Kinetics of the Volume Shrinkage of a Magnetite/Carbon Composite Pellet during Solid-State Carbothermic Reduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experimental Procedure
3. Results
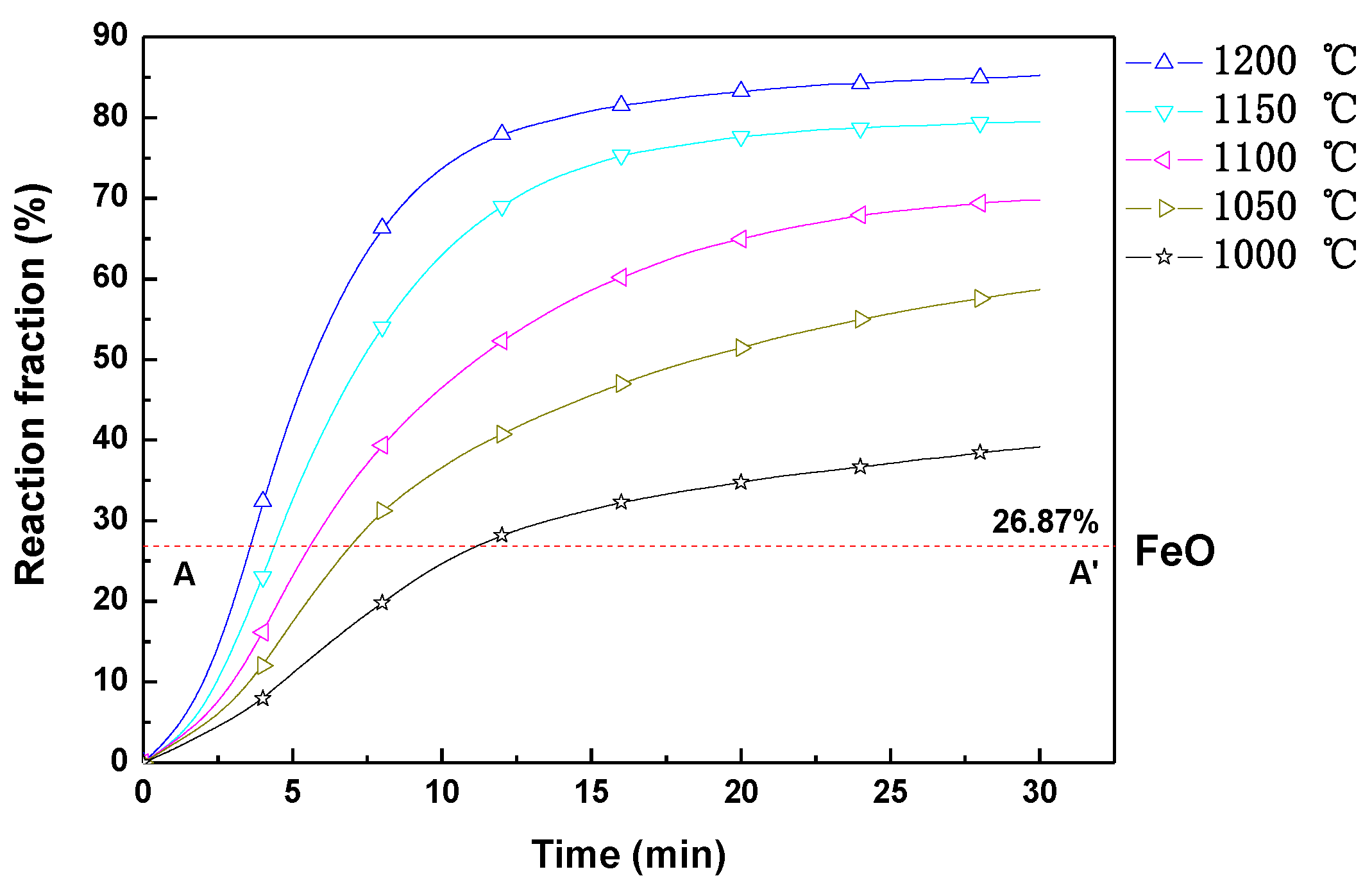
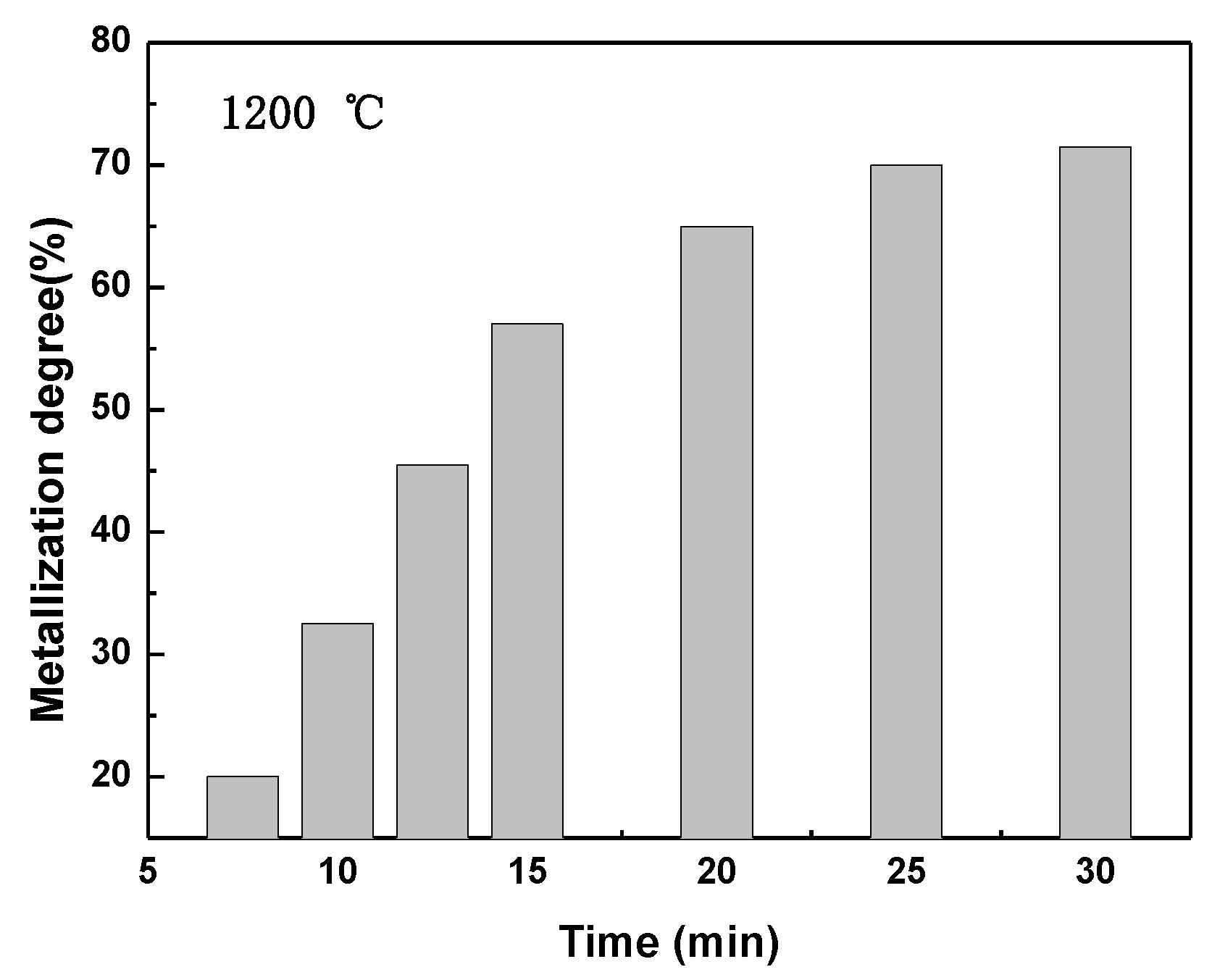
3.1. Variation of the Reaction Fraction and Mineral Phase Composition of the Pellet during Reduction
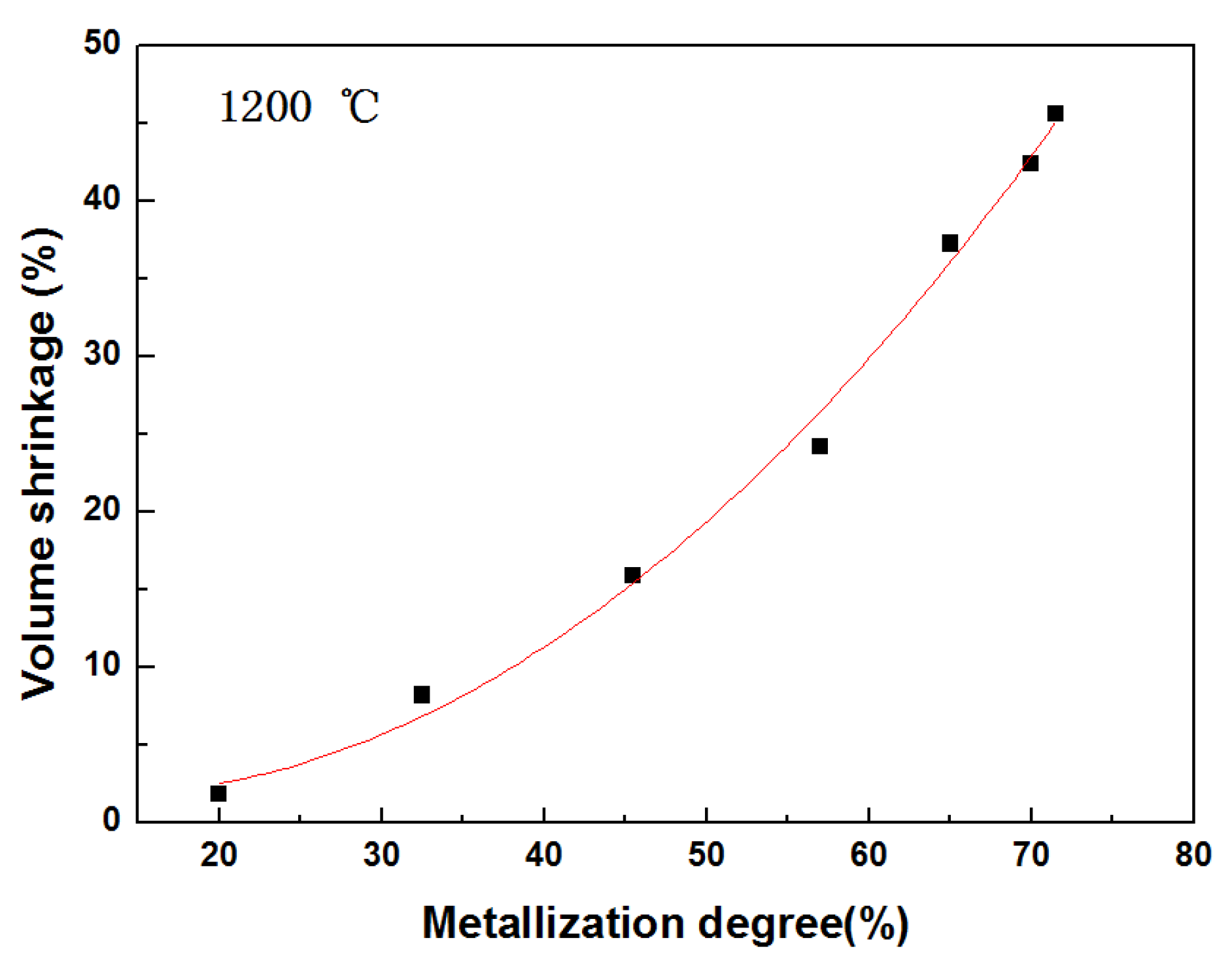
3.2. Volume Shrinkage of Pellets during Reduction
3.3. Shrinkage Kinetics Analysis
4. Discussion
4.1. Shrinkage Driving Forces
4.2. Time-Dependent Phenomena of Shrinkage Kinetics
4.3. Sensitivity Analysis for Shrinkage Kinetics Results
5. Conclusions
- (1)
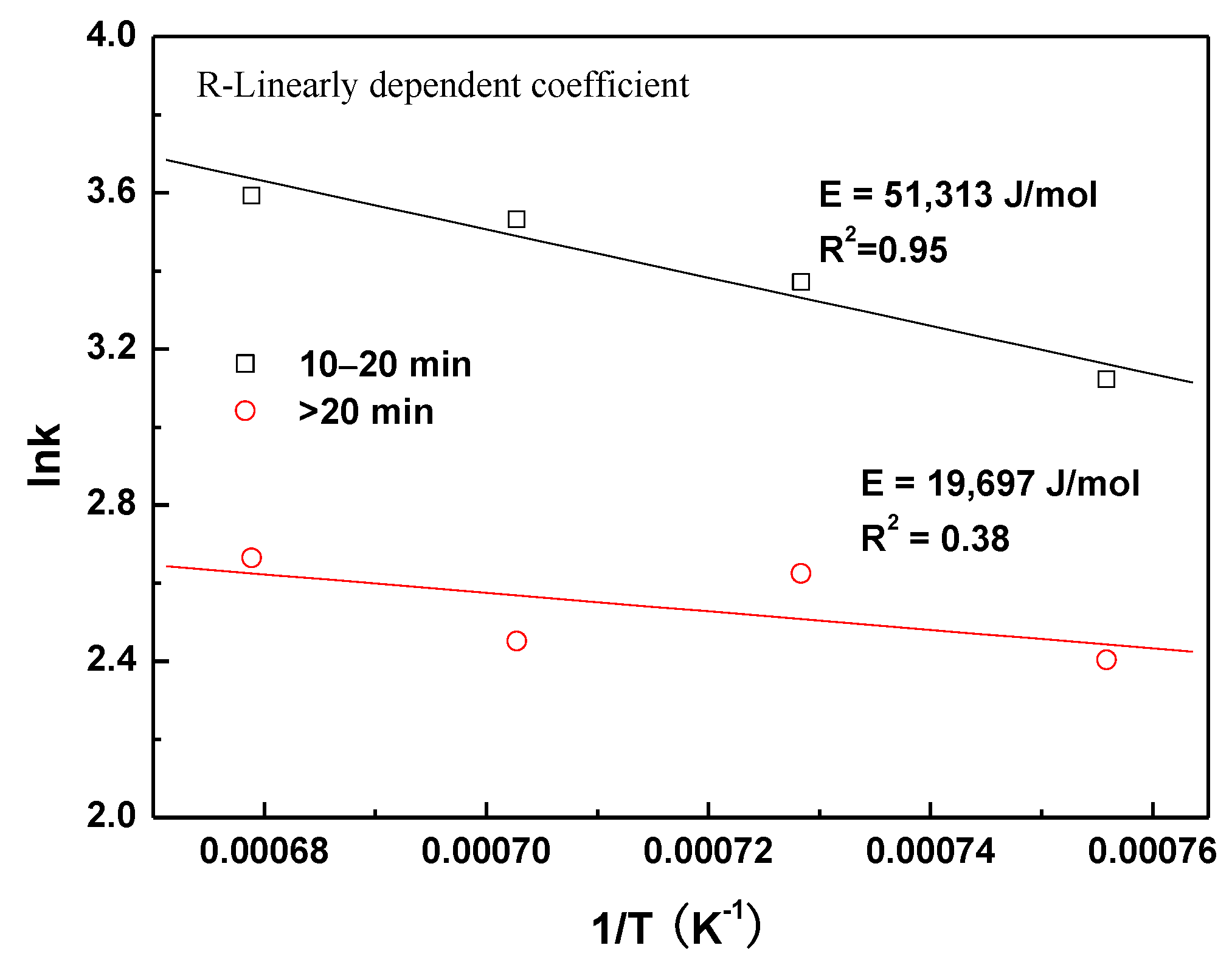
