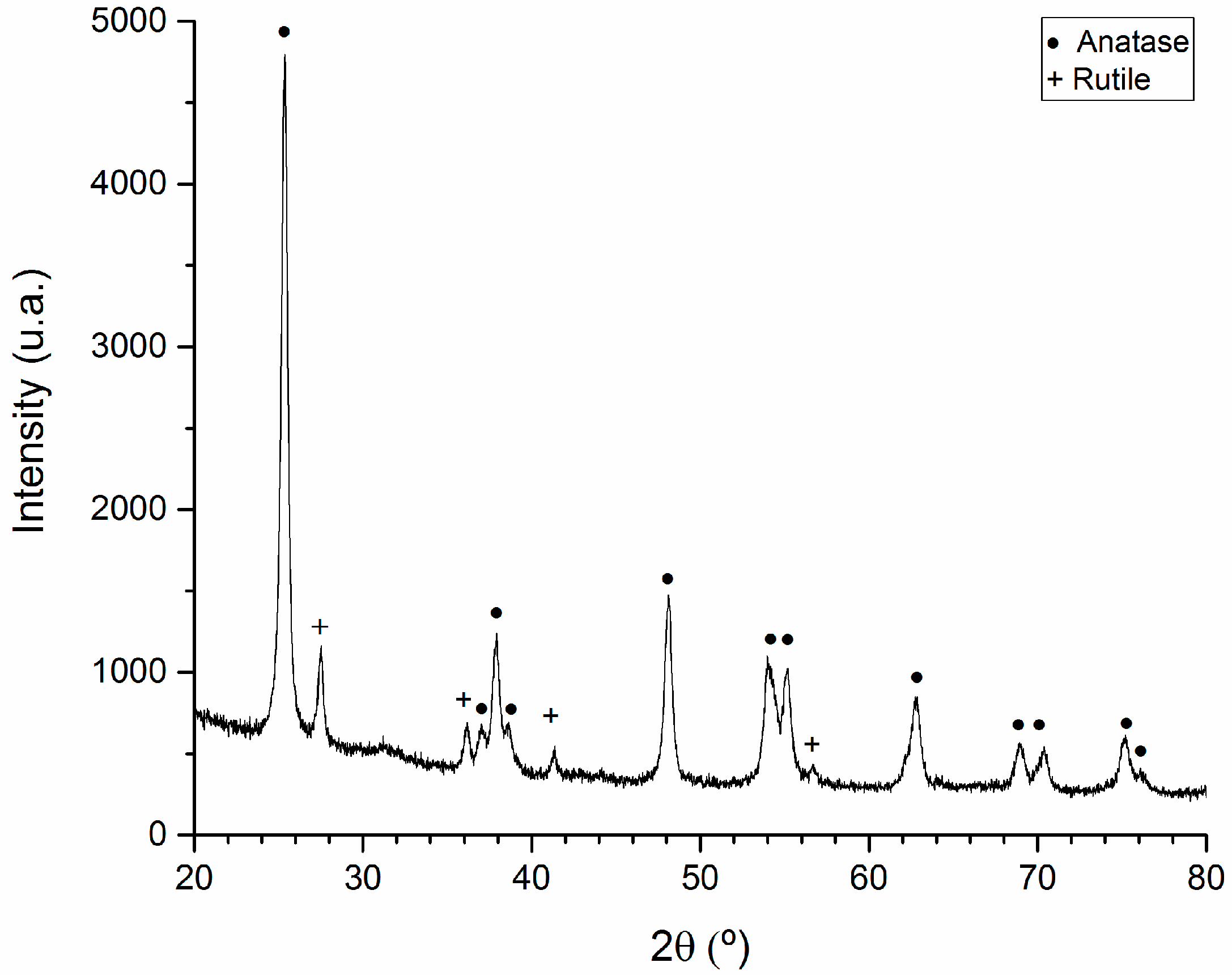
2.2. X-Ray Diffraction Analysis
2.3. Deposition Process of the Coatings
2.4. Characterization of the Sol-Gel Hybrid Coatings
2.5. Electrochemical Corrosion Tests
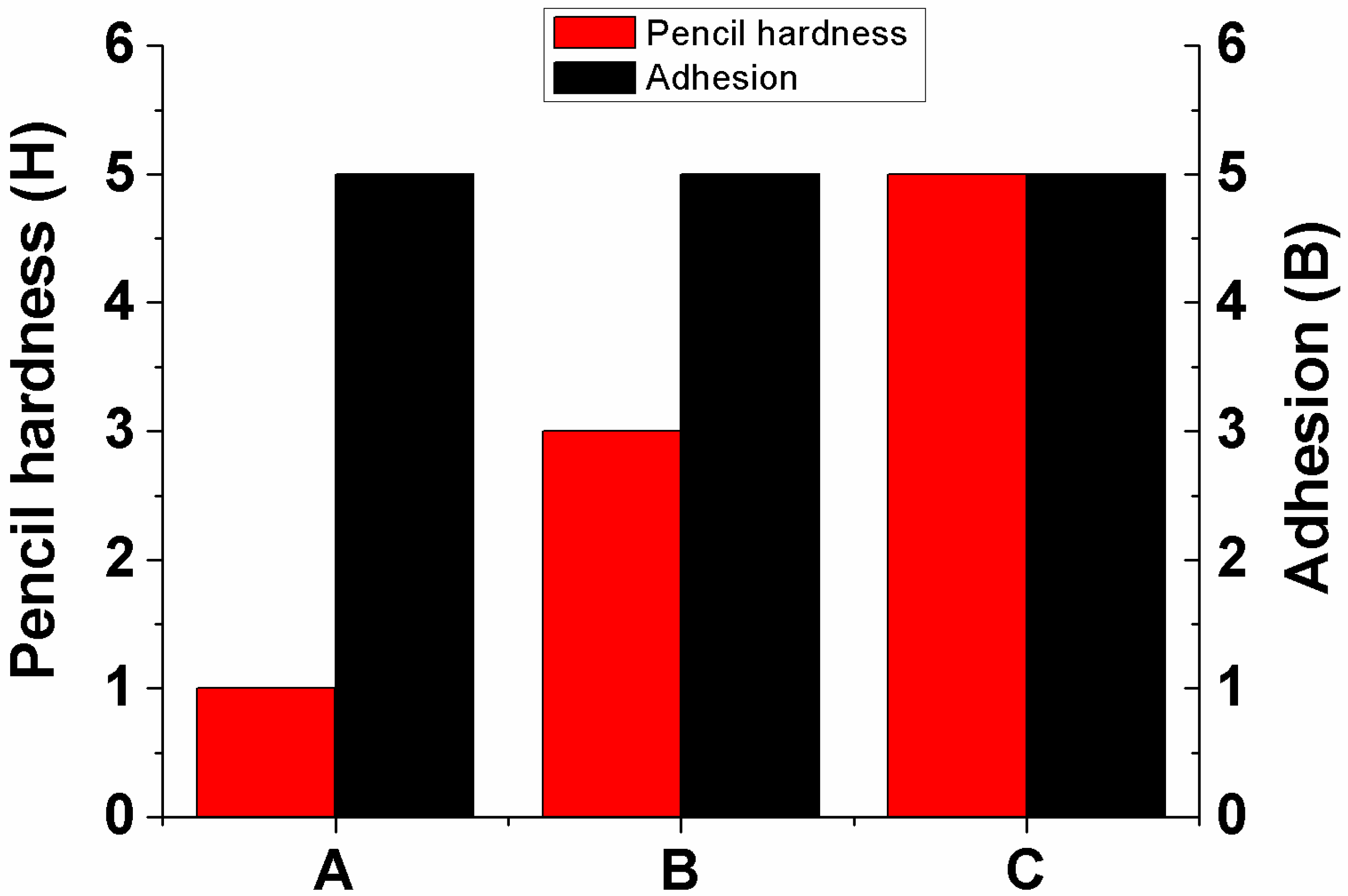
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Immarigeon, J.P.; Holt, R.T.; Koul, A.K.; Zhao, L.; Wallace, W.; Beddoes, J.C. Lightweight Materials for Aircraft Applications. Mater. Charact. 1995, 35, 41–67. [Google Scholar] [CrossRef]
- Antunes, R.A.; Oliveira, M.C.L.; Ett, G.; Ett, V. Corrosion of Metal Bipolar Plates for PEM Fuel Cells: A Review. Int. J. Hydrogen Energy 2010, 35, 3632–3647. [Google Scholar] [CrossRef]
- Deyab, M.A. Corrosion Protection of Aluminum Bipolar Plates with Polyaniline Coating Containing Carbon Nanotubes in Acidic Medium Inside the Polymer Electrolyte Membrane Fuel Cell. J. Power Sour. 2014, 268, 50–55. [Google Scholar] [CrossRef]
- Fan, Z.; Zhu, C.; Chang, F. Effect of Thiourea on the Corrosion Behavior of Aluminum Anode Foil of Aluminum Electrolytic Capacitor. J. Mater. Eng. Perform. 2018, 27, 4168–4175. [Google Scholar] [CrossRef]
- Watanabe, M.; Dokko, K.; Ueno, K.; Thomas, M.L. From Ionic Liquids to Solvate Ionic Liquids: Challenges and Opportunities for Next Generation Battery Electrolytes. Bull. Chem. Soc. Jpn. 2018, 91, 1660–1682. [Google Scholar] [CrossRef]
- Yamada, Y.; Chiang, C.H.; Sodeyama, K.; Wang, J.; Tateyama, Y.; Yamada, A. Corrosion Prevention Mechanism of Aluminum Metal in Superconcentrated Electrolytes. Chem. Electro. Chem. 2015, 2, 1687–1694. [Google Scholar]
- Wang, R.; Li, W.; Liu, L.; Qian, Y.; Liu, F.; Chen, M.; Guo, Y.; Liu, L. Carbon black/graphene-Modified Aluminum Foil Cathode Current Collectors for Lithium Ion Batteries with Enhanced Electrochemical Performances. J. Electroanal. Chem. 2019, 833, 63–69. [Google Scholar] [CrossRef]
- Guillaumin, V.; Mankowski, G. Localized Corrosion of 2024 T351 Aluminium Alloy in Chloride Media. Corros. Sci. 1998, 41, 421–438. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting Corrosion of Aluminum. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Kendig, M.; Jeanjaquet, S.; Addison, R.; Waldrop, J. Role of Hexavalent Chromium in the Inhibition of Corrosion of Aluminum Alloys. Surf. Coat. Technol. 2001, 140, 58–66. [Google Scholar] [CrossRef]
