Change of the Chemical and Mineralogical Composition of the Slag during Oxygen Blowing in the Oxygen Converter Process
Abstract
:1. Introduction
2. Materials and Methods
3. Results
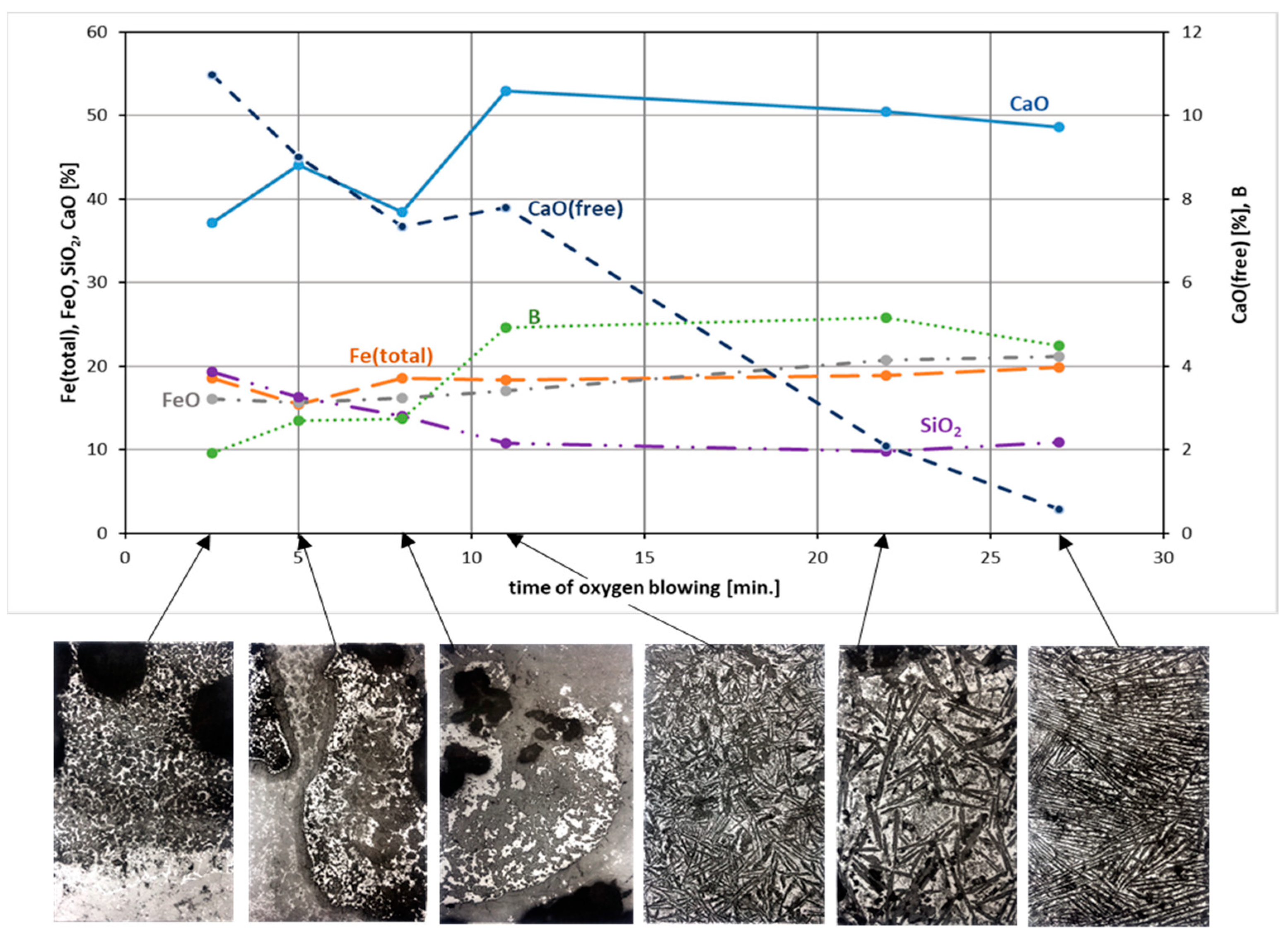
3.1. Changes in the Chemical Composition of Slag during Oxygen Blowing
