Passivity of Spring Steels with Compressive Residual Stress
Abstract
1. Introduction
2. Experimental Procedures
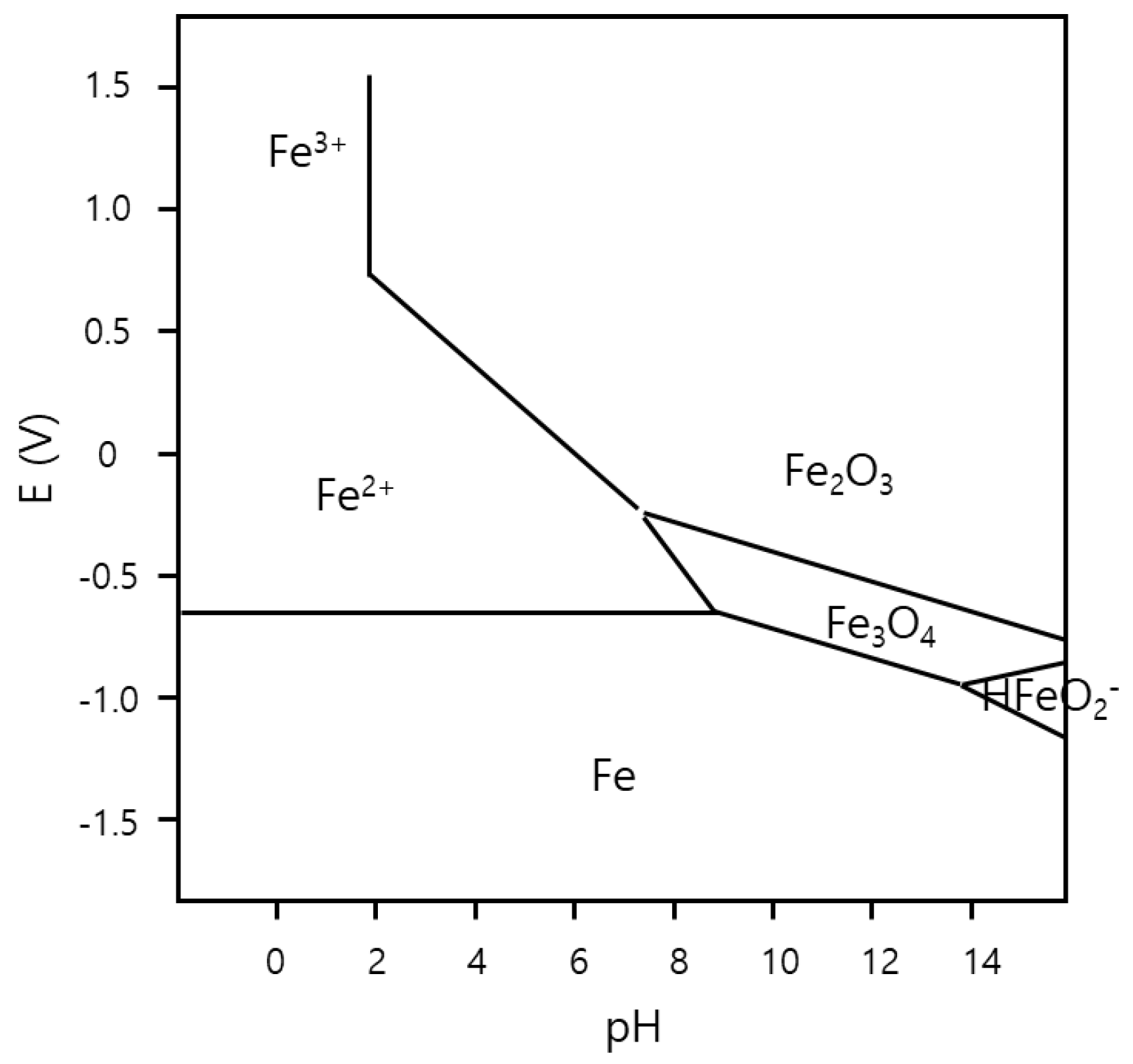
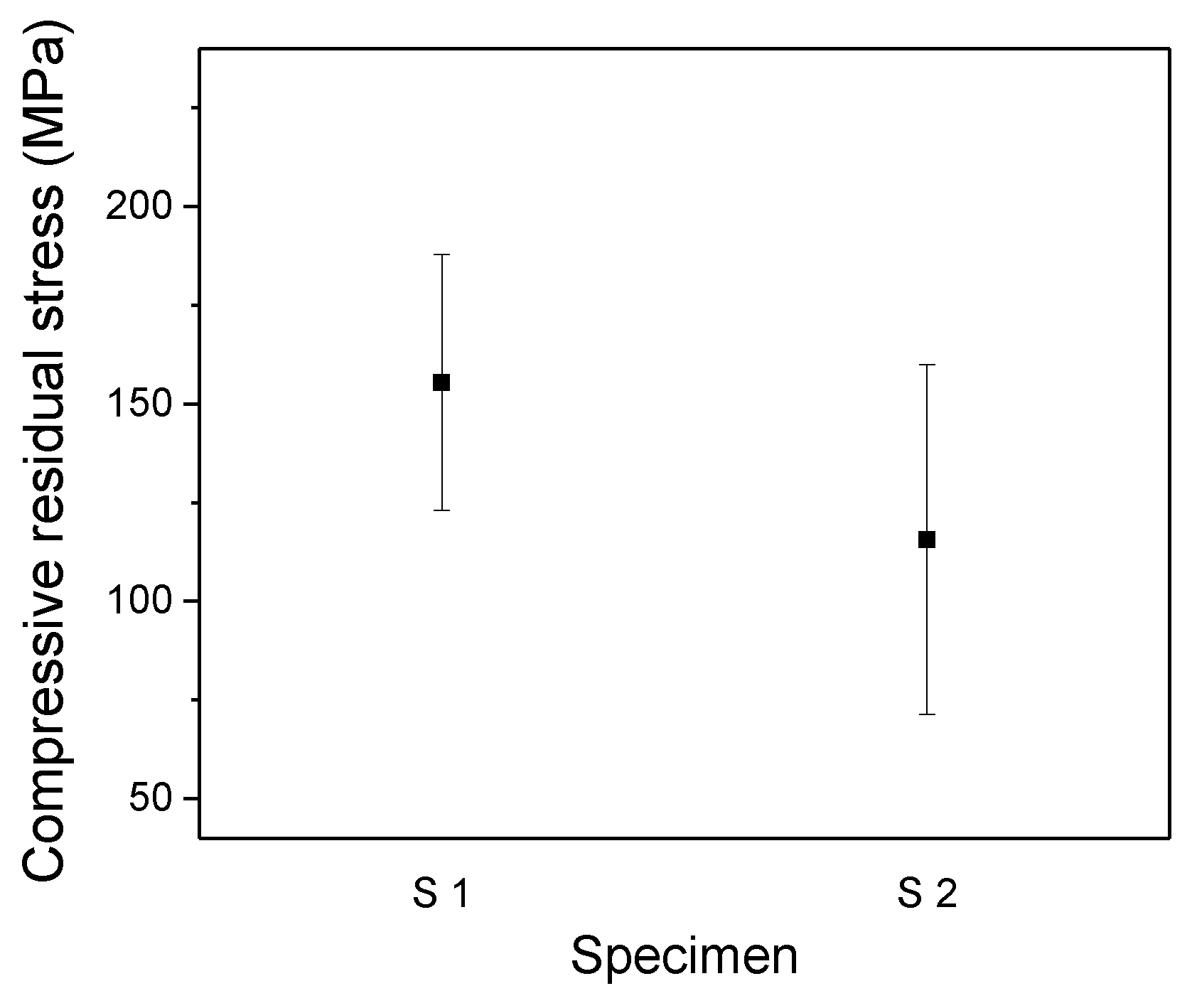
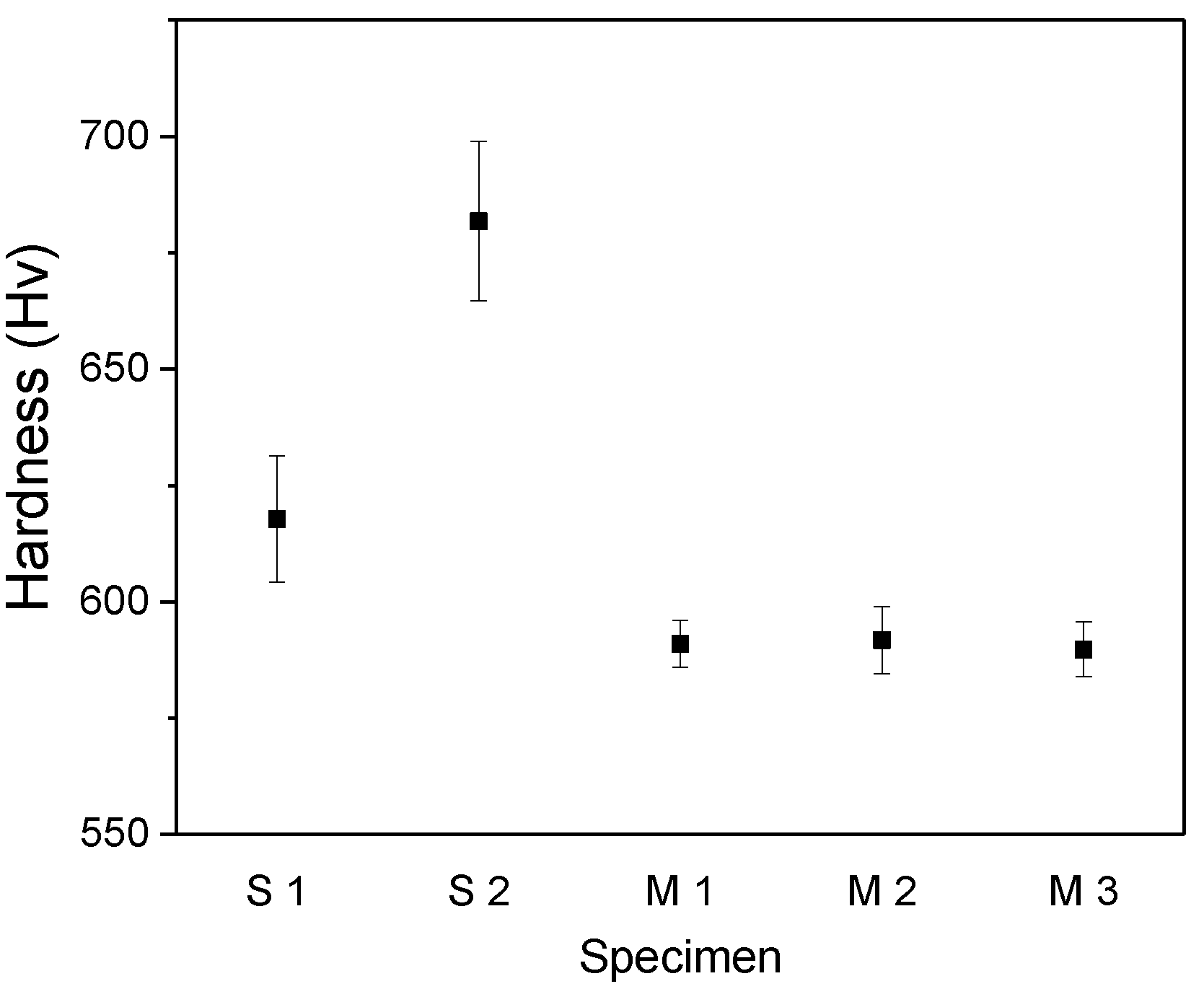
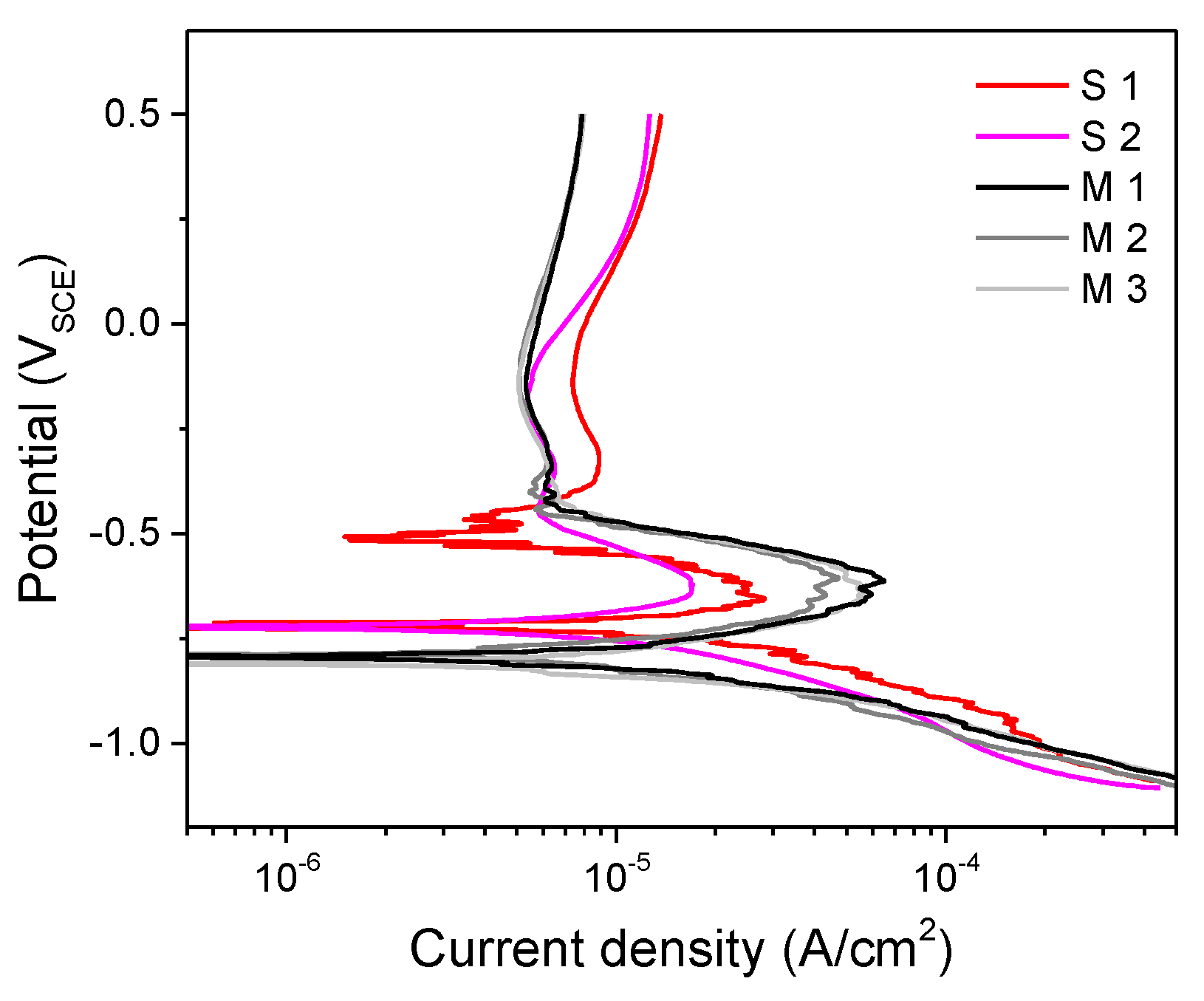
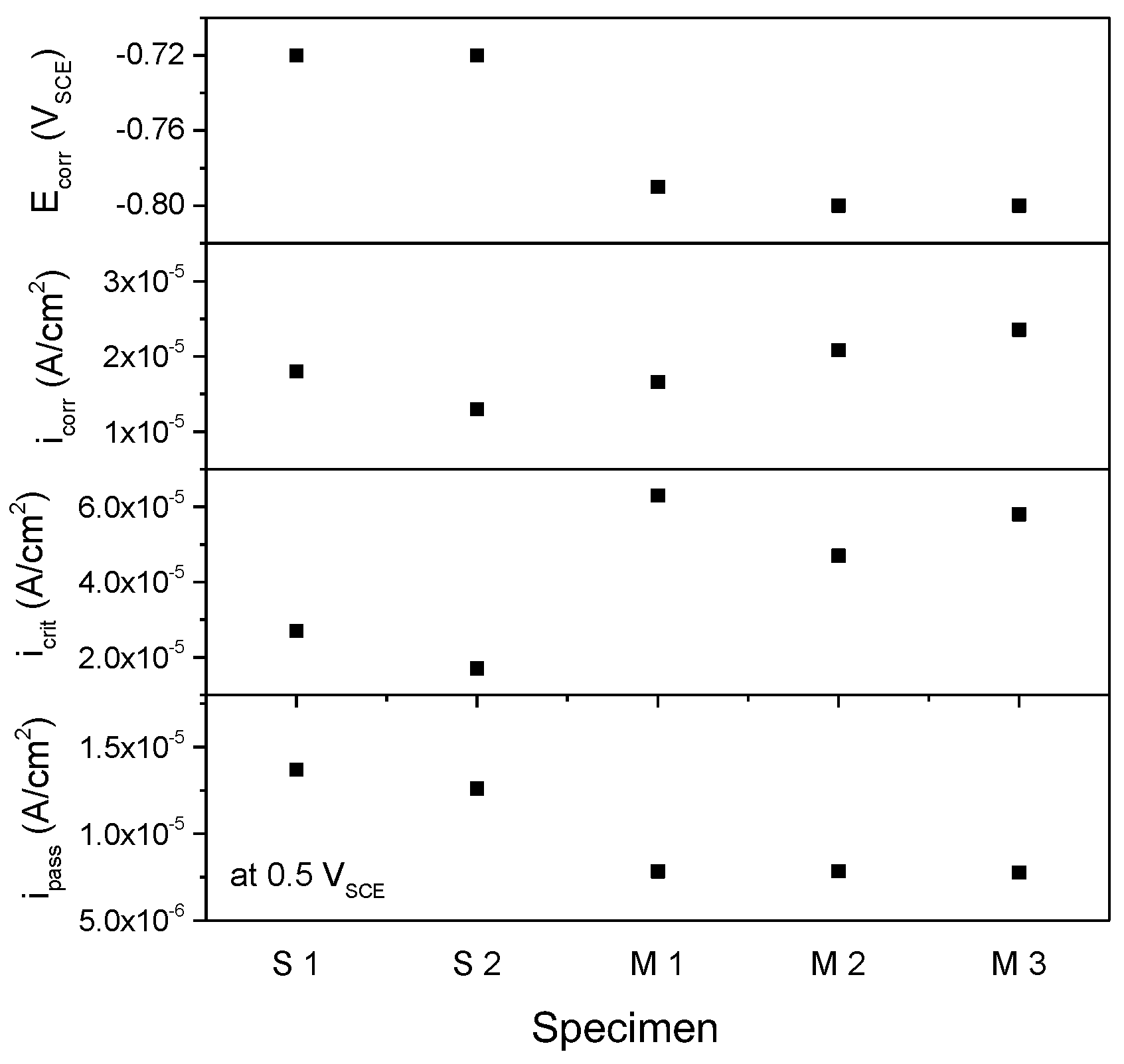
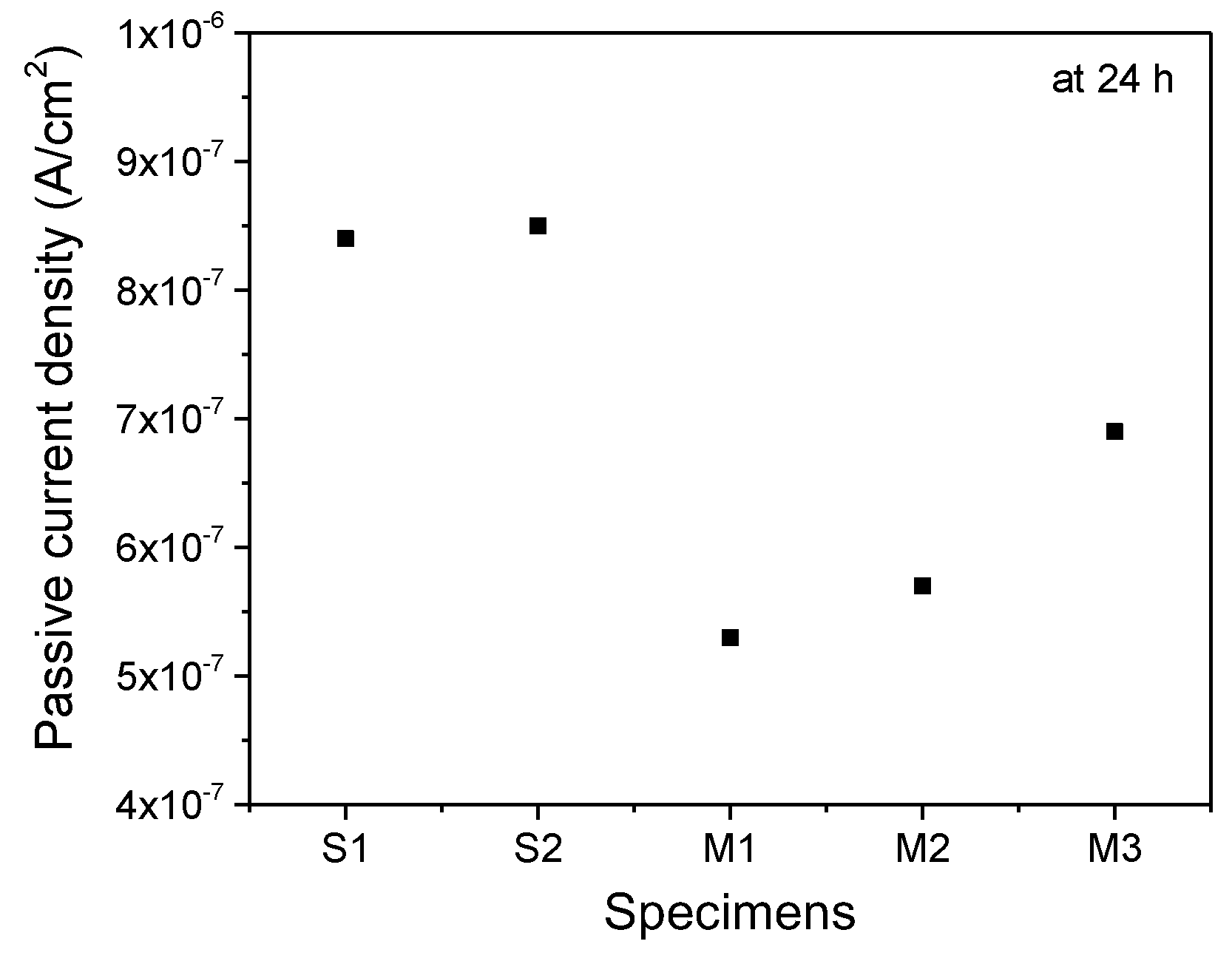
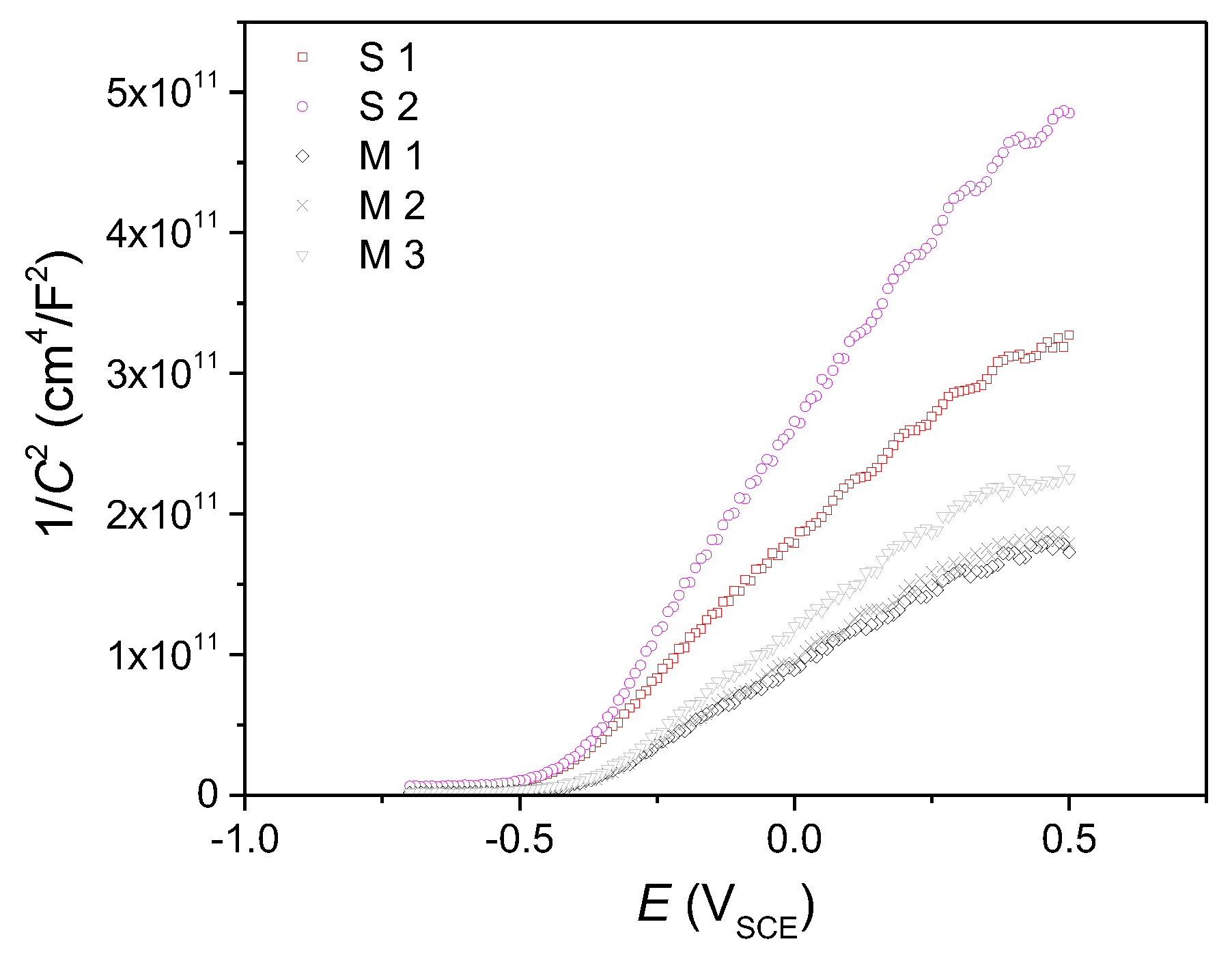
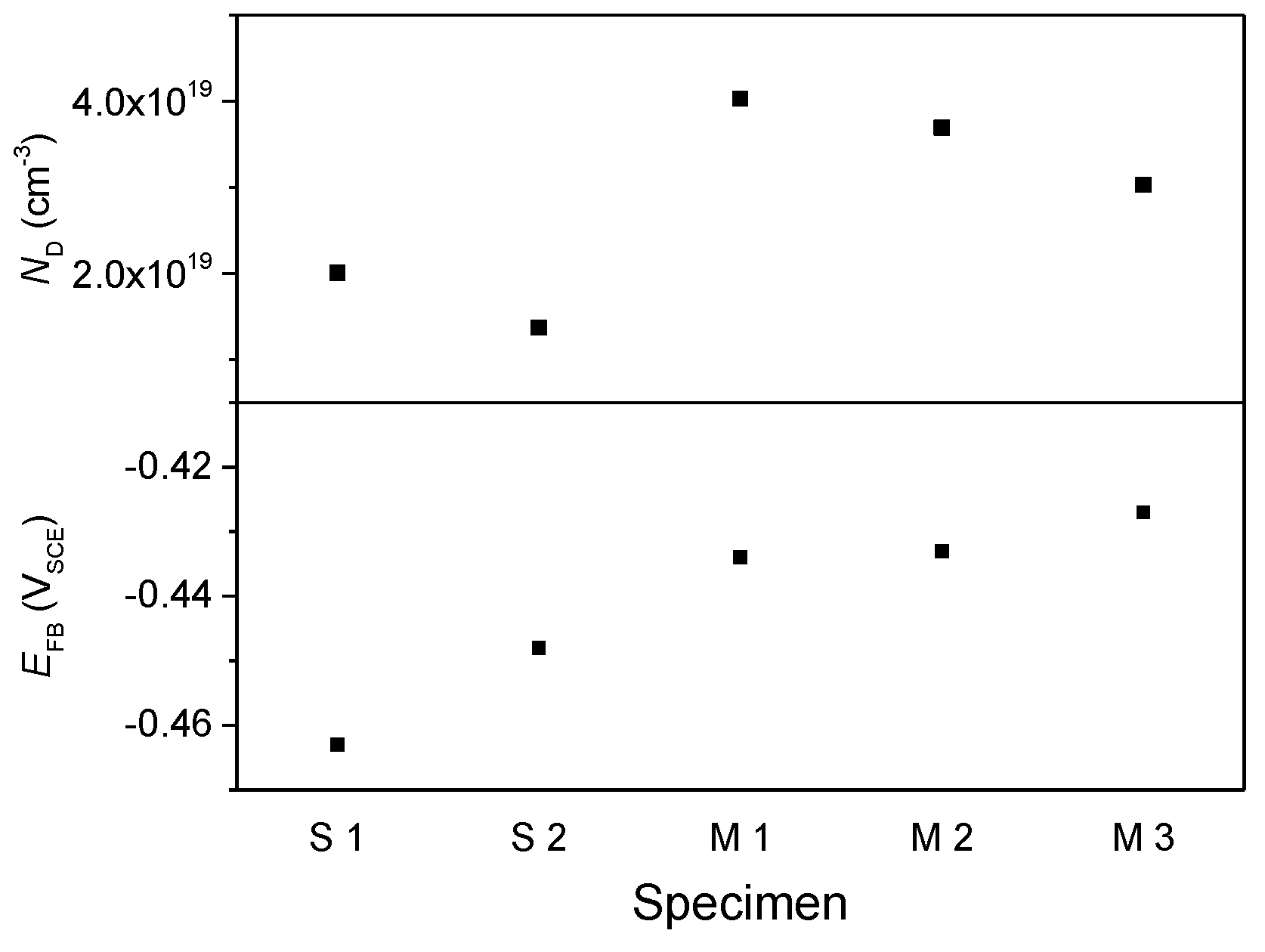
3. Results and Discussion
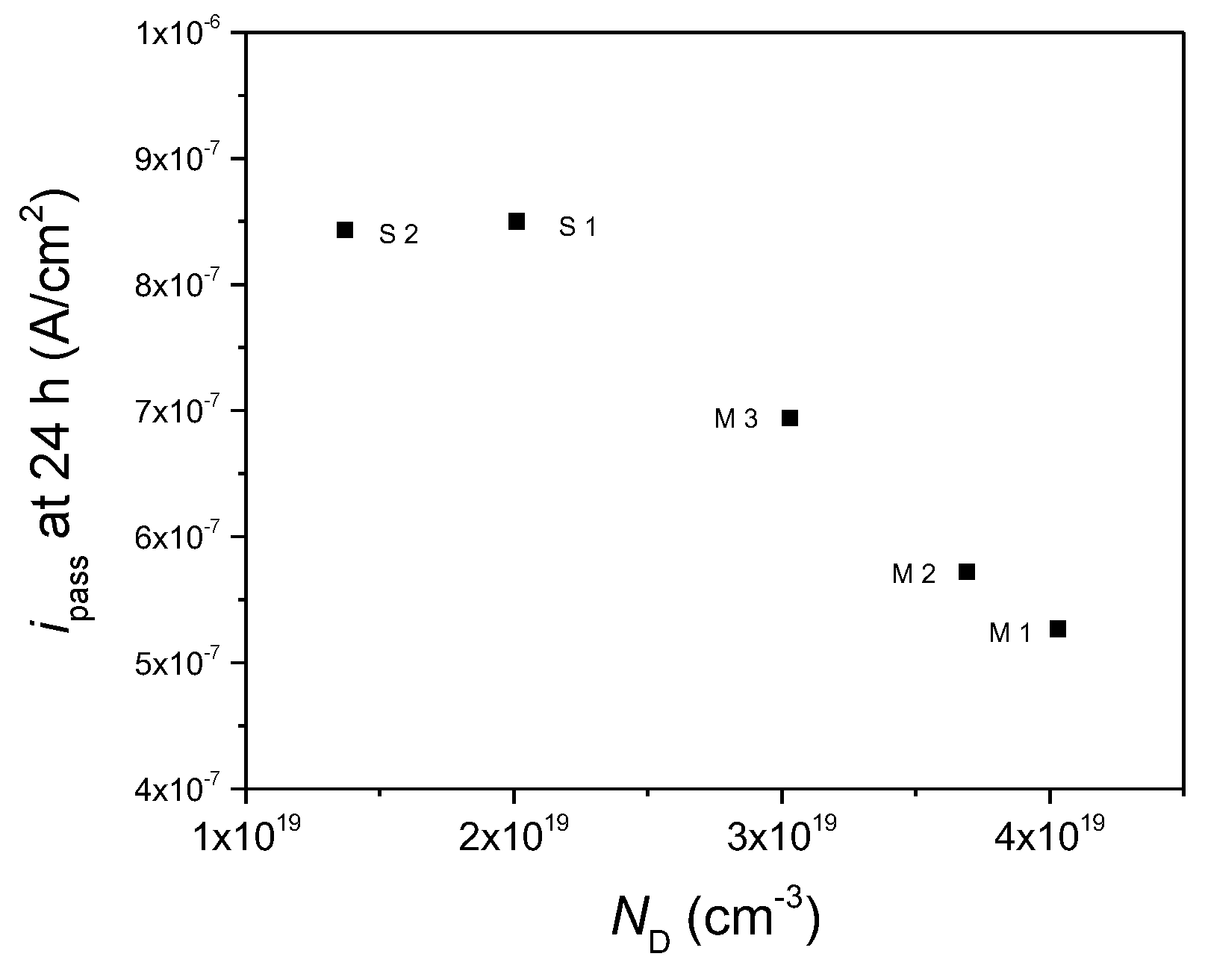
Experimental Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Park, K.D.; Ki, W.T.; Sin, Y.J. An Evaluation on Corrosion Fatigue Life of Spring Steel by Compressive Residual Stress. Trans. Korean Soc. Automot. Eng. 2007, 15, 1–7. [Google Scholar]
- Jung, J.W.; Park, W.J.; Huh, S.C.; Lee, K.Y.; Ha, K.J. The Effect of Compressive Residual Stress on Fracture Toughness of SUP-9 Spring Steel for Automobile. Proc. KSAE Conf. 2003, 1624–1629. [Google Scholar]
- Lim, M.S. Surface treatment method of coil spring. KR10-2011-0116842, 10 November 2011. [Google Scholar]
- Bae, D.H.; Lee, G.Y.; Jung, W.S. Stress Analysis of the Automobile’s Coil Spring Including Residual Stresses by Shot Peening. Key Eng. Mater. 2006, 459–464. [Google Scholar] [CrossRef]
- Akira, T. Coil spring for a suspension of an automobile and manufacturing method thereof. WO2010146898, 23 December 2010. [Google Scholar]
- Kim, W.J.; Kim, J.G.; Kim, Y.S.; Ismail, O.; Tsunekawa, Y. Wear-corrosion of cast iron thermal spray coating on Al alloy for automotive components. Met. Mater. Int. 2017, 13, 317. [Google Scholar] [CrossRef]