- Volume shrinkage of the composite pellet increases with the increasing of reduction temperature, and the shrinkage rate is faster within the initial 20 min. The values of shrinkage apparent activation energy are different at different time ranges. During pellet reduction before the 20-min timepoint, the shrinkage apparent activation energy is 51,313 J/mol due to the complicated reactions. After the 20-min timepoint, the apparent activation energy of volume shrinkage is only 19,697 J/mol.
- (2)
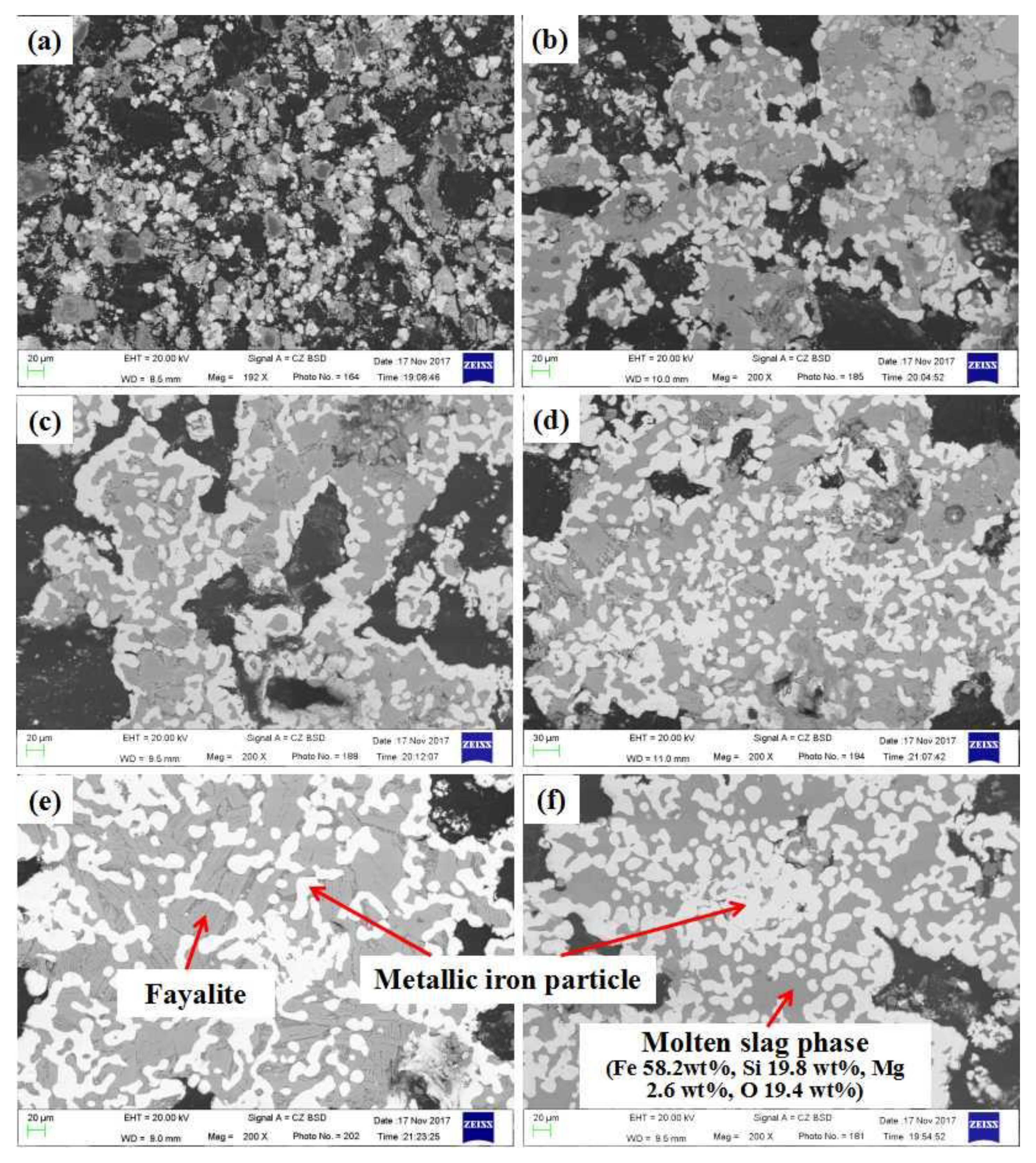
- The volume shrinkage of the composite pellet during reduction mainly results from the mass loss of carbon and oxygen and the sintering of the metallic iron particles and gangue oxides. With the increasing of reduction temperature and time, the formation of large metallic iron crystals and molten slag phase plays an important role in the further shrinkage of the reduced pellet in the final reduction stage.