3.1. Austenite Grain Growth Behavior
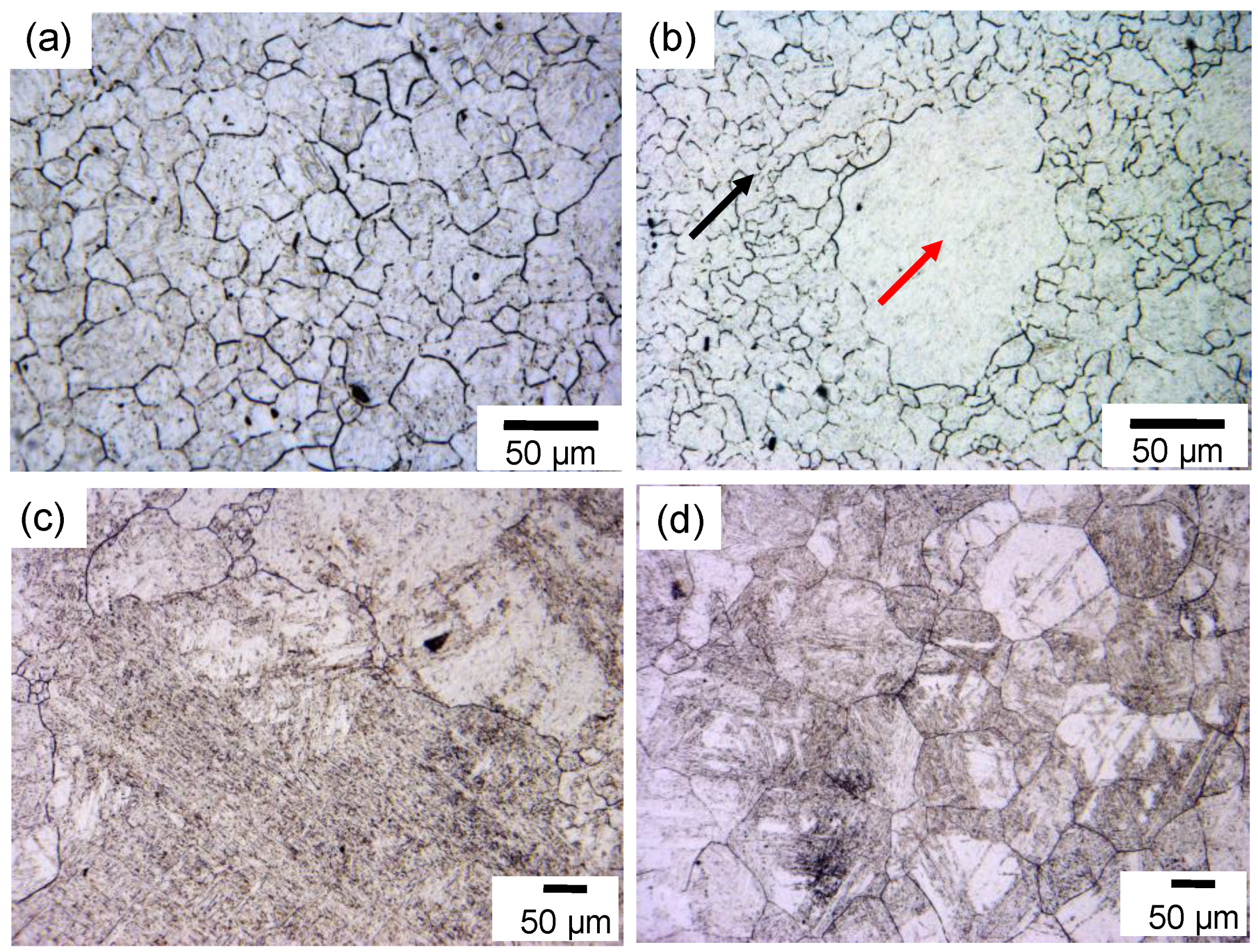
Figure 2 shows the prior austenite grain structures (now martensite after quenching) of the solution-treated (ST) specimens after annealing at different temperatures for various periods of time. Microstructure observation revealed that the austenite grain growth during annealing at 1298 K is very slow and the prior austenite grain size almost has no change. Hence, only the microstructure after annealing at 1298 K for 1800 s is shown
Figure 2a. It is clear that fine and uniform prior austenite grain structures were formed, which is attributed to the occurrence of NGG. However, mixed prior austenite grain structures were formed after the specimen was annealed at 1323 K for 60 s, as shown in
Figure 2b, where abnormal coarse grain (as indicated by the red arrow) mixed with fine matrix grain structures (as indicated by the black arrow) were observed. After a prolonged holding time of up to 1800 s, extremely coarse grain structures were developed (
Figure 2c). Hence, typical AGG occurred at this temperature. After the sample was annealed at 1423 K for 1800 s, slightly coarse but uniform grain structures were formed (
Figure 2d). This indicates that the grain growth switched to NGG mode again.
