One-Step Preparation of Super-Hydrophobic Micro-Nano Dendrites on Al Alloy for Enhanced Corrosion Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.3. Characterization
3. Results and Discussion
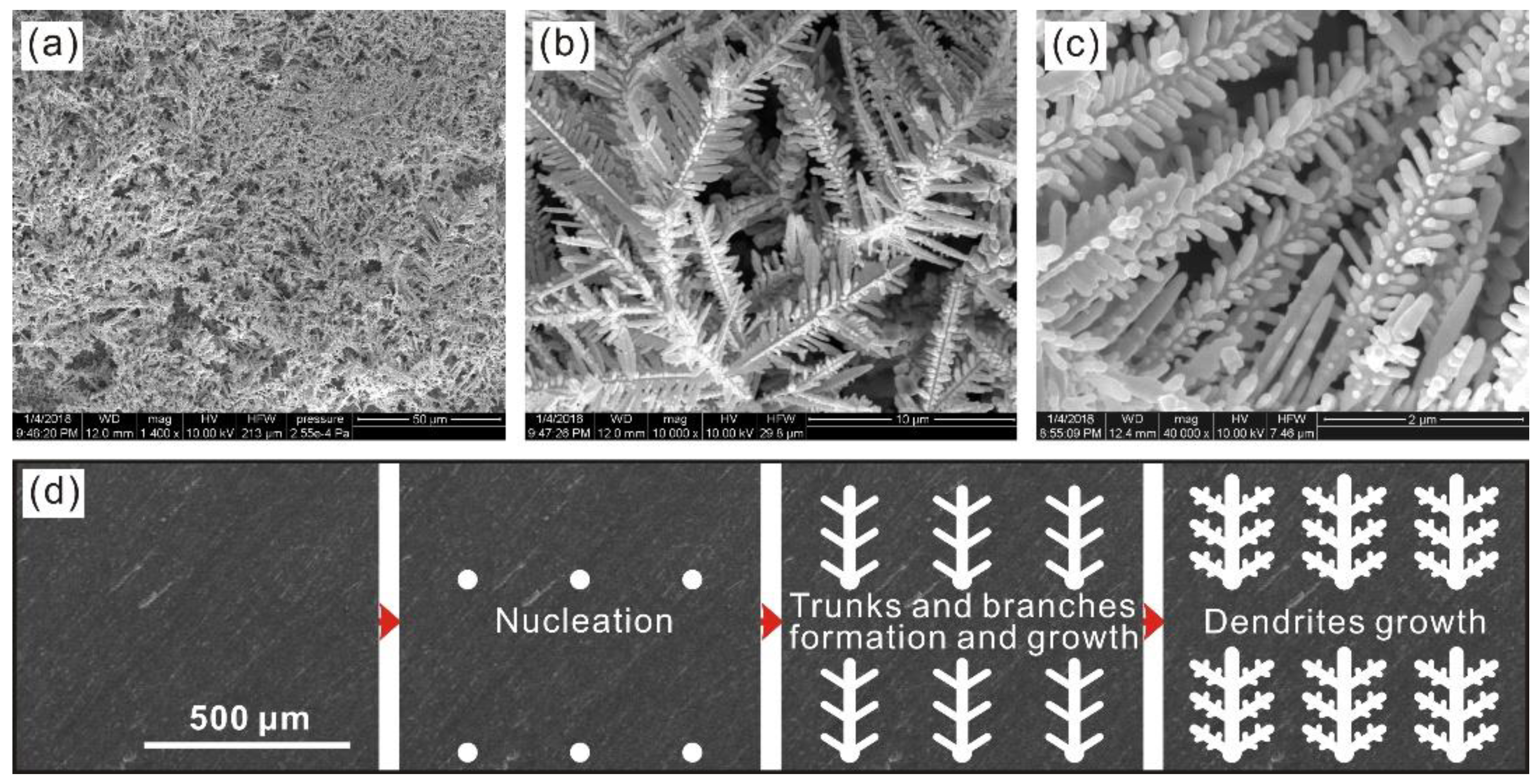