- Kimbrough, D.E.; Cohen, Y.; Winer, A.M.; Creelman, L.; Mabuni, C. A Critical Assessment of Chromium in the Environment. Crit. Rev. Environ. Sci. Technol. 1999, 29, 1–46. [Google Scholar] [CrossRef]
- Costa, M.; Klein, C.B. Toxicity and Carcinogenicity of Chromium Compounds in Humans. Crit. Rev. Toxicol. 2006, 36, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Kulinich, S.A.; Akhtar, A.S. On Conversion Coating Treatments to Replace Chromating for Al Alloys: Recent Developments and Possible Future Directions. Russ. J. Non-ferrous Met. 2012, 53, 176–203. [Google Scholar] [CrossRef]
- Twite, R.L.; Bierwagen, G.P. Review of Alternatives to Chromate for Corrosion Protection of Aluminum Aerospace Alloys. Prog. Org. Coat. 1998, 33, 91–100. [Google Scholar] [CrossRef]
- Barranco, J.; Barreras, F.; Lozano, A.; Lopez, A.M.; Roda, V.; Martin, J.; Maza, M.; Fuentes, G.G.; Almandoz, E. Cr and Zr/Cr Nitride CAE-PVD Coated Aluminum Bipolar Plates for Polymer Electrolyte Membrane Fuel Cells. Int. J. Hydrogen Energy 2010, 35, 11489–11498. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol-Gel Coatings on Metals for Corrosion Protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Voevodin, N.; Jeffcoate, C.; Simon, L.; Khobaib, M.; Donley, M. Characterization of Pitting Corrosion in Bare and Sol-Gel Coated Aluminum 2024-T3 Alloy. Surf. Coat. Technol. 2001, 140, 29–34. [Google Scholar] [CrossRef]
- Fedrizzi, L.; Rodriguez, F.J.; Rossi, S.; Deflorian, F.; Di Maggio, R. The use of Electrochemical Techniques to Study the Corrosion Behaviour of Organic Coatings on Steel Pretreated with Sol-Gel Zirconia Films. Electrochim. Acta 2001, 46, 3715–3724. [Google Scholar] [CrossRef]
- Voevodin, N.N.; Balbyshev, V.N.; Khobaib, M.; Donley, M.S. Nanostructured Coatings Approach for Corrosion Protection. Prog. Org. Coat. 2003, 47, 416–423. [Google Scholar] [CrossRef]
- Kirtay, S. Preparation of Hybrid Silica Sol-Gel Coatings on Mild Steel Surfaces and Evaluation of their Corrosion Resistance. Prog. Org. Coat. 2014, 77, 1861–1866. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Montemor, M.F.; Galio, A.F.; Zheludkevich, M.L.; Trindade, C.; Dick, L.F.; Ferreira, M.G.S. Novel Hybrid Sol-Gel Coatings for Corrosion Protection of AZ31B Magnesium Alloy. Electrochim. Acta 2008, 53, 4773–4783. [Google Scholar] [CrossRef]