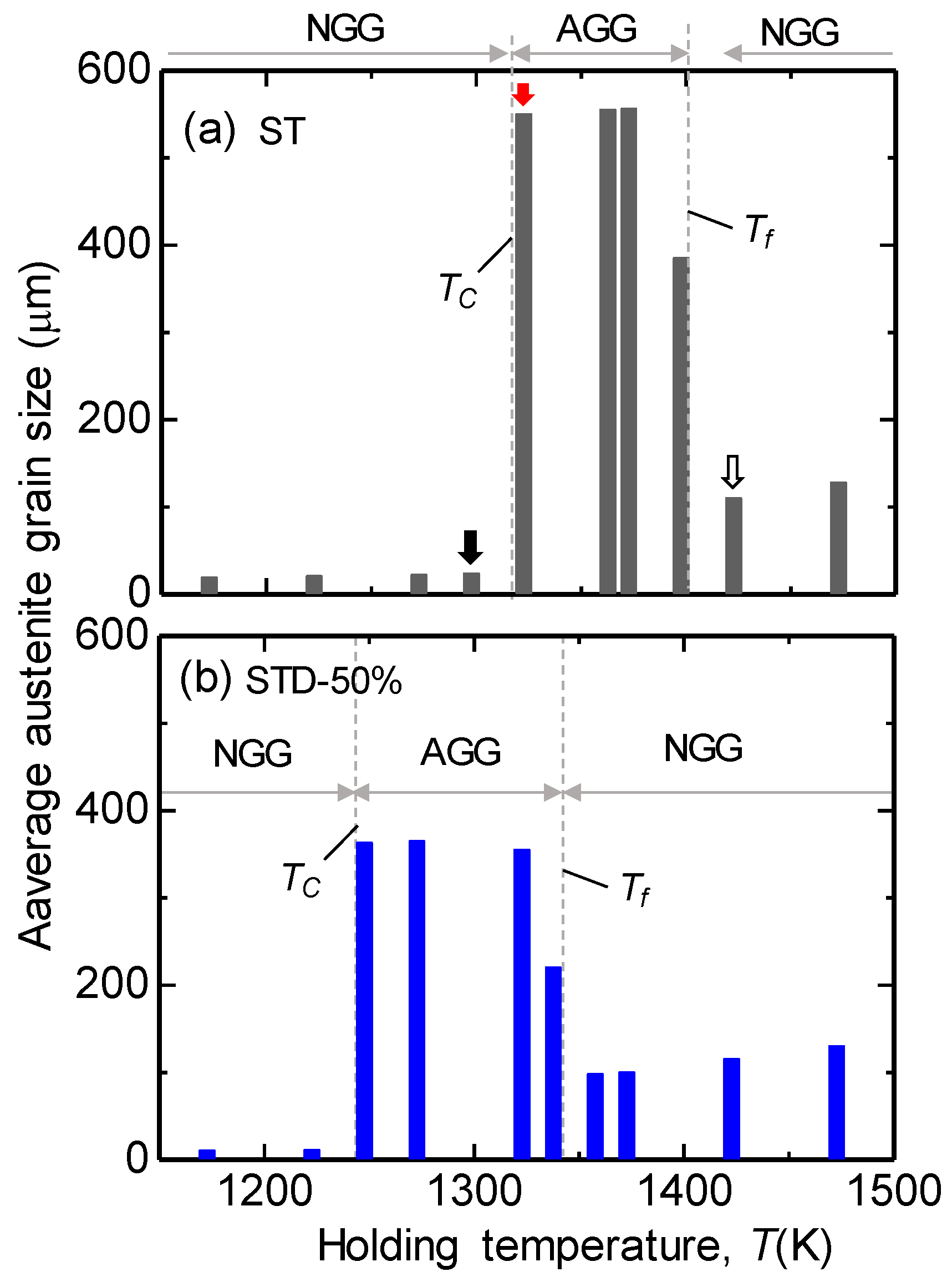
According to the above results, the occurrence AGG led to extremely coarse grain structures, while the NGG led to relatively fine grain structures after annealing. Therefore, the mean austenite grain size of the ST specimens after annealed at various temperatures for 1800 s were plotted in
Figure 3a to show the austenite grain growth behavior during annealing. The average austenite grain sizes after annealing at 1298 K (
Figure 2a), 1323 K (
Figure 2c), and 1423 K (
Figure 2d) are indicated by a black solid, red solid, and open arrows, correspondingly. From
Figure 3a, it can be seen that the prior austenite grain size is fine, and that it has almost no change below 1298 K. This indicates that the austenite grain growth was greatly retarded, and it grew under the NGG mode below 1298 K. The coarse grains that were formed by AGG appeared at intermediate temperatures. Slightly coarse grains that were formed by NGG appeared at high temperatures. Therefore, the ST specimens experienced “normal → abnormal → normal” grain growth modes that were usually observed in the micro-alloyed steels [
5]. The grain growth modes and the transition temperatures of
Tc and
Tf are indicated in
Figure 3a. It is necessary to point out that the grain growth behaviors were examined carefully by changing the annealing temperatures within 25 K intervals, and that only partial results are shown in
Figure 3a.