- Komazaki, I.; Kobayashi, K.; Misawa, T.; Fukuzumi, T. Environmental embrittlement of automobile spring steels caused by wet–dry cyclic corrosion in sodium chloride solution. Corros. Sci. 2005, 47, 2450–2460. [Google Scholar] [CrossRef]
- Ramamurthy, A.C.; Lorenzen, W.I.; Bless, S.J. Stone impact damage to automotive paint finishes: An introduction to impact physics and impact induced corrosion. Prog. Org. Coat. 1994, 25, 43–71. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, J.G.; Kim, Y.S.; Huh, J.Y. Corrosion characteristics of coated automotive parts subjected to field and proving ground tests. Int. J. Automot. Technol. 2008, 9, 625–631. [Google Scholar] [CrossRef]
- Matejicek, J.; Brand, P.C.; Drews, A.R.; Krause, A.; Lowe-Ma, C. Residual stresses in cold-coiled helical compression springs for automotive suspensions measured by neutron diffraction. Mater. Sci. Eng. A 2004, 367, 306–311. [Google Scholar] [CrossRef]
- Das, S.K.; Mukhioadhyay, N.K.; Kumar, B.R.; Bhattacharya, D.K. Failure analysis of a passenger car coil spring. Eng. Fail. Anal. 2007, 14, 158–163. [Google Scholar] [CrossRef]
- Del Llano-Vizcaya, L.; Rubio-Gonzalez, C.; Mesmacque, G.; Banderas-Hernandez, A. Stress relief effect on fatigue and relaxation of compression springs. Mater. Des. 2007, 28, 1130–1134. [Google Scholar] [CrossRef]
- Mano, H.; Kondo, S.; Matsumuro, A. Microstructured surface layer induced by shot peening and its effect on fatigue strength. In Proceedings of the International Symposium on Micro-Nano Mechatronics and Human Science, Nagoya, Japan, 5–8 November 2006; IEEE: Nagoya, Japan, 2006. [Google Scholar]
- Lim, M.S. Cold coil spring heat treatment method for improvement of inner side residual stress and fatigue life. KR10-2015-0048273, 7 May 2015. [Google Scholar]
