Electrochemical Investigation of Corrosion of X80 Steel under Elastic and Plastic Tensile Stress in CO2 Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Solution
2.2. Experimental Set-Up
2.3. Electrochemical Measurements
2.4. Surface Observation
3. Results
3.1. Open-Circuit Potential with Time
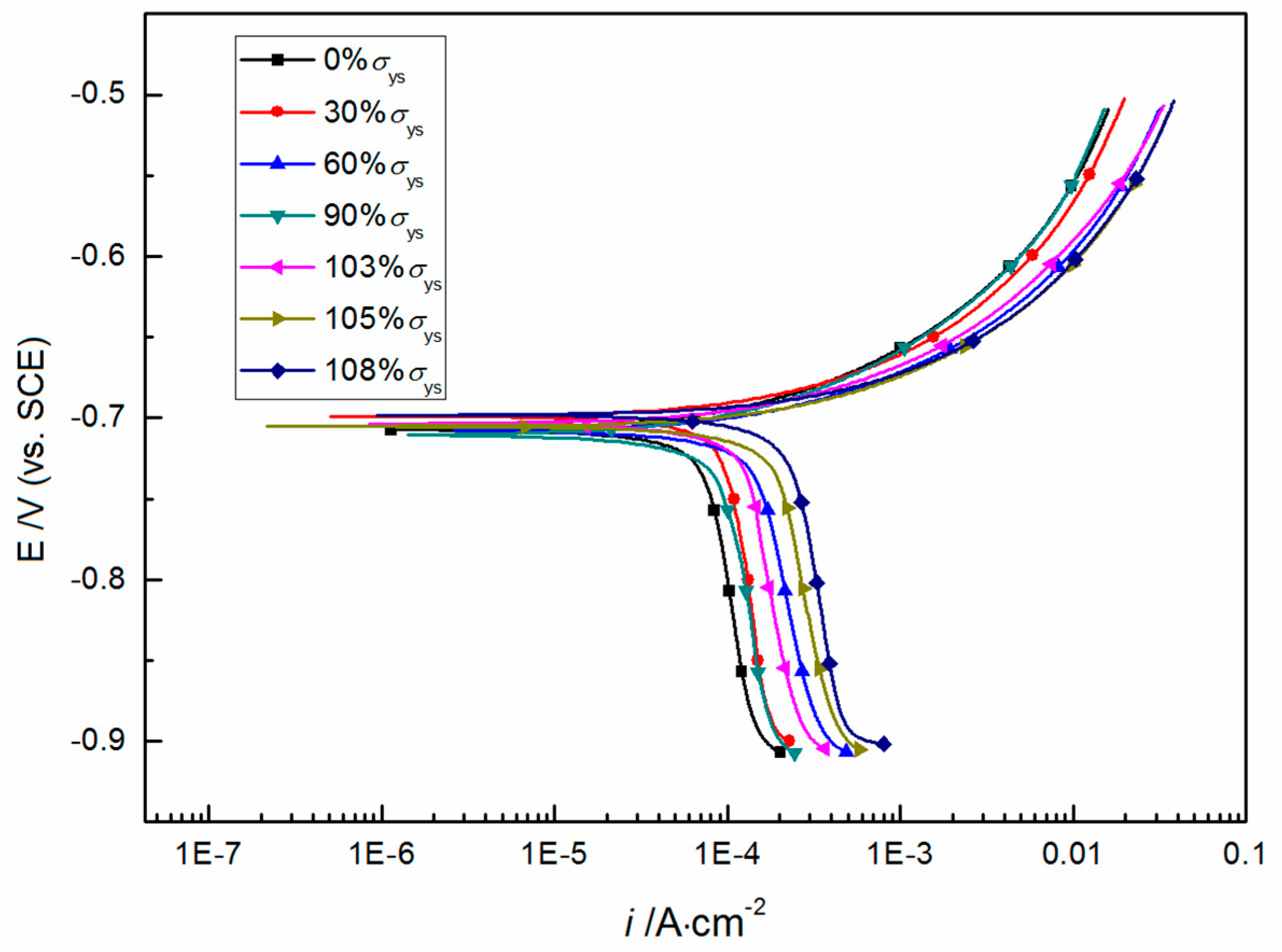
3.2. Potentiodynamic Polarization
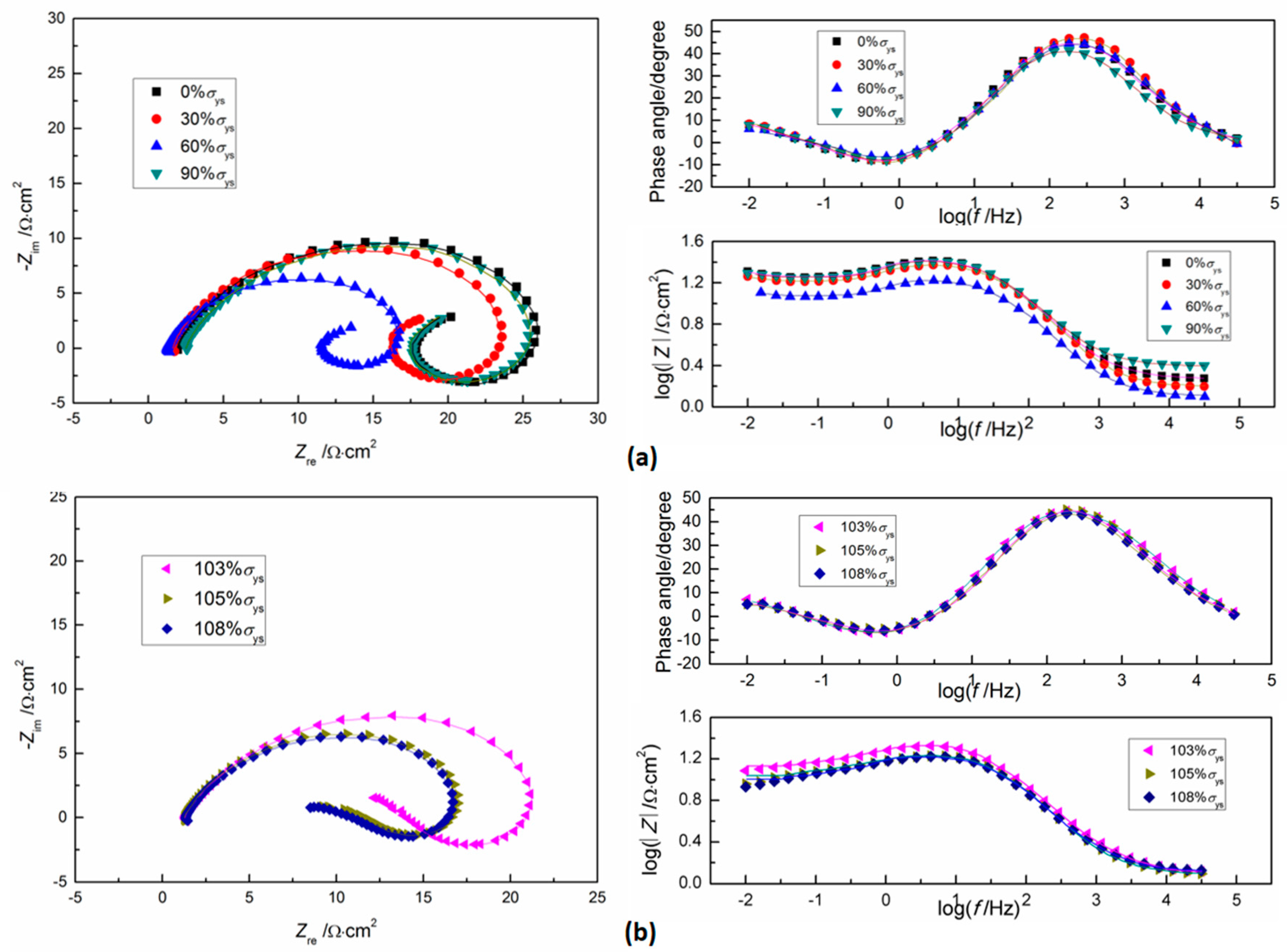
3.3. Electrochemical Impedance Spectroscopy
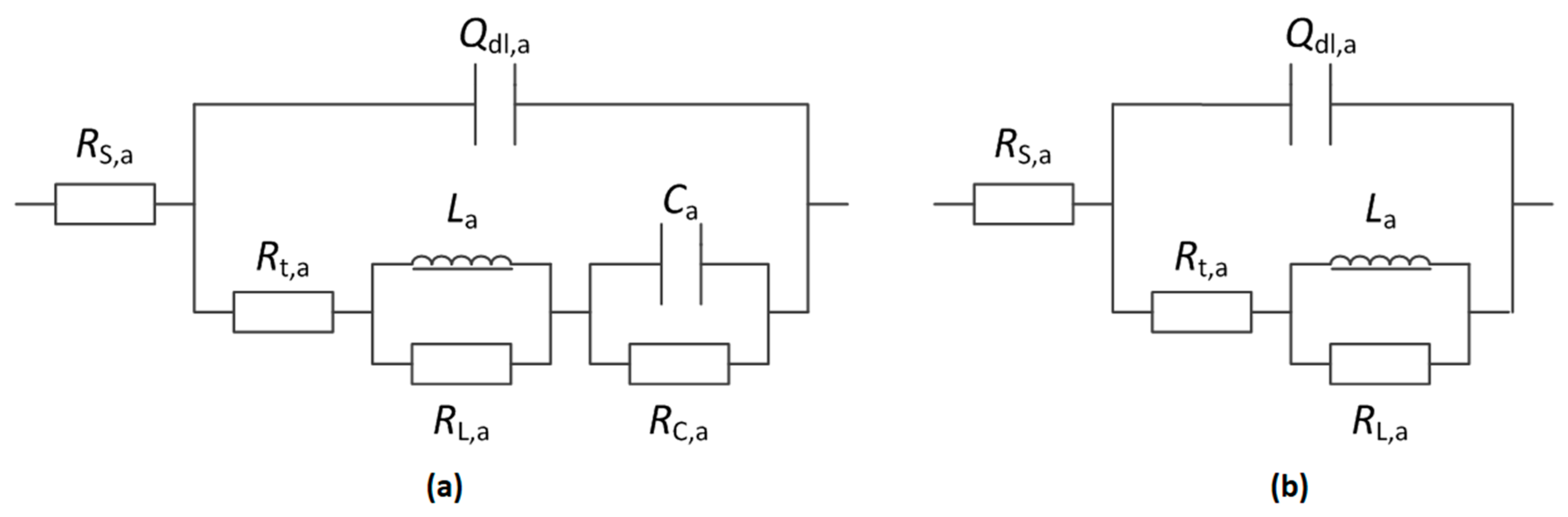
3.3.1. Anodic EIS