To understand the effects of cold-deformation on the austenite grain growth behavior, the average austenite grain sizes at various temperatures in the solution-treated and cold-deformed by 50% (STD-50%) specimens were examined. The measured austenite grain size as a function of annealing temperature is summarized in
Figure 3b. A similar grain structure changing tendency was observed as in that of the ST specimens; viz., a fine grain structure at low temperatures, a coarse grain structure at intermediate temperatures, and slightly coarse and uniform grain structures at high temperatures. Therefore, the STD-50% specimen experienced “normal → abnormal → normal” grain growth modes as well. The prior austenite grain structure evolution process was similar to that of the ST specimens, but at different temperatures; therefore, the microstructures are not shown here again. Importantly, comparing
Figure 3a,b shows that the AGG temperature range was shifted toward a low temperature by cold-deformation.
To gain an overall image of the effect of cold-deformation on austenite grain growth behavior, the austenite grain growth modes against annealing temperatures in the specimens deformed with various reduction ratios are summarized in
Figure 4. The “normal → abnormal → normal” grain growth modes occurred in all the specimens. Importantly, the AGG temperature range was gradually depressed toward low temperatures with the increase in the reduction ratio, which was almost saturated up to 70%. The decreased
Tc and
Tf temperatures, induced by the cold-deformation, can be explained by its influences on the evolution of the AlN precipitates, which is discussed in the following section.
3.2. The Evolution of AlN Precipitates
As reviewed in the Introduction, the stability of the second-phase particles during annealing plays a key role in the austenite grain growth behavior. To understand the reasons for the suppression of the AGG temperature range by the cold-deformation, the evolution of the AlN particles were carefully studied by TEM observations. The volume fractions and sizes of the AlN particles in both the ST and STD-50% specimens quenched from various temperatures from 1073 to 1353 K are summarized in
Figure 5a,b, respectively. For comparison, the equilibrium volume fraction of the AlN particles calculated from Equation (1) is also plotted in
Figure 5a, as shown by the blue broken line.
It should be pointed out that after the solution treatment, the AlN precipitates should have been fully dissolved into the austenite. It was reported that the precipitation of AlN is difficult during the cooling process, and that it is sensitive to the cooling rate [
18,
19,
20]. The precipitation of AlN can be entirely suppressed at a cooling rate larger than 1 K/s [
21], which is approximately equivalent to air cooling a 50 mm diameter steel bar [
22]. Therefore, the AlN precipitation during the air-cooling process is negligible in this study. The AlN precipitates should mainly be re-precipitated during the reheating process in both the ST and STD specimens.
According to the precipitation-time-temperature (PTT) diagram of AlN [
23], the AlN precipitation should have been completed below 1073 K in the current steel during the annealing process. Therefore, the AlN particles within the specimens quenched from 1073 K (ferrite + austenite two phase region) were examined. According to
Figure 5a,b, fine- and near-equilibrium AlN particles precipitated in the STD-50% specimen at 1073 K. Meanwhile, a much lower volume fraction of the AlN particles than the equilibrium was precipitated in the ST specimen. These indicate that the AlN particles have been almost fully precipitated in the STD-50% specimen, while the AlN particles were only partially been precipitated in the ST specimen. The near-equilibrium precipitation in the STD-50% specimen should result from the dislocation-induced precipitation of AlN [
11]. It needs to point out that the slightly overestimated AlN volume fraction in the STD-50% specimen compared to the equilibrium prediction should be caused by the differences between the metallographic observation and theoretical calculation.
