Micro-Galvanic Corrosion of Steam Generator Materials within Pores of Magnetite Flakes in Alkaline Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrodeposition of the Mmagnetite Film
2.3. Microstructural Analysis
2.4. Electrochemical Tests
3. Results and Discussion
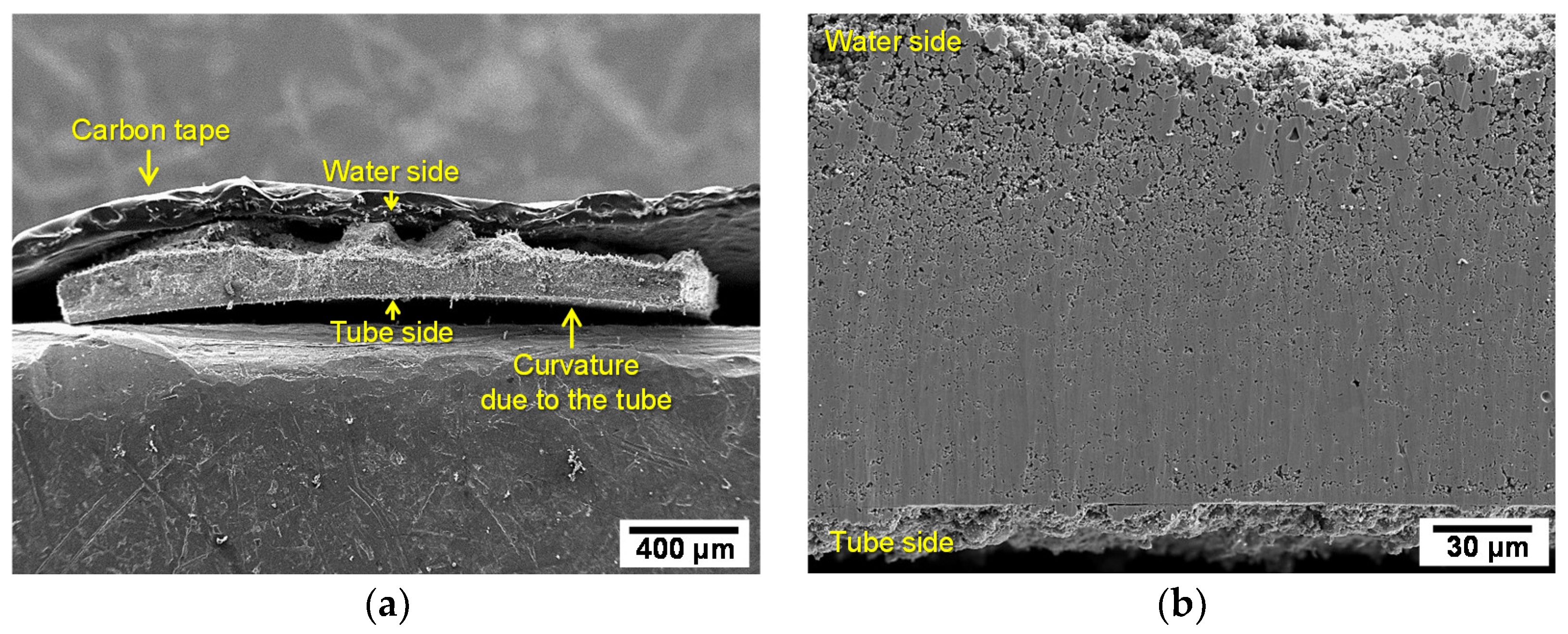
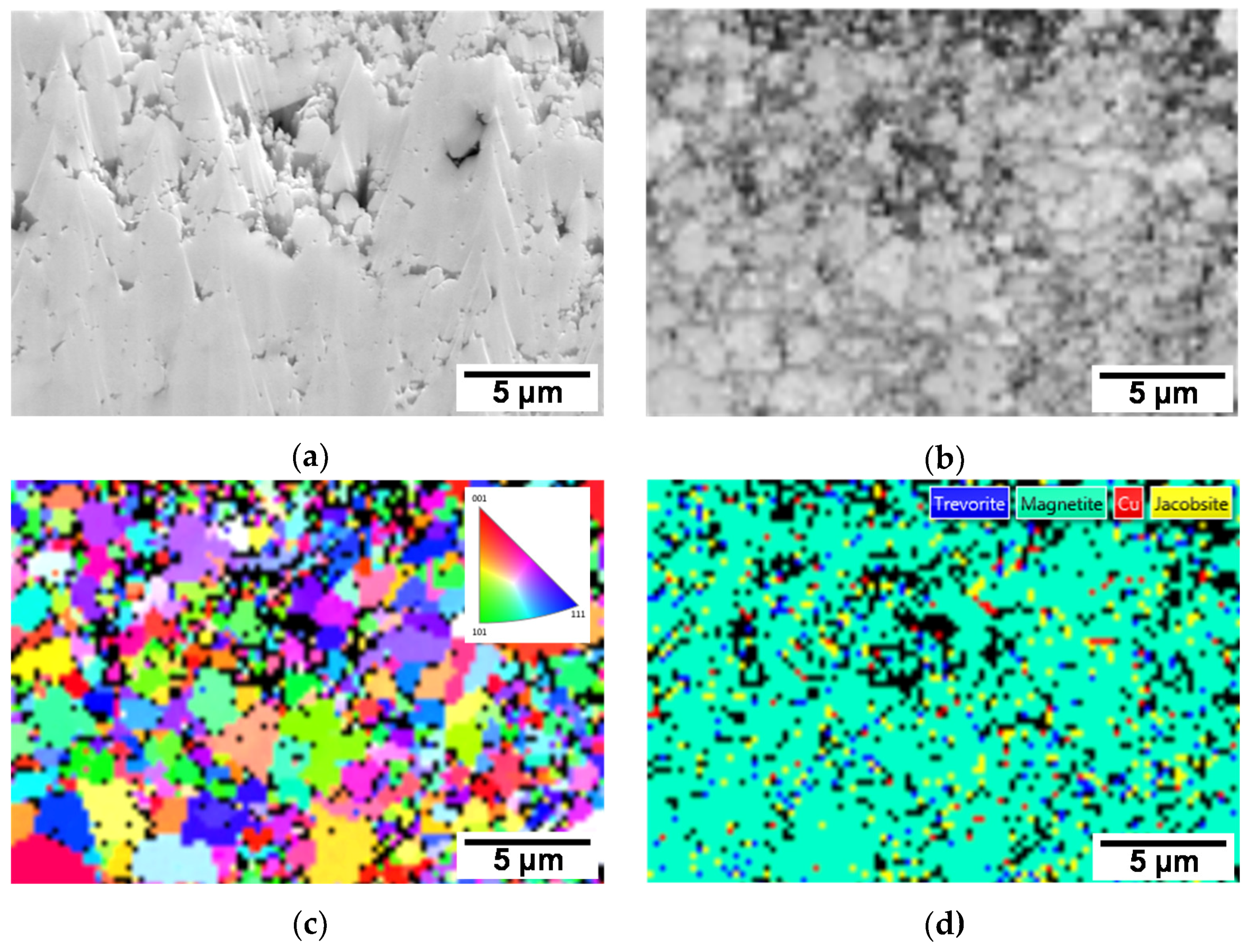
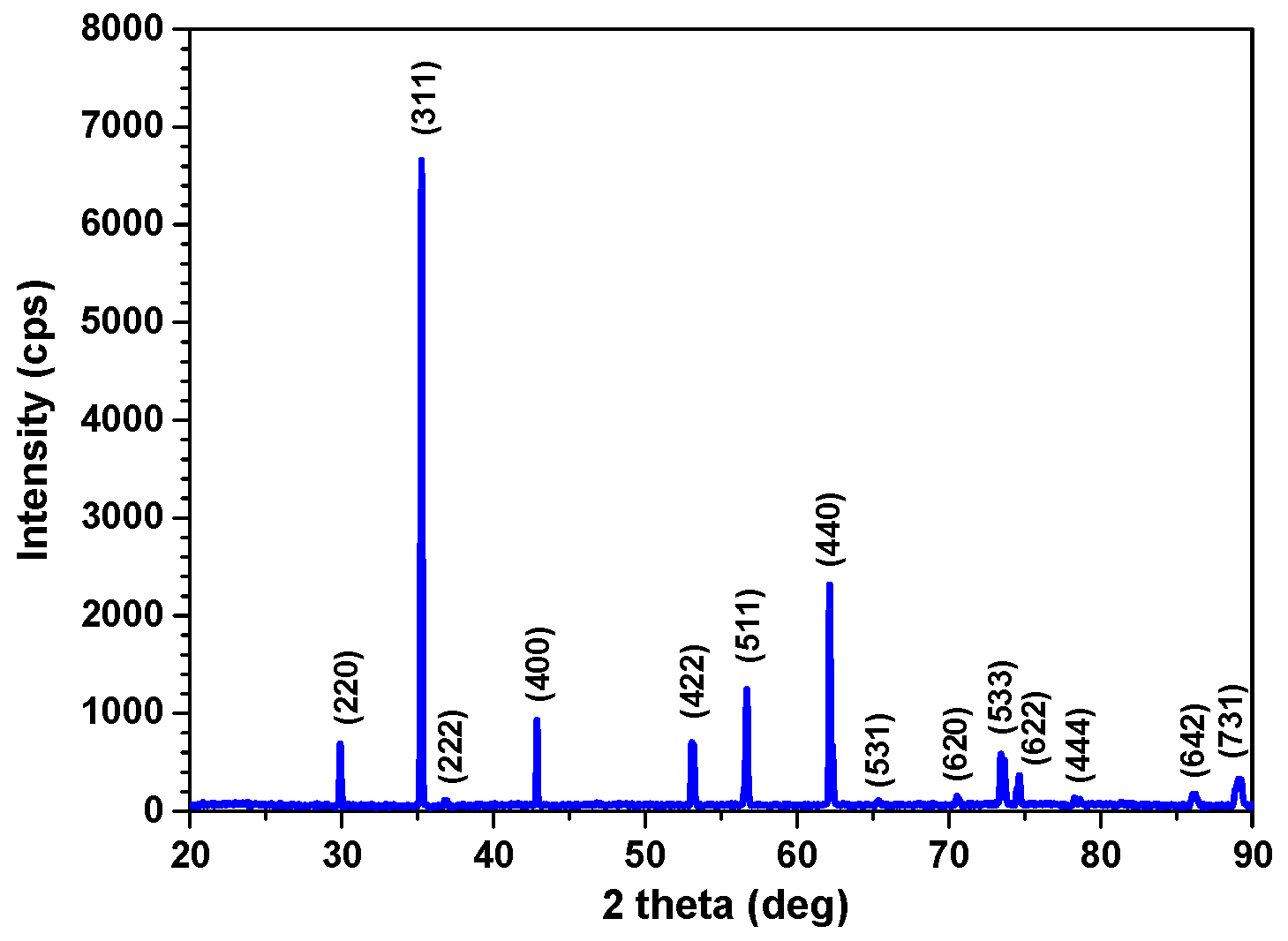
3.1. Characterization of the SG Tube Deposit Flakes Collected From the SG