3.1. Structure Characterization
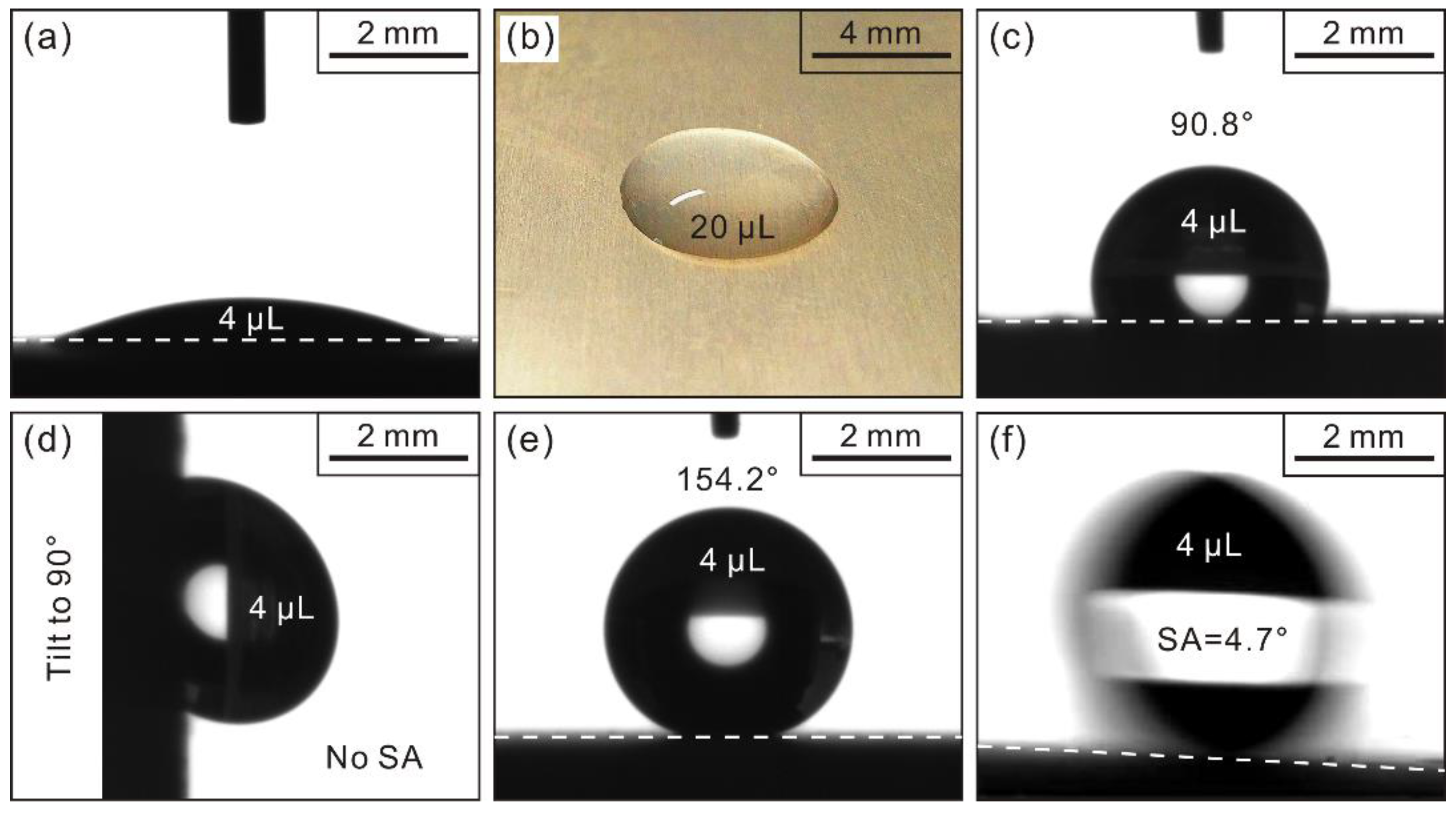
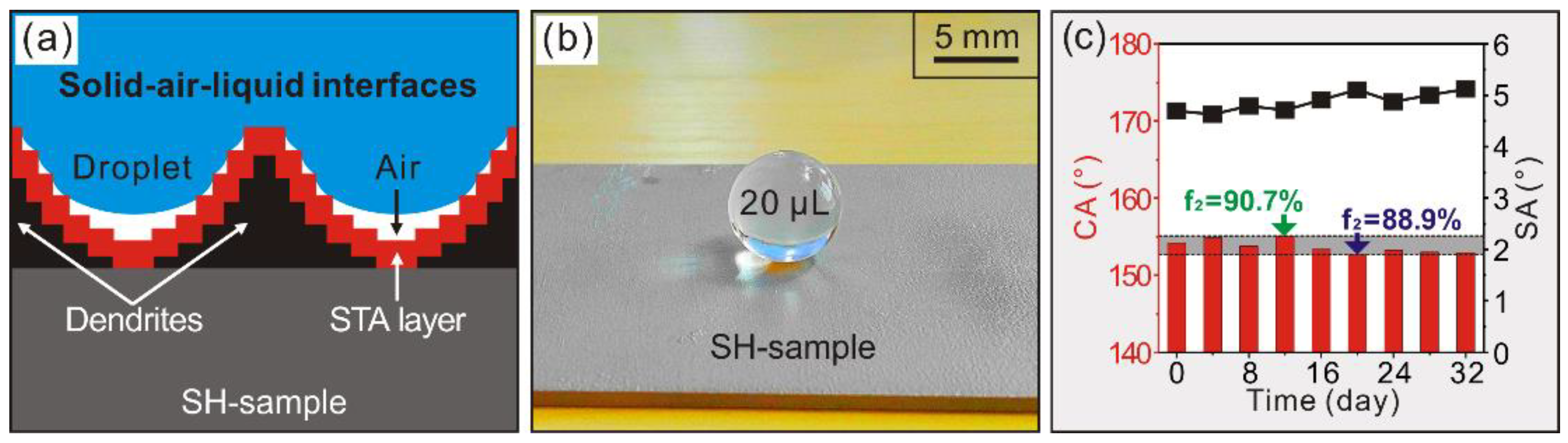
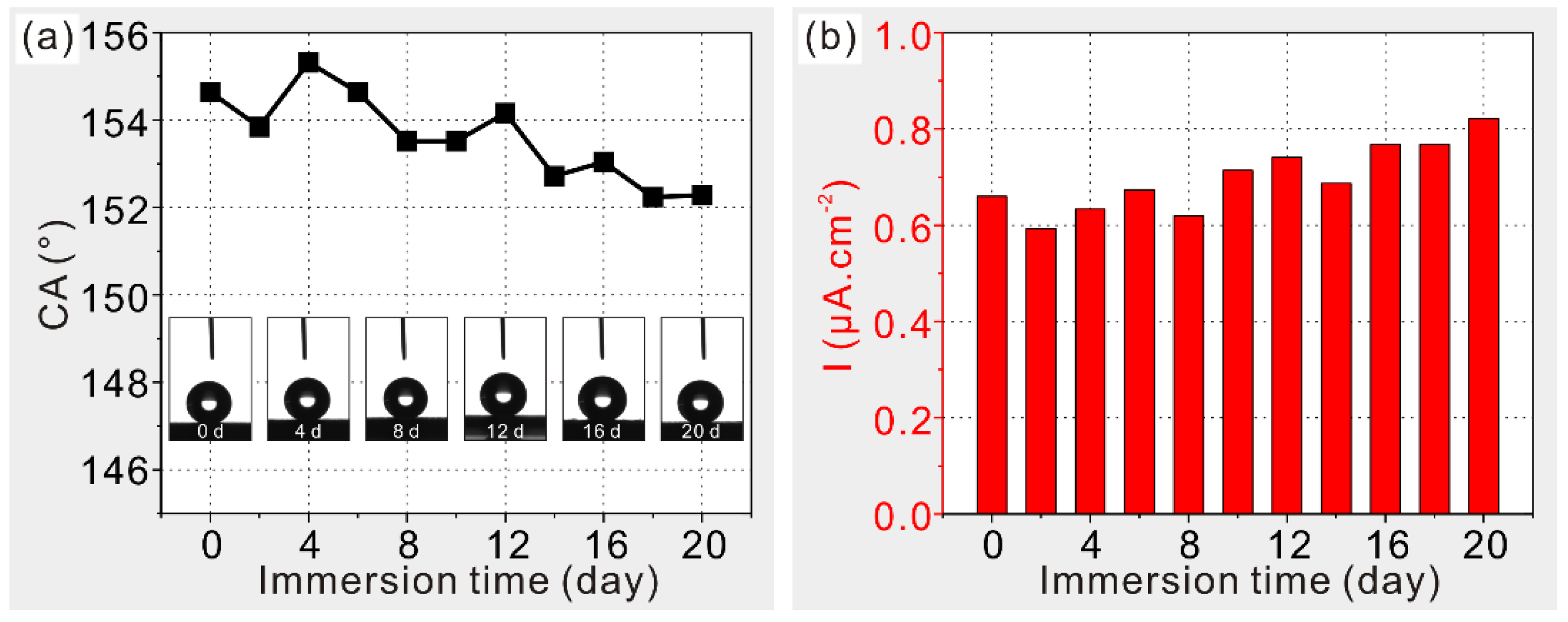
3.2. Wettability Characterization
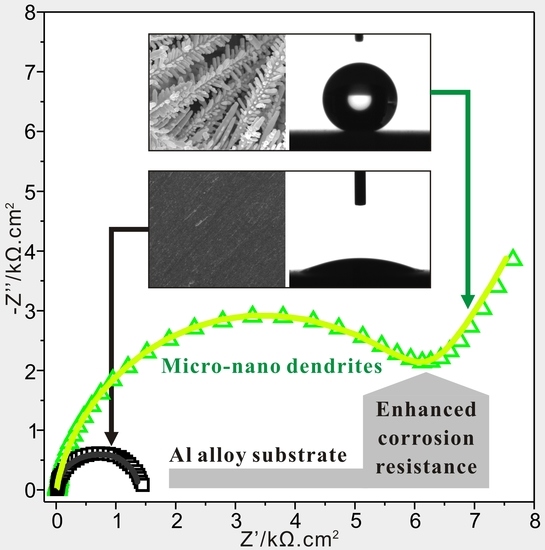