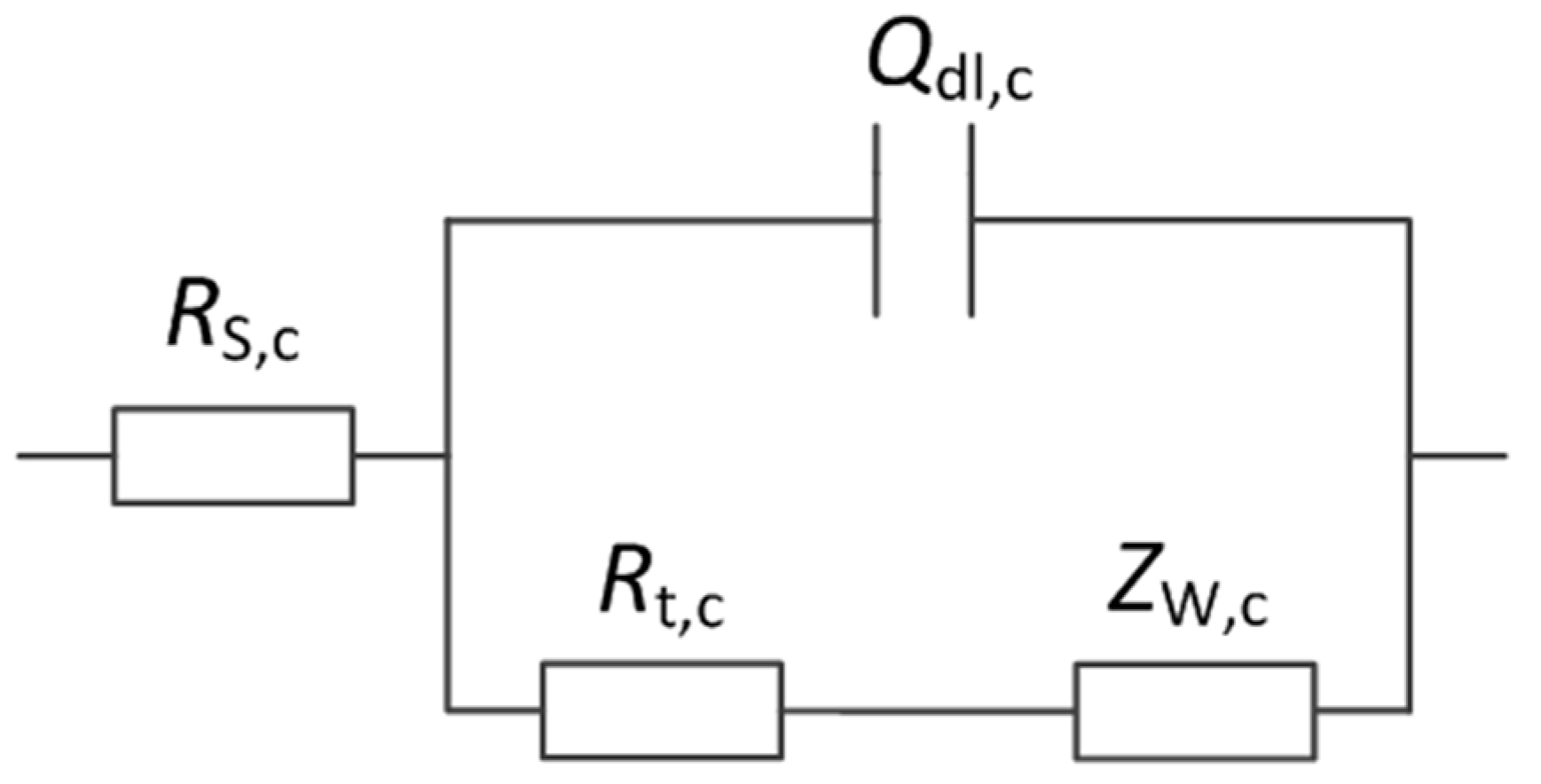
3.3.2. Cathodic EIS
3.4. Characterizations of Corroded Samples
4. Discussion
4.1. Electrochemical Reactions for CO2 Corrosion
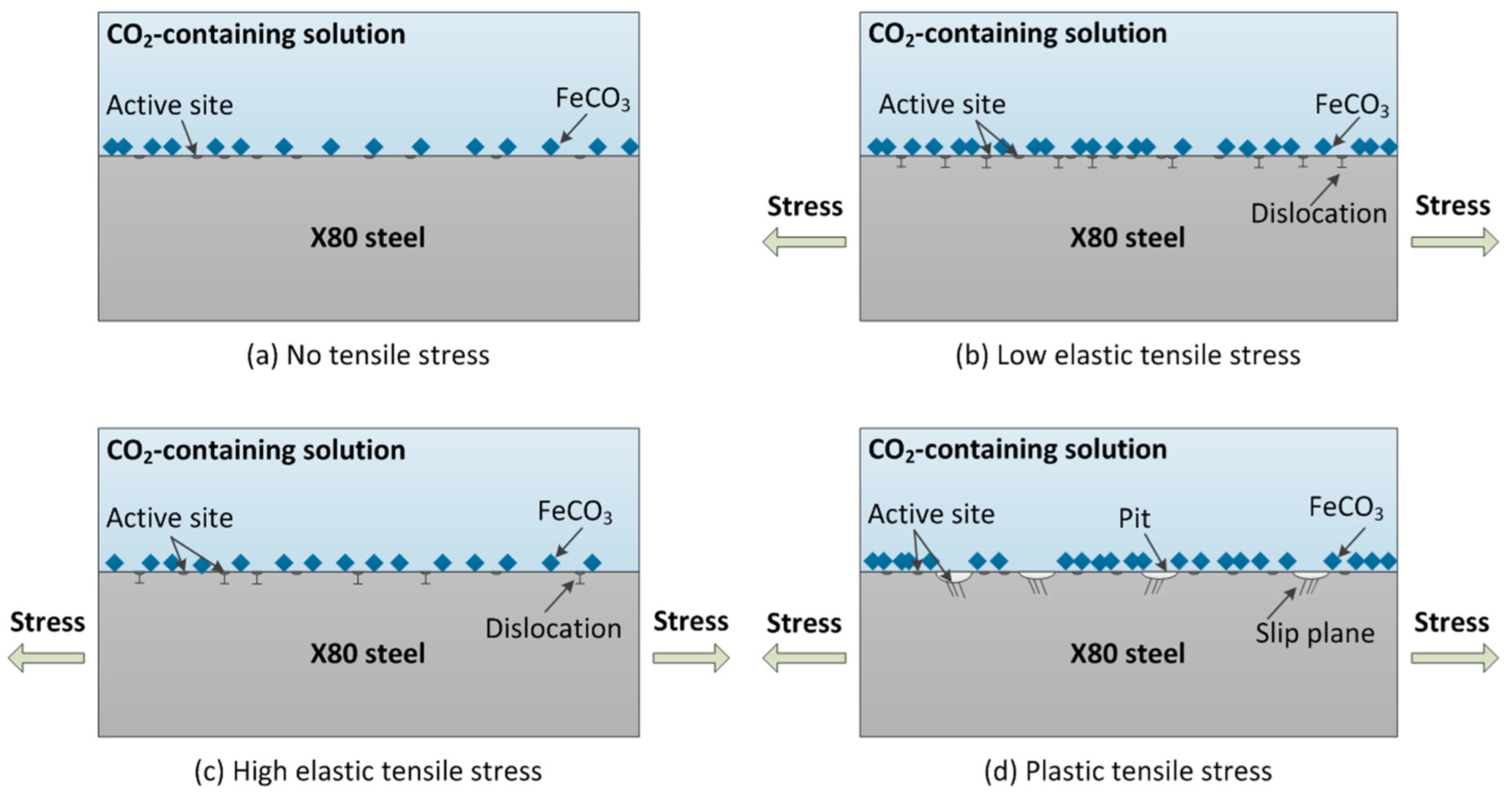
4.2. Mechanoelectrochemical Effect
4.3. Corrosion of Stressed Pipeline Steels in CO2 Environment
5. Conclusions
- (1)
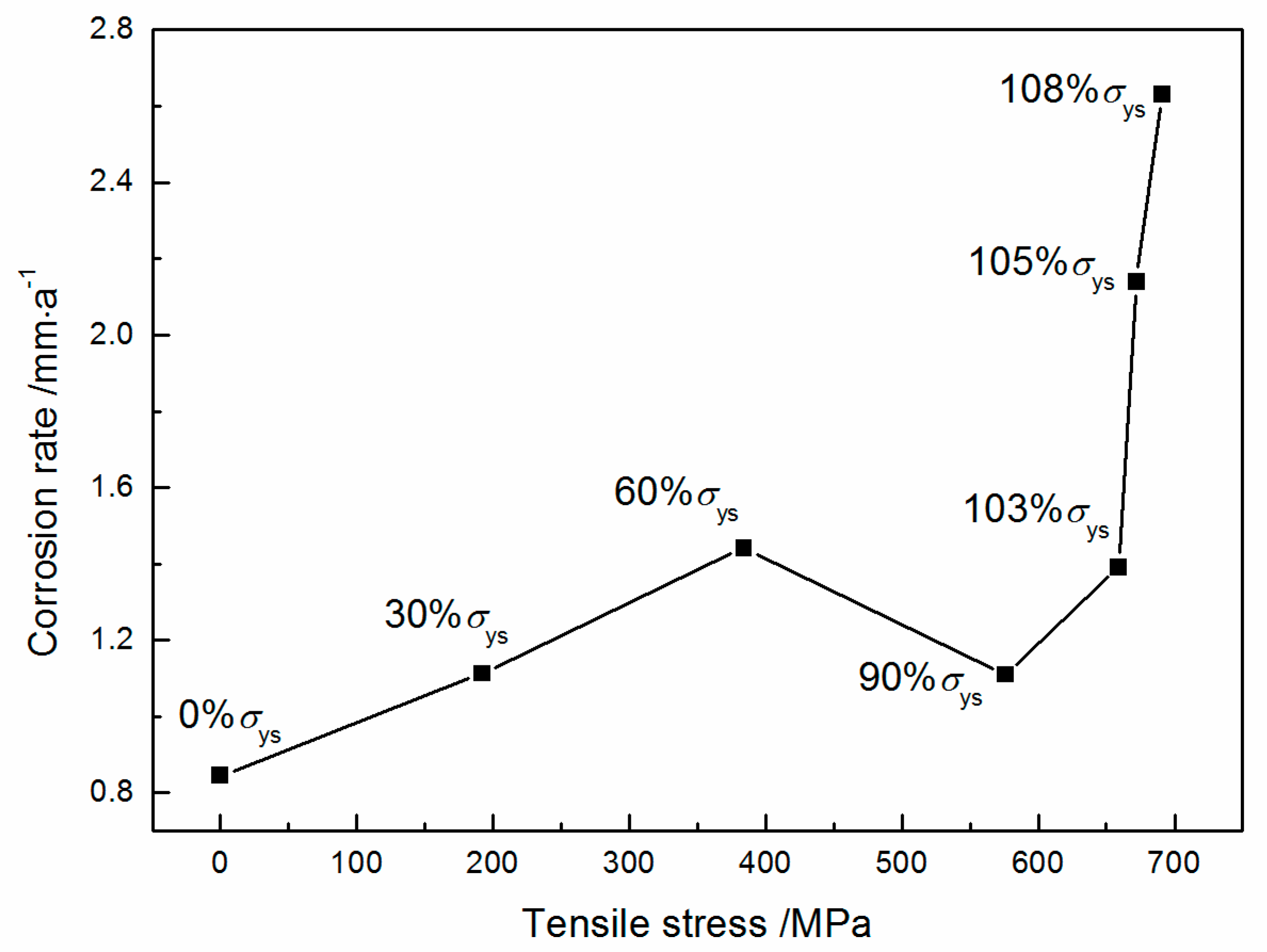
- Corrosion rate of stressed X80 steel in 3.5 wt% NaCl aqueous solution with saturated CO2 at 30 °C increases from 0.845 mm·a−1 at 0%σys to 1.442 mm·a−1 at 60%σys, and then slightly decreases to 1.110 mm·a−1 at 90%σys due to partial stress relaxation and dislocation annihilation at very high elastic stress. In the plastic stress range, corrosion rate increases sharply up to 2.631 mm·a−1 at 108%σys, which is about three times higher than that without tensile stress.
- (2)
- Both elastic tensile stress and plastic tensile stress can decrease the electrode potential and improve the electrochemical corrosion activity of X80 steel in CO2-saturated NaCl aqueous solution. Moreover, plastic tensile stress has a more significant effect than elastic tensile stress.
- (3)
- According to potentiodynamic polarization and EIS analysis, both anodic and cathodic reactions are accelerated by tensile stress.
- (4)
- According to the mechano-electrochemical theory, the corrosion of stressed X80 steel is significantly affected by the surface activity, which has much to do with the microscopic defect (like dislocation and slip plane) on the surface caused by tensile stress.