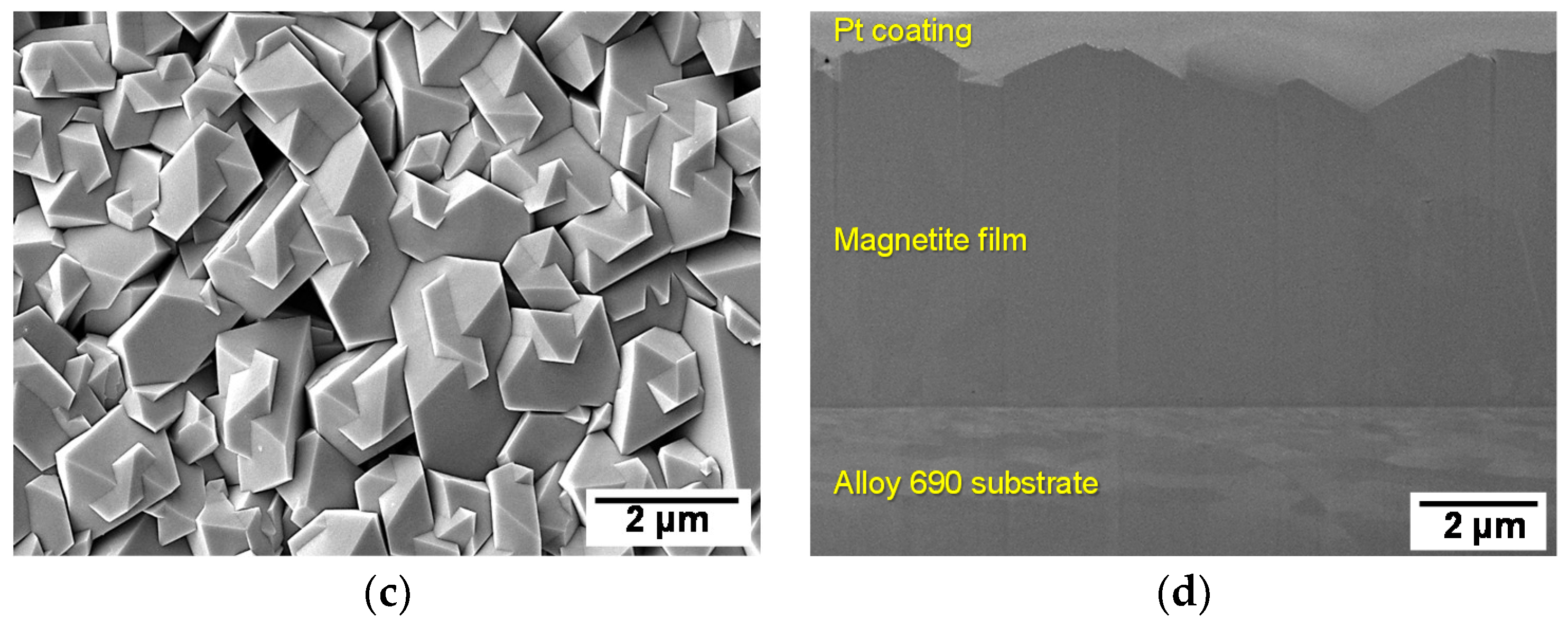
3.2. Microstructural Analysis of the Electrodeposited Magnetite Films
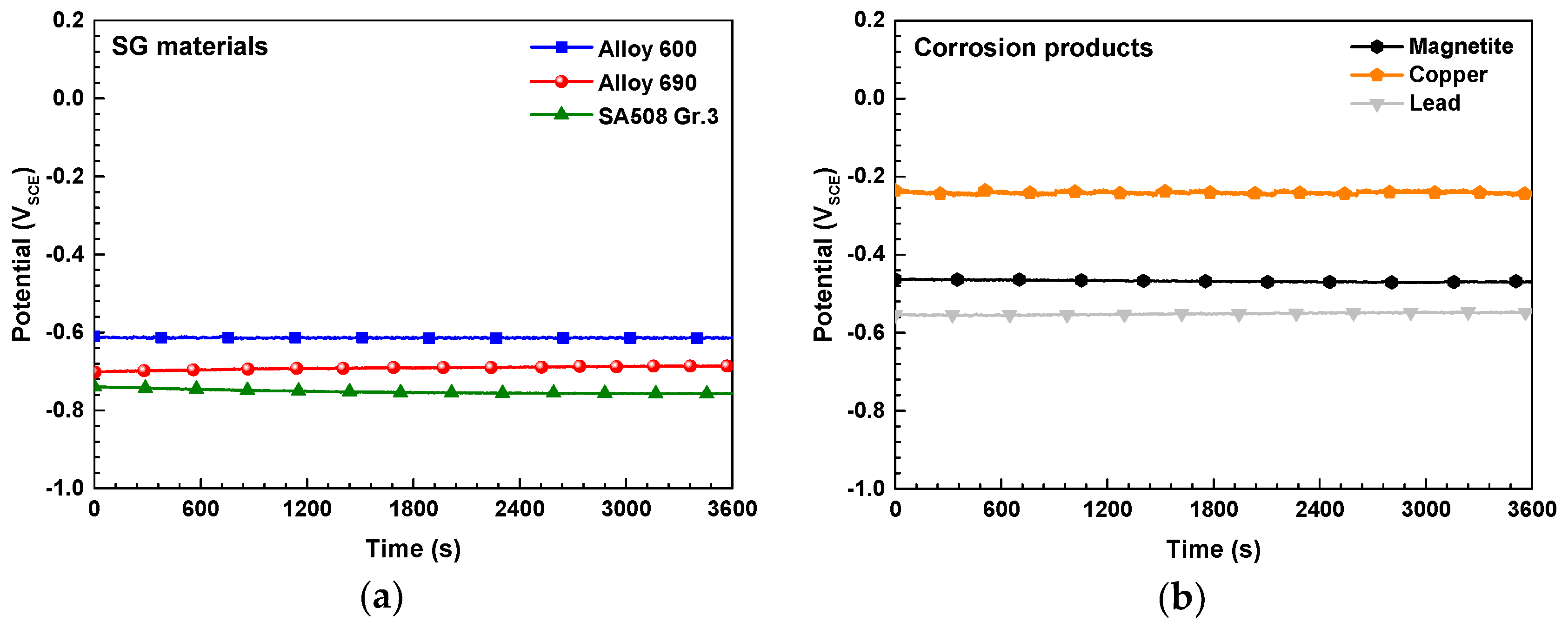
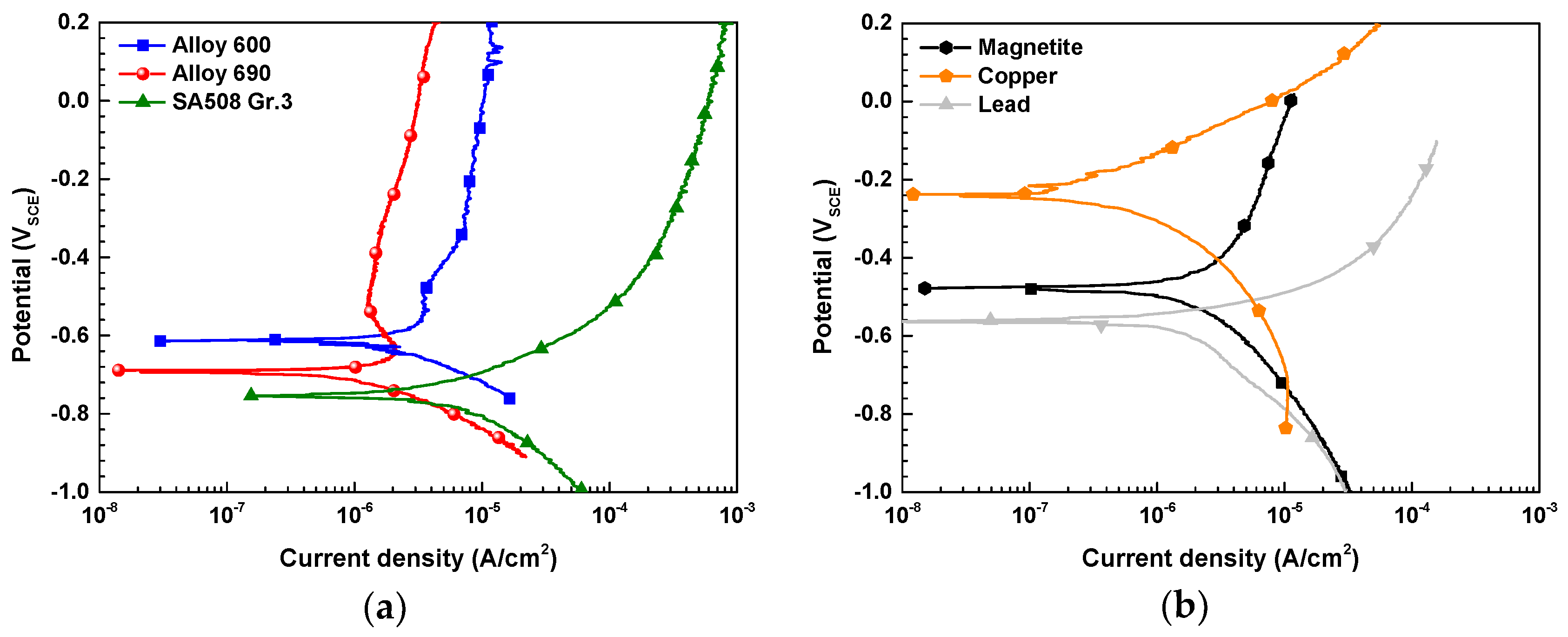
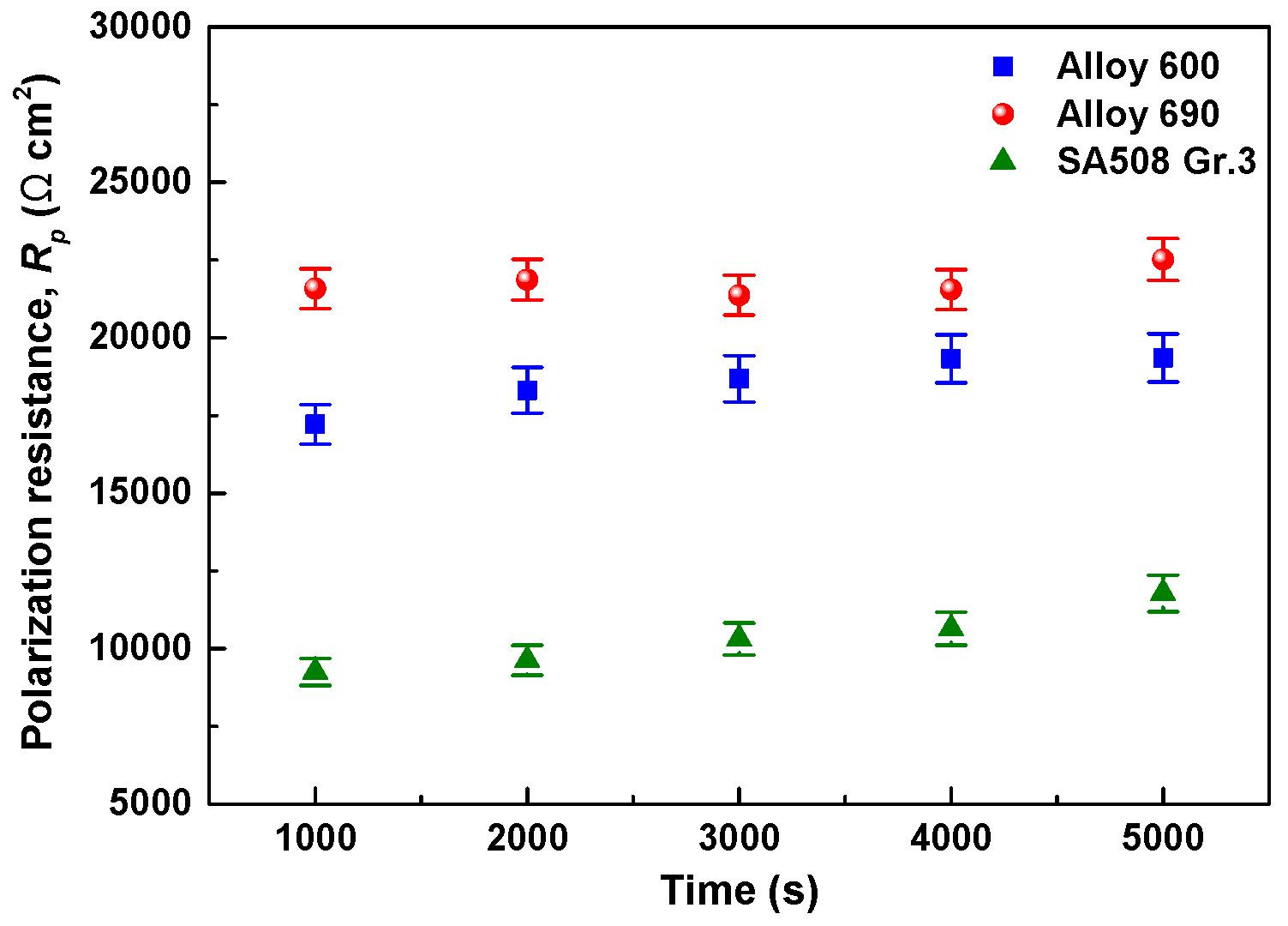
3.3. Electrochemical Corrosion Behavior of the SG Materials
4. Conclusions
- Based on the EBSD results, the flake samples were mainly composed of magnetite and contained only small amounts of trevorite, jacobsite, and metallic Cu particle.