- Raps, D.; Hack, T.; Wehr, J.; Zheludkevich, M.L.; Bastos, A.C.; Ferreira, M.G.S.; Nuyken, O. Electrochemical Study of Inhibitor-Containing Organic-Inorganic Hybrid Coatings on AA2024. Corros. Sci. 2009, 51, 1012–1021. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing. In Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 2013; pp. 1–908. [Google Scholar]
- Chico, B.; De La Fuente, D.; Pérez, M.L.; Morcillo, M. Corrosion Resistance of Steel Treated with Different silane/paint Systems. J. Coat. Tech. Res. 2012, 9, 3–13. [Google Scholar] [CrossRef]
- Altube, A.; García-Lecina, E.; Imaz, N.; Díez, J.A.; Ferrón, P.; Aizpurua, J.M. Influence of Deposition Conditions on the Protective Behavior of Tetraethyl Orthosilicate Sol-Gel Films on AA5754 Aluminum Alloy. Prog. Org. Coat. 2012, 74, 281–287. [Google Scholar] [CrossRef]
- Collinson, M.M.; Wang, H.; Makote, R.; Khramov, A. The Effects of Drying Time and Relative Humidity on the Stability of Sol-Gel Derived Silicate Films in Solution. J. Electroanal. Chem. 2002, 519, 65–71. [Google Scholar] [CrossRef]
- Vasconcelos, D.C.L.; Carvalho, J.A.N.; Mantel, M.; Vasconcelos, W.L. Corrosion Resistance of Stainless Steel Coated with Sol-Gel Silica. J. Non Cryst. Solids 2000, 273, 135–139. [Google Scholar] [CrossRef]
- Chou, T.P.; Chandrasekaran, C.; Limmer, S.J.; Seraji, S.; Wu, Y.; Forbess, M.J.; Nguyen, C.; Cao, G.Z. Organic-Inorganic Hybrid Coatings for Corrosion Protection. J. Non Cryst. Solids 2001, 290, 153–162. [Google Scholar] [CrossRef]
- Zand, B.N.; Mahdavian, M. Corrosion and Adhesion Study of Polyurethane Coating on Silane Pretreated Aluminum. Surf. Coat. Technol. 2009, 203, 1677–1681. [Google Scholar] [CrossRef]
- Zheng, S.; Li, J. Inorganic-Organic Sol Gel Hybrid Coatings for Corrosion Protection of Metals. J. Sol Gel Sci. Technol. 2010, 54, 174–187. [Google Scholar] [CrossRef]
- Du, Y.J.; Damron, M.; Tang, G.; Zheng, H.; Chu, C.J.; Osborne, J.H. Inorganic/organic Hybrid Coatings for Aircraft Aluminum Alloy Substrates. Prog. Org. Coat. 2001, 41, 226–232. [Google Scholar]
- Poznyak, S.K.; Zheludkevich, M.L.; Raps, D.; Gammel, F.; Yasakau, K.A.; Ferreira, M.G.S. Preparation and Corrosion Protective Properties of Nanostructured Titania-Containing Hybrid Sol-Gel Coatings on AA2024. Prog. Org. Coat. 2008, 62, 226–235. [Google Scholar] [CrossRef]
