Anodic Bubble Behavior in a Laboratory Scale Transparent Electrolytic Cell for Aluminum Electrolysis
Abstract
1. Introduction
2. Experimental Details
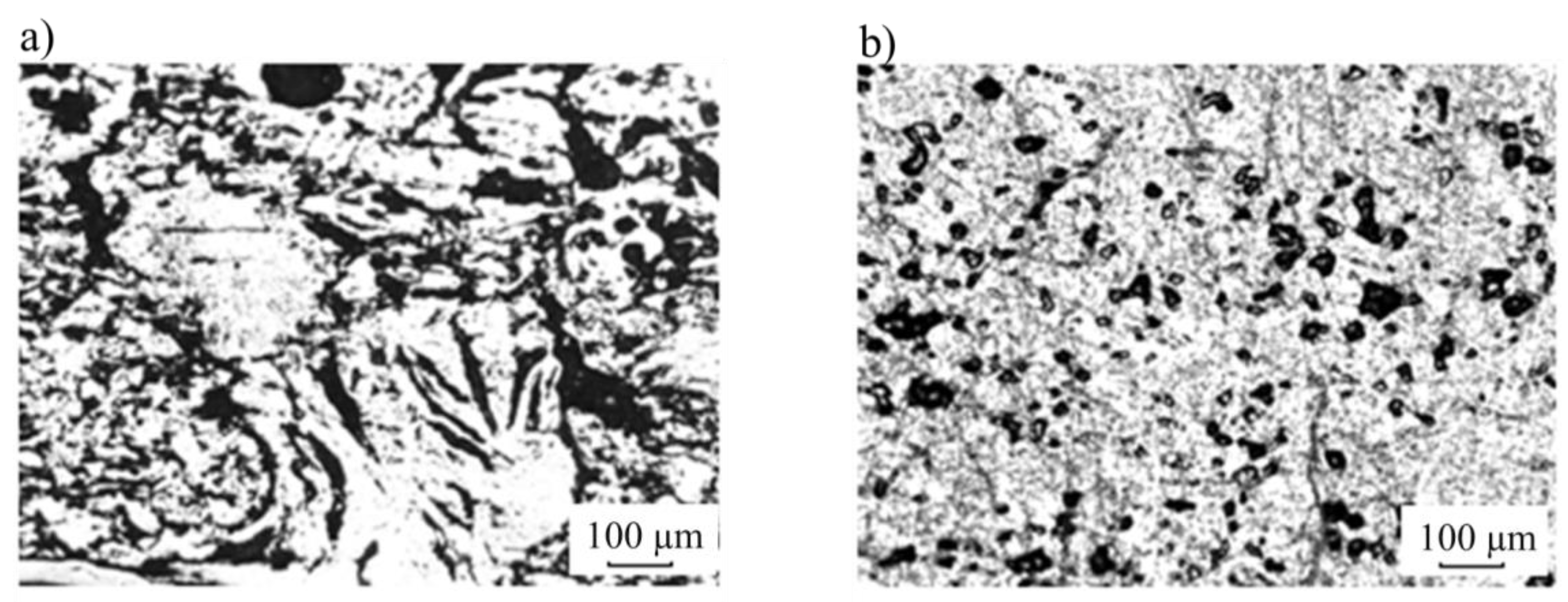
2.1. Materials and Chemicals
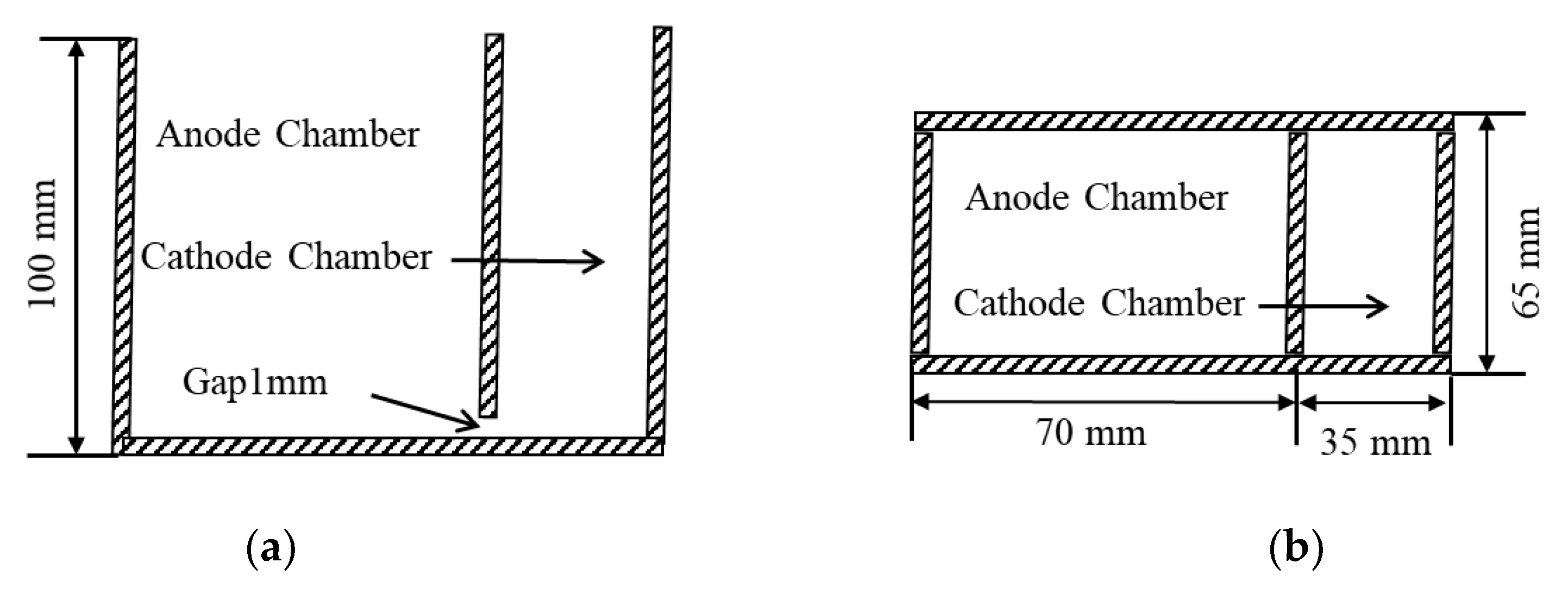
2.2. Transparent Aluminum Electrolysis Cell
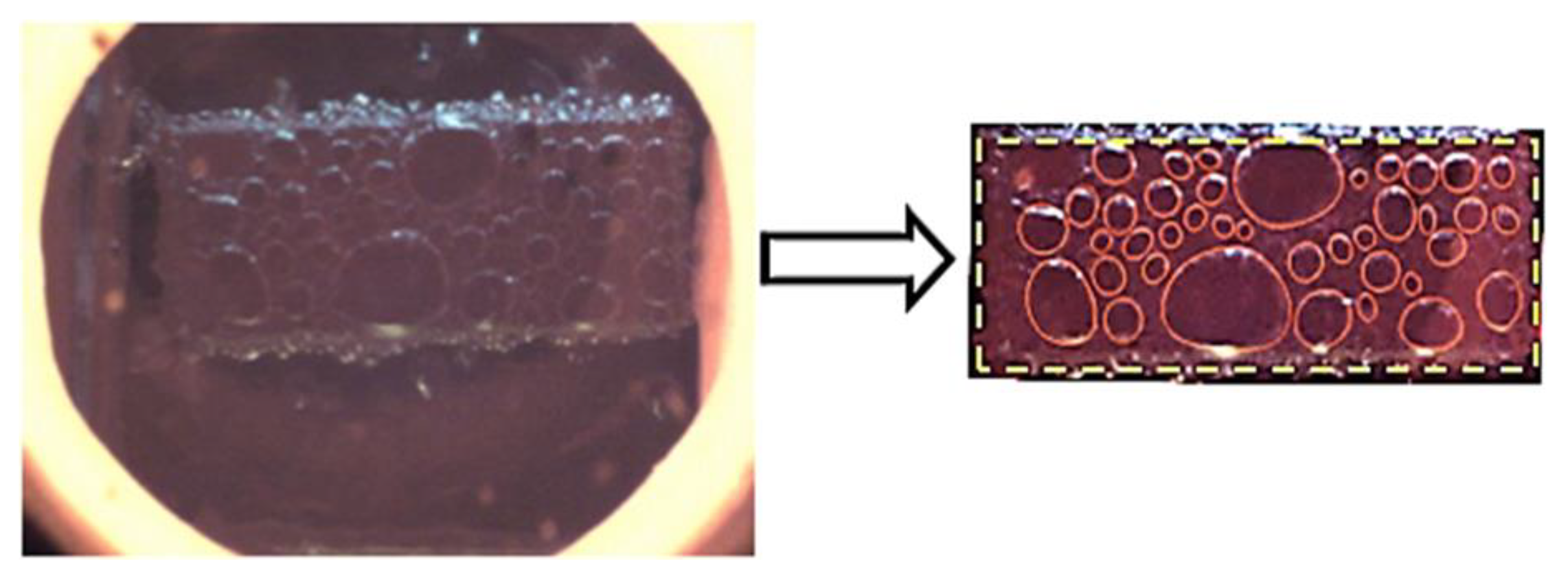
2.3. Image Processing
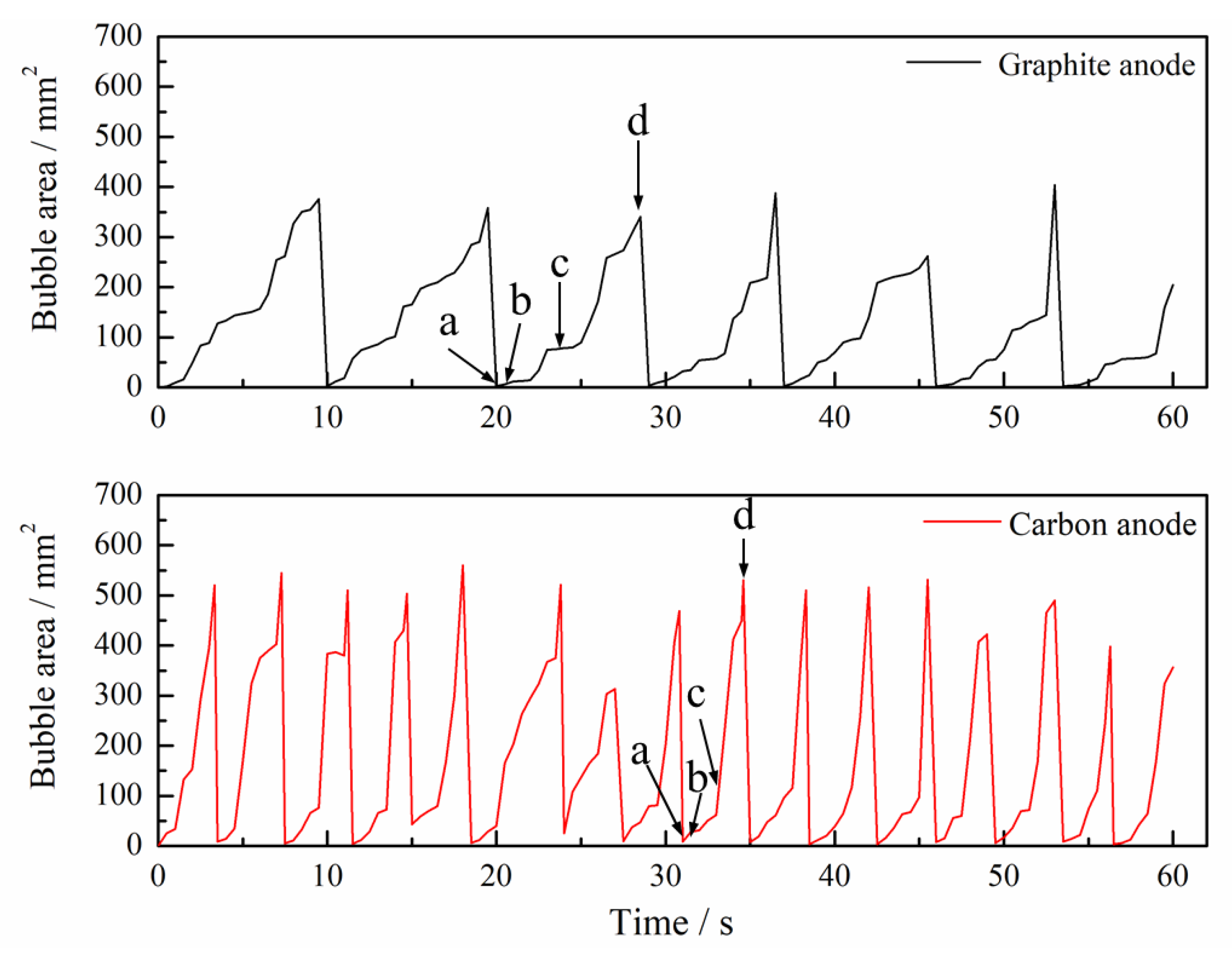
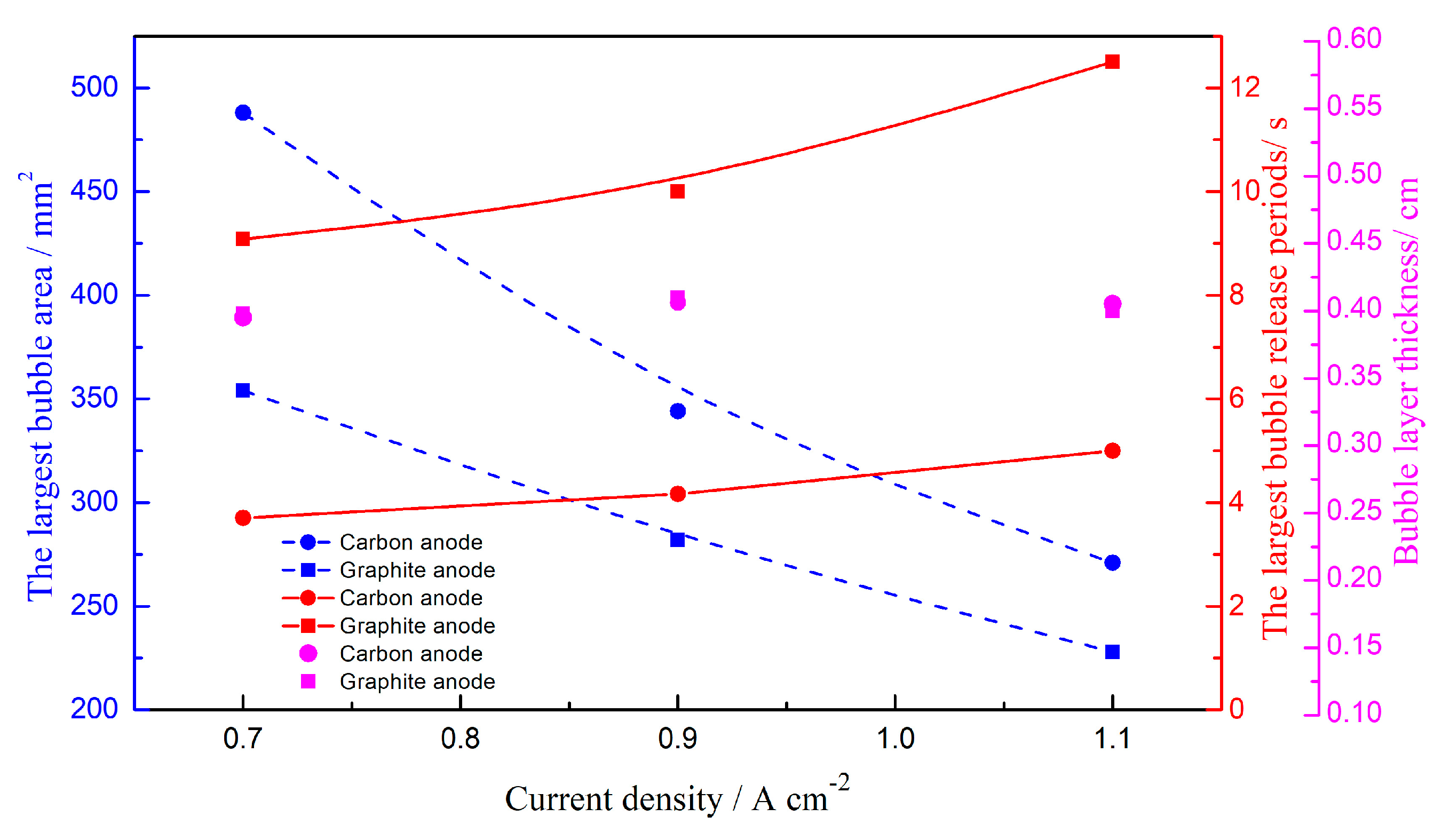
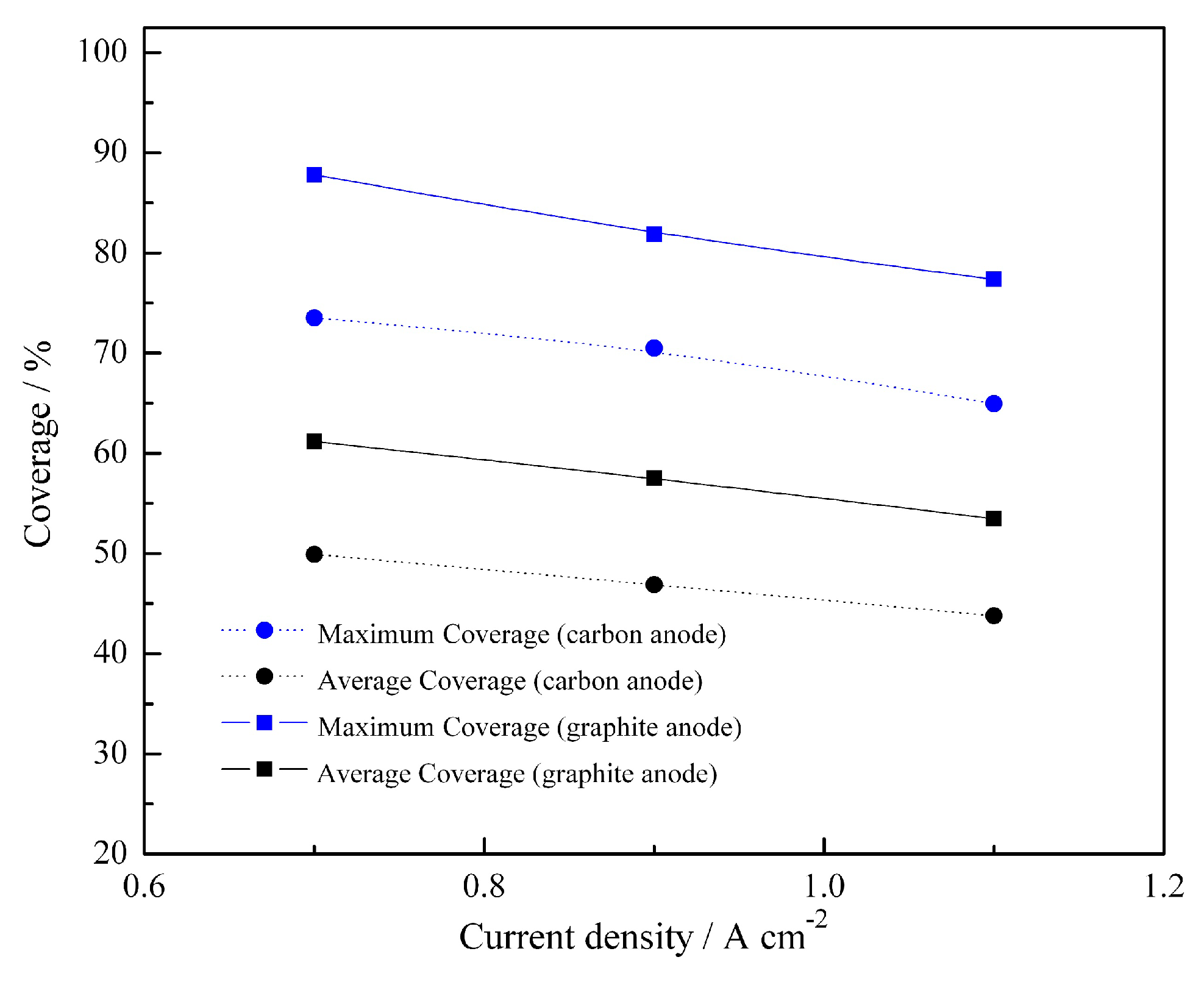
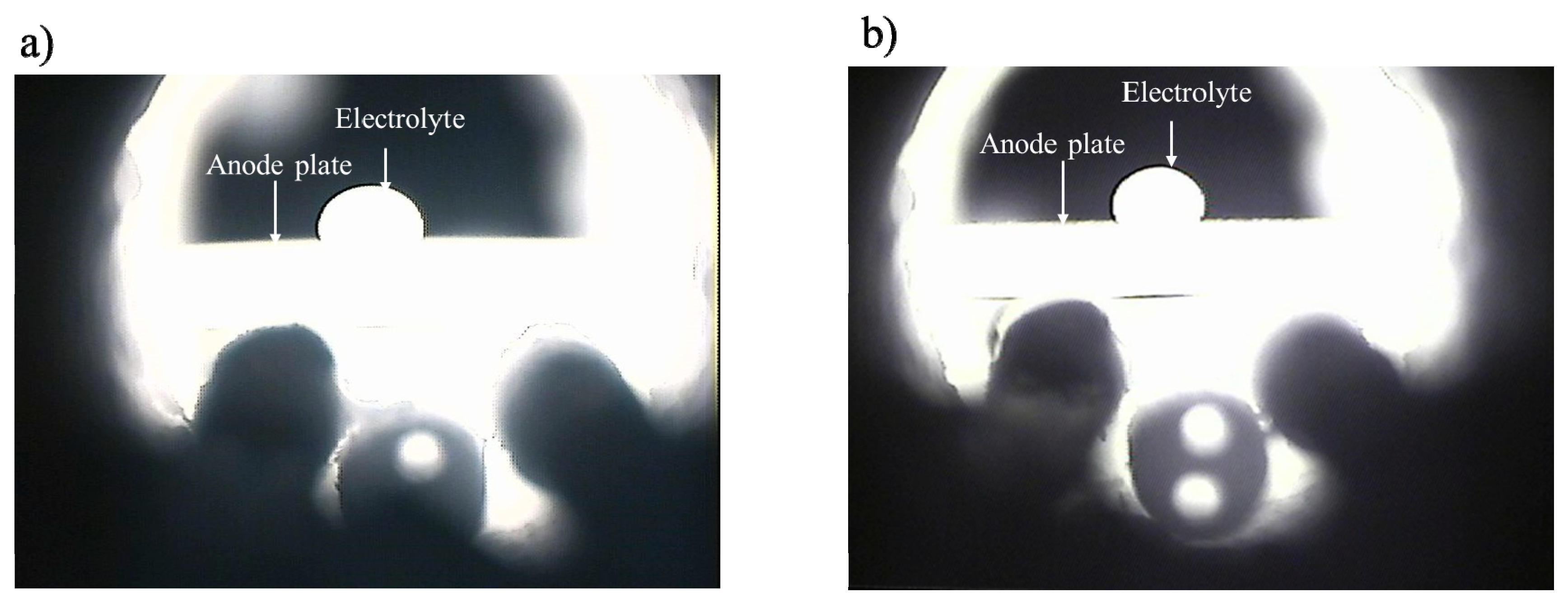
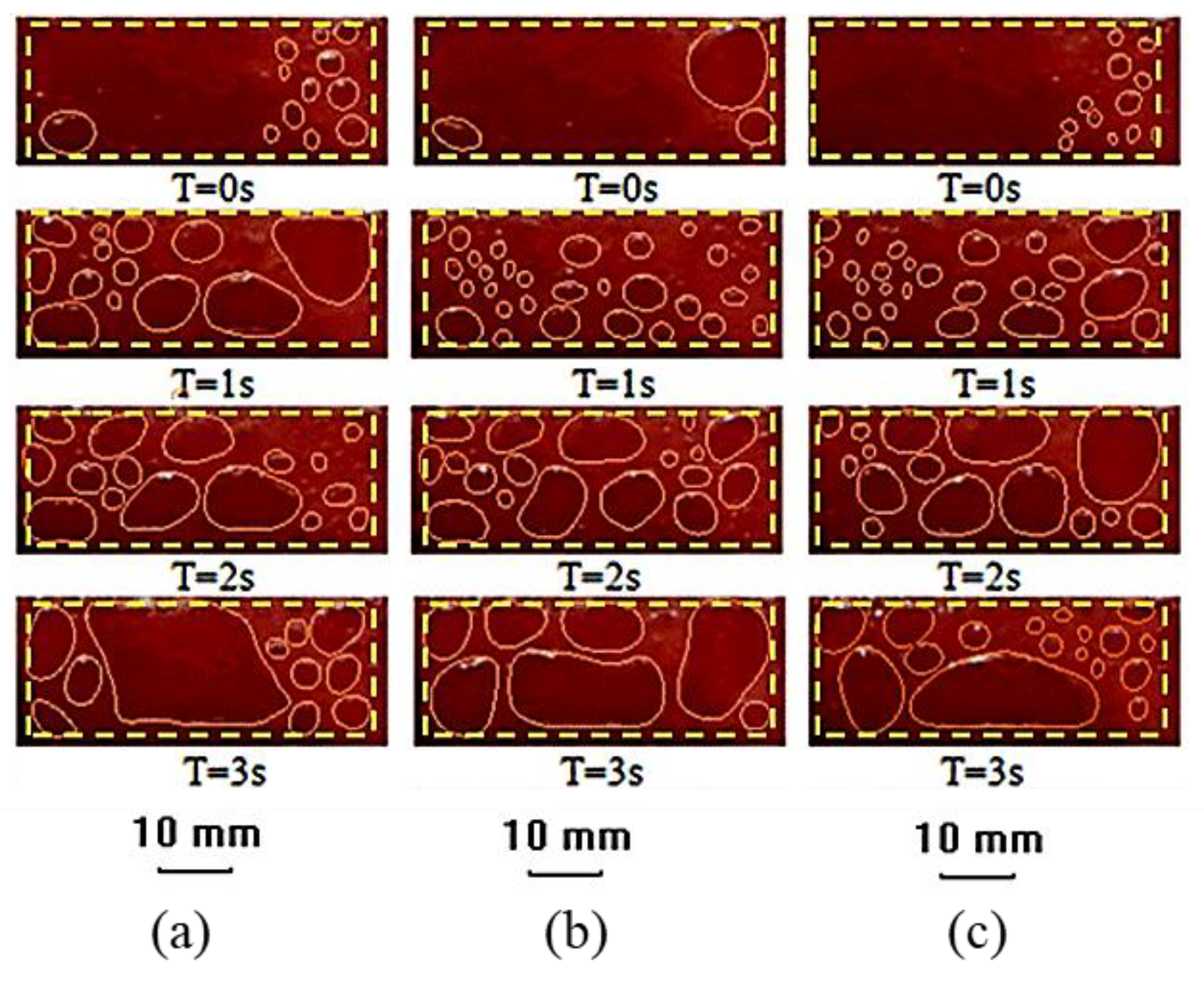
3. Results and Discussions
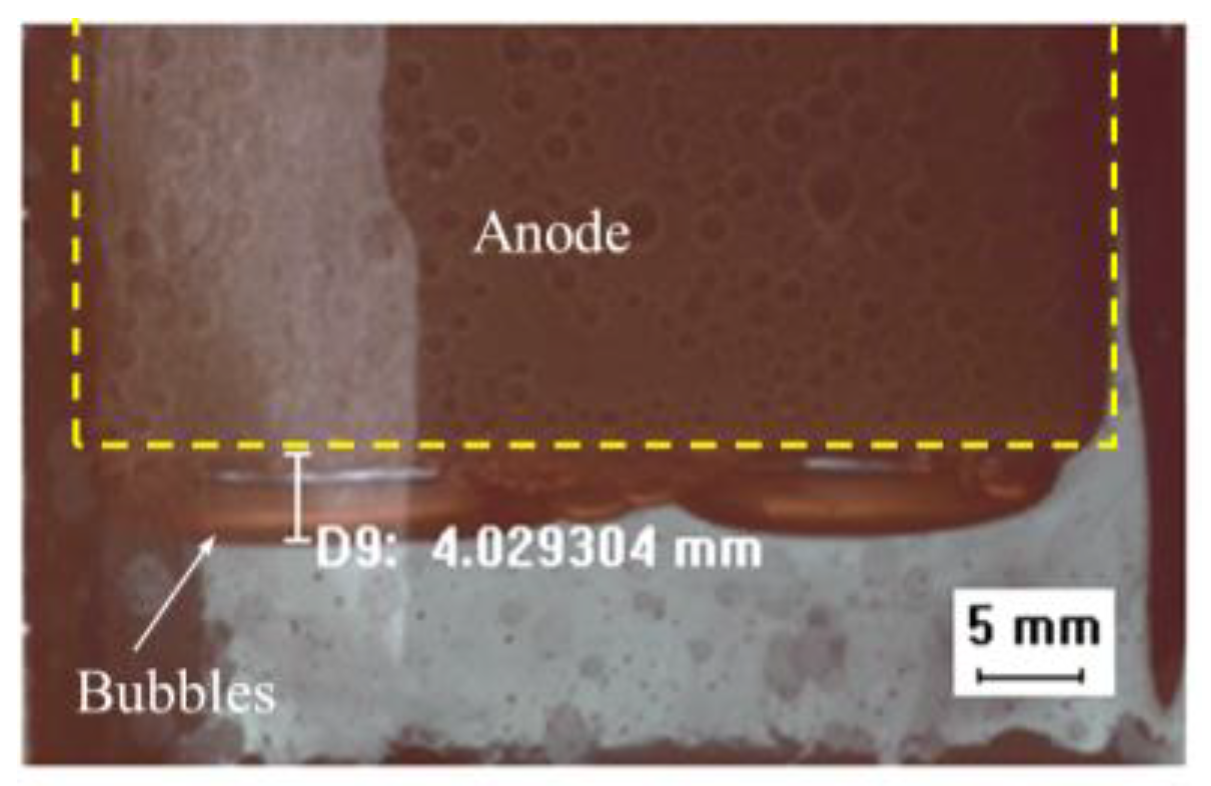
3.1. Bubble Generation-Growth-Releasing Process
3.2. Bubble Nucleation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grjotheim, K. Textbook of Aluminum Electrolysis: Fundamentals of the Hall- Héroult Process, 2nd ed.; Aluminum-Verlag: Dusseldorf, German, 1982; p. 443. [Google Scholar]