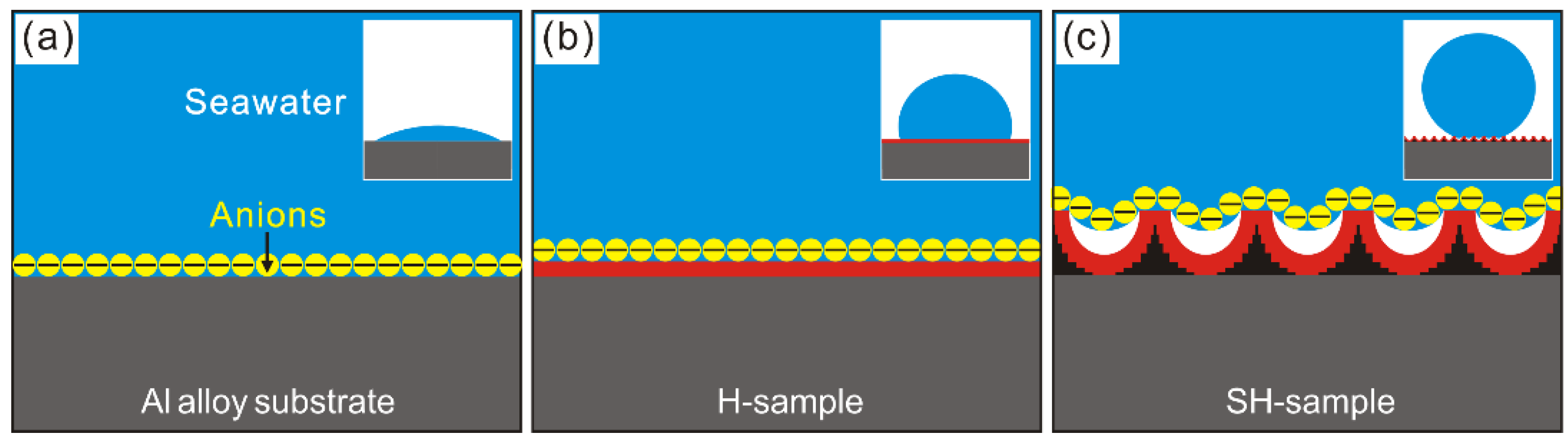
3.3. Anticorrosion Characterization
4. Conclusions
- (1)
- Uniformly dispersed micro-nano dendrites have been fabricated on 5005 Al alloy surface using a facile potentiostatic deposition treatment.
- (2)
- After stearic acid modification, the as-prepared dendrites have displayed greatly amplified and durable water repellence with super-hydrophobic apparent contact angle of 154.2° and sliding angle of 4.7°.