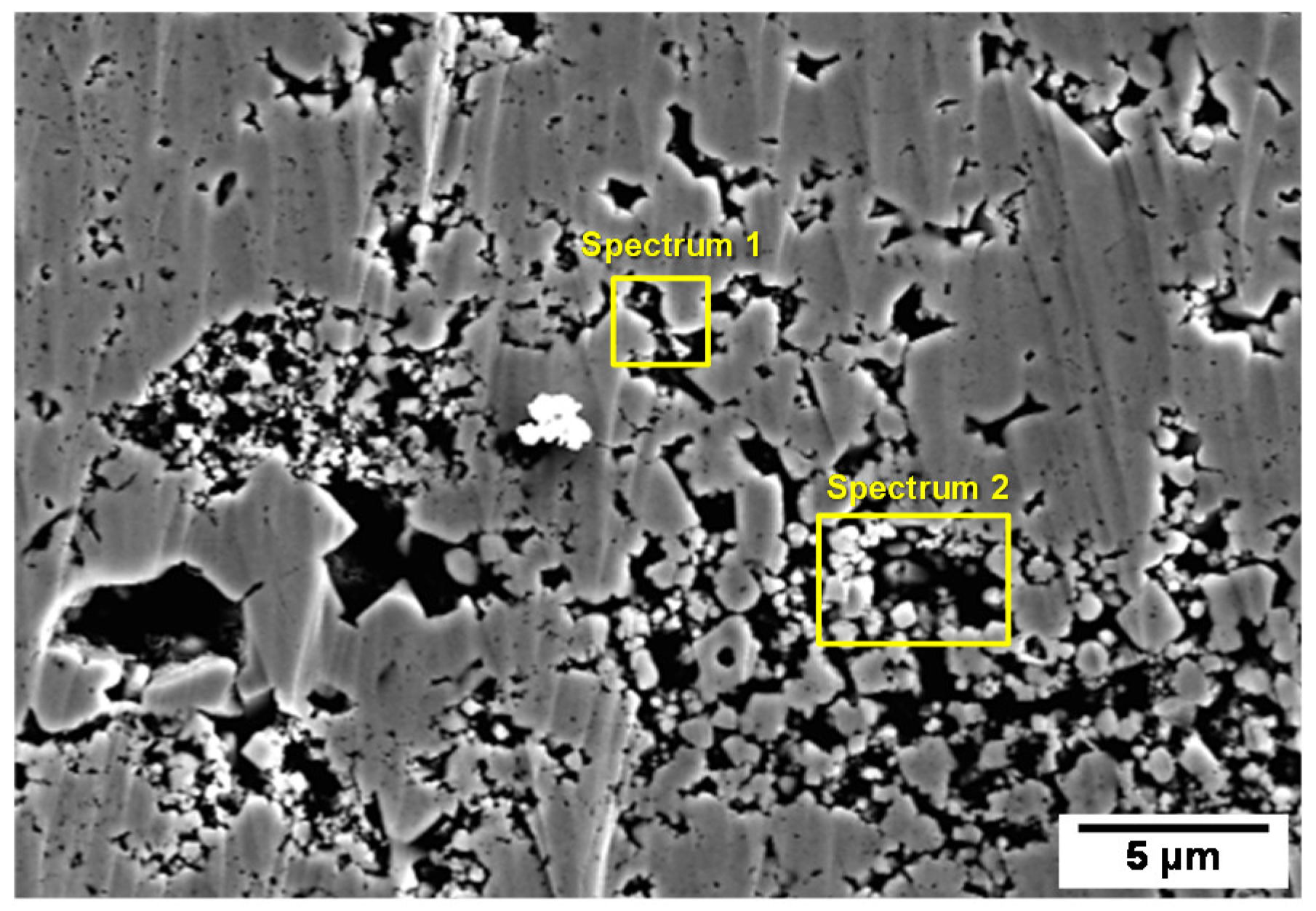
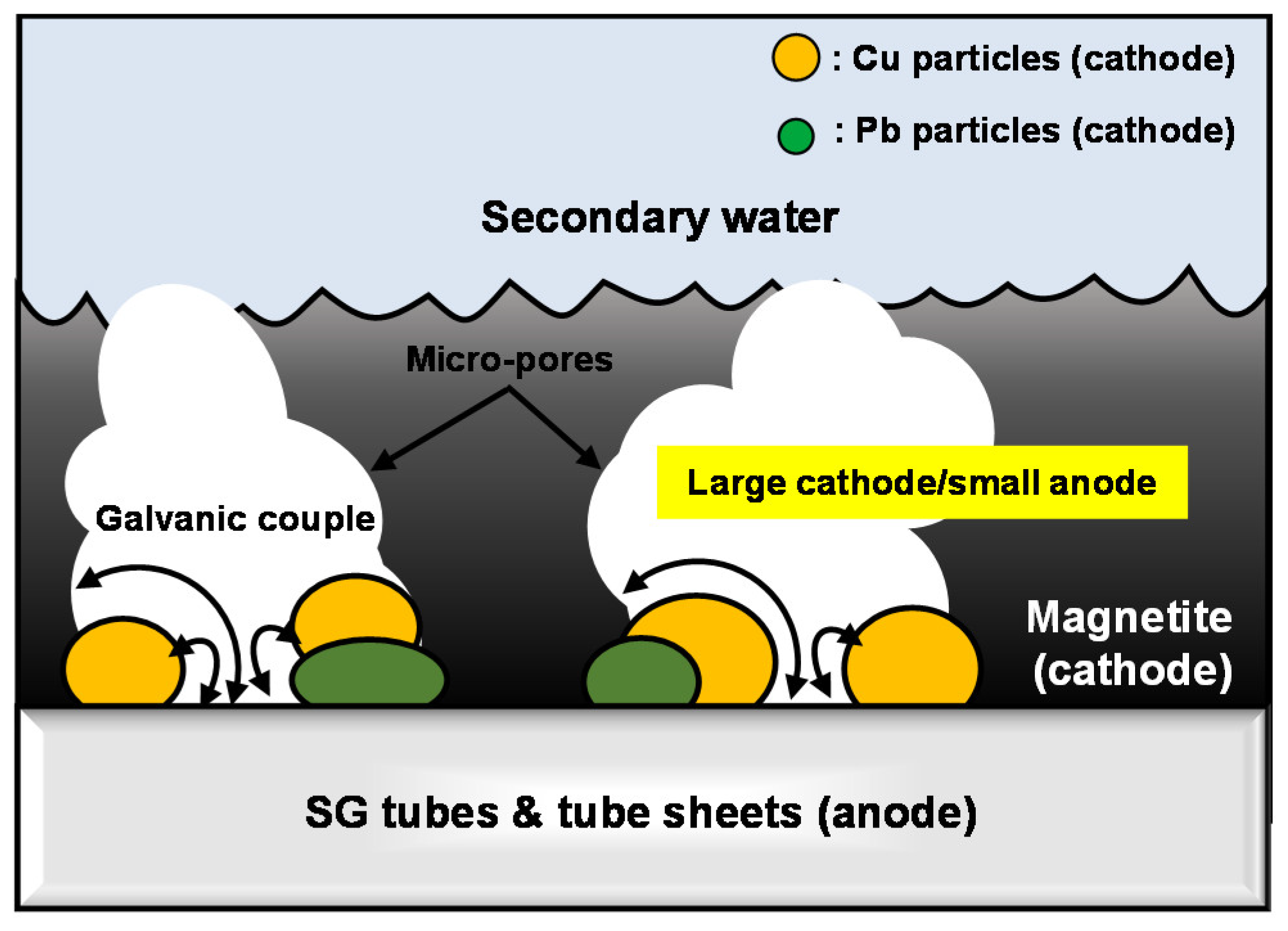
- SEM-EDS results showed that some impurities such as Cu, Pb, P, S, and Cl were concentrated in the micro-pores of the deposits. In particular, Cu and Pb are expected to exist in a metallic form in the micro-pores of the magnetite flakes because the formation reactions of metallic Cu and Pb are thermodynamically spontaneous in the secondary system of SG.