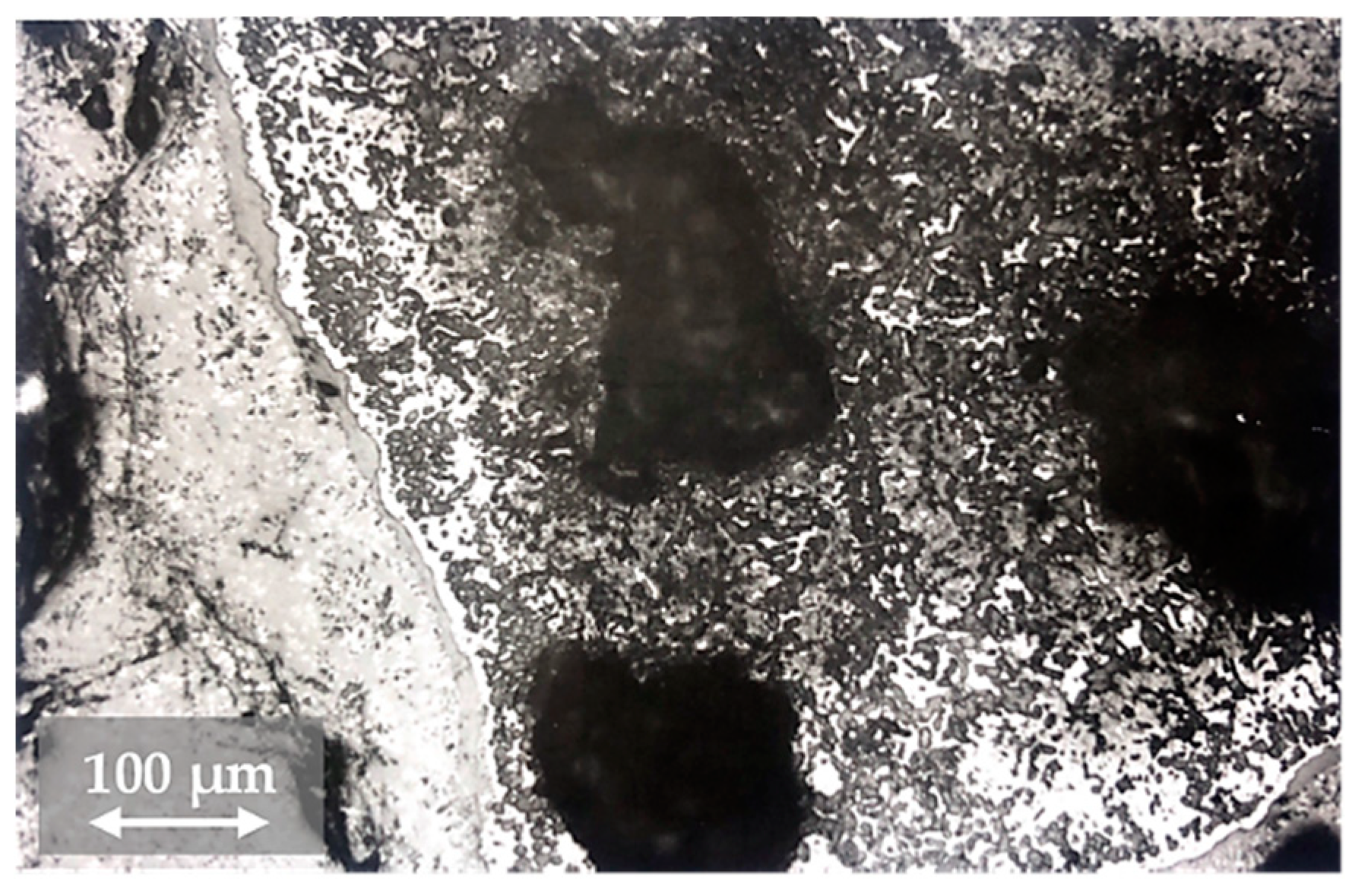
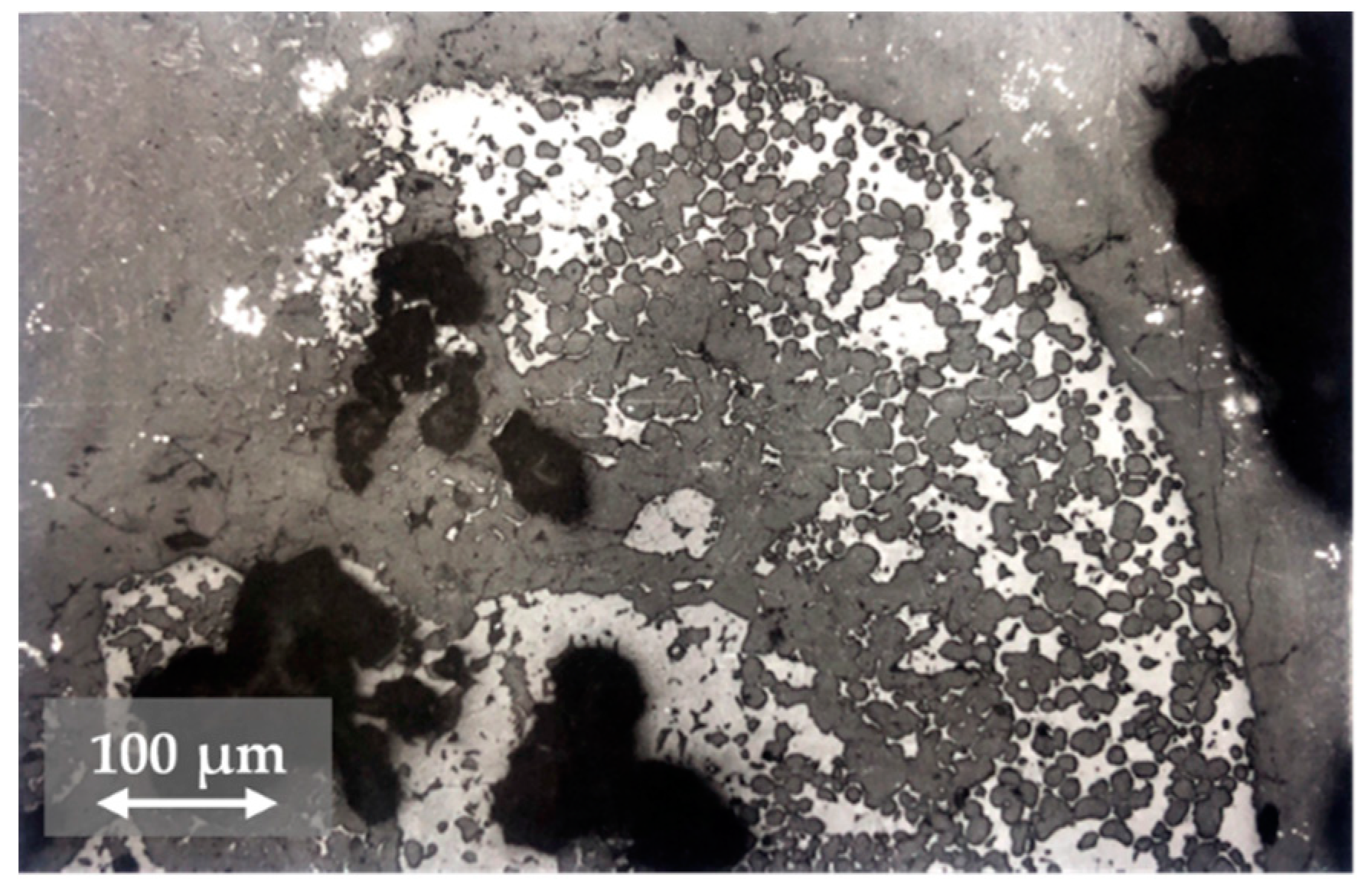
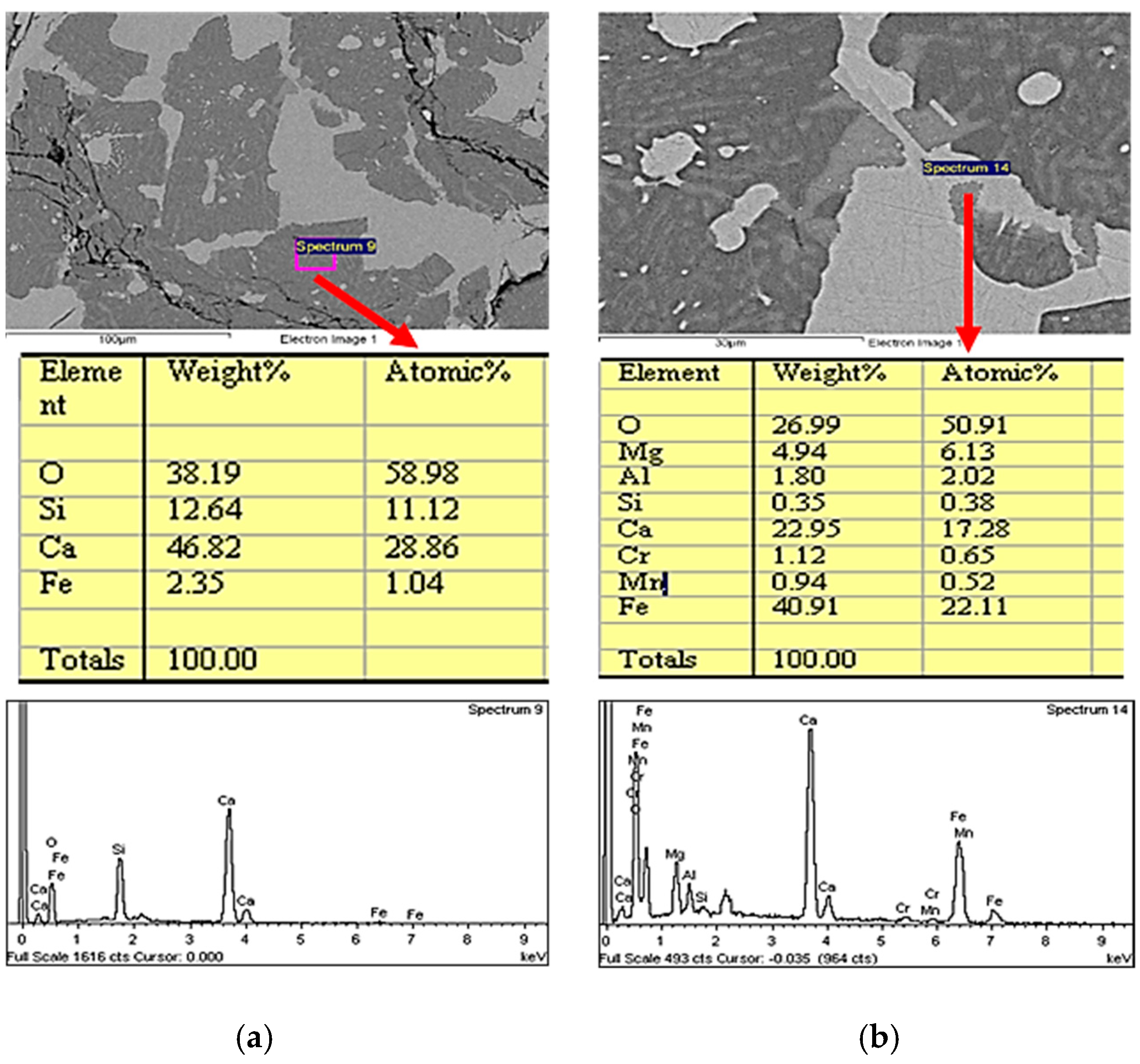
3.2. Changes in the Mineralogical Composition of Slag during Oxygen Blowing
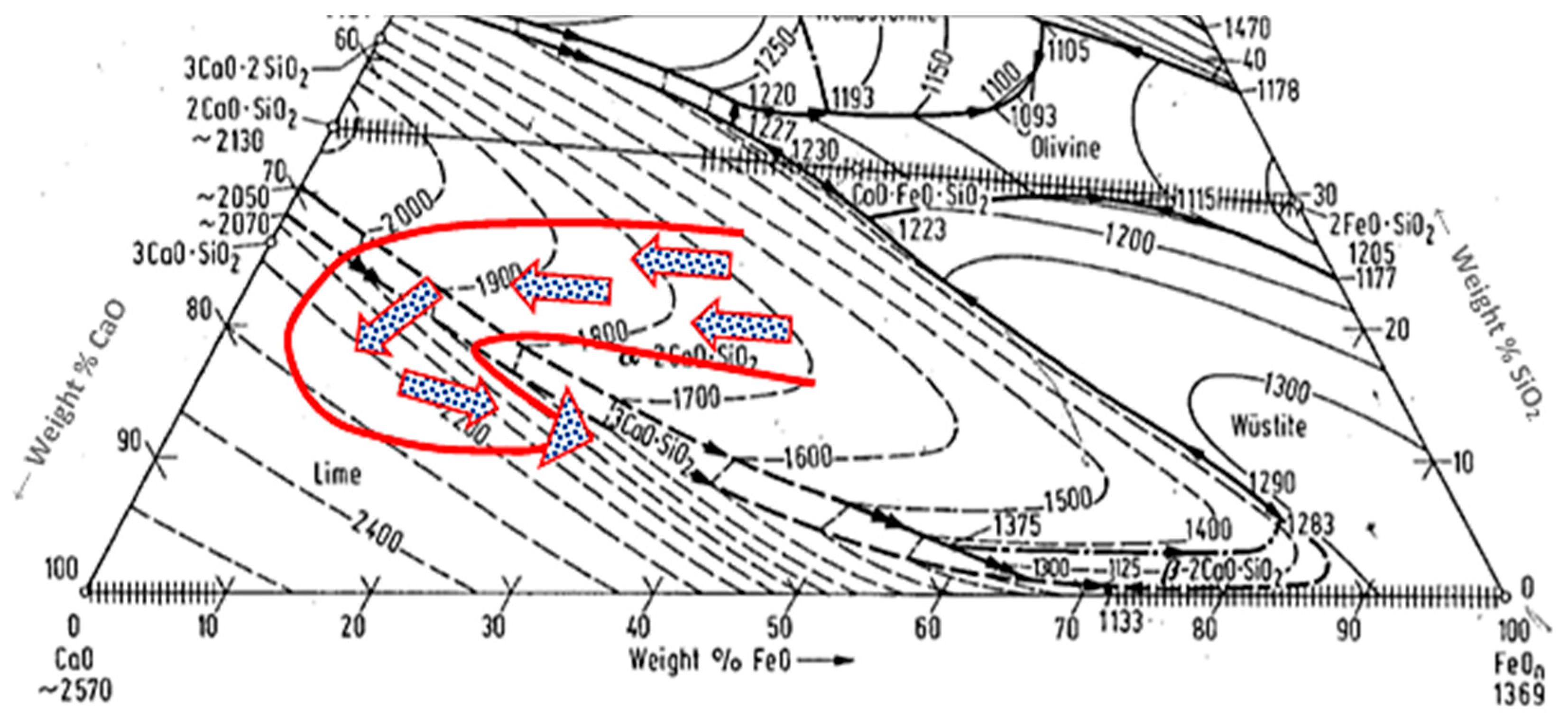
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yugov, I.P. Mechanism and kinetics of optimized slag formation in an oxygen converter. Metallurgist 2005, 49, 307–310. [Google Scholar] [CrossRef]
- Role of Slag in Converter Steelmaking. Available online: http://ispatguru.com/role-of-slag-in-converter-steelmaking/ (accessed on 3 July 2018).
- Jalkanen, H.; Holappa, L. On the role of slag in the oxygen converter process. In Proceedings of the VII International Conference on Molten Slags Fluxes and Salts, Johannesburg, South Africa, 25–28 January 2004; pp. 71–76. [Google Scholar]
- Analysis of Metallurgical Processes and Slag Utilisation in an Integrated Steel Plant Producing Advanced High Strength Steels. Available online: https://www.diva-portal.org/smash/get/diva2:1013704/FULLTEXT01.pdf (accessed on 3 August 2018).