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Mori, T.; Kuwabara, M. Mechanism of carbothermic reduction of hematite in hematite-carbon composite pellets. ISIJ Int. 2007, 47, 1394–1400. [Google Scholar] [CrossRef]
- Bauer, K.; Huette, D.; Lehmkuehler, H.; Schmauch, H. Recycling of iron and steelworks wastes using the Inmetco direct reduction process. Metall. Plant. Technol. 1990, 13, 74–87. [Google Scholar]
- McClelland, J.M.; Metius, G.E. Recycling ferrous and nonferrous waste streams with FASTMET. JOM 2003, 55, 30–34. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.S.; Ding, Y.G.; Ma, S.; Xue, Q.G. New separation method of boron and iron from ludwigite based on carbon bearing pellet reduction and melting technology. ISIJ Int. 2012, 52, 45–51. [Google Scholar] [CrossRef]
- Ding, Y.G.; Wang, J.S.; Wang, G.; Xue, Q.G. Innovative methodology for separating of rare earth and iron from Bayan Obo complex iron ore. ISIJ Int. 2012, 52, 1772–1777. [Google Scholar] [CrossRef]
- Chen, Y.; Hwang, T.; Marsh, M.; Williams, J.S. Mechanically activated carbothermic reduction of ilmenite. Metall. Mater. Trans. A 1997, 28, 1115–1121. [Google Scholar] [CrossRef]
- Anameric, B.; Kawatra, S.K. Laboratory study related to the production and properties of pig iron nuggets. Miner. Metall. Proc. 2006, 23, 52–56. [Google Scholar] [CrossRef]
- Kikuchi, S.; Ito, S.; Kobayashi, I.; Tsuge, O.; Tokuda, K. ITmk3 process. Kobelco. Techno. Rev. 2010, 29, 77–84. [Google Scholar]
- Halder, S.; Fruehan, R.J. Reduction of iron-oxide-carbon composites: part III. shrinkage of composite pellets during reduction. Metall. Mater. Trans. B 2008, 39, 809–817. [Google Scholar] [CrossRef]