- Kazuya, I. Compression coil spring and method for producing same. KR10-2015-7009184, 4 October 2015. [Google Scholar]
- Go, H.G. Spring manufacturing method. KR10-2014-0133138, 2 October 2014. [Google Scholar]
- Macdonald, D.D. Passivity–the key to our metals-based civilization. Pure Appl. Chem. 1999, 71, 951–978. [Google Scholar] [CrossRef]
- Yang, M.Z.; Luo, J.L.; Patchet, B.M. Correlation of hydrogen-facilitated pitting of AISI 304 stainless steel to semiconductivity of passive films. Thin Soild Films 1999, 354, 142–147. [Google Scholar] [CrossRef]
- Lv, J.L.; Luo, H.Y. Comparison of corrosion properties of passive films formed on phase reversion induced nano/ultrafine-grained 321 stainless steel. Appl. Surf. Sci. 2013, 280, 124–131. [Google Scholar]
- Park, K.J.; Ahn, S.J.; Kwon, H.S. Effects of solution temperature on the kinetic nature of passive film on Ni. Electrochim. Acta 2011, 56, 1662–1669. [Google Scholar] [CrossRef]
- Jang, H.J.; Kwon, H.S. Effects of Film Formation Conditions on the Chemical Composition and the Semiconducting Properties of the Passive Film on Alloy 690. Corros. Sci. Technol. 2006, 5, 141–148. [Google Scholar]
- Fattah-Alhosseini, A.; Vafaeian, S. Influence of grain refinement on the electrochemical behavior of AISI 430 ferritic stainless steel in an alkaline solution. Appl. Surf. Sci. 2016, 360, 921–928. [Google Scholar] [CrossRef]
- Jang, H.J.; Kwon, H.S. In situ study on the effects of Ni and Mo on the passive film formed on Fe–20Cr alloys by photoelectrochemical and Mott–Schottky techniques. J. Electroanal. Chem. 2006, 590, 120–125. [Google Scholar] [CrossRef]