- Deo, B.; Overbosch, A.; Snoeijer, B.; Das, D.; Srinivas, K. Control of slag formation, foaming, flopping, and chaos in BOF. Trans. Indian Inst. Met. 2013, 66, 543–554. [Google Scholar] [CrossRef]
- Yildirim, I.Z.; Prezzi, M. Chemical, mineralogical, and morphological properties of steel slag. Adv. Civ. Eng. 2011, 2011. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, P.; Wang, D. Research on mineral characteristics of converter steel slag and its comprehensive utilization of internal and external recycle. J. Clean. Prod. 2017, 156, 50–61. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, T.C.; Shi, C.; Pan, S.Y. Characteristics of steel slags and their use in cement and concrete. Resour. Conserv. Recycl. 2018, 136, 187–197. [Google Scholar] [CrossRef]
- Wan, J.; Wu, S.; Xiao, Y.; Chen, Z.; Zhang, D. Study on the effective composition of steel slag for asphalt mixture induction heating purpose. Constr. Build. Mater. 2018, 178, 542–550. [Google Scholar] [CrossRef]
- Baricová, D.; Pribulová, A.; Demeter, P.; Buľko, B.; Rosová, A. Utilizing of the metallurgical slag for production of cementless concrete mixtures. Metalurgija 2012, 51, 465–468. [Google Scholar]
- Morata, M.; Saborido, C.; Fontserè, V. Slag aggregates for railway track bed layers: Monitoring and maintenance. In Proceedings of the 15th International Conference on Railway Engineering Design and Operation, Madrid, UK, 19–21 July 2016; pp. 283–294. [Google Scholar] [CrossRef]
- Rex, M. The use of BF, converter and ladle slags in European agriculture-benefits or risks? Slag-Providing solutions for global construction and other markets. In Proceedings of the 4th European Slag Conference, Oulu, Finland, 20–21 June 2005; pp. 51–55. [Google Scholar]
- Diao, J.; Zhou, W. System assessment of recycling of steel slag in converter steelmaking. J. Clean. Prod. 2016, 125, 159–167. [Google Scholar] [CrossRef]
- Mihok, Ľ.; Fedičová, D. Recycling of demetallized steelmaking slag into charge of basic oxygen converter. Metalurgija 2000, 39, 93–99. [Google Scholar]
- Thermochemical and Mineralogical Tables for Geochemical Modeling. Available online: http://thermoddem.brgm.fr/species/larnitegamma (accessed on 12 October 2018).
- Kijac, J. Vysokoteplote procesy výroby ocele I. In High temperature processes in steelmaking I, 1st ed.; Hutnícka fakulta TU v Košiciach: Košice, Slovakia, 2006; ISBN 80-8073-515-8. [Google Scholar]
- Allibert, M. Slag atlas, 2nd ed.; Verein Deutscher Eisenhuttenleute: Dusseldorf, Germany, 1995; p. 126. ISBN 3-514-00457-9. [Google Scholar]






| Sample | Pig Iron (t) | Scrap (t) | Lime (kg) | Dolomitic Lime (kg) | Demetallized Slag (kg) | Magnesite (kg) |
|---|---|---|---|---|---|---|
| Melt A | 150.1 | 40.0 | 5500 | 2000 | 1 000 | - |
| 1000 | - | - | - | |||
| Melt B | 149.0 | 40.0 | 9000 | 1500 | - | 985 |
| 1500 | 1000 | - | - | |||