- Donskoi, E.; McElwain, D.L.S. Mathematical modelling of non-isothermal reduction in highly swelling iron ore-coal char composite pellet. Ironmak. Steelmak. 2001, 28, 384. [Google Scholar] [CrossRef]
- Donskoi, E.; McElwain, D.L.S. Estimation and modeling of parameters for direct reduction in iron ore/coal composites: Part I. Physical parameters. Metall. Mater. Trans. B 2003, 34, 93. [Google Scholar] [CrossRef]
- Wang, G.; Xue, Q.G.; Wang, J.S. Volume shrinkage of ludwigite/coal composite pellet during isothermal and non-isothermal reduction. Thermochim. Acta 2015, 621, 90–98. [Google Scholar] [CrossRef]
- Prakash, S. Reduction and sintering of fluxed iron ore pellets-a comprehensive review. J. South. Afr. Inst. Min. Metall. 1996, 96, 3–16. [Google Scholar]
- McAdam, G.; O’Brien, D.; Marshall, T. Rapid reduction of New Zealand ironsand. Ironmak. Steelmak. 1977, 4, 1–9. [Google Scholar]
- Sun, Y.S.; Gao, P.; Han, Y.X.; Ren, D.Z. Reaction behavior of iron minerals and metallic iron particles growth in coal-based reduction of an oolitic iron ore. Ind. Eng. Chem. Res. 2013, 52, 2323–2329. [Google Scholar] [CrossRef]
- Vu-Bac, N.; Lahmer, T.; Zhuang, X.; Nguyen-Thoi, T.; Rabczuk, T. A software framework for probabilistic sensitivity analysis for computationally expensive models. Adv. Eng. Softw. 2016, 100, 19–31. [Google Scholar] [CrossRef]
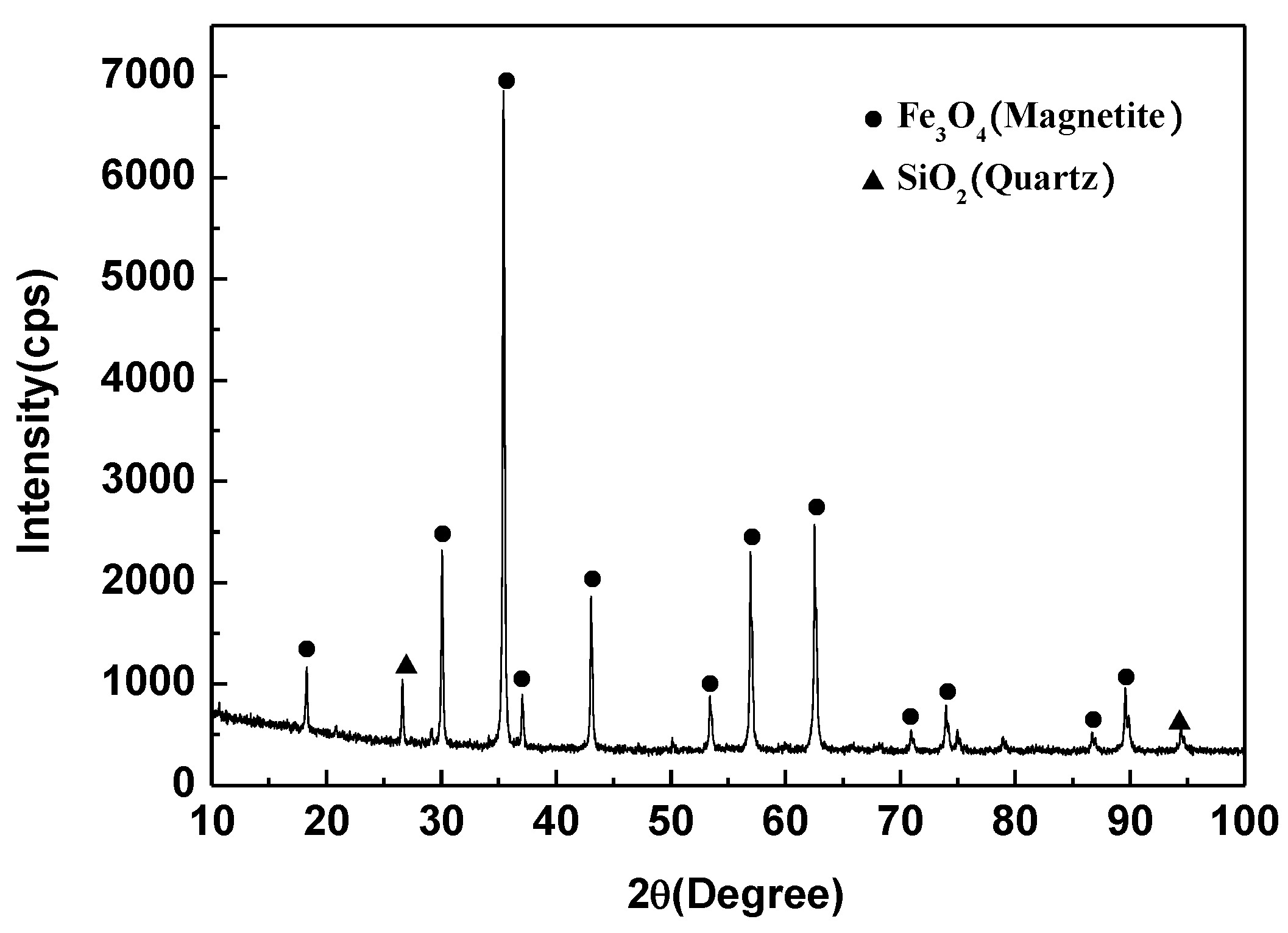
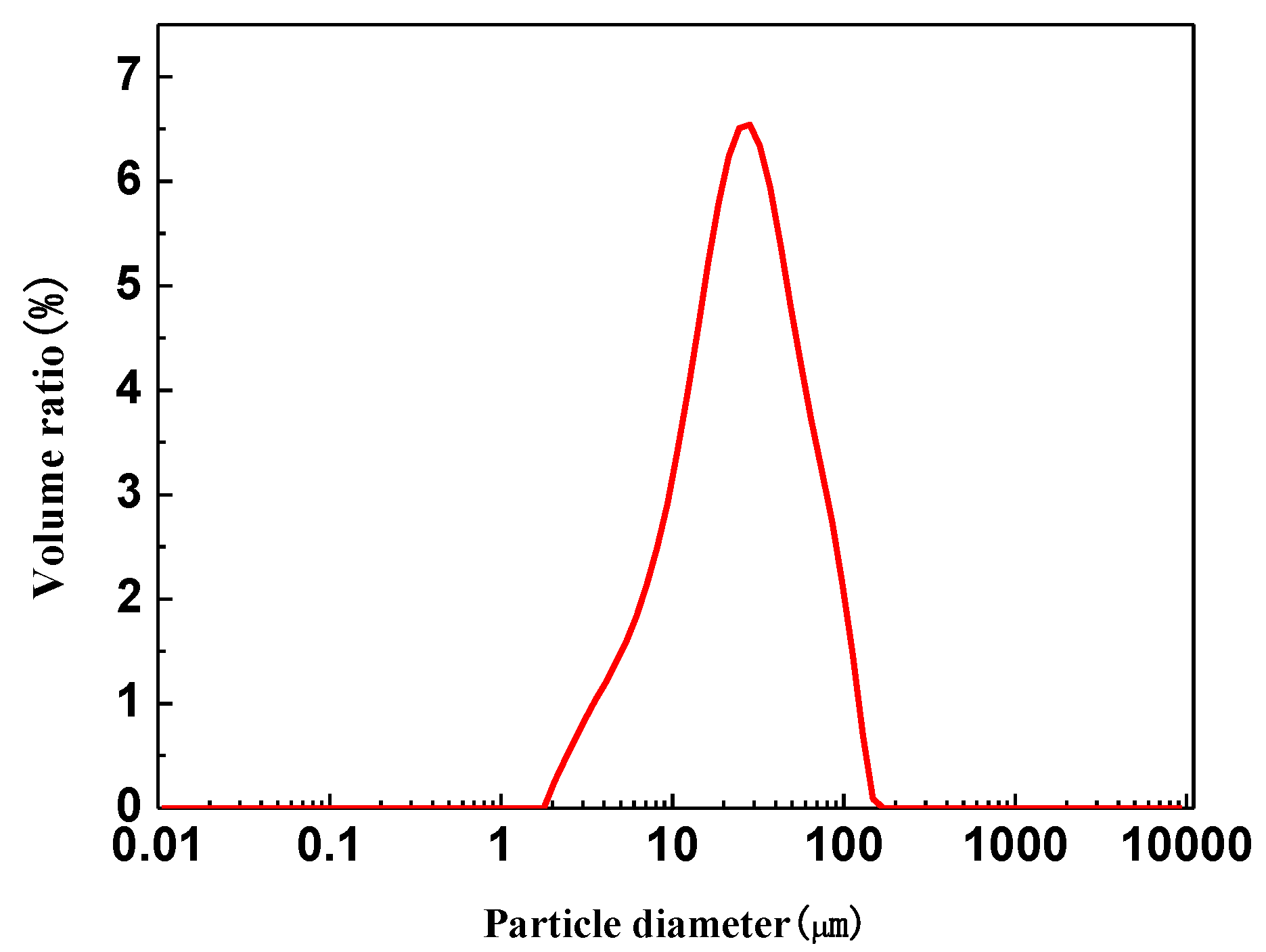
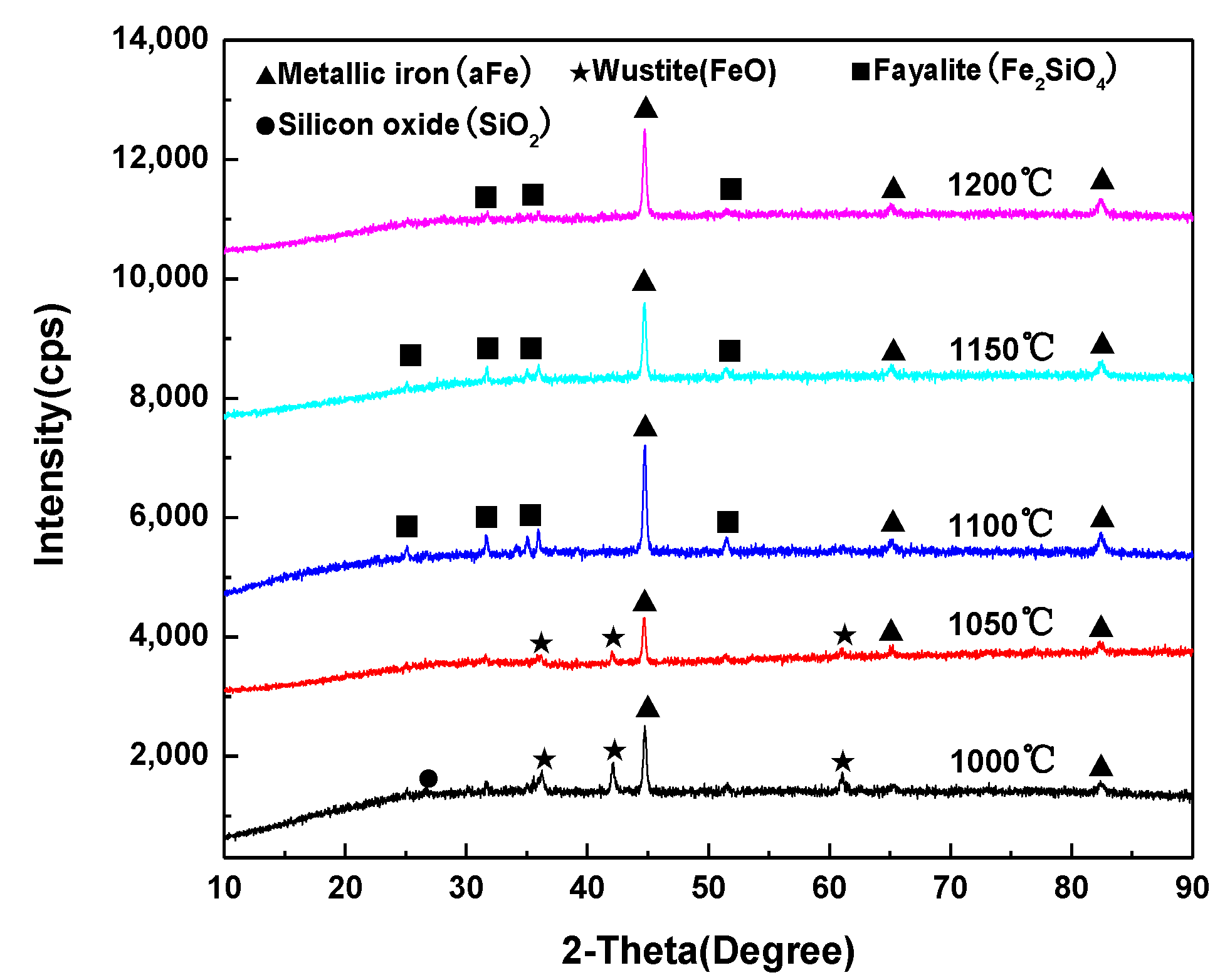

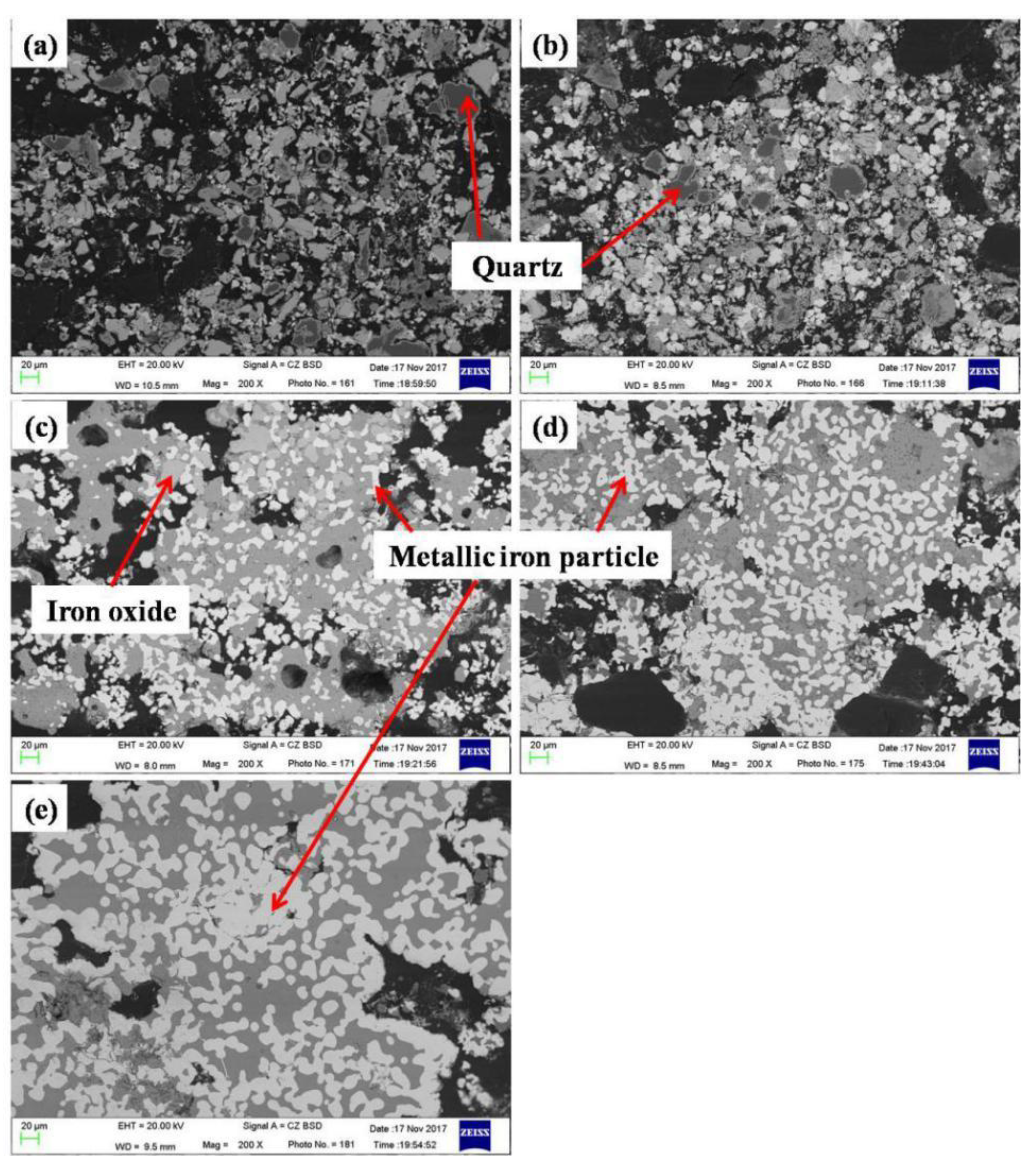
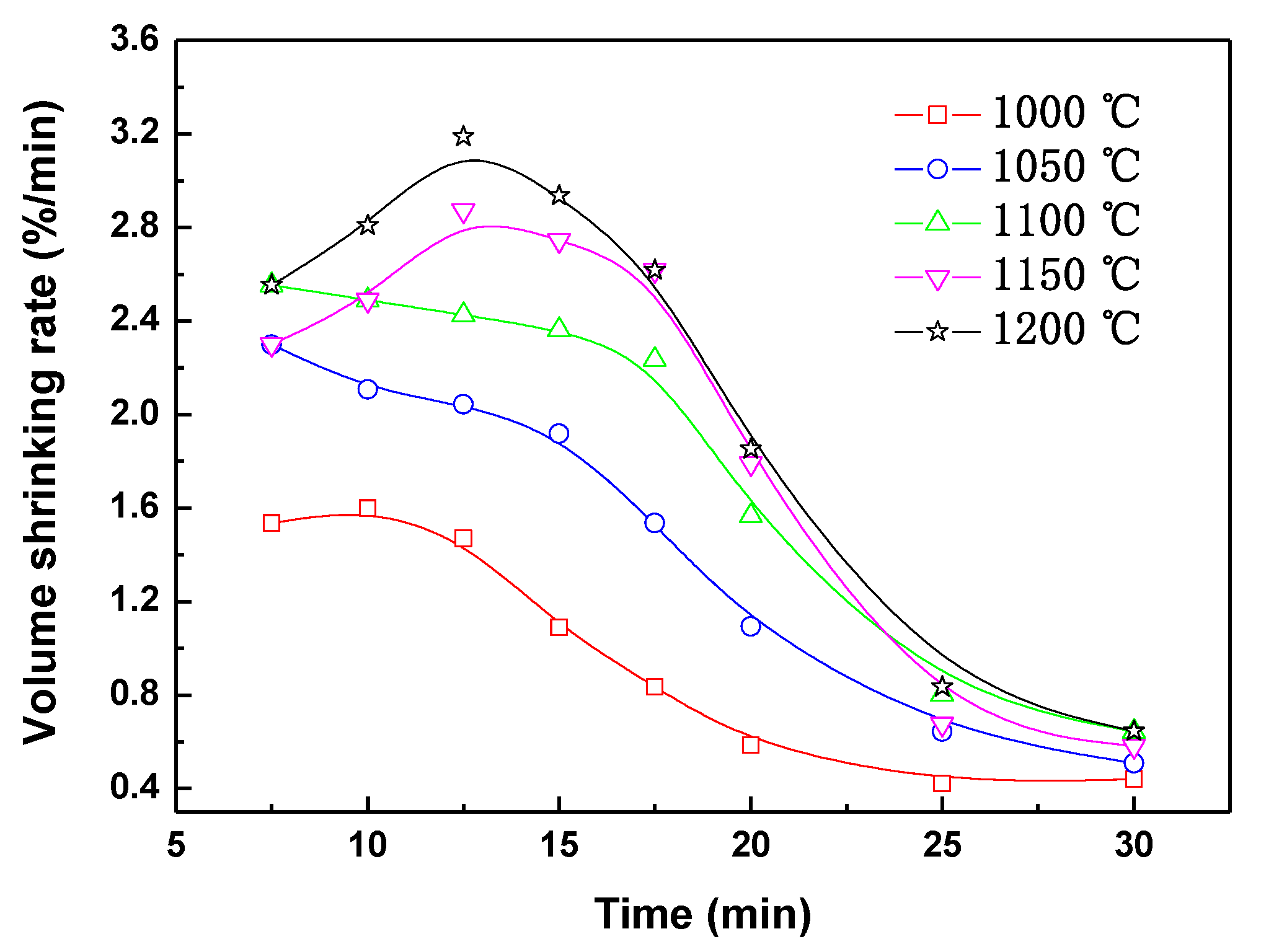
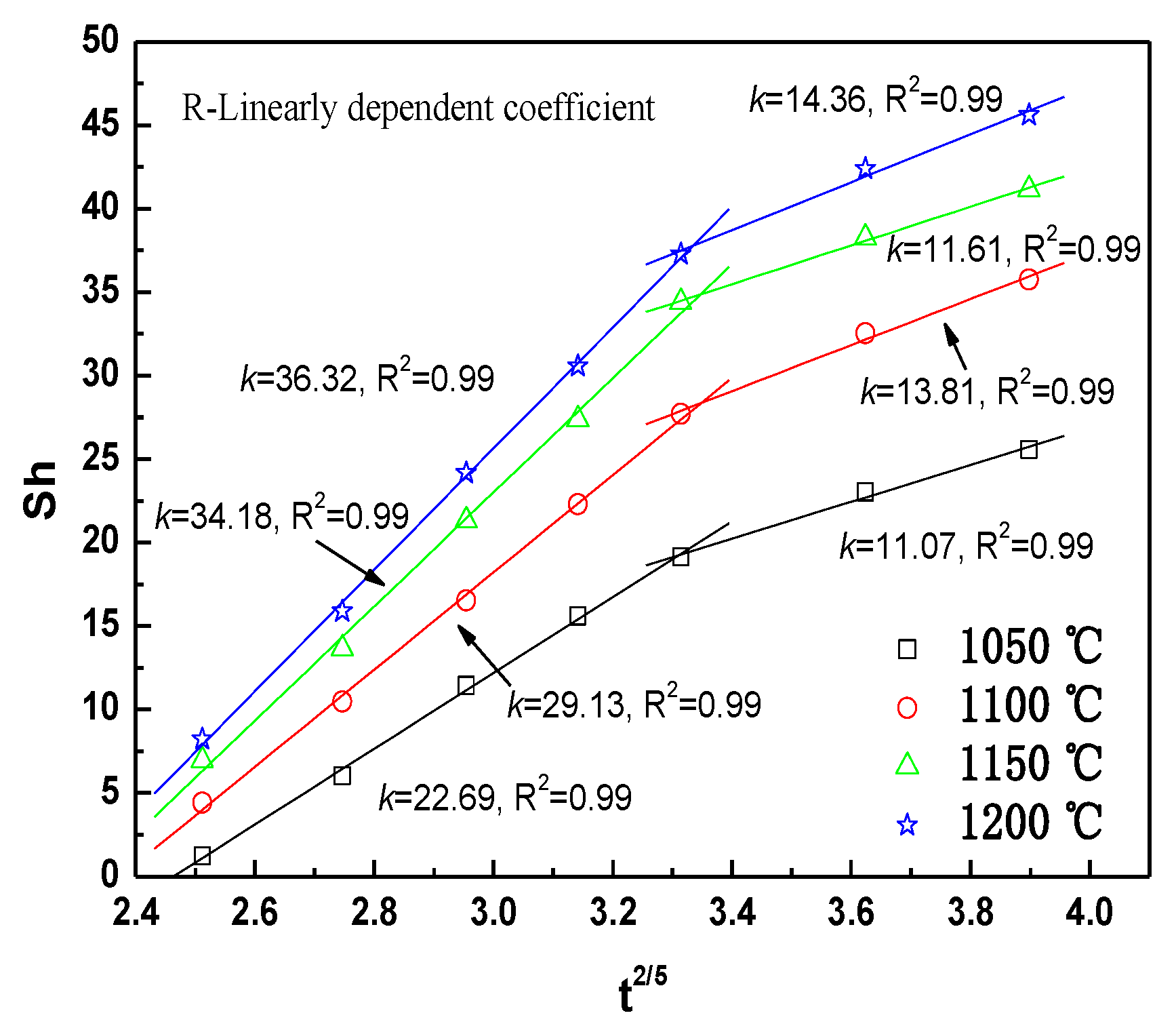