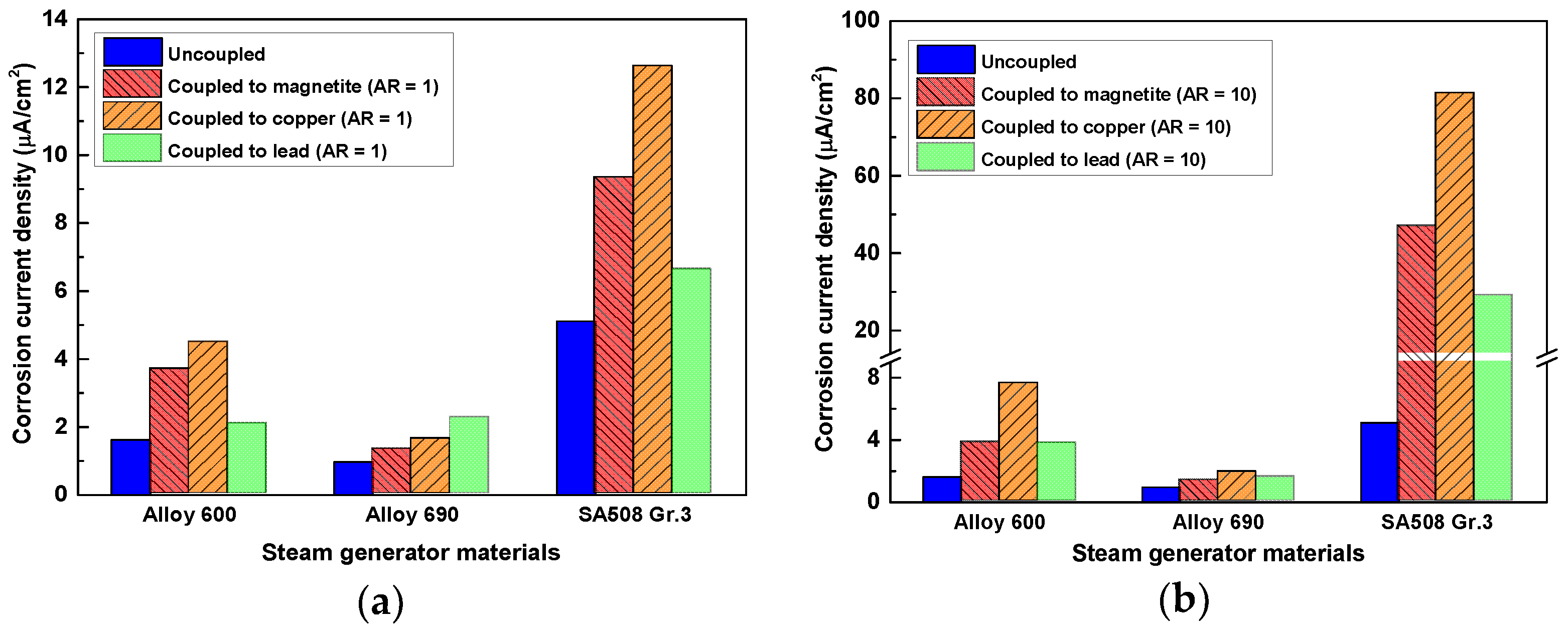
- The all SG materials considered in this study were act as an anode of the galvanic couple with the each corrosion products (magnetite, copper, and lead) and the corrosion rate of all SG materials was significantly increased. When the AR was increased 1 to 10, Cu has the largest galvanic corrosion effect to all SG materials.