| 1300 | 3000 | - | 990 | |||
| Melt C | 152.9 | 36.0 | 6750 | 1000 | - | - |
| 4500 | 1000 | - | - | |||
| Melt D | 152.5 | 42.0 | 5000 | 2000 | - | - |
| 3100 | - | - | - | |||
| Melt E | 153.0 | 42.0 | 5000 | 1000 | - | - |
| 3100 | 1000 | - | - |
| Sample | Time (min) | Fe(total) (%) | FeO (%) | SiO2 (%) | CaO (%) | MgO (%) | MnO (%) | P2O5 (%) | S (%) | B | CaO (free) (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A2.5 | 2.5 | 22.00 | 20.80 | 19.06 | 27.06 | 4.43 | 6.49 | 1.47 | 0.04 | 1.42 | 7.42 |
| A5 | 5.0 | 23.12 | 23.91 | 15.34 | 35.67 | 4.94 | 6.41 | 1.94 | 0.03 | 2.33 | 4.63 |
| A8 | 8.0 | 20.43 | 16.09 | 16.98 | 42.15 | 7.05 | 4.36 | 1.64 | 0.04 | 2.48 | 4.93 |
| A11 | 11.0 | 8.60 | 10.92 | 18.36 | 47.95 | 7.46 | 4.10 | 0.98 | 0.03 | 2.61 | 2.97 |
| A24 | 24.0 | 12.29 | 10.78 | 13.26 | 49.40 | 6.65 | 3.11 | 0.96 | 0.03 | 3.73 | 1.83 |
| A27 | 27.0 | 15.00 | 18.13 | 12.24 | 50.20 | 5.89 | 5.62 | 1.21 | 0.09 | 4.10 | 0.95 |
| B2.5 | 2.5 | 23.80 | 25.87 | 19.10 | 33.37 | 4.03 | 7.06 | 1.40 | 0.04 | 1.75 | 8.39 |
| B5 | 5.0 | 14.52 | 14.80 | 18.27 | 40.66 | 4.78 | 7.39 | 1.59 | 0.03 | 2.23 | 6.38 |
| B8 | 8.0 | 11.28 | 11.77 | 18.53 | 43.82 | 4.85 | 9.39 | 1.34 | 0.04 | 2.36 | 5.45 |
| B11 | 11.0 | 14.31 | 14.87 | 10.64 | 55.21 | 5.56 | 4.67 | 1.32 | 0.03 | 5.19 | 6.59 |
| B24 | 24.0 | 15.77 | 15.52 | 12.20 | 47.26 | 6.03 | 5.78 | 1.32 | 0.04 | 3.87 | 4.81 |
| B27 | 27.0 | 19.06 | 23.15 | 10.06 | 51.76 | 5.08 | 4.24 | 1.04 | 0.13 | 5.15 | 1.77 |
| C2.5 | 2.5 | 20.22 | 24.14 | 18.06 | 35.05 | 4.63 | 8.09 | 1.00 | 0.03 | 1.94 | 7.86 |
| C5 | 5.0 | 17.10 | 18.68 | 16.25 | 43.52 | 6.65 | 8.00 | 1.30 | 0.03 | 2.68 | 6.22 |
| C8 | 8.0 | 6.48 | 8.05 | 15.60 | 57.76 | 5.44 | 4.55 | 1.09 | 0.04 | 3.70 | 5.42 |
| C11 | 11.0 | 7.80 | 3.38 | 15.24 | 63.37 | 4.84 | 3.34 | 1.14 | 0.04 | 4.16 | 4.42 |
| C24 | 24.0 | 12.71 | 4.31 | 14.16 | 47.53 | 5.44 | 3.86 | 1.00 | 0.08 | 3.36 | 1.84 |
| C27 | 27.0 | 15.01 | 15.09 | 11.82 | 50.98 | 5.87 | 4.66 | 1.36 | 0.10 | 4.31 | 0.89 |
| D2.5 | 2.5 | 18.54 | 16.09 | 19.30 | 37.11 | 5.44 | 4.71 | 1.12 | 0.00 | 1.92 | 10.97 |
| D5 | 5.0 | 15.40 | 15.66 | 16.32 | 44.03 | 8.26 | 3.90 | 0.75 | 0.03 | 2.70 | 9.02 |
| D8 | 8.0 | 18.54 | 16.14 | 14.00 | 38.41 | 5.64 | 3.93 | 0.87 | 0.00 | 2.74 | 7.35 |
| D11 | 11.0 | 18.38 | 17.04 | 10.76 | 52.99 | 7.86 | 3.66 | 1.19 | 0.08 | 4.92 | 7.80 |
| D24 | 24.0 | 18.87 | 20.69 | 9.80 | 50.47 | 7.96 | 3.73 | 1.39 | 0.07 | 5.15 | 2.10 |
| D27 | 27.0 | 19.84 | 21.13 | 10.83 | 48.62 | 6.75 | 4.21 | 1.28 | 0.12 | 4.49 | 0.58 |
| E2.5 | 2.5 | 27.96 | 27.39 | 18.19 | 33.37 | 4.86 | 3.12 | 0.50 | 0.00 | 1.83 | 8.97 |
| E5 | 5.0 | 26.37 | 30.46 | 13.73 | 37.23 | 4.66 | 6.23 | 2.00 | 0.03 | 2.71 | 5.79 |
| E8 | 8.0 | 19.10 | 22.80 | 14.97 | 39.54 | 4.86 | 5.77 | 2.01 | 0.03 | 2.64 | 6.30 |
| E11 | 11.0 | 14.32 | 10.63 | 12.12 | 48.03 | 4.86 | 2.17 | 0.99 | 0.00 | 3.96 | 6.98 |