- Chou, T.P.; Chandrasekaran, C.; Cao, G.Z. Sol-Gel-Derived Hybrid Coatings for Corrosion Protection. J. Sol Gel Sci. Technol. 2003, 26, 321–327. [Google Scholar] [CrossRef]
- Khramov, A.N.; Voevodin, N.N.; Balbyshev, V.N.; Donley, M.S. Hybrid Organo-Ceramic Corrosion Protection Coatings with Encapsulated Organic Corrosion Inhibitors. Thin Solid Films 2004, 447–448, 549–557. [Google Scholar] [CrossRef]
- Dalmoro, V.; Dos Santos, J.H.Z.; Baibich, I.M.; Butler, I.S.; Armelin, E.; Alemán, C.; Azambuja, D.S. Improving the Corrosion Performance of Hybrid Sol-Gel Matrix by Modification with Phosphonic Acid. Prog. Org. Coat. 2015, 80, 49–58. [Google Scholar] [CrossRef]
- Pepe, A.; Aparicio, M.; Ceré, S.; Durán, A. Preparation and Characterization of Cerium Doped Silica Sol-Gel Coatings on Glass and Aluminum Substrates. J. Non Cryst. Solids 2004, 348, 162–171. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Serra, R.; Montemor, M.F.; Ferreira, M.G.S. Oxide Nanoparticle Reservoirs for Storage and Prolonged Release of the Corrosion Inhibitors. Electrochem. Commun. 2005, 7, 836–840. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Serra, R.; Montemor, M.F.; Yasakau, K.A.; Salvado, I.M.M.; Ferreira, M.G.S. Nanostructured Sol-Gel Coatings Doped with Cerium Nitrate as Pre-Treatments for AA2024-T3 Corrosion Protection Performance. Electrochim. Acta 2005, 51, 208–217. [Google Scholar] [CrossRef]
- Khramov, A.N.; Voevodin, N.N.; Balbyshev, V.N.; Mantz, R.A. Sol-Gel-Derived Corrosion-Protective Coatings with Controllable Release of Incorporated Organic Corrosion Inhibitors. Thin Solid Films 2005, 483, 191–196. [Google Scholar] [CrossRef]
- Montemor, M.F. Functional and Smart Coatings for Corrosion Protection: A Review of Recent Advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Abdullah, A.M.; Younan, N.A. Corrosion Behavior of Superhydrophobic Surfaces: A Review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef]
- Yu, Q.; Xu, J. Structure and Surface Properties of Fluorinated Organic-Inorganic Hybrid Films. J. Sol Gel Sci. Technol. 2012, 61, 243–248. [Google Scholar] [CrossRef]