- Ge, H.H.; Zhou, G.D.; Wu, W.Q. Passivation model of 316 stainless steel in simulated cooling water and the effect of sulfide on the passive film. Appl. Surf. Sci. 2003, 211, 321–334. [Google Scholar] [CrossRef]
- Li, D.; Wang, J.; Chen, H.; Chen, D. Investigation on electronic property of passive film on nickel in bicarbonate/carbonate buffer solution. Chin. J. Chem. 2011, 29, 243–253. [Google Scholar] [CrossRef]
- Freire, L.; Carmezim, M.J.; Ferreira, M.G.S.; Monremor, M.F. The passive behaviour of AISI 316 in alkaline media and the effect of pH: A combined electrochemical and analytical study. Electrochim. Acta 2010, 55, 6174–6181. [Google Scholar] [CrossRef]
- Sarlak, H.; Atapour, M.; Esmailzadeh, M. Corrosion behavior of friction stir welded lean duplex stainless steel. Mater. Des. 2015, 66, 209–216. [Google Scholar] [CrossRef]
- Lv, J.; Luo, H. Effects of strain and strain-induced α′-martensite on passive films in AISI 304 austenitic stainless steel. Mater. Sci. Eng. C 2014, 34, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.A.; Cheng, Y.F. Micro-electrochemical characterization and Mott–Schottky analysis of corrosion of welded X70 pipeline steel in carbonate/bicarbonate solution. Electrochim. Acta 2009, 55, 316–324. [Google Scholar] [CrossRef]
- Guo, H.X.; Lu, B.T.; Luo, J.L. Study on passivation and erosion-enhanced corrosion resistance by Mott-Schottky analysis. Electrochim. Acta 2006, 52, 1108–1116. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Nakanishi, T.; Hasegawa, Y.; Fushimi, K. Passivity of Dual-Phase Carbon Steel with Ferrite and Martensite Phases in pH 8.4 Boric Acid-Borate Buffer Solution. J. Electrochem. Soc. 2015, 162, C322–C326. [Google Scholar] [CrossRef]
