Research on Reaction Mechanism of Vacuum Carbon Thermal Reduction and Dephosphorization in High Phosphate Iron Ore
Abstract
:1. Introduction
2. Experiment
2.1. Materials
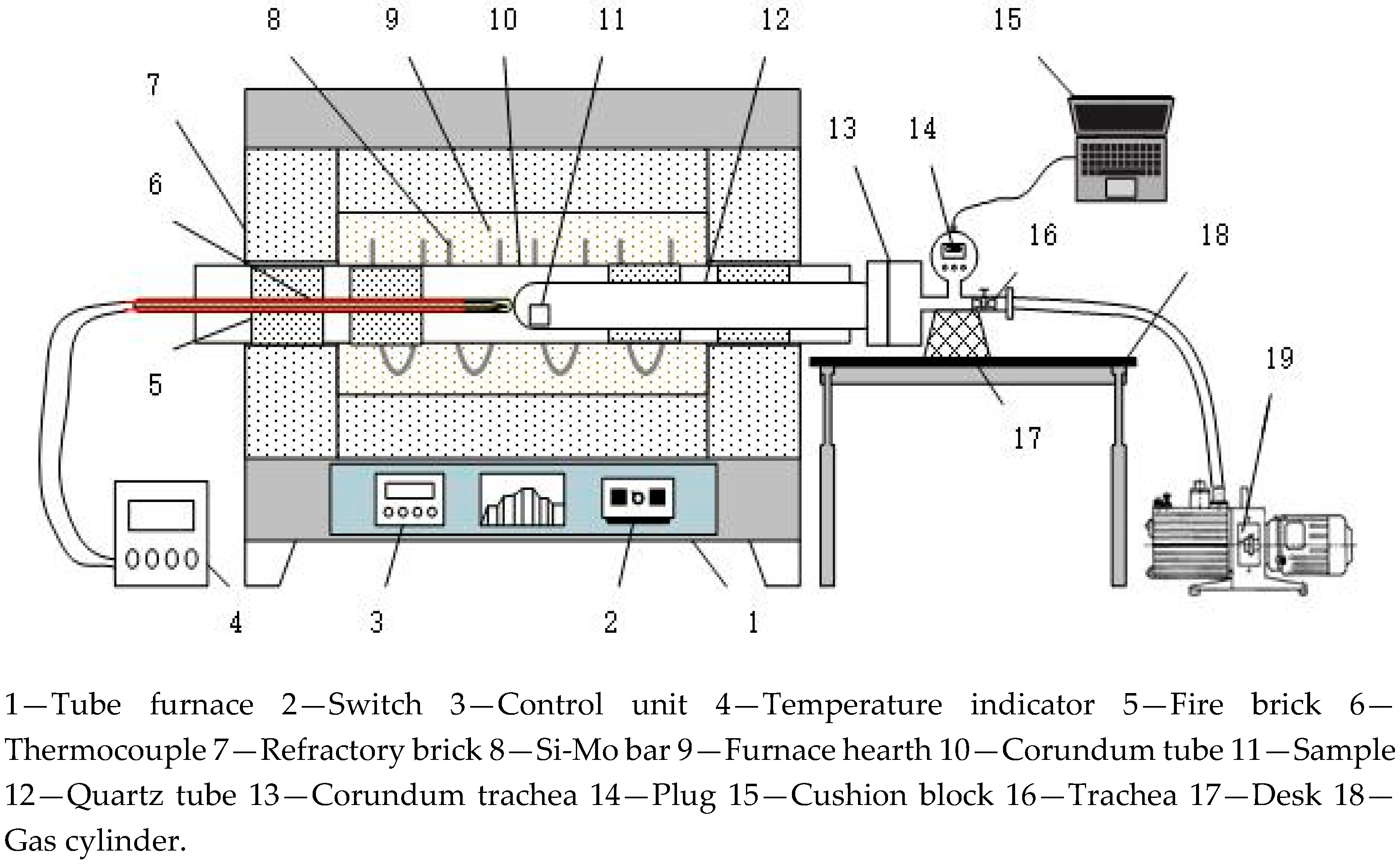
2.2. Procedures
2.3. Data Treatment
- Pt: the phosphorus content in the pellets after reduction;