- Maeztu, J.D.; Rivero, P.J.; Berlanga, C.; Bastidas, D.M.; Palacio, J.F.; Rodriguez, R. Effect of Graphene Oxide and Fluorinated Polymeric Chains Incorporated in a Multilayered Sol-Gel Nanocoating for the Design of Corrosion Resistant and Hydrophobic Surfaces. Appl. Surf. Sci. 2017, 419, 138–149. [Google Scholar] [CrossRef]
- Wankhede, R.G.; Morey, S.; Khanna, A.S.; Birbilis, N. Development of Water-Repellent Organic-Inorganic Hybrid Sol-Gel Coatings on Aluminum using Short Chain Perfluoro Polymer Emulsion. Appl. Surf. Sci. 2013, 283, 1051–1059. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Kim, D.-K.; Lee, S.-B.; Kwon, S.-H.; Kadono, K. Preparation of Water-Repellent Glass by Sol-Gel Process using Perfluoroalkylsilane and Tetraethoxysilane. J. Colloid Interface Sci. 2001, 235, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, R.V.; Bera, P.; Anandan, C.; Basu, B.J. Effect of the Size of Silica Nanoparticles on Wettability and Surface Chemistry of Sol-Gel Superhydrophobic and Oleophobic Nanocomposite Coatings. Appl. Surf. Sci. 2014, 320, 780–786. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Bharathidasan, T.; Basu, B.J. Superhydrophobic Sol-Gel Nanocomposite Coatings with Enhanced Hardness. Appl. Surf. Sci. 2011, 257, 10421–10426. [Google Scholar] [CrossRef]
- Im, H.G.; Park, G.U.; Park, H.Y.; Jin, J.; Kang, D.J. A Robust Transparent Protective Hard-Coating Material using Physicochemically-Incorporated Silica Nanoparticles and Organosiloxanes. Prog. Org. Coat. 2017, 105, 330–335. [Google Scholar] [CrossRef]
- Basu, B.J.; Hariprakash, V.; Aruna, S.T.; Lakshmi, R.V.; Manasa, J.; Shruthi, B.S. Effect of Microstructure and Surface Roughness on the Wettability of Superhydrophobic Sol-Gel Nanocomposite Coatings. J. Sol Gel Sci. Technol. 2010, 56, 278–286. [Google Scholar] [CrossRef]
- Zaharescu, M.; Predoana, L.; Barau, A.; Raps, D.; Gammel, F.; Rosero-Navarro, N.C.; Castro, Y.; Durán, A.; Aparicio, M. SiO2 Based Hybrid Inorganic-Organic Films Doped with TiO2-CeO2 Nanoparticles for Corrosion Protection of AA2024 and Mg-AZ31B Alloys. Corros. Sci. 2009, 51, 1998–2005. [Google Scholar] [CrossRef]
- Radoman, T.S.; Džunuzovic, J.V.; Jeremic, K.B.; Grgur, B.N.; Milicevic, D.S.; Popovic, I.G.; Džunuzovic, E.S. Improvement of Epoxy Resin Properties by Incorporation of TiO2 Nanoparticles Surface Modified with Gallic Acid Esters. Mater. Des. 2014, 62, 158–167. [Google Scholar] [CrossRef]