- (3)
- The Super-hydrophobic Al alloy surface has been prepared with greatly enhanced corrosion resistance in seawater by reducing interfacial interactions between corrosive ions and modified deposited surface, owing to the newly-generated composite solid-air-liquid interfaces.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seikh, A.H.; Baig, M.; Ammar, H.R.; Alam, M.A. The influence of transition metals addition on the corrosion resistance of nanocrystalline Al alloys produced by mechanical alloying. Metals 2016, 6, 140. [Google Scholar] [CrossRef]
- Wan, H.L.; Lin, J.P.; Min, J.Y. Effect of laser ablation treatment on corrosion resistance of adhesive-bonded Al alloy joints. Surf. Coat. Technol. 2018, 345, 13–21. [Google Scholar] [CrossRef]
- Ou, J.F.; Fang, X.Z.; Zhao, W.J.; Lei, S.; Xue, M.S.; Wang, F.J.; Li, C.Q.; Lu, Y.L.; Li, W. Influence of hydrostatic pressure on corrosion behavior of superhydrophobic surfaces on bare and oxidized aluminum substrates. Langmuir 2018, 34, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Olajire, A.A. Recent advances on organic coating system technologies for corrosion protection of offshore metallic structures. J. Mol. Liq. 2018, 269, 572–606. [Google Scholar] [CrossRef]
- Li, J.C.; Dang, J. A Summary of corrosion properties of Al-rich solid solution and secondary phase particles in Al alloys. Metals 2017, 7, 84. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, Z.P.; Fu, B.G.; Long, L.; Zhang, X.L.; Cui, H.X. Effects of Mg content on the mechanical properties and corrosion resistance of Al-Cu-Mg-Ag alloy. J. Alloy. Compd. 2016, 685, 209–215. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Zhan, L.H. Effect of creep aging process on microstructures and properties of the retrogressed Al-Zn-Mg-Cu alloy. Metals 2016, 6, 189. [Google Scholar] [CrossRef]