- Liu, C.Y.; Jing, R.; Wang, Q.; Zhang, B.; Jia, Y.Z.; Ma, M.Z.; Liu, R.P. Fabrication of Al/Al3Mg2 composite by vacuum annealing and accumulative roll-bonding process. Mater. Sci. Eng. A 2012, 558, 510–516. [Google Scholar] [CrossRef]
- Lee, J.B.; Yoon, S.I. Effect of nitrogen alloying on the semiconducting properties of passive films and metastable pitting susceptibility of 316L and 316LN stainless steels. Mater. Chem. Phys. 2010, 122, 194–199. [Google Scholar] [CrossRef]
- Ningshen, S.; Mudali, U.K.; Mittal, V.K.; Khatak, H.S. Semiconducting and passive film properties of nitrogen-containing type 316LN stainless steels. Corros. Sci. 2007, 49, 481–496. [Google Scholar] [CrossRef]
- Amri, J.; Souier, T.; Malki, B.; Baroux, B. Effect of the final annealing of cold rolled stainless steels sheets on the electronic properties and pit nucleation resistance of passive films. Corros. Sci. 2008, 50, 431–435. [Google Scholar] [CrossRef]
- Yan, M.L.; Zhao, W.Z. Influence of temperature on corrosion behavior of PbCaSnCe alloy in 4.5 M H2SO4 solution. J. Power Sources 2010, 195, 631–637. [Google Scholar]
- Cheng, Y.F.; Luo, J.L. A comparison of the pitting susceptibility and semiconducting properties of the passive films on carbon steel in chromate and bicarbonate solutions. Appl. Surf. Sci. 2000, 167, 113–121. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Li, J.; Liu, Y.; Chen, D.; Li, Y.; Yang, K.; Wang, F. The effect of Cu addition on the electrochemical corrosion and passivation behavior of stainless steels. Electrochim. Acta 2010, 55, 5028–5535. [Google Scholar] [CrossRef]