- Praw: the phosphorus content in raw pellets.
- MFe: the metal iron content in the pellets after reduction;
- TFe: the total iron content in the pellets after reduction.
3. Results and Discussion
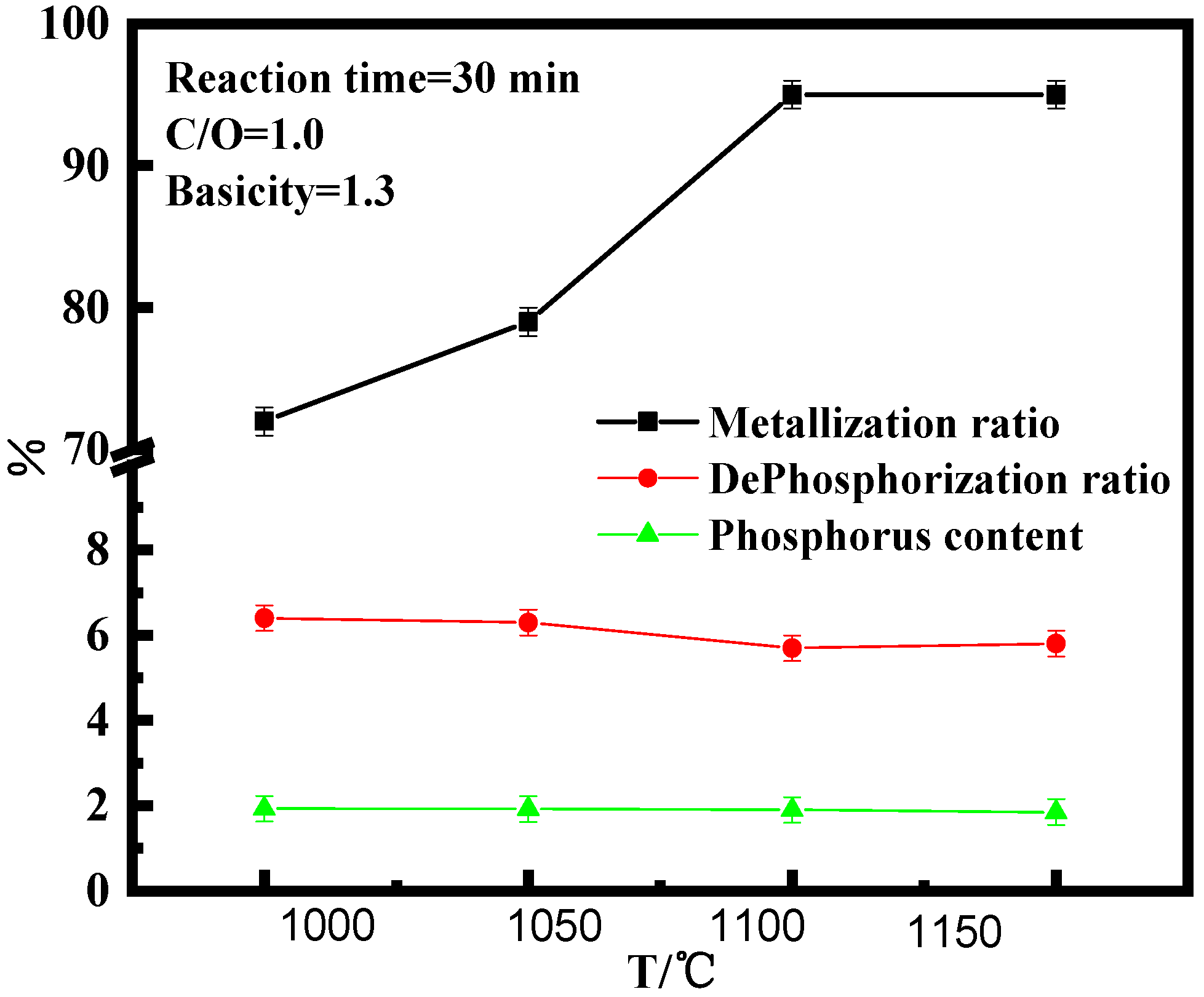
3.1. Effect of Reduction Temperature on High-Phosphorus Iron Ore in Vacuum Reduction