- Qi, H.; Liu, X.Y.; Liang, S.X.; Zhang, X.L.; Cui, H.X.; Zheng, L.Y.; Gao, F.; Chen, Q.H. Mechanical properties and corrosion resistance of Al-Cu-Mg-Ag heat-resistant alloy modified by interrupted aging. J. Alloy. Compd. 2016, 657, 318–324. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Zhang, J.X.; Gu, X.H.; Qin, P.; Dai, N.W.; Li, X.P.; Kruth, J.P.; Zhang, L.C. Improved corrosion behavior of ultrafine-grained eutectic Al-12Si alloy produced by selective laser melting. Mater. Des. 2018, 146, 239–248. [Google Scholar] [CrossRef]
- Fouladi, S.; Ghasemi, A.H.; Abbasi, M.; Abedini, M.; Khorasani, A.M.; Gibson, I. The effect of vibration during friction stir welding on corrosion behavior, mechanical properties, and machining characteristics of stir zone. Metals 2017, 7, 421. [Google Scholar] [CrossRef]
- Kaseem, M.; Lee, Y.H.; Ko, Y.G. Incorporation of MoO2, and ZrO2, particles into the oxide film formed on 7075 Al alloy via micro-arc oxidation. Mater. Lett. 2016, 182, 260–263. [Google Scholar] [CrossRef]
- Ji, S.P.; Weng, Y.C.; Wu, Z.Z.; Ma, Z.Y.; Tian, X.B.; Fu, R.K.Y.; Lin, H.; Wu, G.S.; Chu, P.K.; Pan, F. Excellent corrosion resistance of P and Fe modified micro-arc oxidation coating on Al alloy. J. Alloy. Compd. 2017, 710, 452–459. [Google Scholar] [CrossRef]
- Watson, G.S.; Green, D.W.; Cribb, B.W.; Brown, C.L.; Meritt, C.R.; Tobin, M.J.; Vongsvivut, J.; Sun, M.X.; Liang, A.P.; Watson, J.A. Insect analogue to the lotus leaf: A planthopper wing membrane incorporating a low-adhesion, nonwetting, superhydrophobic, bactericidal, and biocompatible surface. ACS Appl. Mater. Interfaces 2017, 9, 24381. [Google Scholar] [CrossRef] [PubMed]
- Frankiewicz, C.; Attinger, D. Texture and wettability of metallic lotus leaves. Nanoscale 2016, 8, 3982–3990. [Google Scholar] [CrossRef] [PubMed]
- Marmur, A.; Volpe, C.D.; Siboni, S.; Amirfazli, A.; Drelich, J.W. Contact angles and wettability: Towards common and accurate terminology. Surf. Innov. 2017, 5, 1–24. [Google Scholar] [CrossRef]
- Nosonovsky, M. Multiscale roughness and stability of superhydrophobic biomimetic interfaces. Langmuir 2007, 23, 3157–3161. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Kong, J.Y.; Wang, X.D.; Li, X.W. Preparation of multifunctional Al-Mg alloy surface with hierarchical micro/nanostructures by selective chemical etching processes. Appl. Surf. Sci. 2016, 389, 335–343. [Google Scholar] [CrossRef]