- Solheim, A.; Johansen, S.T.; Rolseth, S.; Thonstad, J. Gas driven flow in hall-Héroult cells. JOM 1988, 40, 57. [Google Scholar]
- Doheim, M.A.; El-Kersh, A.M.; Ali, M.M. Computational modeling of flow in aluminum reduction cells due to gas bubbles and electromagnetic forces. Metall. Mater. Trans. B 2007, 38, 113–119. [Google Scholar] [CrossRef]
- Feng, Y.Q.; Cooksey, M.A.; Schwarz, M.P. CFD Modelling of Alumina Mixing in Aluminium Reduction Cells. In Light Metals 2011; Lindsay, S.J., Ed.; Springer: Berlin, Germany, 2011; pp. 543–548. [Google Scholar]
- Haupin, W.E.; McGrew, W.C. A scanning reference electrode for voltage contours in aluminum smelting cells. JOM 1971, 23, 46–51. [Google Scholar] [CrossRef]
- Cooksey, M.A.; Taylor, M.P.; Chen, J.J.J. Resistance due to gas bubbles in alumiumum reduction cells. JOM 2008, 60, 51–57. [Google Scholar] [CrossRef]
- Haarberg, T.; Solheim, A.; Johansen, S.T. Effect of anodic gas release on current efficiency in Hall-Héroult cells. In Light Metals 1998; Welch, B., Ed.; Springer: Berlin, Germany, 1998; pp. 475–481. [Google Scholar]
- Einarsrud, K.E. The effect of detaching bubbles on aluminum-cryolite interfaces: An experimental and numerical investigation. Metall. Mater. Trans. B 2010, 41, 560–573. [Google Scholar] [CrossRef]
- Fortin, S.; Gerhardt, M.; Gesing, A.J. Physical modelling of bubble behavior and gas release from aluminum reduction cell anodes. In Essential Readings in Light Metals; Bearne, G., Dupuis, M., Tarcy, G., Eds.; Springer: Berlin, Germany, 1984; pp. 385–395. [Google Scholar]
- Vekony, K.; Kiss, K.L. Morphology of two-phase layers with large bubble. Metall. Mater. Trans. B 2010, 41, 1006–1017. [Google Scholar] [CrossRef]