Author Contributions
Funding
Conflicts of Interest
References
- Liang, P.; Li, X.; Du, C.; Chen, X. Stress corrosion cracking of X80 pipeline steel in simulated alkaline soil solution. Mater. Des. 2009, 30, 1712–1717. [Google Scholar] [CrossRef]
- Nešić, S. Key issues related to modelling of internal corrosion of oil and gas pipelines—A review. Corros. Sci. 2007, 49, 4308–4338. [Google Scholar] [CrossRef]
- Song, F.M. A comprehensive model for predicting CO2, corrosion rate in oil and gas production and transportation systems. Electrochim. Acta 2010, 55, 689–700. [Google Scholar] [CrossRef]
- Eliyan, F.F.; Mahdi, E.S.; Alfantazi, A. Electrochemical evaluation of the corrosion behaviour of API-X100 pipeline steel in aerated bicarbonate solutions. Corros. Sci. 2012, 58, 181–191. [Google Scholar] [CrossRef]
- Xue, H.B.; Cheng, Y.F. Passivity and pitting corrosion of X80 pipeline steel in carbonate/bicarbonate solution studied by electrochemical measurements. J. Mater. Eng. Perform. 2010, 19, 1311–1317. [Google Scholar] [CrossRef]
- Mohammadi, F.; Eliyan, F.F.; Alfantazi, A. Corrosion of simulated weld HAZ of API X-80 pipeline steel. Corros. Sci. 2012, 63, 323–333. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, G.X.; Wu, W.; Qiao, Q.; Li, Y.; Li, X.F. Effect of pH and chloride on the micro-mechanism of pitting corrosion for high strength pipeline steel in aerated NaCl solutions. Appl. Surf. Sci. 2015, 349, 746–756. [Google Scholar] [CrossRef]
- Kermani, M.B.; Morshed, A. Carbon dioxide corrosion in oil and gas Production—A compendium. Corrosion 2013, 59, 659–683. [Google Scholar] [CrossRef]
- Eduok, U.; Ohaeri, E. Electrochemical and surface analyses of X70 steel corrosion in simulated acid pickling medium: Effect of poly (N-vinyl imidazole) grafted carboxymethyl chitosan additive. Electrochim. Acta 2018, 278, 302–312. [Google Scholar] [CrossRef]
- Eduok, U.; Jossou, E.; Szpunar, J. Enhanced surface protective performance of chitosanic hydrogel via nano-CeO2, dispersion for API 5L X70 alloy: Experimental and theoretical investigations of the role of CeO2. J. Mol. Liq. 2017, 241, 684–693. [Google Scholar] [CrossRef]