- Balaskas, A.C.; Kartsonakis, I.A.; Tziveleka, L.A.; Kordas, G.C. Improvement of Anti-Corrosive Properties of Epoxy-Coated AA 2024-T3 with TiO2 Nanocontainers Loaded with 8-Hydroxyquinoline. Prog. Org. Coat. 2012, 74, 418–426. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Du, Y. Effect of Nano-Sized Titanium Powder Addition on Corrosion Performance of Epoxy Coatings. Surf. Coat. Technol. 2007, 201, 7241–7245. [Google Scholar] [CrossRef]
- Radetic, M. Functionalization of Textile Materials with TiO2 nanoparticles. J. Photochem. Photobiol. C Photochem. Rev. 2013, 16, 62–76. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lou, Y.; Zhang, C.; Yin, S.; Li, H.; Sun, D.; Sun, X. Improved Corrosion Resistance and Antibacterial Properties of Composite Arch-Wires by N-Doped TiO2 coating. RSC Adv. 2017, 7, 43938–43949. [Google Scholar] [CrossRef]
- De Falco, G.; Ciardiello, R.; Commodo, M.; Del Gaudio, P.; Minutolo, P.; Porta, A.; D’Anna, A. TiO2 Nanoparticle Coatings with Advanced Antibacterial and Hydrophilic Properties Prepared by Flame Aerosol Synthesis and Thermophoretic Deposition. Surf. Coat. Technol. 2018, 349, 830–837. [Google Scholar] [CrossRef]
- Cravanzola, S.; Jain, S.M.; Cesano, F.; Damin, A.; Scarano, D. Development of a Multifunctional TiO2/MWCNT Hybrid Composite Grafted on a Stainless Steel Grating. RSC Adv. 2015, 5, 103255–103264. [Google Scholar] [CrossRef]
- Bell, N.J.; Ng, Y.H.; Du, A.; Coster, H.; Smith, S.C.; Amal, R. Understanding the Enhancement in Photoelectrochemical Properties of Photocatalytically Prepared TiO2-Reduced Graphene Oxide Composite. J. Phys. Chem. C 2011, 115, 6004–6009. [Google Scholar] [CrossRef]
- Cairns, D.R.; Kessman, A.J.; Richter, P.J.; Bottari, F.J.; Randall, N.X. Mechanical and Tribological Investigations of Sol-Gel Derived SiO2 Optical Coatings. Wear 2008, 265, 411–416. [Google Scholar] [CrossRef]
- Sermon, P.A.; Leadley, J.G. Fluoroalkylsilane Modification of Sol-Gel SiO2-TiO2 Coatings. J. Sol Gel Sci. Technol. 2004, 32, 293–296. [Google Scholar] [CrossRef]
- Liu, W.; Luo, Y.; Sun, L.; Wu, R.; Jiang, H.; Liu, Y. Fabrication of the Superhydrophobic Surface on Aluminum Alloy by Anodizing and Polymeric Coating. Appl. Surf. Sci. 2013, 264, 872–878. [Google Scholar] [CrossRef]