- Shekhar, R.; Evans, J.W. Physical modeling studies of electrolyte flow due to gas evolution and some aspects of bubble behavior in advanced Hall cells Part I. Flow in cells with a flat anode. Metall. Mater. Trans. B 1994, 25, 333–340. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zhang, L.F.; Zuo, X.J. Fluid flow and bubble behavior in the aluminum electrolysis cell. In Light Metals 2009; Bearne, G., Ed.; Springer: Berlin, Germany, 2009; pp. 581–586. [Google Scholar]
- Solheim, A.; Johansen, S.T. Gas induced bath circulation in aluminum reduction cells. J. Appl. Electrochem. 1989, 19, 703–712. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zhang, L.F. Numerical modeling on the fluid flow-related phenomena in an aluminum electrolysis cell. In Light Metals 2010; Li, B.Q., Thomas, B.G., Zhang, L.F., Doyle, F.M., Campbell, A.P., Eds.; Springer: Berlin, Germany, 2010; pp. 207–214. [Google Scholar]
- Xue, J.L.; Oye, H.A. Bubble behavior-cell voltage oscillation during aluminium electrolysis and the effects of sound and ultrasound. In Light Metals 1995; Cutshall, E., Ed.; Springer: Berlin, Germany, 1995; pp. 265–271. [Google Scholar]
- Cassayre, L.; Utigard, T.A.; Bouvet, S. Visualizing gas evolution on graphite and oxygen-evolving anode. JOM 2002, 54, 41–45. [Google Scholar] [CrossRef]
- Utigard, T.; Toguri, J.M. Anode gas behavior during electrolysis. In Light Metals 1986; Miller, R.E., Ed.; Springer: Berlin, Germany, 1986; pp. 405–413. [Google Scholar]
- Zhao, Z.B.; Wang, Z.W.; Gao, B.L.; Feng, Y.Q.; Shi, Z.N.; Hu, X.W. Anodic bubble behavior and voltage drop in a laboratory transparent aluminum electrolysis cell. Metall. Mater. Trans. B 2016, 47, 1962–1976. [Google Scholar] [CrossRef]