- Ohaeri, E.; Omale, J.; Eduok, U.; Szpunar, J. Effect of Thermomechanical Processing and Crystallographic Orientation on the Corrosion Behavior of API 5L X70 Pipeline Steel. Metall. Mater. Trans. A 2018, 49, 2269–2280. [Google Scholar] [CrossRef]
- Xu, L.Y.; Cheng, Y.F. An experimental investigation of corrosion of X100 pipeline steel under uniaxial elastic stress in a near-neutral pH solution. Corros. Sci. 2012, 59, 103–109. [Google Scholar] [CrossRef]
- Xu, L.Y.; Cheng, Y.F. Corrosion of X100 pipeline steel under plastic strain in a neutral pH bicarbonate solution. Corros. Sci. 2012, 64, 145–152. [Google Scholar] [CrossRef]
- Boven, G.V.; Chen, W.; Rogge, R. The role of residual stress in neutral pH stress corrosion cracking of pipeline steels. Part I: Pitting and cracking occurrence. Acta Mater. 2007, 55, 29–42. [Google Scholar] [CrossRef]
- Bao, M.; Ren, C.; Lei, M.; Wang, X.; Singh, A.; Guo, X. Electrochemical behavior of tensile stressed P110 steel in CO2 environment. Corros. Sci. 2016, 112, 585–595. [Google Scholar] [CrossRef]
- Li, M.C.; Cheng, Y.F. Corrosion of the stressed pipe steel in carbonate–bicarbonate solution studied by scanning localized electrochemical impedance spectroscopy. Electrochim. Acta 2008, 53, 2831–2836. [Google Scholar] [CrossRef]
- Li, K.; Wu, W.; Cheng, G.X.; Li, Y.; Hu, H.J.; Zhang, H. Corrosion behavior of X70 pipeline steel and corrosion rate prediction under the combination of corrosive medium and applied pressure. In Proceedings of the ASME 2017 Pressure Vessels and Piping Conference, Waikoloa, HI, USA, 16–20 July 2017. [Google Scholar]
- Wang, Y.; Zhao, W.; Ai, H.; Zhou, X.; Zhang, T. Effects of strain on the corrosion behaviour of X80 steel. Corros. Sci. 2011, 53, 2761–2766. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer: New York, UK, 2014; ISBN 978-1461489320. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, UK, 2001; ISBN 978-0471043720. [Google Scholar]