- Rivero, P.; Yurrita, D.; Berlanga, C.; Palacio, J.F.; Rodriguez, R. Functionalized electrospun fibers for the design of novel hydrophobic and anticorrosive surfaces. Coatings 2018, 8, 300. [Google Scholar] [CrossRef]
- Liang, J.; Wang, L.; Bao, J.; He, L. SiO2-g-PS/fluoroalkylsilane Composites for Superhydrophobic and Highly Oleophobic Coatings. Colloids Surf. A Physicochem. Eng. Asp. 2016, 507, 26–35. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, F.; Hao, J.; Liu, W. Effects of System Parameters on Making Aluminum Alloy Lotus. J. Colloid Interface Sci. 2006, 303, 298–305. [Google Scholar] [CrossRef] [PubMed]


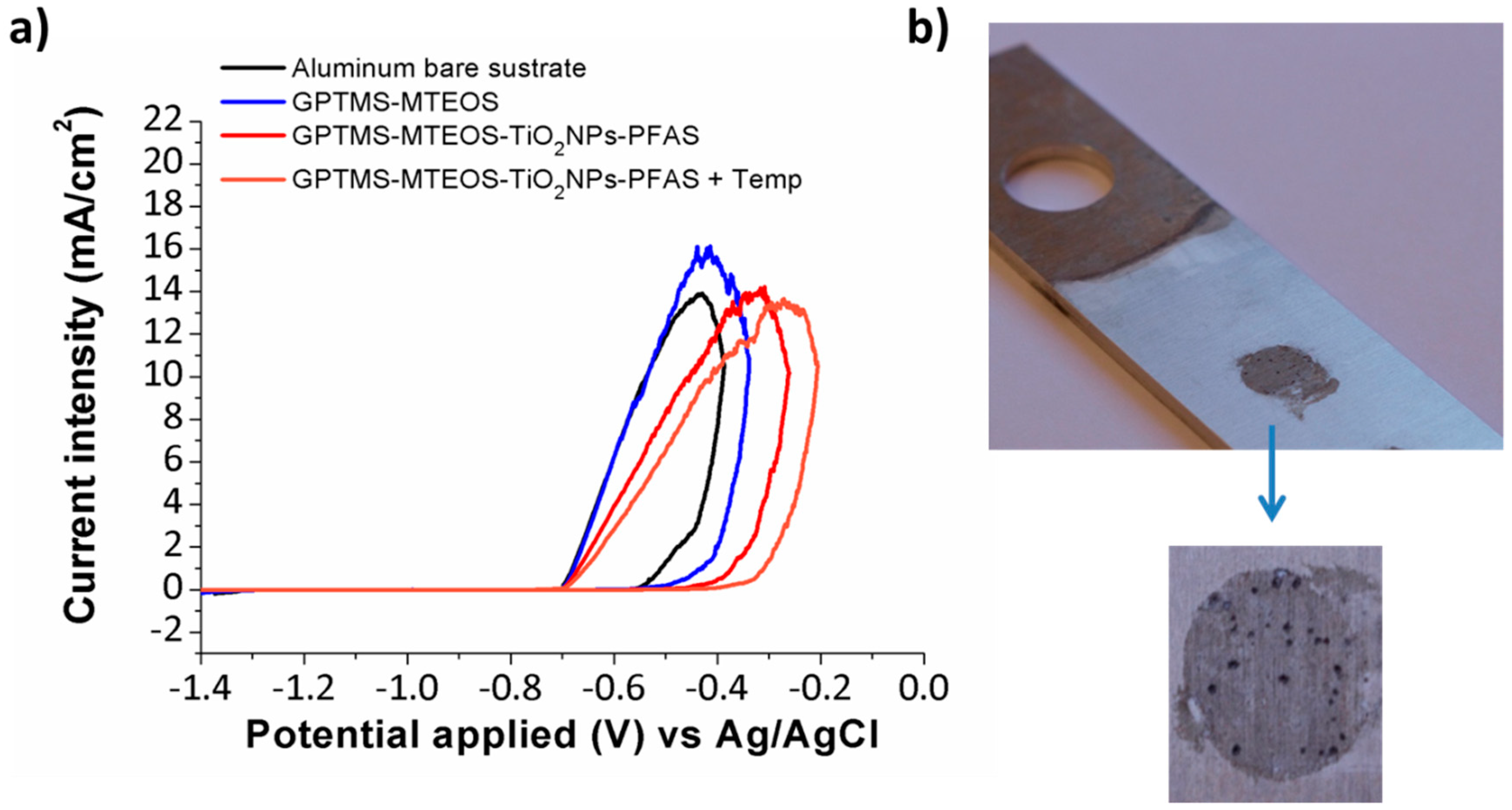
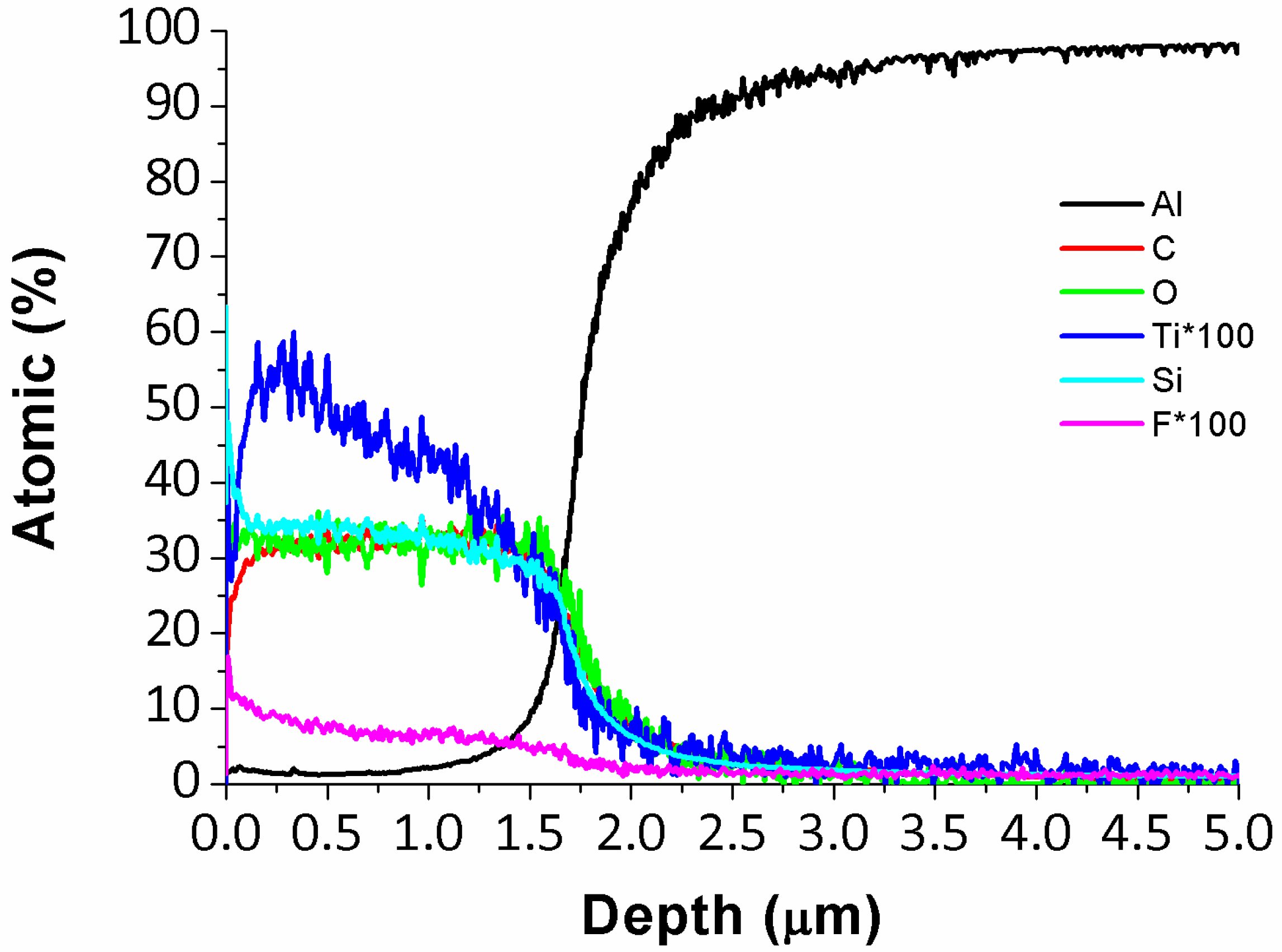
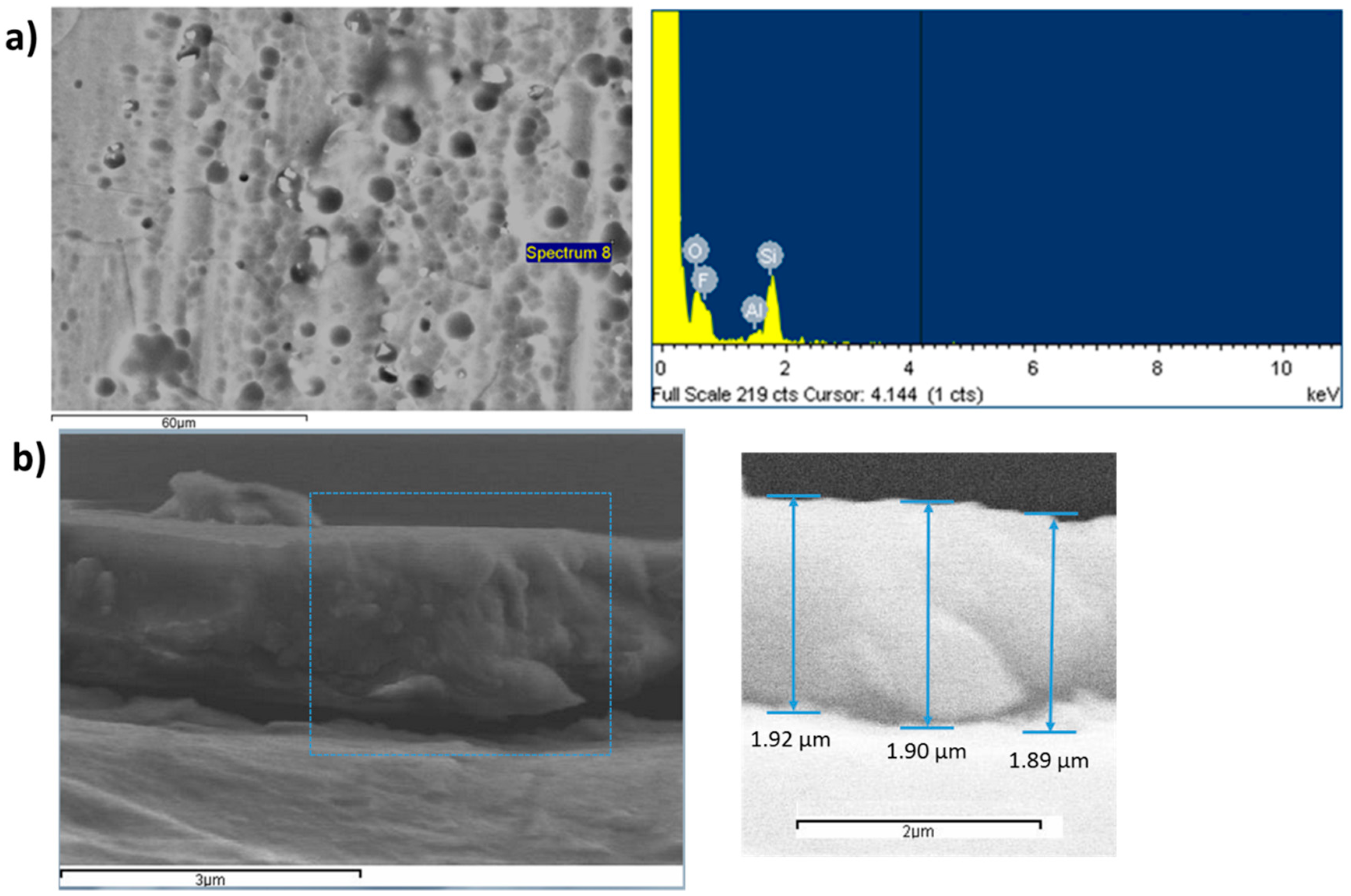
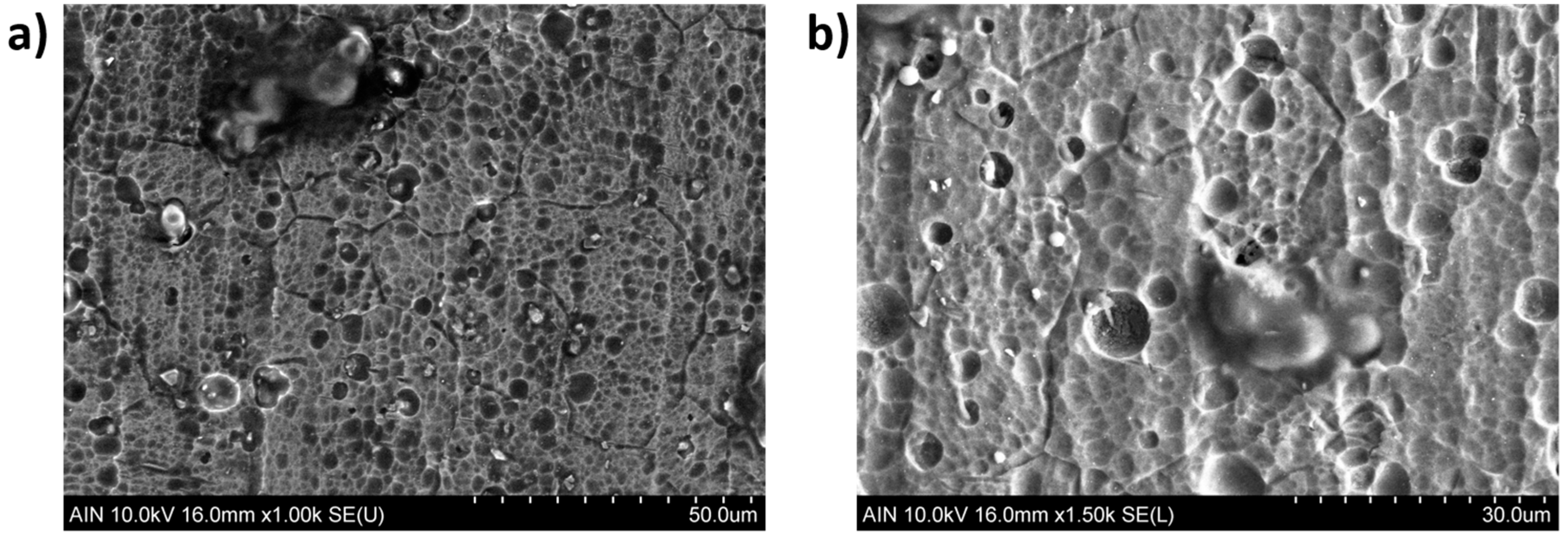
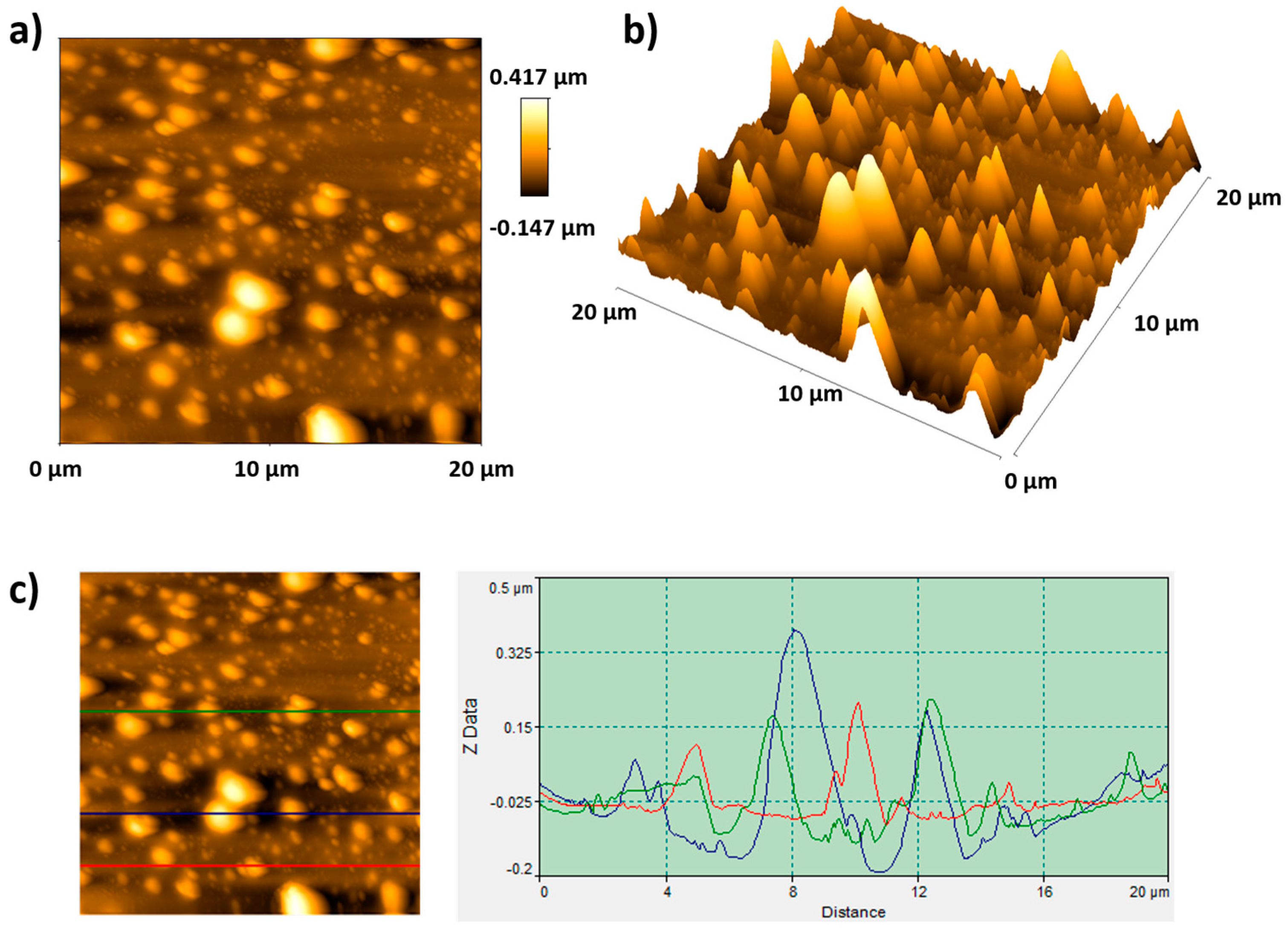
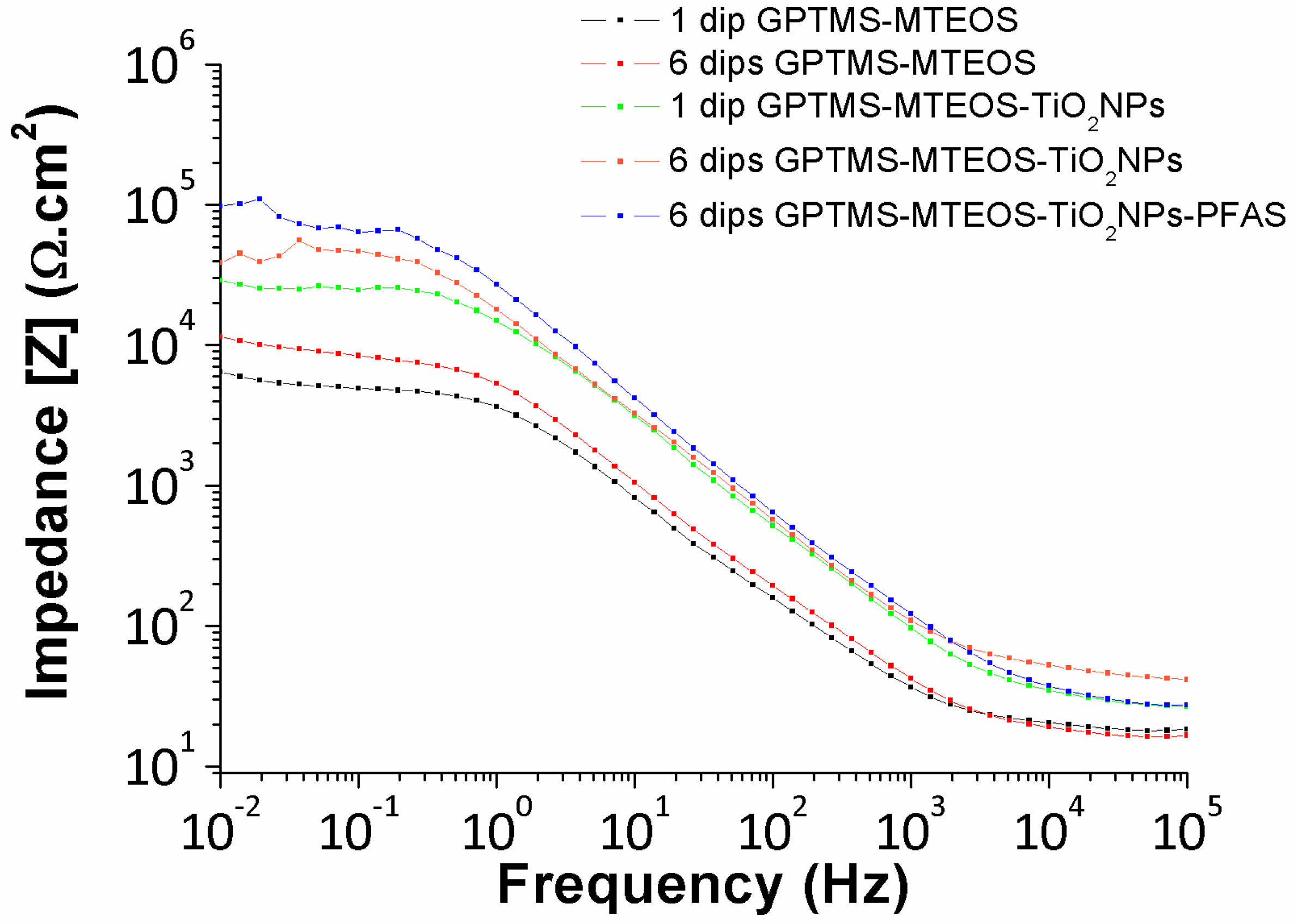
| Metallic Substrate | Precursors for the Fabrication of the Sol-Gel Coating | Active Agent | Ref. |
|---|---|---|---|
| AA2024-T3 | Tetramethylortosilicate | - | [17] |
| Carbon steel | Zirconium tetrabutoxide | - | [18] |
| Mild steel | 3-glycidoxypropyltrimethoxysilane (GPTMS) and aminopropylethoxysilane | - | [20] |
| AA5754 | Tetraethylorthosilicate (TEOS) | - | [25] |
| AISI 304 | Tetraethylorthosilicate (TEOS) | - | [27] |
| AISI 304 | Tetraethylorthosilicate (TEOS) and 3-methacryloxypropyltrimethoxysilane (MPS) | - | [28] |
| AA2024-T3 | 3-glycidoxypropyltrimethoxysilane (GPTMS) and titanium organic compounds | - | [32] |
| AA2024-T3 | Tetramethoxysilane (TMOS) and 3-glycidoxypropyltrimethoxysilane (GPTMS) | Organic corrosion inhibitors | [34] |
| AA2024-T3 | Vinyltrimethoxysilane (VTMS) and tetraethylorthosilicate (TEOS) | Ethylenediamine tetra (methylene phosponic acid) | [35] |
| AA3005 | Tetraethylorthosilicate (TEOS) and methyltriethoxysilane (MTES) | Cerium salts | [36] |
| AA2024-T3 | Tetramethoxysilane (TMOS) and 3-glycidoxypropyltrimethoxysilane (GPTMS) | Organic corrosion inhibitors | [39] |
| AA6061T6 | Methyltriethoxysilane (MTEOS), 3-glycidoxypropyltrimethoxysilane (GPTMS), and perfluoroalkylsilane | Graphene oxide | [43] |
| AA2024 | Tetraethylorthosilicate (TEOS) and 3-methoxysilylpropylmethacrylate (TSPM) | TiO2-CeO2 nanoparticles | [50] |