- Jang, H.J.; Oh, K.N.; Ahn, S.J.; Kwon, H.S. Determination of the diffusivity of cation vacancy in a passive film of Ni using Mott-Schottky analysis and in-situ ellipsometry. Met. Mater. Int. 2014, 20, 277–283. [Google Scholar] [CrossRef]
- Jang, H.J.; Kwon, H.S. Effects of Cr on the structure of the passive films on Ni-(15, 30) Cr. ECS Trans. 2007, 3, 1–11. [Google Scholar]
- Liu, B.; Zhang, T.; Shao, Y.; Meng, G.; Wang, F. Effect of hydrostatic pressure on the nature of passive film of pure nickel. Mater. Corros. 2011, 62, 269–274. [Google Scholar] [CrossRef]
- Jang, H.J.; Kwon, H.S. Effects of Mo on the Passive Films Formed on Ni-(15, 30) Cr-5Mo Alloys in pH 8.5 Buffer Solution. J. Korean Electrochem. Soc. 2009, 12, 258–262. [Google Scholar] [CrossRef]
- Park, K.; Kwon, H. Effects of manganese on the passivity of Fe-18Cr-xMn (x = 0, 6, 12). Korea Adv. Inst. Sci. Technol. Daejeon 2005, 32–40. [Google Scholar]
- Ahn, S.J.; Kwon, H.S.; Macdonald, D.D. Role of chloride ion in passivity breakdown on iron and nickel. J. Electrochem. Soc. 2005, 152, B482–B490. [Google Scholar] [CrossRef]
- Li, D.G.; Wang, J.D.; Chen, D.R.; Liang, P. The role of passive potential in ultrasonic cavitation erosion of titanium in 1 M HCl solution. Ultrason. Sonochem. 2016, 29, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Hakiki, N.B.; Boudin, S.; Rondot, B.; Belo, M.D.C. The electronic structure of passive films formed on stainless steels. Corros. Sci. 1995, 37, 1809–1822. [Google Scholar] [CrossRef]
- Paola, A.D. Semiconducting properties of passive films on stainless steels. Electrochim. Acta 1989, 34, 203–210. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Luo, J.L. Electronic structure and pitting susceptibility of passive film on carbon steel. Electrohim. Acta 1999, 44, 2947–2957. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; National Association of Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar]