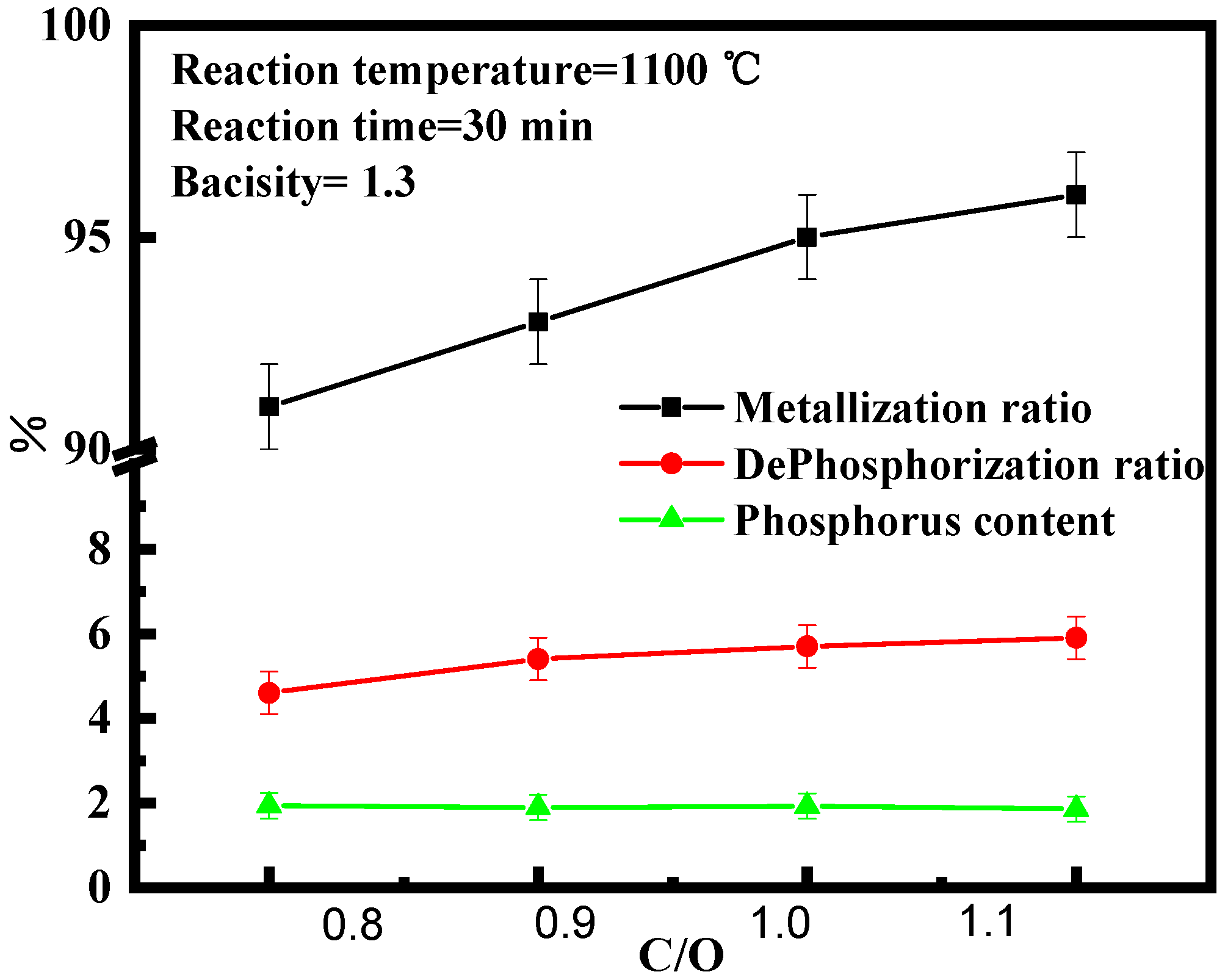
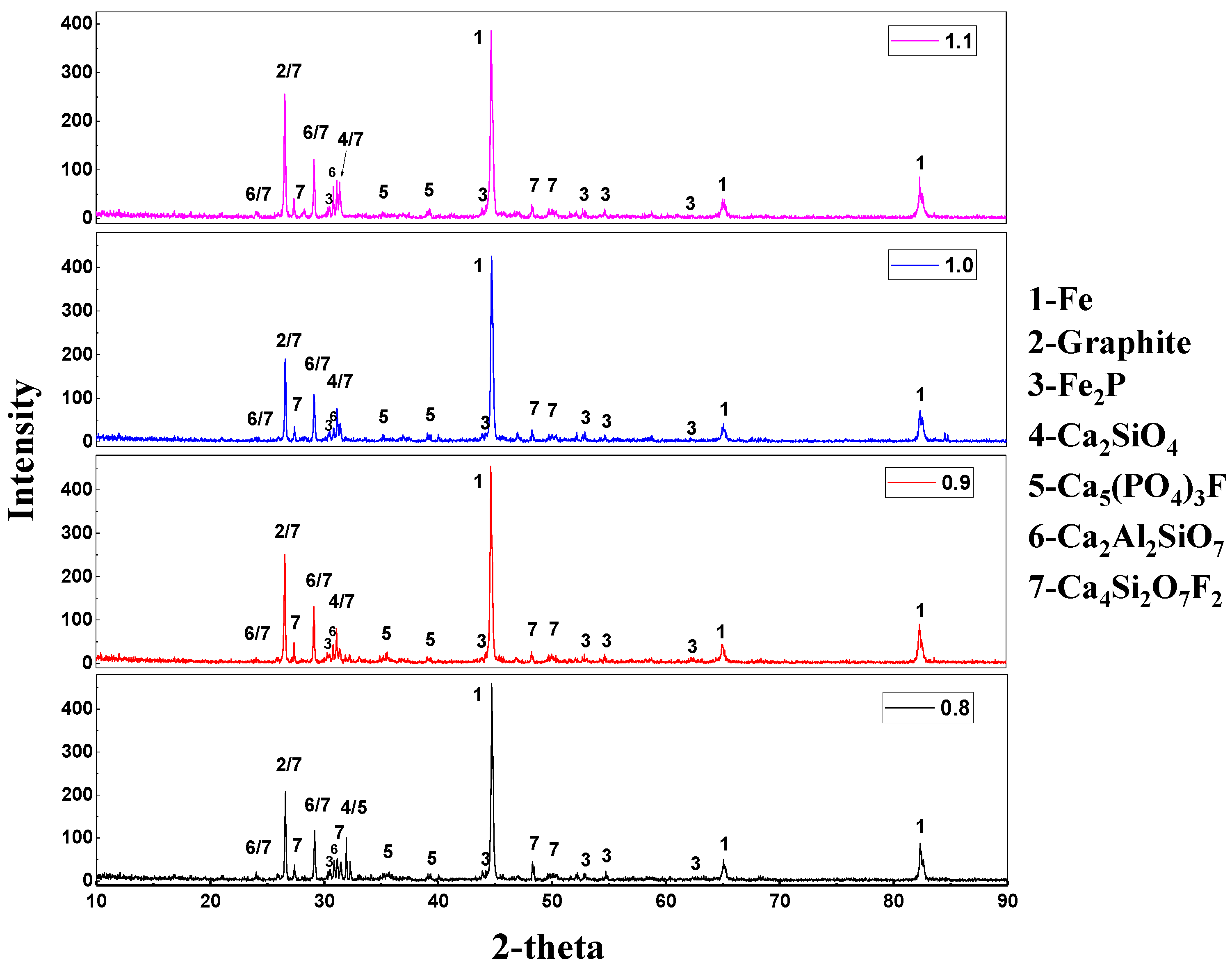
3.2. Effect of Carbon on High Phosphate Iron Ore in Vacuum Reduction
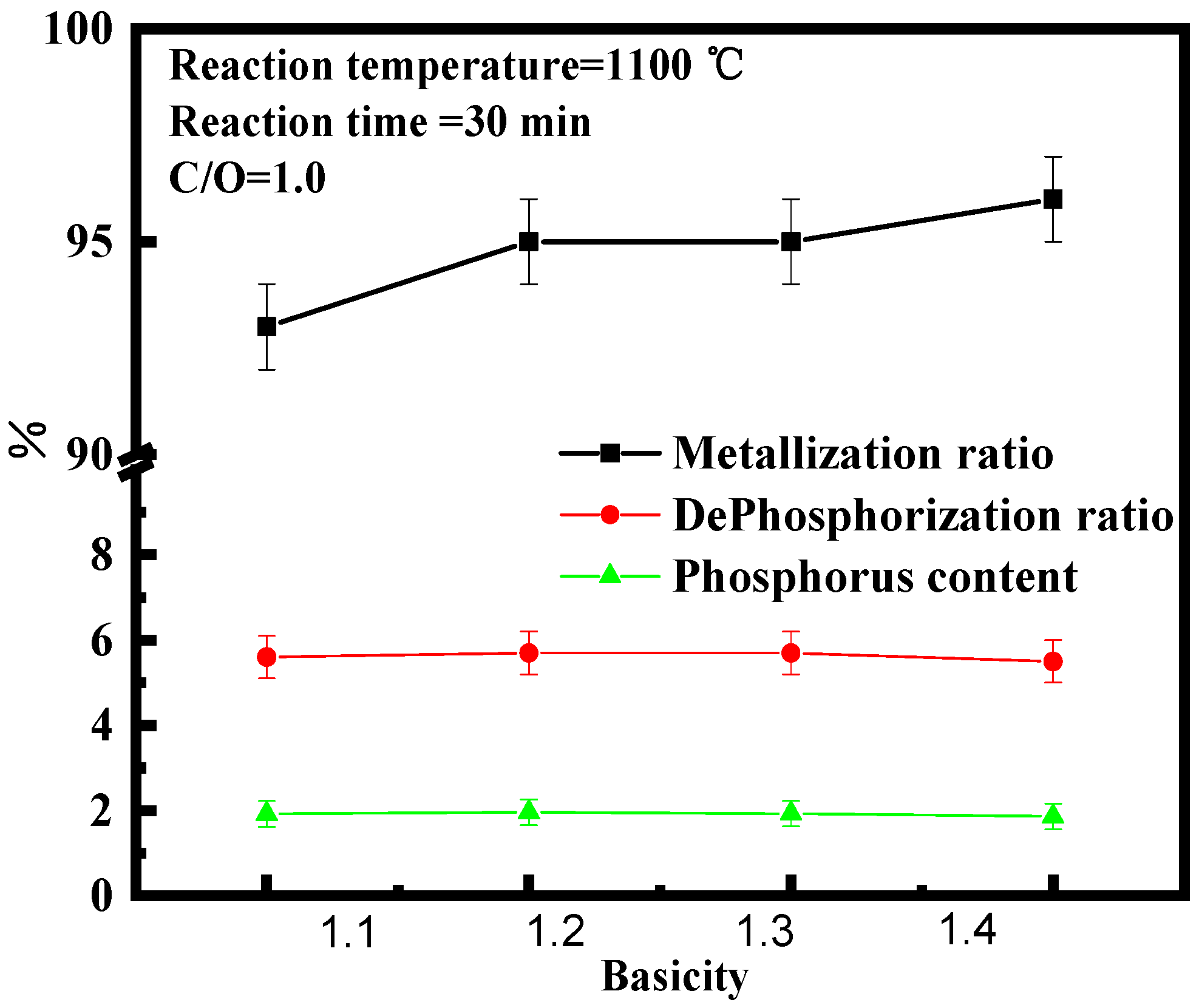
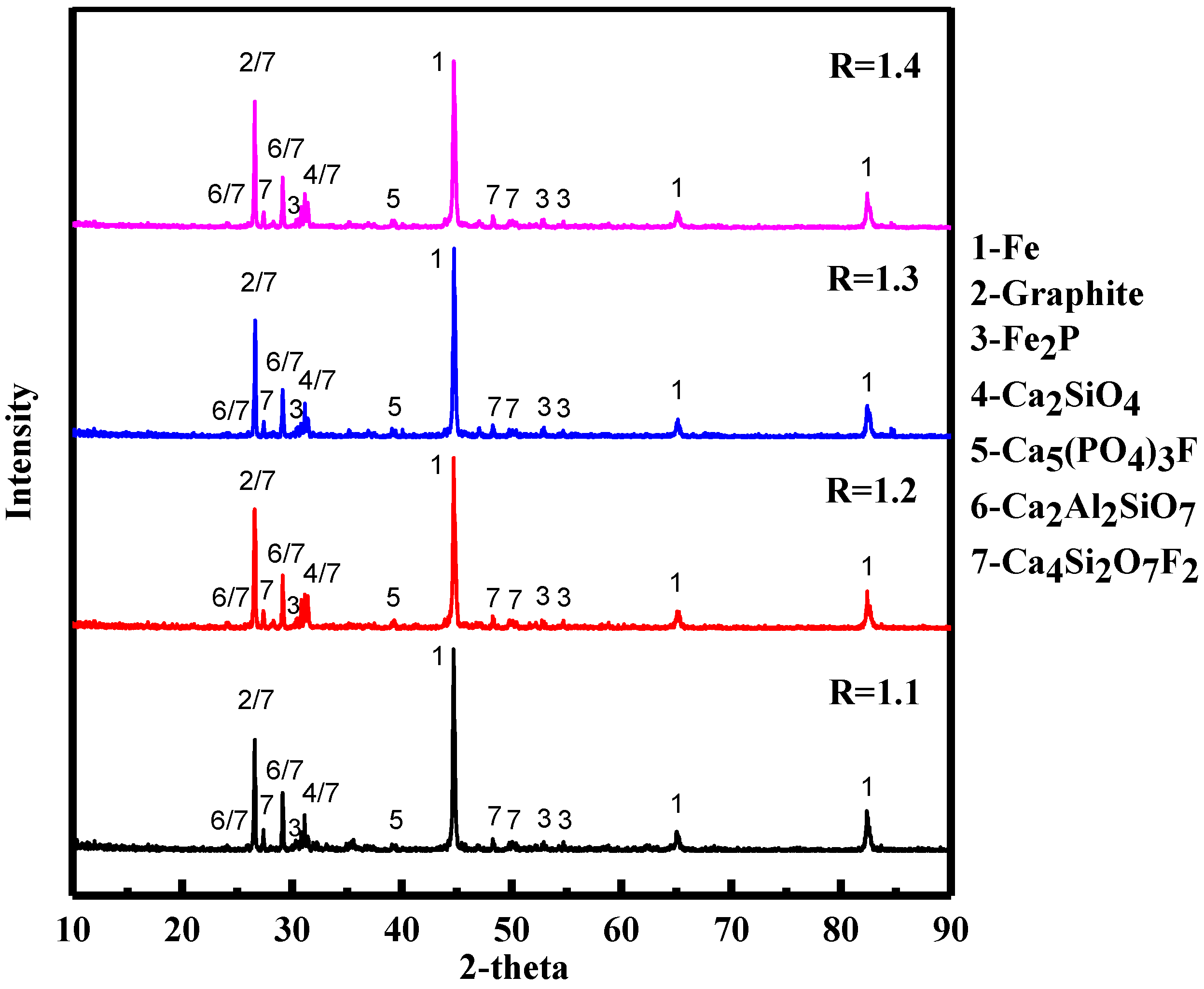
3.3. Effect of Basicity on High Phosphate Iron Ore in Vacuum Reduction
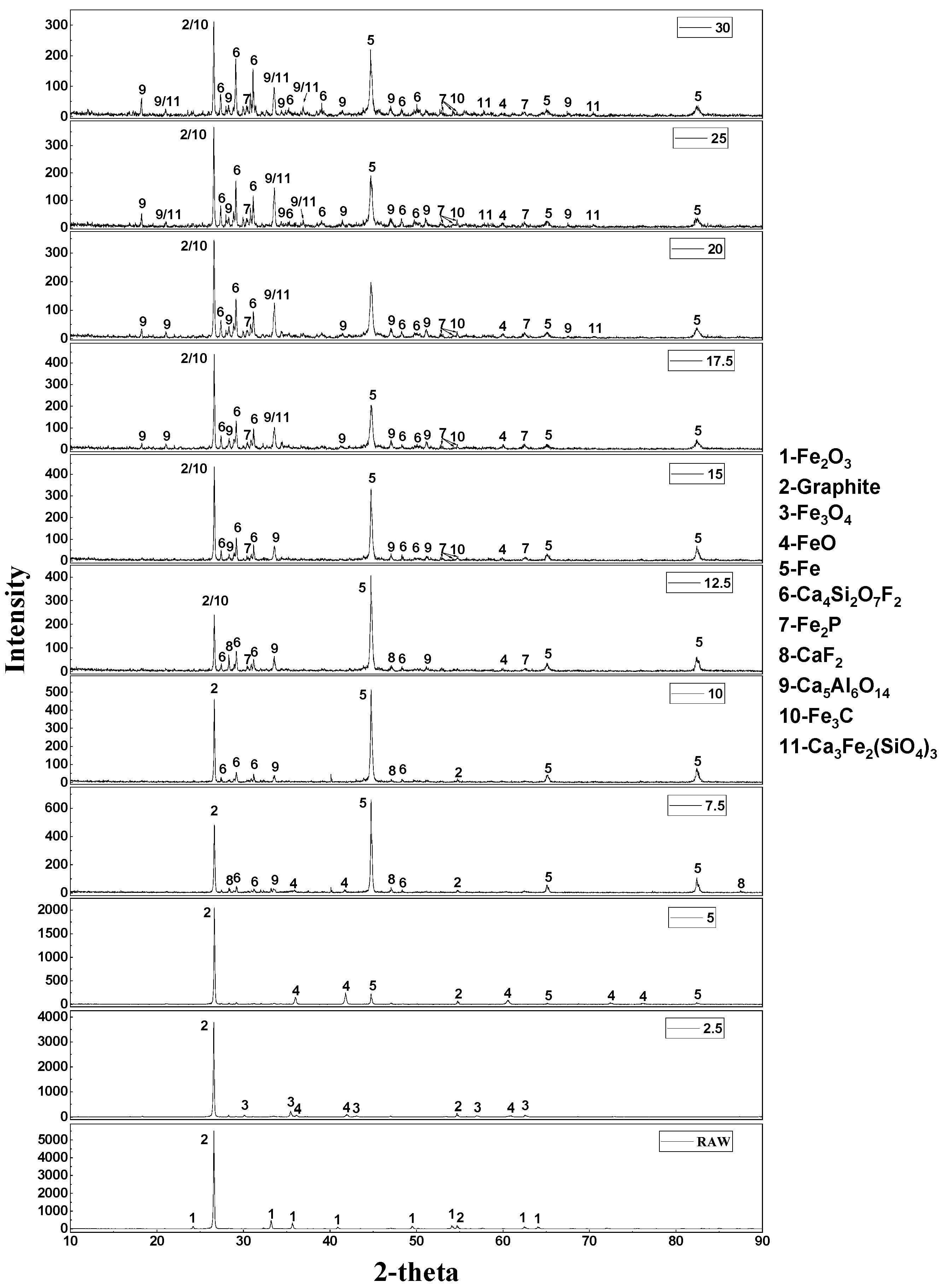