- Nordsveen, M.; Nešić, S.; Nyborg, R.; Stangeland, A. A mechanistic model for carbon dioxide corrosion of mild steel in the presence of protective iron carbonate films—Part 1: Theory and verification. Corrosion 2012, 59, 616–628. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Mao, L.J.; Zhou, S.W. Effects of chloride content on CO2 corrosion of carbon steel in simulated oil and gas well environments. Corros. Sci. 2014, 84, 165–171. [Google Scholar] [CrossRef]
- Bockris, J.O.; Drazic, D.; Despic, A.R. The electrode kinetics of the deposition and dissolution of iron. Electrochim. Acta 1961, 4, 325–361. [Google Scholar] [CrossRef]
- Nešić, S.; Postlethwaite, J.; Olsen, S. An electrochemical model for prediction of corrosion of mild steel in aqueous carbon dioxide solutions. Corrosion 1996, 52, 280–294. [Google Scholar] [CrossRef]
- Gutman, E.M. Mechanochemistry of Materials; Cambridge International Science Publishing: Cambridge, UK, 1998; ISBN 1-898326-32-0. [Google Scholar]
- Xu, L.Y.; Cheng, Y.F. Development of a finite element model for simulation and prediction of mechanoelectrochemical effect of pipeline corrosion. Corros. Sci. 2013, 73, 150–160. [Google Scholar] [CrossRef]
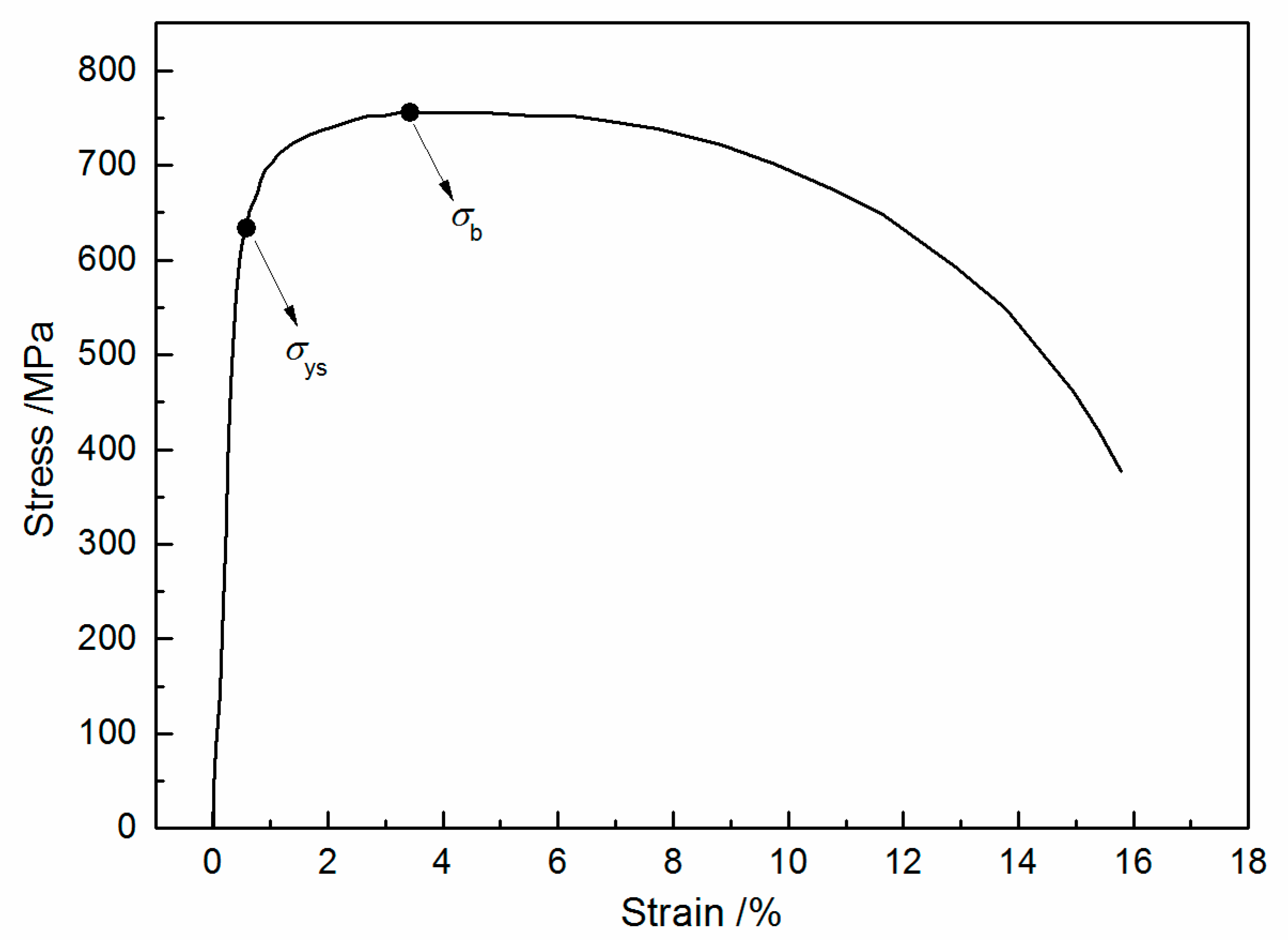
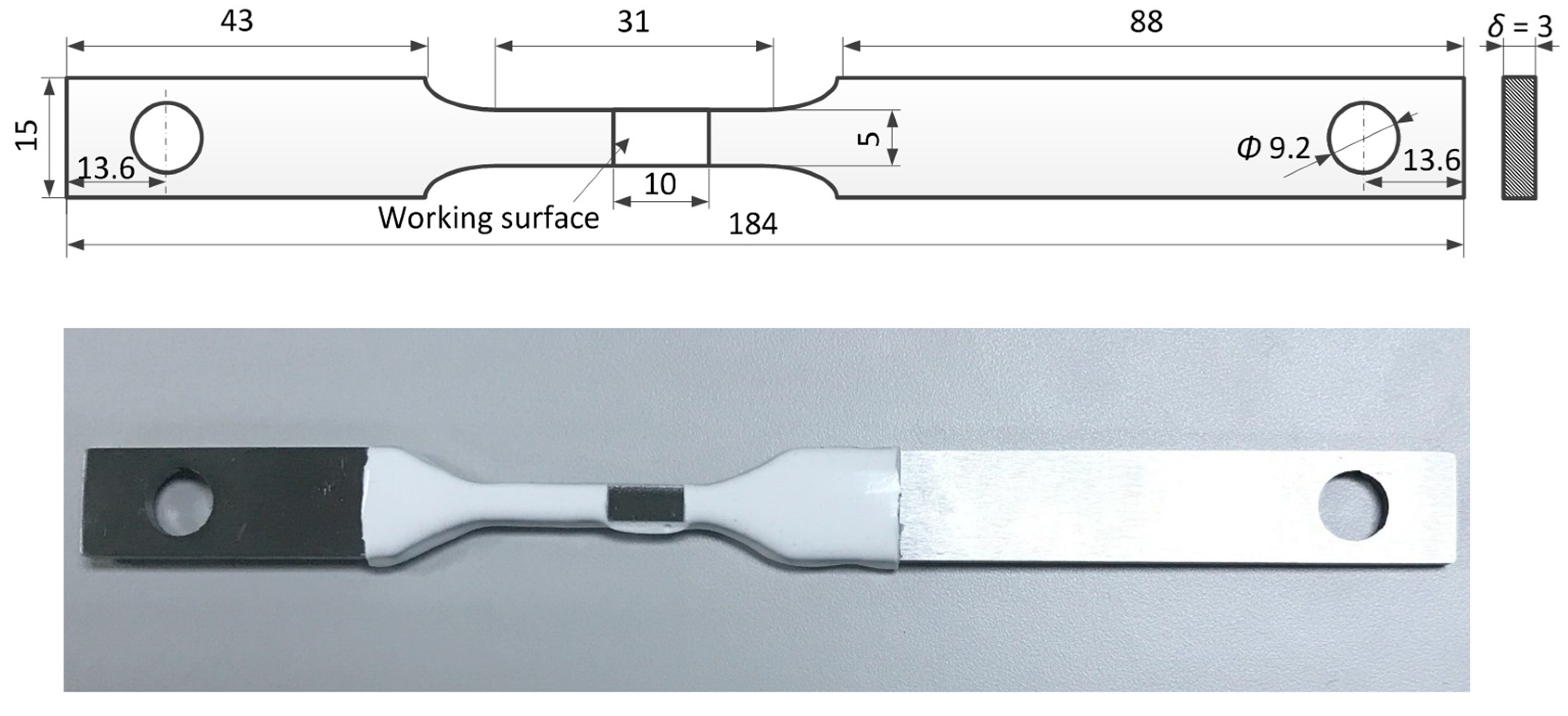
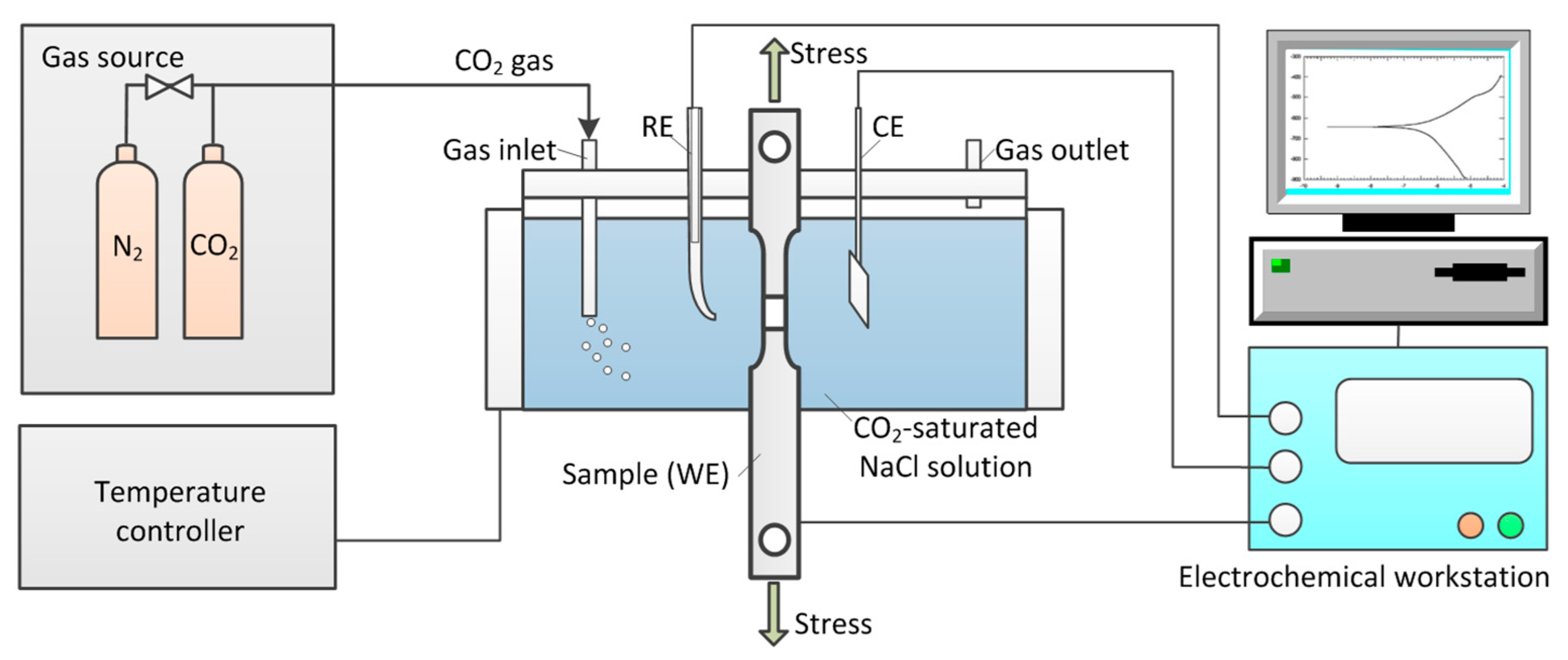
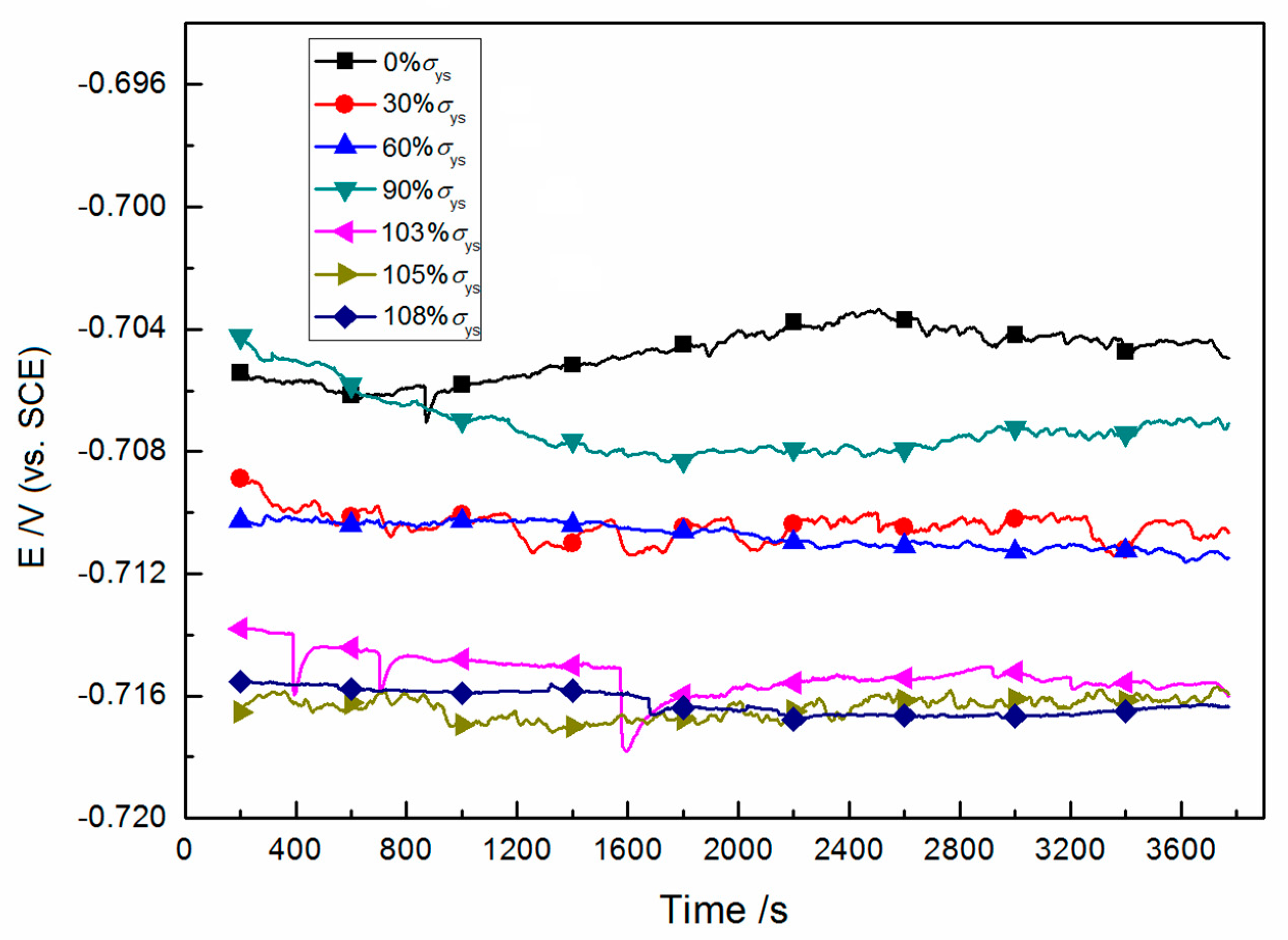


| Steel | C | Si | Mn | P | Nb | Ti | Al | Mo | Ni | Cu | Cr | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X80 | 0.05 | 0.22 | 1.78 | 0.01 | 0.055 | 0.015 | 0.044 | 0.26 | 0.256 | 0.143 | 0.03 | Bal. |
| Tensile Stress | 0%σys | 30%σys | 60%σys | 90%σys | 103%σys | 105%σys | 108%σys |
|---|---|---|---|---|---|---|---|
| ba/mV·dec−1 | 40.29 | 34.54 | 42.02 | 35.28 | 38.52 | 42.06 | 39.69 |
| Tensile Stress | RS,a/Ω·cm2 | Ydl,a/Ω−1·cm−2·sn | ndl,a | Rt,a/Ω·cm2 | La/H·cm2 | RL,a/Ω·cm2 | Ca/F·cm-2 | RC,a/Ω·cm2 |
|---|---|---|---|---|---|---|---|---|
| 0 | 1.858 | 0.0005508 | 0.7866 | 16.30 | 1.5200 | 9.809 | 3.870 | 6.825 |
| 30%σys | 1.575 | 0.0004375 | 0.8186 | 14.95 | 1.3050 | 8.360 | 4.046 | 7.034 |