- Ahn, S.J.; Kwon, H.S. Effects of solution temperature on electronic properties of passive film formed on Fe in pH 8.5 borate buffer solution. Electrochim. Acta 2004, 49, 3347–3353. [Google Scholar] [CrossRef]
- Smith, W.F. Structure and Properties of Engineering Alloys, 2nd ed.; McGraw-Hill: New York, NY, USA, 1990. [Google Scholar]
- Han, M.S.; Hyun, G.Y.; Kim, S.J. Effects of Shot Peening Time on Microstructure and Electrochemical Characteristics for Cu Alloy. J. Korean Soc. Mar. Environ. Saf. 2013, 19, 545–551. [Google Scholar] [CrossRef]
- Di Franco, F.; Santamaria, M.; Massro, G.; Di Quarto, F. Photoelectrochemical monitoring of rouging and de-rouging on AISI 316L. Corros. Sci. 2017, 116, 74–87. [Google Scholar] [CrossRef]
- Tranchida, G.; Clesi, M.; Di Franco, F.; Di Quarto, F.; Santamaria, M. Electronic properties and corrosion resistance of passive films on austenitic and duplex stainless steels. Electrochim. Acta 2018, 273, 412–423. [Google Scholar] [CrossRef]
- Di Quarto, F.; Di Franco, F.; Miraghaei, S.; Santamaria, M.; La Mantia, F. The amorphous semiconductor Schottky barrier approach to study the electronic properties of anodic films on Ti. J. Electrochem. Soc. 2017, 164, C516–C525. [Google Scholar] [CrossRef]
- Park, K.D.; Sin, Y.J.; Kim, D.U. The effect of shot peening on the corrosion and fatigue crack to SAE5155 steel. J. Korean Soc. Mar. Eng. 2006, 30, 731–739. [Google Scholar]
- Park, K.D.; Ha, K.J. Influence of shot peening on the corrosion of spring steel. J. Ocean Eng. Technol. 2003, 17, 39–45. [Google Scholar]