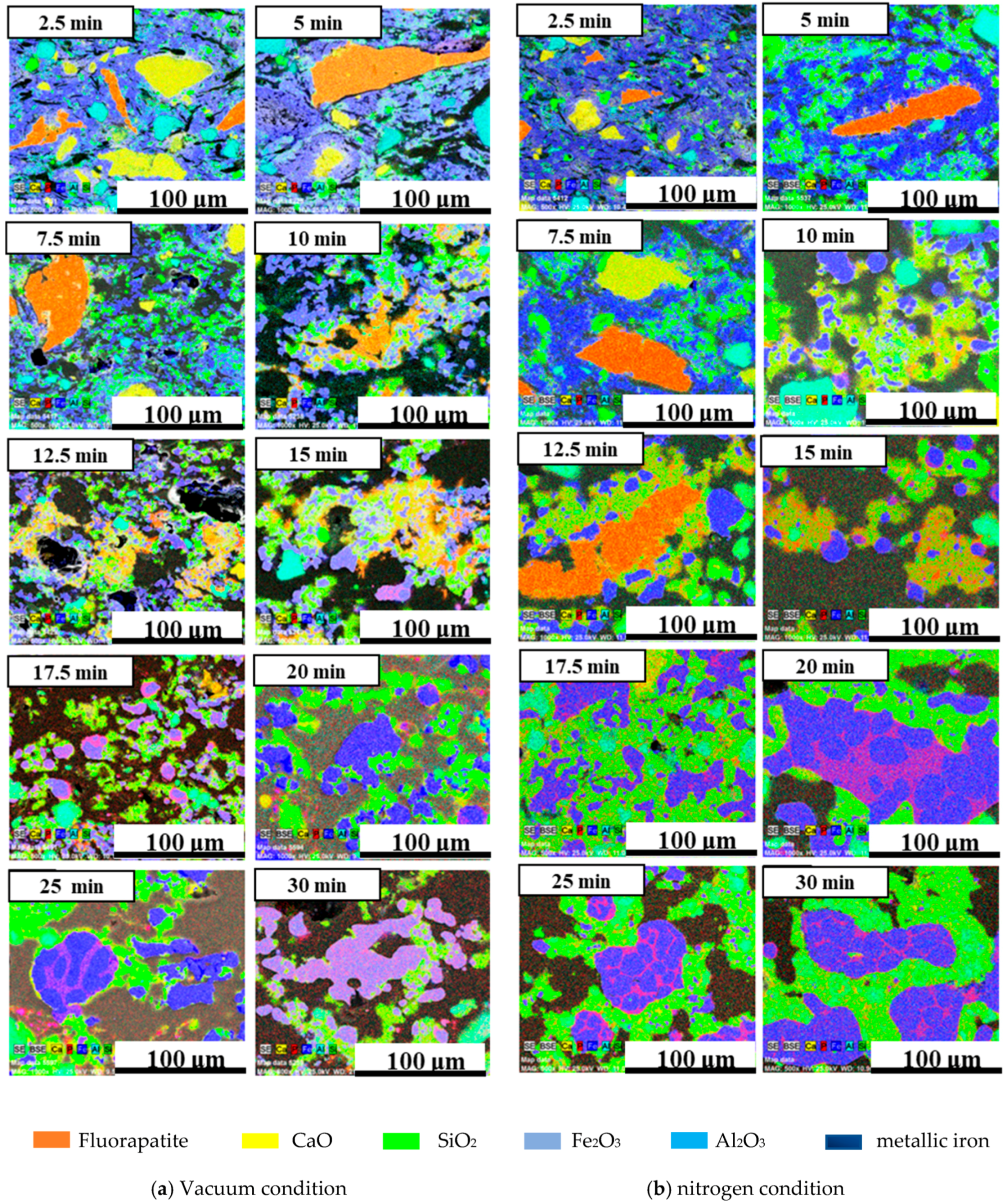
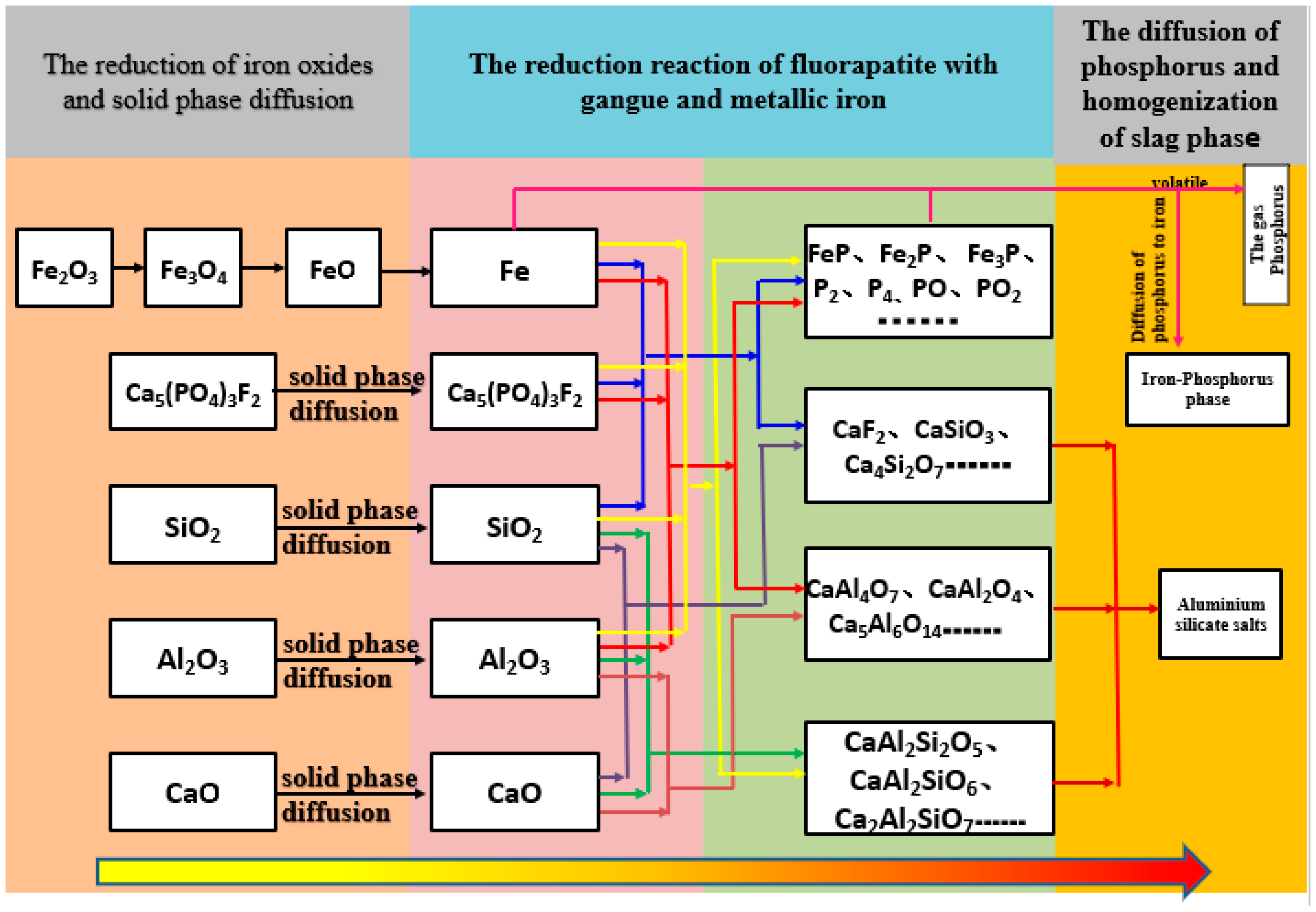
3.4. The Reaction Mechanism of High-Phosphate Iron Ore in Vacuum Reduction
4. Conclusions
- The effects of reduction temperature, basicity, and C/O ratio, on the metallization ratio, dephosphorization ratio, and phosphorus content of pellets were studied under the vacuum carbon thermal reduction (VCTR) condition. It was found that the metallization ratio of pellets reached maximum (95%); however, the dephosphorization ratio of only 5.6% showed an adverse result, when the reduction temperature, C/O ratio, and basicity were 1100 °C, 0.8–1.0, and 1.3, respectively.