It is necessary to point out that as the annealing temperature was raised up to 1173 K (just after the ferrite-to-austenite transformation), the AlN particles in the STD-50% specimen were less coarsened than that of the ST specimen (
Figure 5b). This could be more clearly seen from the TEM images of the AlN particles in both the undeformed and cold-deformed specimens after annealing at 1173 K for 1800 s, as shown in
Figure 5c,d, respectively. Smaller AlN particles were formed in the STD-50% specimen, than that of the ST specimen. Therefore, the AlN particles in the prior cold-deformed specimen had sluggish growth kinetics. This may strongly influence the dissolution behavior of the precipitates at high temperatures. Similar phenomena have also been reported by Furubayashi et al. [
11] and Kesternich [
12], while the mechanism(s) for this are still not very clear.
Regarding the pinning effect of the second-phase particles, there is a maximum radius of the precipitates,
rcrit, for pinning the grain growth [
6]. That is, only the fine second phase particles with a radius smaller than
rcrit, can effectively pin the grain boundaries. The
rcrit can be expressed as [
6]:
where
R0 is the average grain size,
Z is a parameter that is used to represents the inhomogeneity of the initial grains, and
f is the volume fraction of second-phase particles. It is obvious that for a given uniform initial grain structure,
rcrit linearly decreases with the decrease of
f [
6].
According to
Figure 5a, the fraction of the AlN particles is stable in the STD-50% specimen below 1223 K (as indicated by symbol ① in
Figure 5a). Therefore, both the
rcrit and the pinning force, which is proportional to
f/
r [
6] (here,
r is the average radius of the pinning particles), may have no obvious change, and the grain boundaries can be well-pinned. This results in the sluggish austenite grain growth kinetics in the STD-50% specimens below 1223 K, as shown in
Figure 3b. However, when the annealing temperature was increased up to 1248 K (position ② in
Figure 5a), the fraction of the AlN particles in the STD-50% specimen decreased obviously. This may be caused by the accelerated diffusion rates of the elements at elevated temperatures, the increased solubility of the AlN in austenite, and the easy dissolution of the fine AlN particles in the STD-50% specimen. Hence, the
rcrit may decrease, according to Equation (4). Then, the volume faction of the AlN precipitates available to pin the grain boundaries may decrease steeply at 1248 K. This may cause the occurrence of AGG at this temperature.
As for the ST specimen, the fraction of AlN particles were almost constant until 1298 K (position ③ in
Figure 5a). Therefore, both
rcrit and the pinning force were stable, and thus the grain growth could be effectively retarded. This agrees with the observed sluggish grain growth kinetics below 1298 K in
Figure 3a. However, as the annealing temperature was raised up to 1323 K (position ④ in
Figure 5a), the fractions of the AlN particles were decreased, and the
rcrit may decrease accordingly. This may result in the occurrence of AGG in the ST specimen, as shown in
Figure 2b,c.
According to the discussion above, it can be concluded that the change in the volume fraction of the AlN particles during annealing plays a key role in the austenite grain growth behavior. The rapid decrease in the AlN volume fraction may greatly change the rcrit, and then the effective volume fraction of the second phase particles to pin the gain boundaries may decrease dramatically, which may result in the occurrence of AGG.
As reviewed in the Introduction, the transition from AGG to NGG mode occurs at high temperatures when the AlN precipitates are nearly completely dissolved, and the grain growth becomes free. According to
Figure 5a, the fractions of the AlN particles in both the deformed and undeformed specimens were low at 1353 K. Experimental results revealed that AGG occurred within 60 s when the ST specimen was annealed at 1353 K, while NGG occurred in the STD-50% specimen, even after a longer holding time (
Figure 3b). The average diameters of the AlN particles in ST and STD-50% specimens after annealing at 1353 K for 0 s were evaluated to be around 60 and 30 nm, correspondingly. It was reported that the dissolution time and the size of the precipitates had a parabolic relationship [
23]. Accordingly, the dissolution time of the AlN particles in the ST specimen was around four times that in the STD-50% specimen. Therefore, it was possible that the fine AlN precipitates in the STD-50% specimen dissolved very quickly during annealing, which made the grain growth become free and change to the NGG mode. However, since the dissolution of the coarse precipitates in the ST specimen took a longer time, AGG could occur. To quickly dissolve the relatively large AlN precipitates in the ST specimen, this required higher temperatures. This may promote the AGG-to-NGG transition temperature to the higher temperature in the ST specimen.
According to the above discussion, cold-deformation results in the fine and near-equilibrium precipitation of the AlN particles, which are readily dissolved during the heating process, due to the increase in the solubilities of Al and N in austenite. However, non-equilibrium and coarse AlN precipitates formed in the undeformed specimen, which were resistant to dissolution during the heating process. These should be the reasons for the suppression of the AGG temperature range by the prior cold-deformation.