- Xiao, X.; Xie, W.; Ye, Z. Preparation of corrosion-resisting superhydrophobic surface on aluminium substrate. Surf. Eng. 2018, 1–7. [Google Scholar] [CrossRef]
- Lee, J.W.; Hwang, W. Exploiting the silicon content of aluminum alloys to create a superhydrophobic surface using the sol-gel process. Mater. Lett. 2016, 168, 83–85. [Google Scholar] [CrossRef]
- Yu, H.D.; Zhang, X.R.; Wan, Y.L.; Xu, J.K.; Yu, Z.J.; Li, Y.Q. Superhydrophobic surface prepared by micromilling and grinding on aluminium alloy. Surf. Eng. 2016, 32, 108–113. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Modin, E.B.; Sayfutdinova, A.R.; Emelyanenko, K.A.; Vasiliev, A.L.; Emelyanenko, A.M. Combination of functional nanoengineering and nanosecond laser texturing for design of superhydrophobic aluminum alloy with exceptional mechanical and chemical properties. ACS Nano 2017, 11, 10113. [Google Scholar] [CrossRef] [PubMed]
- Anitha, C.; Azim, S.S.; Mayavan, S. Salvinia inspired fluroine free superhydrophobic coatings. Appl. Surf. Sci. 2018, 449, 250–260. [Google Scholar]
- Wu, Y.H.; Zhao, W.J.; Wang, W.R.; Sui, W.J. Fabricating binary anti-corrosion structures containing superhydrophobic surfaces and sturdy barrier layers for Al alloys. RSC Adv. 2016, 6, 5100–5110. [Google Scholar] [CrossRef]
- Wei, Z.B.; Jiang, D.Y.; Chen, J.; Guo, X.G. Combination of chemical etching and electrophoretic deposition for the fabrication of multi-scale superhydrophobic Al films. Mater. Lett. 2017, 196, 115–118. [Google Scholar] [CrossRef]
- Li, J.Y.; Lu, S.X.; Xu, W.G.; He, G.; Yu, T.L.; Cheng, Y.Y.; Wu, B. Fabrication of stable Ni-Al4Ni3-Al2O3 superhydrophobic surface on aluminum substrate for self-cleaning, anti-corrosive and catalytic performance. J. Mater. Sci. 2018, 53, 1097–1109. [Google Scholar] [CrossRef]
- Saravanan, G.; Mohan, S. Formation of silver nanorod arrays in an anodised aluminium oxide membrane. Trans. IMF 2015, 93, 248–254. [Google Scholar] [CrossRef]