- Zhao, Z.B.; Gao, B.L.; Feng, Y.Q.; Huang, Y.P.; Wang, Z.W.; Shi, Z.N.; Hu, X.W. Effects of anode wettability and slots on anodic bubble behavior using transparent aluminium electrolytic cells. JOM 2017, 69, 281–291. [Google Scholar] [CrossRef]
- Kasherman, D.; Kazacos, M.S. Effect of anode material and bath composition on bubble layer resistivity and gaseous volume fraction in an aluminium electrolysis cell with sloping anode and cathode. J. Appl. Electrochem. 1991, 21, 716–720. [Google Scholar] [CrossRef]
- Thorne, R.T.; Sommerseth, C.; Ratvik, A.P.; Rørvik, S.; Sandnes, E.; Lossius, L.P.; Linga, H.; Svensson, A.M. Bubble evolution and anode surface properties in aluminium electrolysis. J. Electrochem. Soc. 2015, 162, 104–114. [Google Scholar] [CrossRef]
- Franklin, R.E. The structure of graphitic carbons. Acta Cryst. 1951, 4, 253–261. [Google Scholar] [CrossRef]
- Einarsrud, K.E.; Johansen, S.T.; Eick, I. Anodic bubble behavior in Hall-Héroult cells. In Light Metals 2012; Suarez, C.E., Ed.; Springer: Berlin, Germany, 2012; pp. 875–880. [Google Scholar]