| Iron Concentrate | TFe | FeO | SiO2 | Al2O3 | CaO | MgO | P | S |
| 64.6 | 29.3 | 7.21 | 0.18 | 0.17 | 0.20 | 0.01 | 0.21 | |
| Reducing Agent | FCd | Vd | Ad | S | ||||
| 81.40 | 6.40 | 11.10 | 0.34 | |||||
| Temperature (°C) | Metallic Iron Particle Size (μm) | Fayalite Fraction (vol %) | Density (g/cm3) |
|---|---|---|---|
| 1000 | 3.5 | not detected/no data | 2.36 |
| 1050 | 6.1 | not detected/no data | 2.58 |
| 1100 | 7.3 | 36.9 | 2.81 |
| 1150 | 9.8 | 34.2 | 2.91 |
| 1200 | 16.5 | 29.9 | 3.02 |
| Temperature(°C) | 10–20 min (a(T)1) | >20 min (a(T)2) |
|---|---|---|
| 1050 | −55.89 | −17.41 |
| 1100 | −69.17 | −17.88 |
| 1150 | −79.54 | −4.00 |
| 1200 | −83.33 | −10.11 |
| 1050 °C | 1100 °C | 1150 °C | 1200 °C |
|---|---|---|---|
| 2.15 | 4.50 | 4.88 | 3.85 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Wang, J.; Xue, Q. Kinetics of the Volume Shrinkage of a Magnetite/Carbon Composite Pellet during Solid-State Carbothermic Reduction. Metals 2018, 8, 1050. https://doi.org/10.3390/met8121050
Wang G, Wang J, Xue Q. Kinetics of the Volume Shrinkage of a Magnetite/Carbon Composite Pellet during Solid-State Carbothermic Reduction. Metals. 2018; 8(12):1050. https://doi.org/10.3390/met8121050
Chicago/Turabian StyleWang, Guang, Jingsong Wang, and Qingguo Xue. 2018. "Kinetics of the Volume Shrinkage of a Magnetite/Carbon Composite Pellet during Solid-State Carbothermic Reduction" Metals 8, no. 12: 1050. https://doi.org/10.3390/met8121050
APA StyleWang, G., Wang, J., & Xue, Q. (2018). Kinetics of the Volume Shrinkage of a Magnetite/Carbon Composite Pellet during Solid-State Carbothermic Reduction. Metals, 8(12), 1050. https://doi.org/10.3390/met8121050