- The galvanic effect of metallic copper and lead as well as the magnetite should be considered in the corrosion behavior of SG tube contacted to the corrosion products.
Author Contributions
Funding
Conflicts of Interest
References
- Goujon, C.; Pauporté, T.; Bescond, A.; Mansour, C.; Delaunay, S.; Bretelle, J.-L. Effects of curative and preventive chemical cleaning processes on fouled steam generator tubes in nuclear power plants. Nucl. Eng. Des. 2017, 323, 120–132. [Google Scholar] [CrossRef]
- Fujiwara, K.; Kawamura, H.; Kanbe, H.; Hirano, H.; Takiguchi, H.; Yoshino, K.; Yamamoto, S.; Shibata, T.; Ishigura, K. Applicability of chemical cleaning process to steam generator secondary side, (I) outline of the investigation and cleaning effectiveness. J. Nucl. Sci. Techonol. 2004, 41, 44–54. [Google Scholar] [CrossRef]
- Kawamura, H.; Fujiwara, K.; Kanbe, H.; Hirano, H.; Takiguchi, H.; Yoshino, K.; Yamamoto, S.; Shibata, T.; Ishigura, K. Applicability of chemical cleaning process to steam generator secondary side, (III) effect of chemical cleaning on long term integrity of steam generator tube after chemical cleaning process. J. Nucl. Sci. Techonol. 2006, 43, 665–668. [Google Scholar]
- Jeon, S.H.; Song, G.D.; Hur, D.H. Corrosion behavior of Alloy 600 coupled with electrodeposited magnetite in simulated secondary water of PWRs. Mater. Trans. 2015, 56, 2078–2083. [Google Scholar] [CrossRef]
- Jeon, S.H.; Song, G.D.; Hur, D.H. Galvanic corrosion between Alloy 690 and magnetite in alkaline aqueous solutions. Metals 2015, 5, 2372–2382. [Google Scholar] [CrossRef]
- Song, G.D.; Jeon, S.H.; Kim, J.G.; Hur, D.H. Synergistic effect of chloride ions and magnetite on the corrosion of Alloy 690 in alkaline solutions. Corrosion 2017, 73, 216–220. [Google Scholar] [CrossRef]
- Jeon, S.H.; Song, G.D.; Hur, D.H. Electrodeposition of magnetite on carbon steel in Fe(III)-triethanolamine solution and its corrosion behavior. Mater. Trans. 2015, 56, 1107–1111. [Google Scholar] [CrossRef]
- Song, G.D.; Jeon, S.H.; Kim, J.G.; Hur, D.H. Effect of polyacrylic acid on the corrosion behavior of carbon steel and magnetite in alkaline aqueous solutions. Corrosion 2017, 72, 1010–1120. [Google Scholar] [CrossRef]
- Song, G.D.; Jeon, S.H.; Kim, J.G.; Son, Y.H.; Hur, D.H. Galvanic effect of magnetite on the corrosion behavior of carbon steel in deaerated alkaline solutions under flowing conditions. Corros. Sci. 2018, 131, 71–80. [Google Scholar] [CrossRef]
- Hur, D.H.; Choi, M.S.; Kim, U.C.; Han, J.H. Magnetite dissolution and corrosion behavior in high temperature EDTA solvents. Nucl. Eng. Des. 2003, 220, 11–16. [Google Scholar] [CrossRef]
- Jeon, S.H.; Son, Y.H.; Choi, W.I.; Song, G.D.; Hur, D.H. Simulating porous magnetite layer deposited on Alloy 690TT steam generator tubes. Materials 2018, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, N.; Nasu, K.; Fujimori, A.; Siratori, K. Electronic Conduction in Oxides; Springer-Verlag: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- EPRI-TR-106048. Characterization of PWR Steam Generator Deposits; EPRI: Palo Alto, CA, USA, 1996. [Google Scholar]
- Jeon, S.H.; Hong, S.; Kwon, H.C.; Hur, D.H. Characteristics of steam generator tube deposits in an operating pressurized water reactor. J. Nucl. Mater. 2018, 507, 371–380. [Google Scholar] [CrossRef]
- Diego, G.D.; Merino, S. Microstructural characterization of sludge steam generator from Spanish NPP’s. In Proceedings of the 20th International Conference on Water Chemistry in Nuclear Reactor System, Brighton, UK, 2–7 October 2016. No. 139. [Google Scholar]
- Turner, C.W.; Klimas, S.J.; Brideau, M.G. Thermal resistance of steam-generator tube deposits under single-phase forced convection and flow-boiling heat transfer. Can. J. Chem. Eng. 2000, 78, 53–60. [Google Scholar] [CrossRef]
- EPRI-TR-3002002794. Steam Generator Management Program: PWR Steam Generator Deposit Characterization Sourcebook; EPRI: Palo Alto, CA, USA, 2014. [Google Scholar]
- Wright, S.I.; Nowell, M.M.; Lindeman, S.P.; Camus, P.P.; Graef, M.D.; Jackson, M.A. Introduction and comparison of new EBSD post-processing methodologies. Ultramicroscopy 2015, 159, 81–94. [Google Scholar] [CrossRef] [PubMed]
- HSC Chemistry 6, version 6.12; Outotec Research Oy: Pori, Filand, 2006.