- Vogt, H. Contribution to the interpretation of the anode effect. Electrochim. Acta 1997, 42, 2695–2705. [Google Scholar] [CrossRef]
- Liu, S.Y.; Li, W.Z.; Ban, Y.G.; Wang, Z.W. Study on the wettability of electrolyte for graphite cathode blocks. China Nonferr. Metal. 2008, 6, 20–22. [Google Scholar]


| Name | Carbon Anode | Graphite Anode | |
|---|---|---|---|
| Bulk density (g·cm−3) a | 1.570 | 1.820 | |
| Porosity (%) b | 21 | 6.8 | |
| Surface roughness (μm) c | 9.31 | 2.55 | |
| Graphitization (%) d | 27.0 | 78.0 | |
| Impurities (%) a | S | 3.011 | 0.009 |
| Ca | 0.058 | 0.126 | |
| Na | 0.040 | <0.001 | |
| Al | 0.037 | 0.148 | |
| V | 0.040 | <0.001 | |
| Fe | 0.027 | 0.063 | |
| Mn | <0.001 | 0.004 | |
| Ni | 0.012 | <0.001 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wang, Z.; Yang, Y.; Gao, B.; Shi, Z.; Hu, X. Anodic Bubble Behavior in a Laboratory Scale Transparent Electrolytic Cell for Aluminum Electrolysis. Metals 2018, 8, 806. https://doi.org/10.3390/met8100806
Huang Y, Wang Z, Yang Y, Gao B, Shi Z, Hu X. Anodic Bubble Behavior in a Laboratory Scale Transparent Electrolytic Cell for Aluminum Electrolysis. Metals. 2018; 8(10):806. https://doi.org/10.3390/met8100806
Chicago/Turabian StyleHuang, Yipeng, Zhaowen Wang, Youjian Yang, Bingliang Gao, Zhongning Shi, and Xianwei Hu. 2018. "Anodic Bubble Behavior in a Laboratory Scale Transparent Electrolytic Cell for Aluminum Electrolysis" Metals 8, no. 10: 806. https://doi.org/10.3390/met8100806
APA StyleHuang, Y., Wang, Z., Yang, Y., Gao, B., Shi, Z., & Hu, X. (2018). Anodic Bubble Behavior in a Laboratory Scale Transparent Electrolytic Cell for Aluminum Electrolysis. Metals, 8(10), 806. https://doi.org/10.3390/met8100806