- The vacuum condition played an insignificant role in evaluating the dephosphorization ratio, resulting in a higher phosphorus content of iron bead in the reduction process. This was attributed to the fact that during the vacuum reduction process fluorapatite reacted not only with carbon to form gaseous phosphide, but also with iron to form the FexP phase.
- Compared to the nitrogen condition, the metallization ratio of pellets was significantly improved, while the dephosphorization ratio of pellets showed an opposite result.
- In the VCTR condition, metallic iron was involved in the reduction of fluorapatite, thus a new mechanism of reduction of fluorapatite was proposed: 2Ca5(PO4)3F + 12Fe + 9SiO2 + 15C = 9CaSiO3 + 6Fe2P + 15CO + CaF2.
- The expected result that a vacuum condition promotes dephosphorization of fluorapatite during the reduction stage was not achieved, and phosphorus was not reduced to gas phase, but to iron-phosphorus phase. Based on the new mechanism of fluorapatite reduction, the reduction of fluorapatite which was difficult to separate from the iron phase should be inhibited. Therefore, a new idea was proposed: the reduction temperature of high-phosphorus ore should be less than the temperature of the rapid reduction of fluoroapatite. Decreasing the reduction time and carbon addition would promote the iron increase and phosphorus decrease. Less reducing agent after the reduction of iron oxide should be more beneficial to the inhibition of fluorapatite reduction due to the reduction of iron oxide prior to the reduction of fluoroapatite. These systematic explorations will be carried out in our next study.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Steel Association. Steel Statistical Yearbook 2015 of Report; World steel Committee on Economic Studies: Brussels, Belgium, 2015; Volume 1, pp. 1–122. [Google Scholar]
- Ministry of Land and Resources of PRC. China Land and Resources Bulletin 2015; Ministry of Land and Resources of PRC: Beijing, China, 25 September 2016; pp. 6–8.
- Cao, X.Y.; Lv, G.X.; Zhu, Y.S. The characteristics of regional distribution of the major metal minerals resources in China. Resour. Ind. 2004, 6, 20–22. [Google Scholar]
- Wang, Y.; Xing, S.W.; Zhang, Z.J. Reserves analysis of identified low-grade iron resources in China. Multipurp. Util. Miner. Resour. 2014, 5, 15–17. [Google Scholar]
- Ren, Z.B.; Wu, Q.Y. The anlysis and estimation of mineral resources situation in China since new century. China Min. Mag. 2011, 20, 10–13. [Google Scholar]
- Zitzman, A.; Walther, H. The Iron Ore Deposits of Europe and Adjacent Areas; Bundesanstalf für Geowissenschaften und Rohstoffe: Hanover, Germany, 1978.
- Maynard, J.; Van-Houten, F. Descriptive model of oolitic ironstones. US Geol. Surv. Bull. 1992, 2004, 39–40. [Google Scholar]
- Roesler, M. The Iron-Ore Resources of Europe; U.S. Government Publishing Office: Washington, DC, USA, 1921.
- Ai, G.H.; Li, X.B.; Zhou, Y. Research Status and Trend of the Dephosphorization technology of high-phosphorus iron ore. Nonferrous Met. Sci. Eng. 2011, 2, 53–58. [Google Scholar]