- Sasaki, K.; Tenjimbayashi, M.; Manabe, K.; Shiratori, S. Asymmetric superhydrophobic/superhydrophilic cotton fabrics designed by spraying polymer and nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, W.Z.; He, G.J.; Zhang, P.Y.; Zhang, Z.J.; Parkin, I.P. Flexible and mechanically robust superhydrophobic silicone surfaces with stable Cassie-Baxter state. J. Mater. Chem. A 2016, 4, 14180–14186. [Google Scholar] [CrossRef]
- Li, X.W.; Shi, T.; Liu, C.; Zhang, Q.X.; Huang, X.J. Multifunctional substrate of Al alloy based on general hierarchical micro/nanostructures: Superamphiphobicity and enhanced corrosion resistance. Sci. Rep. 2016, 6, 35940. [Google Scholar] [CrossRef] [PubMed]

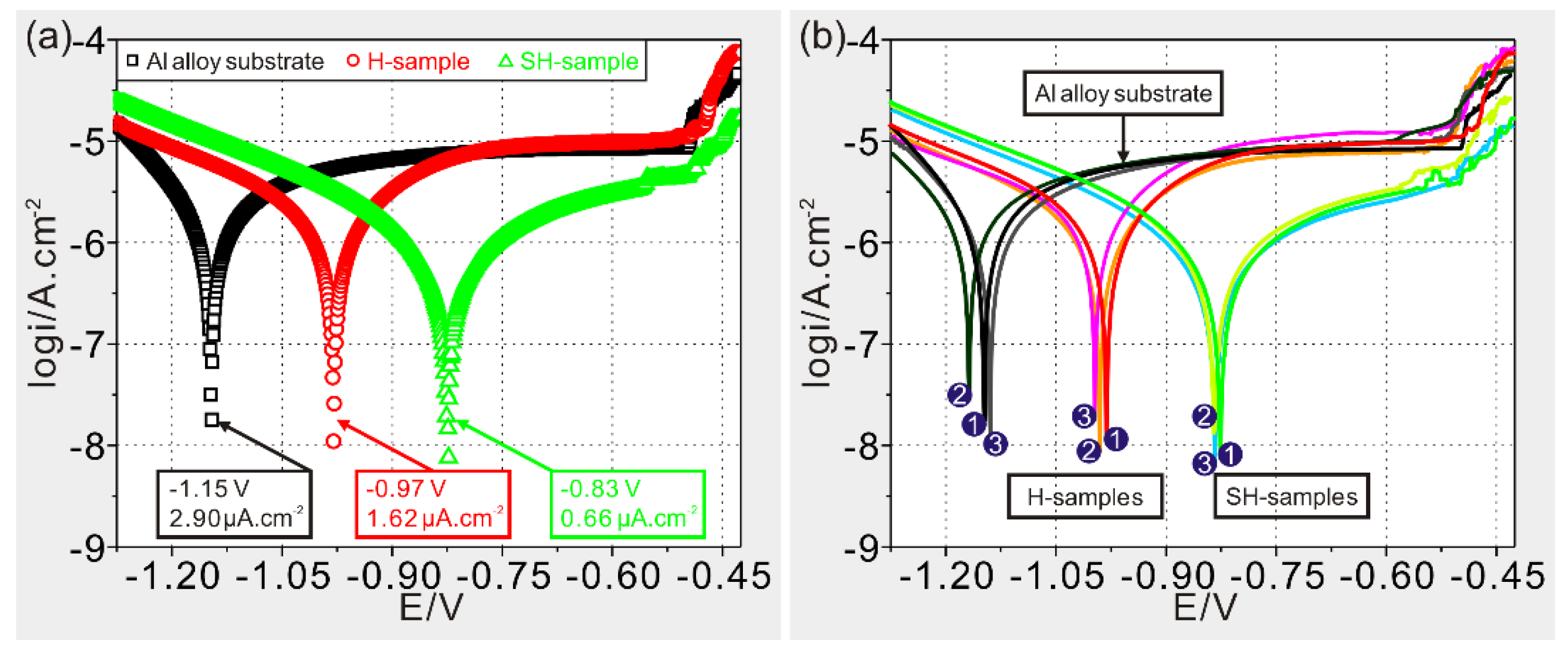
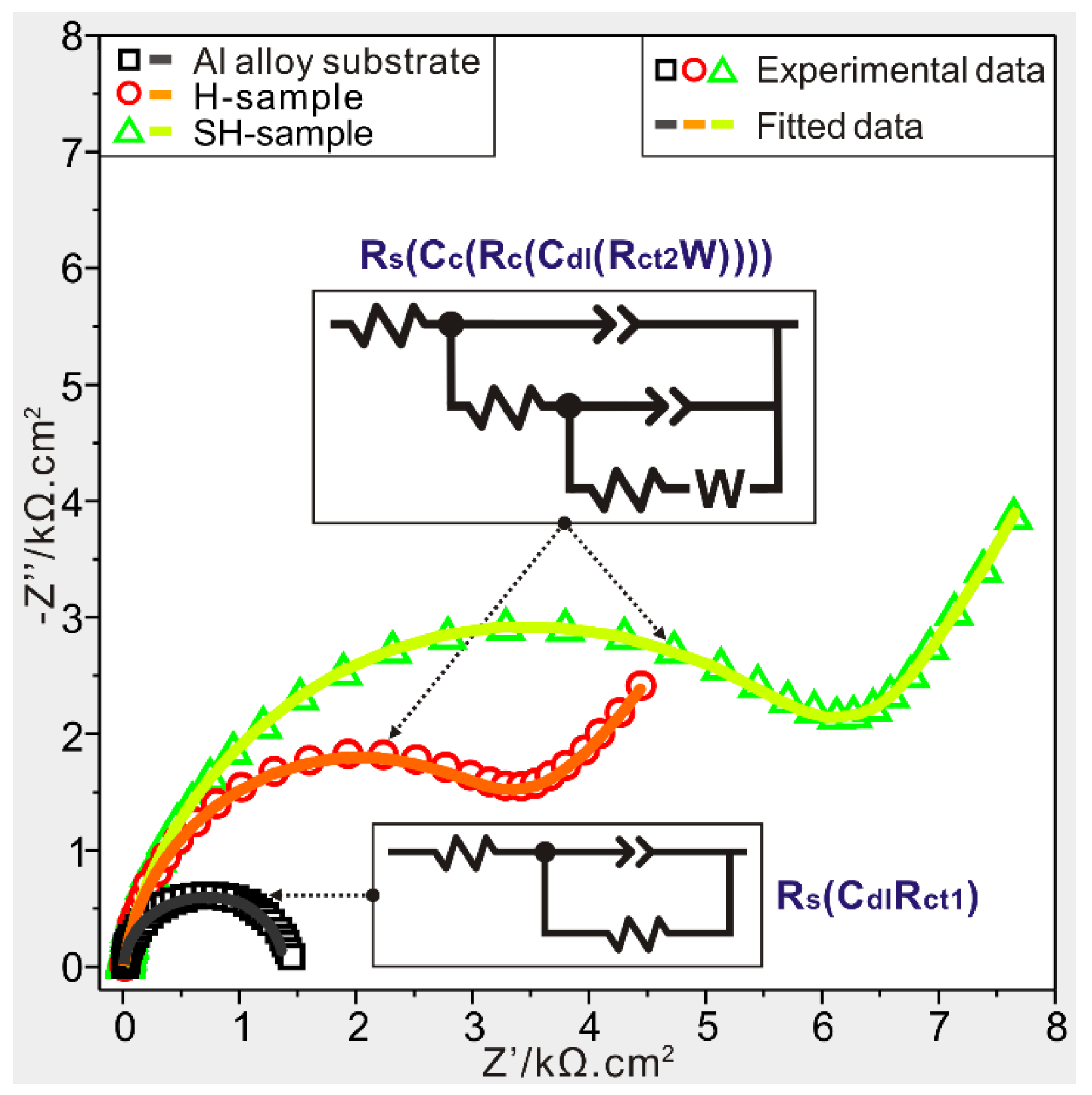
| Sample | Rs (Ω·cm2) | Δ (%) | Rct (Ω·cm2) | Δ (%) | Rc (Ω·cm2) | Δ (%) | W (Ω·cm2) | Δ (%) | Ydl (μF·cm−2) | Δ (%) | Yc (μF·cm−2) | Δ (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 11 | 1.2 | 1428 | 2.5 | - | - | - | - | 61.7 | 3.4 | - | - |
| 2 | 8 | 3.3 | 3306 | 1.9 | 331 | 3.8 | 0.008 | 5.3 | 15.2 | 4.6 | 21.9 | 2.3 |
| 3 | 10 | 0.8 | 6374 | 1.7 | 390 | 5.5 | 0.025 | 7.0 | 4.2 | 8.3 | 3.8 | 6.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Shi, T.; Li, B.; Zhang, C.; Zhong, B.; Lv, Y.; Zhang, Q. One-Step Preparation of Super-Hydrophobic Micro-Nano Dendrites on Al Alloy for Enhanced Corrosion Resistance. Metals 2018, 8, 960. https://doi.org/10.3390/met8110960
Li X, Shi T, Li B, Zhang C, Zhong B, Lv Y, Zhang Q. One-Step Preparation of Super-Hydrophobic Micro-Nano Dendrites on Al Alloy for Enhanced Corrosion Resistance. Metals. 2018; 8(11):960. https://doi.org/10.3390/met8110960
Chicago/Turabian StyleLi, Xuewu, Tian Shi, Ben Li, Chuanwei Zhang, Bin Zhong, Yuan Lv, and Qiaoxin Zhang. 2018. "One-Step Preparation of Super-Hydrophobic Micro-Nano Dendrites on Al Alloy for Enhanced Corrosion Resistance" Metals 8, no. 11: 960. https://doi.org/10.3390/met8110960
APA StyleLi, X., Shi, T., Li, B., Zhang, C., Zhong, B., Lv, Y., & Zhang, Q. (2018). One-Step Preparation of Super-Hydrophobic Micro-Nano Dendrites on Al Alloy for Enhanced Corrosion Resistance. Metals, 8(11), 960. https://doi.org/10.3390/met8110960