- Hosaka, T.; Yoshihara, S.; Amanina, I.; MacDonald, B.J. Influence of grain refinement and residual stress on corrosion behavior of AZ31 magnesium alloy processed by ECAP in RPMI-1640 medium. Procedia Eng. 2017, 184, 432–441. [Google Scholar] [CrossRef]
- Peyre, P.; Scherpereel, X.; Berthe, L.; Carboni, C.; Fabbro, R.; Beranger, G.; Lemaitre, C. Surface modifications induced in 316L steel by laser peening and shot-peening. Influence on pitting corrosion resistance. Mater. Sci. Eng. A 2000, 280, 294–302. [Google Scholar] [CrossRef]
- Jang, H.J.; Park, C.J.; Kwon, H.S. Photoelectrochemical study of the growth of the passive film formed on Fe-20Cr-15Ni in a pH 8.5 buffer solution. Met. Mater. Int. 2010, 16, 247–252. [Google Scholar] [CrossRef]



| Element | C | Si | Mn | P | S | Cr | V |
|---|---|---|---|---|---|---|---|
| Composition (wt%) | 0.45–0.55 | 0.15–0.35 | 0.65–0.95 | <0.035 | <0.035 | 0.80–1.10 | 0.15–0.25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-H.; Ahn, S.-H.; Seo, J.-W.; Jang, H. Passivity of Spring Steels with Compressive Residual Stress. Metals 2018, 8, 788. https://doi.org/10.3390/met8100788
Lee K-H, Ahn S-H, Seo J-W, Jang H. Passivity of Spring Steels with Compressive Residual Stress. Metals. 2018; 8(10):788. https://doi.org/10.3390/met8100788
Chicago/Turabian StyleLee, Kyu-Hyuk, Seung-Ho Ahn, Ji-Won Seo, and HeeJin Jang. 2018. "Passivity of Spring Steels with Compressive Residual Stress" Metals 8, no. 10: 788. https://doi.org/10.3390/met8100788
APA StyleLee, K.-H., Ahn, S.-H., Seo, J.-W., & Jang, H. (2018). Passivity of Spring Steels with Compressive Residual Stress. Metals, 8(10), 788. https://doi.org/10.3390/met8100788