- Han, Y.X.; Sun, Y.S.; Gao, P. Exploitation situation and development trend of high phosphorus oolitic hematite. Met. Mine 2012, 41, 1–5. [Google Scholar]
- Bai, L.M.; Niu, F.G.; Wu, G. Experimental study of high intensity magnetic separation-gravity concentration process for oolitic hematite ore. Express Inf. Min. Ind. 2008, 24, 26–28. [Google Scholar]
- Bai, L.M.; Liu, L.N.; Li, M. Test research on roasting-low intensity magnetic separation of oolitic hematite ore in Zhangjiakou area. China Min. Mag. 2009, 18, 83–87. [Google Scholar]
- Chen, W.X.; Hu, W.M.; Wang, B. Research on the combined beneficiation process for dephosphorization of high phosphorus oolitic hematite ore from Wushan, Tao hua County. Met. Mine 2009, 3, 50–53. [Google Scholar]
- Lu, S.W.; Zhang, B.J.; Xiong, D.R. Investigation on the dephosphorization of lingxiang type colloidal phosphorous iron ore by decolloidization leaching. Met. Mine 1994, 8, 30–33. [Google Scholar]
- Fisher-White, M.J.; Lovel, R.R.; Sparrow, G.J. Heat and acid leach treatments to lower phosphorus levels in goethitic iron ores. ISIJ Int. 2012, 52, 1794–1800. [Google Scholar] [CrossRef]
- Fisher-White, M.J.; Lovel, R.R.; Sparrow, G.J. Phosphorus removal from goethitic iron ore with a low temperature heat treatment and a caustic leach. ISIJ Int. 2012, 52, 797–803. [Google Scholar] [CrossRef]
- Shen, H.T.; Huang, X.Y.; Bao, X.L. Research on dephosphorization of a high phosphorus—Bearing iron concentrate. China Min. Mag. 2011, 20, 82–86. [Google Scholar]
- Wang, Y.M.; Li, M.D. High gradient magnetic separation of oolitic hematite. Min. Metall. Eng. 1986, 3, 1–8. [Google Scholar]
- Yin, J.Q.; Xu, A.B.; Zhang, H.Q. Improving the recovery rate of weak magnetic separation of fine grinding artificial magnetite ore. China Min. Mag. 2016, 10. [Google Scholar]
- Yang, D.W.; Sun, B.C.; Xu, C.Y. Beneficiation test on iron increase and phosphorous reduction of high-phosphorus oolitic hematite in western Hubei. Met. Mine 2009, 10, 81–83. [Google Scholar]
- Wang, C. Research on the magnetizing roasting and magnetic separation of an oolitic hematite ore. Met. Mine 2009, 39, 57–59. [Google Scholar]
- Zhang, H.; Jin, Y.L.; He, Z.J. Mechanism research on raising the grade of iron and dephosphorization for high phosphor hematite in microwave field. Gansu Metall. 2011, 33, 1–3. (In Chinese) [Google Scholar]
- Zhou, J.C.; Xue, Z.L.; Zhang, H.F. Present technology of high phosphorus oolitic hematite dephosphorization. Ironmaking 2007, 2, 40–43. [Google Scholar]
- Sun, Y.S.; Han, Y.X.; Gao, P. Recovery of iron from high phosphorus oolitic iron ore using coal-based reduction followed by magnetic separation. Int. J. Miner. Metall. Mater. 2013, 20, 411–419. [Google Scholar] [CrossRef]
- Li, Y.L.; Sun, T.C.; Xu, C.Y. Effects of reductant types oil direct reduction and phosphorus removal of high-phosphorus oolitic hematite ore. Min. Metall. Eng. 2012, 32, 66–69. [Google Scholar]
- Cheng, C.; Xue, Q.G.; Wang, G. Phosphorus migration during direct reduction of coal composite high-phosphorus iron ore pellets. Metall. Mater. Trans. B 2016, 47, 154–163. [Google Scholar] [CrossRef]