| E24 | 24.0 | 16.23 | 20.19 | 9.97 | 42.90 | 4.06 | 4.29 | 1.37 | 0.08 | 4.30 | 2.06 |
| E27 | 27.0 | 21.44 | 22.13 | 10.24 | 46.55 | 4.34 | 5.79 | 1.28 | 0.12 | 4.55 | 0.72 |
| Sample | Time (min) | C (%) | Mn (%) | Si (%) | P (%) | S (%) | Tsteel (°C) | aO (ppm) |
|---|---|---|---|---|---|---|---|---|
| AM0 pig iron after desulphurization | 4.530 | 0.540 | 0.790 | 0.068 | 0.011 | - | - | |
| AM8 | 8 | 2.520 | 0.090 | 0.010 | 0.020 | 0.015 | 1418 | 10.0 |
| AM24 | 24 | 0.036 | 0.114 | 0.010 | 0.004 | 0.019 | 1645 | 882.7 |
| AM27 | 27 | 0.031 | 0.069 | 0.005 | 0.007 | 0.016 | 1665 | 989.5 |
| BM0 pig iron after desulphurization | 4.470 | 0.510 | 0.890 | 0.081 | 0.012 | - | - | |
| BM8 | 8 | 2.240 | 0.140 | 0.010 | 0.033 | 0.033 | 1425 | 10.8 |
| BM24 | 24 | 0.040 | 0.120 | 0.010 | 0.007 | 0.018 | 1630 | 669.4 |
| BM27 | 27 | 0.020 | 0.050 | 0.005 | 0.004 | 0.017 | 1651 | 957.6 |
| CM0 pig iron after desulphurization | 4.600 | 0.610 | 0.760 | 0.066 | 0.014 | - | - | |
| CM8 | 8 | 2.490 | 0.090 | 0.010 | 0.019 | 0.016 | 1480 | 17.8 |
| CM24 | 24 | 0.041 | 0.162 | 0.010 | 0.013 | 0.015 | 1667 | 798.5 |
| CM27 | 27 | 0.036 | 0.135 | 0.005 | 0.010 | 0.014 | 1659 | 931.2 |
| DM0 pig iron after desulphurization | 4.400 | 0.480 | 0.790 | 0.078 | 0.012 | - | - | |
| DM8 | 8 | 2.370 | 0.100 | 0.010 | 0.036 | 0.015 | 1391 | 19.2 |
| DM24 | 24 | 0.055 | 0.119 | 0.010 | 0.010 | 0.020 | 1659 | 759.7 |
| DM27 | 27 | 0.033 | 0.084 | 0.010 | 0.009 | 0.017 | 1664 | 983.2 |
| EM0 pig iron after desulphurization | 4.600 | 0.470 | 0.810 | 0.069 | 0.009 | - | - | |
| EM8 | 8 | 2.290 | 0.090 | 0.010 | 0.018 | 0.022 | 1375 | 18.5 |
| EM24 | 24 | 0.053 | 0.097 | 0.010 | 0.006 | 0.023 | 1590 | 618.8 |
| EM27 | 27 | 0.028 | 0.066 | 0.005 | 0.006 | 0.017 | 1655 | 1191.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baricová, D.; Pribulová, A.; Futáš, P.; Buľko, B.; Demeter, P. Change of the Chemical and Mineralogical Composition of the Slag during Oxygen Blowing in the Oxygen Converter Process. Metals 2018, 8, 844. https://doi.org/10.3390/met8100844
Baricová D, Pribulová A, Futáš P, Buľko B, Demeter P. Change of the Chemical and Mineralogical Composition of the Slag during Oxygen Blowing in the Oxygen Converter Process. Metals. 2018; 8(10):844. https://doi.org/10.3390/met8100844
Chicago/Turabian StyleBaricová, Dana, Alena Pribulová, Peter Futáš, Branislav Buľko, and Peter Demeter. 2018. "Change of the Chemical and Mineralogical Composition of the Slag during Oxygen Blowing in the Oxygen Converter Process" Metals 8, no. 10: 844. https://doi.org/10.3390/met8100844
APA StyleBaricová, D., Pribulová, A., Futáš, P., Buľko, B., & Demeter, P. (2018). Change of the Chemical and Mineralogical Composition of the Slag during Oxygen Blowing in the Oxygen Converter Process. Metals, 8(10), 844. https://doi.org/10.3390/met8100844