| 60%σys | 1.273 | 0.0007162 | 0.7938 | 10.65 | 0.8289 | 5.958 | 6.738 | 2.879 |
| 90%σys | 1.682 | 0.0005311 | 0.7905 | 15.51 | 1.3490 | 9.387 | 3.751 | 97.718 |
| Tensile Stress | RS,a/Ω·cm2 | Ydl,a/Ω−1·cm−1·sn | ndl,a | Rt,a/Ω·cm2 | La/H·cm2 | RL,a/Ω·cm2 |
|---|---|---|---|---|---|---|
| 103%σys | 1.308 | 0.0007835 | 0.7555 | 12.94 | 1.59 | 8.573 |
| 105%σys | 1.268 | 0.0006818 | 0.7937 | 10.25 | 1.274 | 6.282 |
| 108%σys | 1.355 | 0.0007329 | 0.7872 | 9.52 | 1.477 | 6.588 |
| Tensile Stress | RS,c /Ω·cm2 | Ydl,c /Ω−1·cm−2·sn | ndl,c | Rt,c /Ω·cm2 | AW,c /Ω·cm2·s−0.5 |
|---|---|---|---|---|---|
| 0 | 1.914 | 0.0003185 | 0.8375 | 571.3 | 0.002394 |
| 30%σys | 1.599 | 0.0003293 | 0.8463 | 465.5 | 0.003021 |
| 60%σys | 1.449 | 0.0008707 | 0.8009 | 228.0 | 0.006291 |
| 90%σys | 1.446 | 0.0003825 | 0.8295 | 474.6 | 0.003360 |
| 103%σys | 1.319 | 0.0006090 | 0.8200 | 260.7 | 0.004155 |
| 105%σys | 1.278 | 0.0001056 | 0.7876 | 212.0 | 0.007714 |
| 108%σys | 1.314 | 0.0009652 | 0.8065 | 196.9 | 0.006539 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Yin, H.; Zhang, H.; Kang, J.; Li, Y.; Dan, Y. Electrochemical Investigation of Corrosion of X80 Steel under Elastic and Plastic Tensile Stress in CO2 Environment. Metals 2018, 8, 949. https://doi.org/10.3390/met8110949
Wu W, Yin H, Zhang H, Kang J, Li Y, Dan Y. Electrochemical Investigation of Corrosion of X80 Steel under Elastic and Plastic Tensile Stress in CO2 Environment. Metals. 2018; 8(11):949. https://doi.org/10.3390/met8110949
Chicago/Turabian StyleWu, Wei, Hailong Yin, Hao Zhang, Jia Kang, Yun Li, and Yong Dan. 2018. "Electrochemical Investigation of Corrosion of X80 Steel under Elastic and Plastic Tensile Stress in CO2 Environment" Metals 8, no. 11: 949. https://doi.org/10.3390/met8110949
APA StyleWu, W., Yin, H., Zhang, H., Kang, J., Li, Y., & Dan, Y. (2018). Electrochemical Investigation of Corrosion of X80 Steel under Elastic and Plastic Tensile Stress in CO2 Environment. Metals, 8(11), 949. https://doi.org/10.3390/met8110949