- Cheng, C.; Xue, Q.; Zhang, Y.Y. Dynamic migration process and mechanism of phosphorus permeating into metallic iron with carburizing in coal-based direct reduction. ISIJ Int. 2015, 55, 2576–2581. [Google Scholar] [CrossRef]
- Cheng, C.; Xue, Q.G.; Wang, J.S. Carbothermal reduction mechanism of fluorapatite and gangue in high phosphorus iron ore. J. Iron Steel Res. Int. 2016, 28, 8–15. [Google Scholar]
- Zhang, Y.; Xue, Q.; Wang, G. Phosphorus-containing mineral evolution and thermodynamics of phosphorus vaporization during carbothermal reduction of high-phosphorus iron ore. Metals 2018, 8, 451. [Google Scholar] [CrossRef]
- Matinde, E.; Sasaki, Y.; Hino, M. Phosphorus gasification from sewage sludge during carbothermic reduction. ISIJ Int. 2008, 48, 912–917. [Google Scholar] [CrossRef]




| Compositions | TFe | Fe2O3 | FeO | SiO2 | CaO | Al2O3 | MgO | P | S |
|---|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 54.08 | 73.46 | 3.42 | 7.77 | 4.26 | 5.07 | 0.74 | 1.15 | 0.022 |
| Raw Materials | Fe2O3 | Ca5(PO4)3F | SiO2 | Al2O3 | CaO | MgO |
|---|---|---|---|---|---|---|
| Raw ore | 73.46 | 6.23 | 7.77 | 5.07 | 0.79 | 0.74 |
| Approximate ratio | 15 | 1.2 | 1.5 | 1 | 0.15 | 0.15 |
| Simulate ratio | 15 | 1.2 | 1.5 | 1 | 0 | 0 |
| Composition | Fe2O3 | Ca5(PO4)3F | SiO2 | Al2O3 | CaO | CaF2 | Na2CO3 | Graphite |
|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 15 | 1.2 | 1.5 | 1 | 1.95 | 1.4 | 1.4 | 3.3 |
| Sample | Metallization Ratio/% | Dephosphorization Ratio/% |
|---|---|---|
| Vacuum-reduced pellets | 94.7 | 6.2 |
| Nitrogen-reduced pellets | 86.6 | 13.5 |
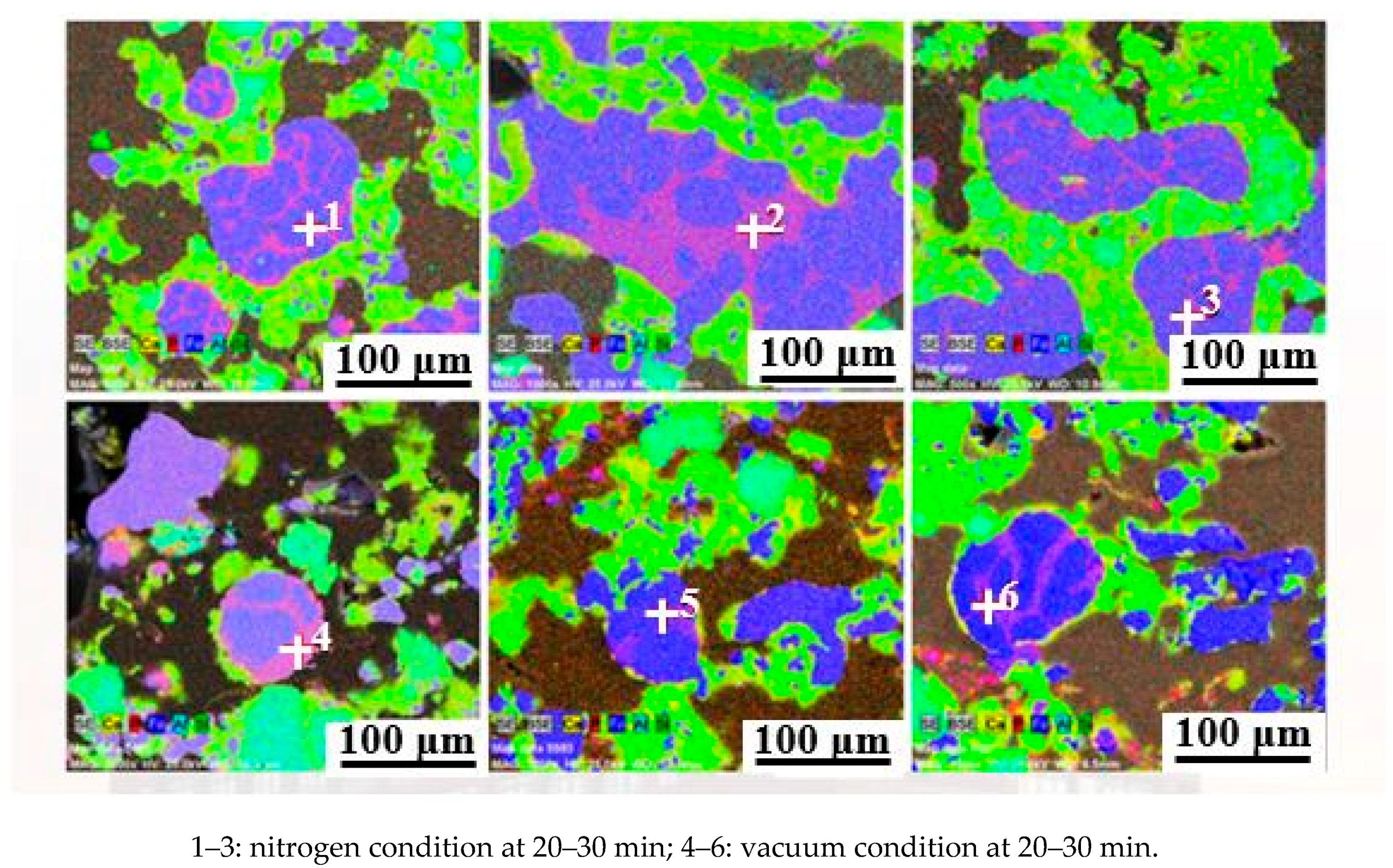
| Element | Fe (at.%) | P (at.%) |
|---|---|---|
| 1 | 86.89 | 13.11 |
| 2 | 88.32 | 11.68 |
| 3 | 88.58 | 11.42 |
| 4 | 86.92 | 13.08 |
| 5 | 87.43 | 12.57 |
| 6 | 85.93 | 14.07 |
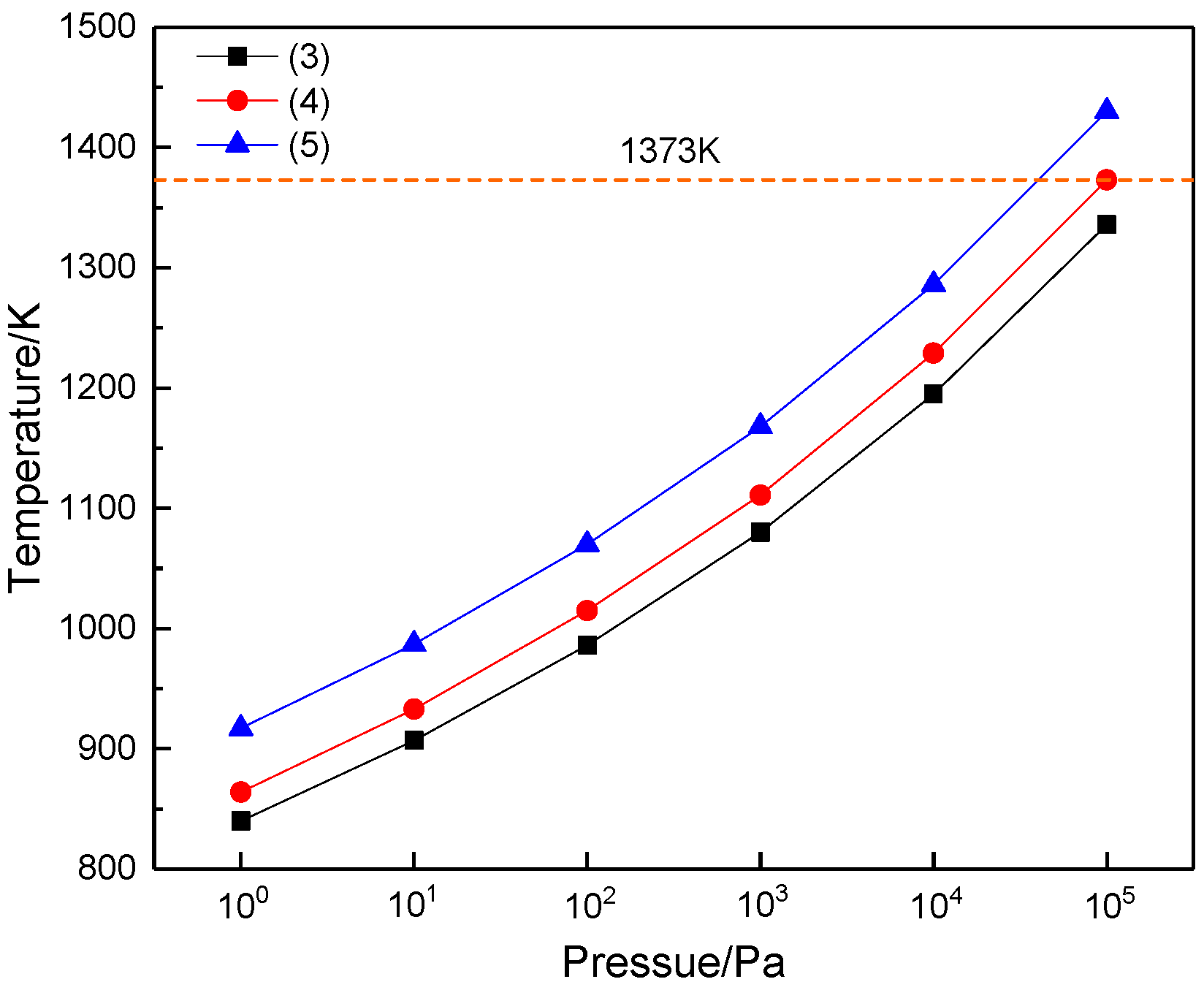
| P/Pa | 105 | 104 | 103 | 102 | 101 | 100 |
|---|---|---|---|---|---|---|
| (3) | 1336 | 1195 | 1080 | 986 | 907 | 840 |
| (4) | 1373 | 1229 | 1111 | 1015 | 933 | 864 |
| (5) | 1430 | 1286 | 1168 | 1070 | 987 | 917 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Chen, Z.; Zuo, H.; Wang, J.; Xue, Q. Research on Reaction Mechanism of Vacuum Carbon Thermal Reduction and Dephosphorization in High Phosphate Iron Ore. Metals 2018, 8, 1003. https://doi.org/10.3390/met8121003
Zhao J, Chen Z, Zuo H, Wang J, Xue Q. Research on Reaction Mechanism of Vacuum Carbon Thermal Reduction and Dephosphorization in High Phosphate Iron Ore. Metals. 2018; 8(12):1003. https://doi.org/10.3390/met8121003
Chicago/Turabian StyleZhao, Jun, Zhijie Chen, Haibin Zuo, Jingsong Wang, and Qingguo Xue. 2018. "Research on Reaction Mechanism of Vacuum Carbon Thermal Reduction and Dephosphorization in High Phosphate Iron Ore" Metals 8, no. 12: 1003. https://doi.org/10.3390/met8121003
APA StyleZhao, J., Chen, Z., Zuo, H., Wang, J., & Xue, Q. (2018). Research on Reaction Mechanism of Vacuum Carbon Thermal Reduction and Dephosphorization in High Phosphate Iron Ore. Metals, 8(12), 1003. https://doi.org/10.3390/met8121003