- Tostmann, K.-H. Korrosion; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]


| SG Materials | C | Cr | Fe | Si | Mn | Ti | Al | Mo | Ni |
|---|---|---|---|---|---|---|---|---|---|
| Alloy 600 | 0.02 | 15.7 | 10.0 | 0.1 | 0.3 | 0.1 | 0.1 | - | Bal. |
| Alloy 690 | 0.02 | 28.0 | 10.2 | 0.1 | 0.3 | 0.1 | 0.1 | - | Bal. |
| SA508 Gr.3 | 0.20 | 0.2 | Bal. | 0.1 | 1.5 | - | - | 0.6 | 1.0 |
| Fe | Mn | Ni | Cu | Ti | Al | Cr | Si | Mg | Ca |
|---|---|---|---|---|---|---|---|---|---|
| 95.97 | 2.23 | 0.84 | 0.43 | 0.23 | 0.12 | 0.08 | 0.04 | 0.04 | 0.02 |
| Structure | Phase Fraction (%) |
|---|---|
| Magnetite (Fe3O4) | 71.03 |
| Jacobsite (Mn2FeO4) | 4.55 |
| Trevorite (Ni2FeO4) | 3.79 |
| Metallic Cu | 1.72 |
| Zero solutions (black regions) | 18.91 |
| Element | Spectrum 1 | Spectrum 2 |
|---|---|---|
| Cu | 3.13 | 0.19 |
| Pb | - | 0.26 |
| Cl | - | 0.03 |
| S | 0.03 | 0.03 |
| P | - | 0.04 |
| Corrosion Parameters | SG Materials | ||
|---|---|---|---|
| Alloy 600 | Alloy 690 | SA508 Gr.3 | |
| βa (V/decade) | 0.100 ± 0.008 | 0.092 ± 0.005 | 0.142 ± 0.007 |
| βc (V/decade) | 0.135 ± 0.009 | 0.130 ± 0.004 | 0.183 ± 0.010 |
| Ecorr (VSCE) | −0.611 ± 0.004 | −0.687 ± 0.005 | −0.756 ± 0.009 |
| icorr (µA/cm2) | 1.62 ± 0.10 | 1.05 ± 0.12 | 5.11 ± 0.18 |
| Rpa (Ω/cm2), | 15880 ± 636 | 21580 ± 647 | 8698 ± 435 |
| icorr. pr (µA/cm2) | 1.57 ± 0.04 | 1.08± 0.12 | 4.00 ± 0.13 |
| Galvanic Coupling | SG Materials | Galvanic Corrosion Parameters | ||
|---|---|---|---|---|
| Ecouple (VSCE) | icouple (μA/cm2) | icouple/icorr (Times) | ||
| Coupled to magnetite | Alloy 600 | −0.564 ± 0.007 | 3.69 ± 0.10 | 2.28 |
| Alloy 690 | −0.507 ± 0.006 | 1.32 ± 0.13 | 1.26 | |
| SA508 Gr.3 | −0.697 ± 0.006 | 9.34 ± 0.20 | 1.83 | |
| Coupled to copper | Alloy 600 | −0.394 ± 0.005 | 4.48 ± 0.11 | 2.77 |
| Alloy 690 | −0.318 ± 0.006 | 1.63 ± 0.12 | 1.55 | |
| SA508 Gr.3 | −0.691 ± 0.007 | 12.62 ± 0.26 | 2.47 | |
| Coupled to lead | Alloy 600 | −0.593 ± 0.009 | 2.08 ± 0.08 | 1.28 |
| Alloy 690 | −0.599 ± 0.008 | 2.26 ± 0.12 | 2.15 | |
| SA508 Gr.3 | −0.713 ± 0.010 | 6.63 ± 0.15 | 1.30 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.-H.; Song, G.D.; Hur, D.H. Micro-Galvanic Corrosion of Steam Generator Materials within Pores of Magnetite Flakes in Alkaline Solutions. Metals 2018, 8, 899. https://doi.org/10.3390/met8110899
Jeon S-H, Song GD, Hur DH. Micro-Galvanic Corrosion of Steam Generator Materials within Pores of Magnetite Flakes in Alkaline Solutions. Metals. 2018; 8(11):899. https://doi.org/10.3390/met8110899
Chicago/Turabian StyleJeon, Soon-Hyeok, Geun Dong Song, and Do Haeng Hur. 2018. "Micro-Galvanic Corrosion of Steam Generator Materials within Pores of Magnetite Flakes in Alkaline Solutions" Metals 8, no. 11: 899. https://doi.org/10.3390/met8110899
APA StyleJeon, S.-H., Song, G. D., & Hur, D. H. (2018). Micro-Galvanic Corrosion of Steam Generator Materials within Pores of Magnetite Flakes in Alkaline Solutions. Metals, 8(11), 899. https://doi.org/10.3390/met8110899