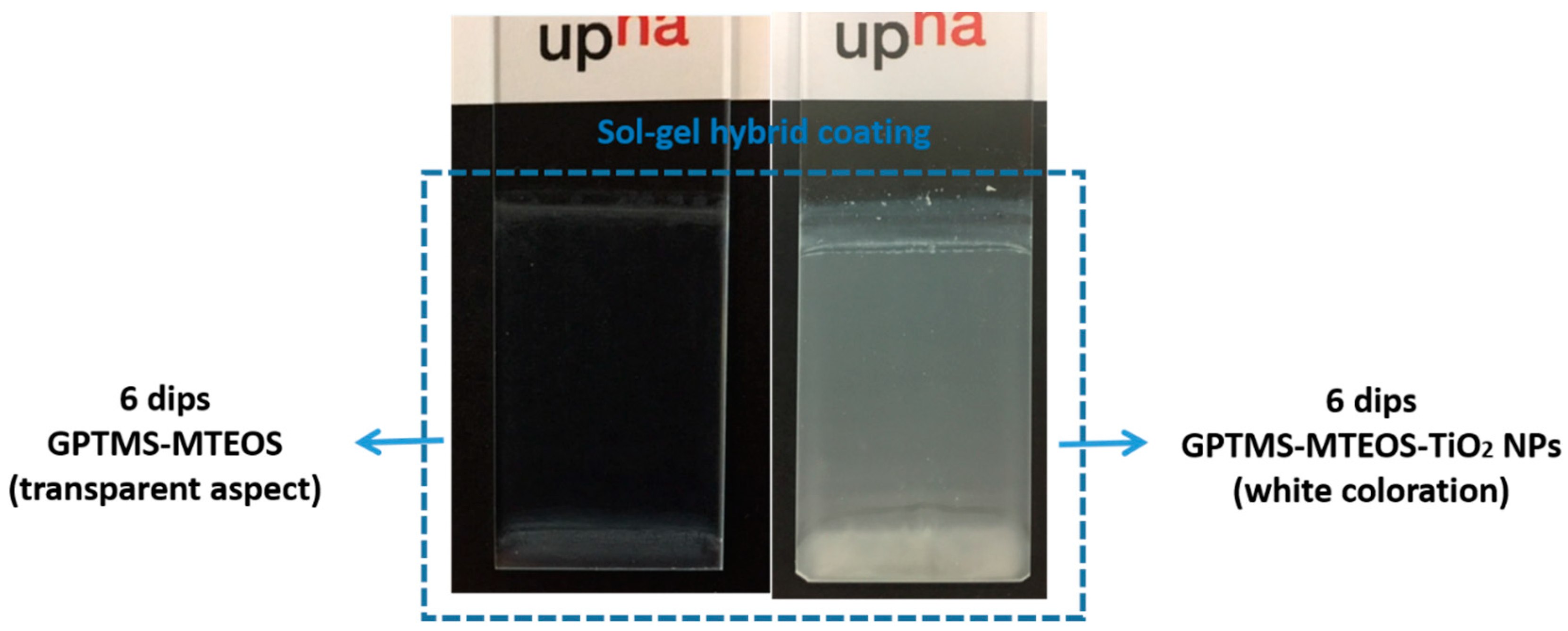
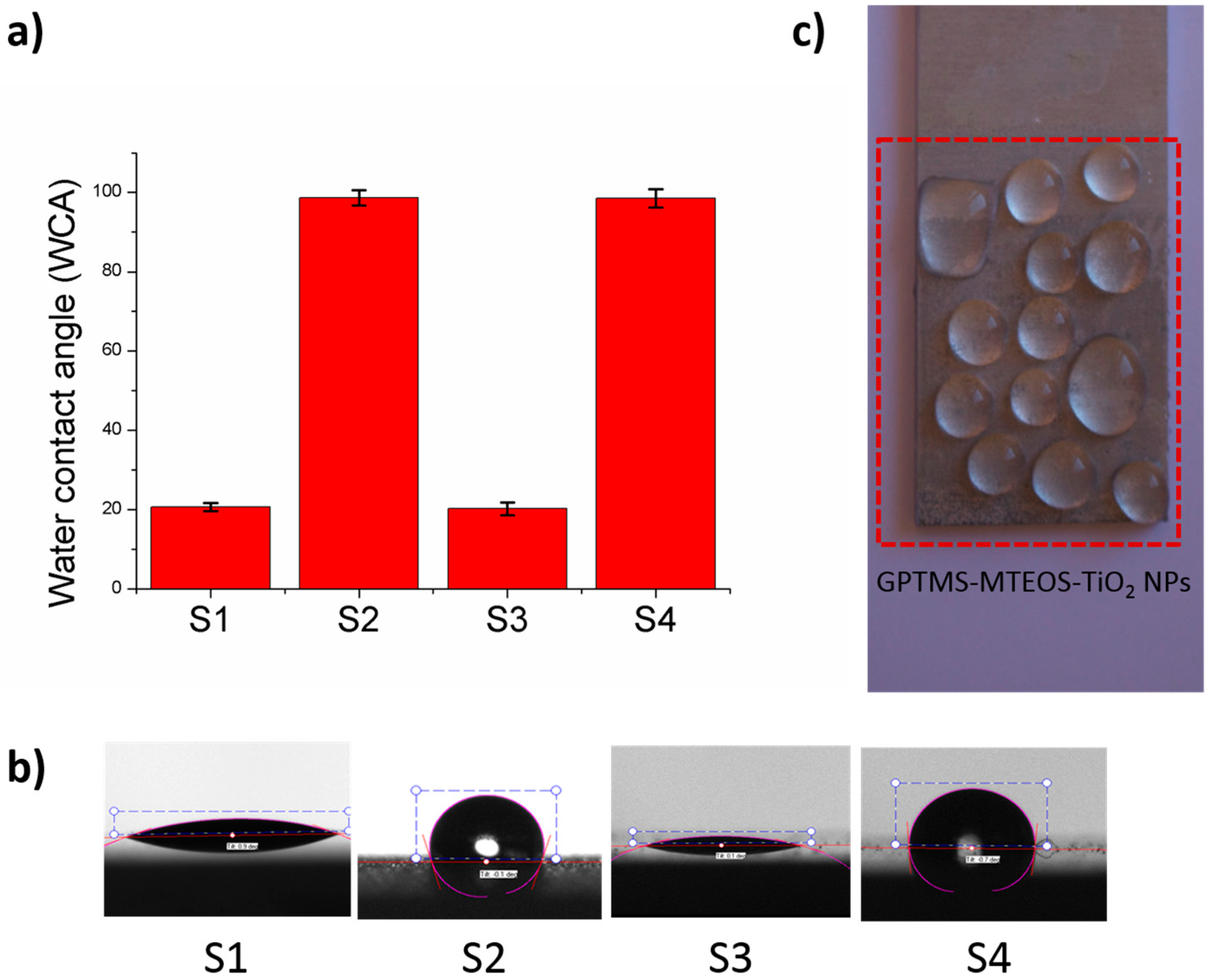
| Sample | Coating | Number of Dips | Water Contact Angle Value (WCA) |
|---|---|---|---|
| 1 | GPTMS-MTEOS | 1 | 20.66 ± 1.17 |
| 2 | GPTMS-MTEOS-TiO2 NPs | 1 | 98.61 ± 2.11 |
| 3 | GPTMS-MTEOS | 6 | 20.28 ± 1.59 |
| 4 | GPTMS-MTEOS-TiO2 NPs | 6 | 98.51 ± 2.32 |
| Coating | Number of Dips | Thermal Treatment | Water Contact Angle Value (WCA) |
|---|---|---|---|
| GPTMS-MTEOS | 6 | No | 20.28 ± 1.59 |
| GPTMS-MTEOS-TiO2 NPs | 6 | No | 98.51 ± 2.32 |
| (GPTMS-MTEOS-TiO2 NPs) + PFAS | 6 | No | 107.11 ± 1.75 |
| (GPTMS-MTEOS-TiO2 NPs) + PFAS | 6 | Yes | 120.76 ± 2.17 |
| Coating | Number of Dips | Thermal Treatment | Pitting Corrosion Potential |
|---|---|---|---|
| Aluminum bare substrate | - | - | −570 mV |
| GPTMS-MTEOS | 6 | No | −520 mV |
| (GPTMS-MTEOS) + PFAS | 6 | No | −480 mV |
| (GPTMS-MTEOS-TiO2 NPs) + PFAS | 6 | No | −450 mV |
| (GPTMS-MTEOS-TiO2 NPs) + PFAS | 6 | Yes | −400 mV |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivero, P.J.; Maeztu, J.D.; Berlanga, C.; Miguel, A.; Palacio, J.F.; Rodriguez, R. Hydrophobic and Corrosion Behavior of Sol-Gel Hybrid Coatings Based on the Combination of TiO2 NPs and Fluorinated Chains for Aluminum Alloys Protection. Metals 2018, 8, 1076. https://doi.org/10.3390/met8121076
Rivero PJ, Maeztu JD, Berlanga C, Miguel A, Palacio JF, Rodriguez R. Hydrophobic and Corrosion Behavior of Sol-Gel Hybrid Coatings Based on the Combination of TiO2 NPs and Fluorinated Chains for Aluminum Alloys Protection. Metals. 2018; 8(12):1076. https://doi.org/10.3390/met8121076
Chicago/Turabian StyleRivero, Pedro J., Juan Deyo Maeztu, Calos Berlanga, Adrian Miguel, José F. Palacio, and Rafael Rodriguez. 2018. "Hydrophobic and Corrosion Behavior of Sol-Gel Hybrid Coatings Based on the Combination of TiO2 NPs and Fluorinated Chains for Aluminum Alloys Protection" Metals 8, no. 12: 1076. https://doi.org/10.3390/met8121076
APA StyleRivero, P. J., Maeztu, J. D., Berlanga, C., Miguel, A., Palacio, J. F., & Rodriguez, R. (2018). Hydrophobic and Corrosion Behavior of Sol-Gel Hybrid Coatings Based on the Combination of TiO2 NPs and Fluorinated Chains for Aluminum Alloys Protection. Metals, 8(12), 1076. https://doi.org/10.3390/met8121076








