Abstract
Pyrometallurgical metal production results in side streams, such as dusts and slags, which are carriers of metals, though commonly containing lower metal concentrations compared to the main process stream. In order to improve the circular economy of metals, selective leaching of copper from an intermediate raw material originating from primary base metal production plant was investigated. The raw material investigated was rich in Cu (12.5%), Ni (2.6%), Zn (1.6%), and Fe (23.6%) with the particle size D80 of 124 µm. The main compounds present were nickel ferrite (NiFe2O4), fayalite (Fe2SiO4), cuprite (Cu2O), and metallic copper. Leaching was studied in 16 different solutions. The results revealed that copper phases could be dissolved with high yield (>90%) and selectivity towards nickel (Cu/Ni > 7) already at room temperature with the following solutions: 0.5 M HCl, 1.5 M HCl, 4 M NaOH, and 2 M HNO3. A concentration of 4 M NaOH provided a superior selectivity between Cu/Ni (340) and Cu/Zn (51). In addition, 1–2 M HNO3 and 0.5 M HCl solutions were shown to result in high Pb dissolution (>98%). Consequently, 0.5 M HCl leaching is suggested to provide a low temperature, low chemical consumption method for selective copper removal from the investigated side stream, resulting in PLS (pregnant leach solution) which is a rich in Cu and lead free residue, also rich in Ni and Fe.
1. Introduction
The growth in metal production has resulted in a gradual decrease in metal grades of ore deposits. Therefore, new technologies and flow-sheets are needed for the more efficient utilization of ore processing tailings, metallurgical slags, flue dusts, etc. In the base metal production, various solid side-streams are generated, such as slags, dusts, and leach residues. Inherently, these side-streams contain valuable base metals.
Thermodynamics determines the distributions of metals between metal and slag in high temperature processing [1,2,3]. In addition, kinetics and physical entrainment cause metal traces ending up to the slag in different steps of the production. About 60% of the world’s copper and 50% of world sulphidic nickel production comes from plants using flash smelting furnace (FSF) technologies [4]. The main advantages of the FSF processes are high sulfur recovery, flexibility to feed materials and the efficient energy utilization [5]. The subsequent converting takes place in two sequential steps:
- (a)
- The FeS elimination or slag making stage2FeS(s) + 3O2(g) + 2SiO2(s) = 2FeO·SiO2(s) + 2SO2(g)
- (b)
- The copper making stageCu2S(s) + 2O2(g) = 2Cu (s) + 2SO2(g)
As the process throughputs are generally high [6,7,8] the slags of the primary production can present a valuable secondary raw materials for metal recovery in future.
The composition of slags in base metal processing vary depending on the process and raw material. Copper flash smelting furnace slag generally consist of 30–50% Fe, 30–40% SiO2, 1–10% Al2O3, 1–16% CaO and 0.2–1.2% of Cu [9]. Copper is mainly entrapped in the slag as chalcocite and metallic copper, as well as trace copper oxide [10]. The converter slag is usually characterized by 20–25% SiO2, 40–45% Fe, and 5% Cu. The slags of anode furnace differs from the converter slags due its very high copper content, containing typically above 50 wt. % CuOx, 30–35 wt. % FeO, 5–15 wt. % SiO2, and minor amounts of As, Sb, and Pb [11,12]. Nickel flash smelting furnace slag has been reported to contain 8.7% Fe2SiO4, 10% Fe3O4, 20.5% SiO2, 3.1% Al2O3, 1.3% MgO, and 1.1% CaO [13]. Generally, the slag former used is SiO2.
Industrial smelting and converting slags are cleaned before discarding them. In most cases an electric furnace settling or reduction is used, but some copper smelters use milling and slag flotation.
In the literature, new methods for slag cleaning have been studied for eliminating trace element or cutting their internal circulations in the smelter. Thus, the impurity levels in the slags and anode copper will be lowered. Roasting of the converter slag with ferric sulphate and selective sulphation roasting are the documented pyrometallurgical methods used for the recovery of nickel, copper and zinc [14,15]. Also, pyro-hydrometallurgical methods involving acid roasting or thermal decomposition followed by water leaching have been suggested [16,17,18]. Various hydrometallurgical methods have been developed using lixiviants such as acids, bases, and salts for base metal extraction. Atmospheric leaching of different slag fractions has been studied in H2SO4, FeSO4, (NH4)2SO4, FeS2, NaCl, and FeCl2 media [19,20,21,22,23]. In addition, pressure leaching of copper slag containing 4.03% Cu, 0.48% Co, and 1.98% Ni at 130 °C have resulted in significant recoveries of Cu, Co, and Ni, amounting to 90% [24]. Leaching with aqueous sulfur dioxide has also proven effective in recovering 77% Co and 35% Ni from a nickel smelter slag [25].
The current study was undertaken to investigate the dissolution behaviour of selected metals, from the Cu, Ni, Fe, and Zn rich intermediate of base metal production. The focus was to dissolve copper selectively in order to produce PLS rich in copper and a residue with Fe and Ni, applicable for recovery of metals. The lixiviants used in the present study were 0.5–0.5 M HCl, 0.5–3.06 M H2SO4, 1–2 M HNO3, 0.5 M NaCl + 0.1 M CuCl2, 4.5 M NaCl + 0.5 M CuCl2, 4.5 M NaCl + 0.1 M CuCl2, and 4 M NaOH.
2. Materials and Methods
Characterization studies by Scanning Electron Microscopy (SEM), X-ray diffraction (XRD), and Particle Size Distribution (PSD) were conducted to determine the morphology, mineralogical composition, and elemental distribution of the raw material.
2.1. The Raw Material
Chemical analysis of the raw material was performed by employing microwave-assisted digestion in aqua regia (ETHOS Touch Control, Milestone Microwave Laboratory Systems, Sorisole, Italy), as aqua regia is one of the strongest and effective solvent used for metal digestion [26], Table 1. The solution analyses were conducted by ICP-OES (Inductively Coupled Plasma Optical Emission Spectroscopy, Perkin Elmer Optima 7100 DV, Waltham, MA, USA) by Milomatic Oy.

Table 1.
Chemical analysis of metals of interest in raw material investigated.
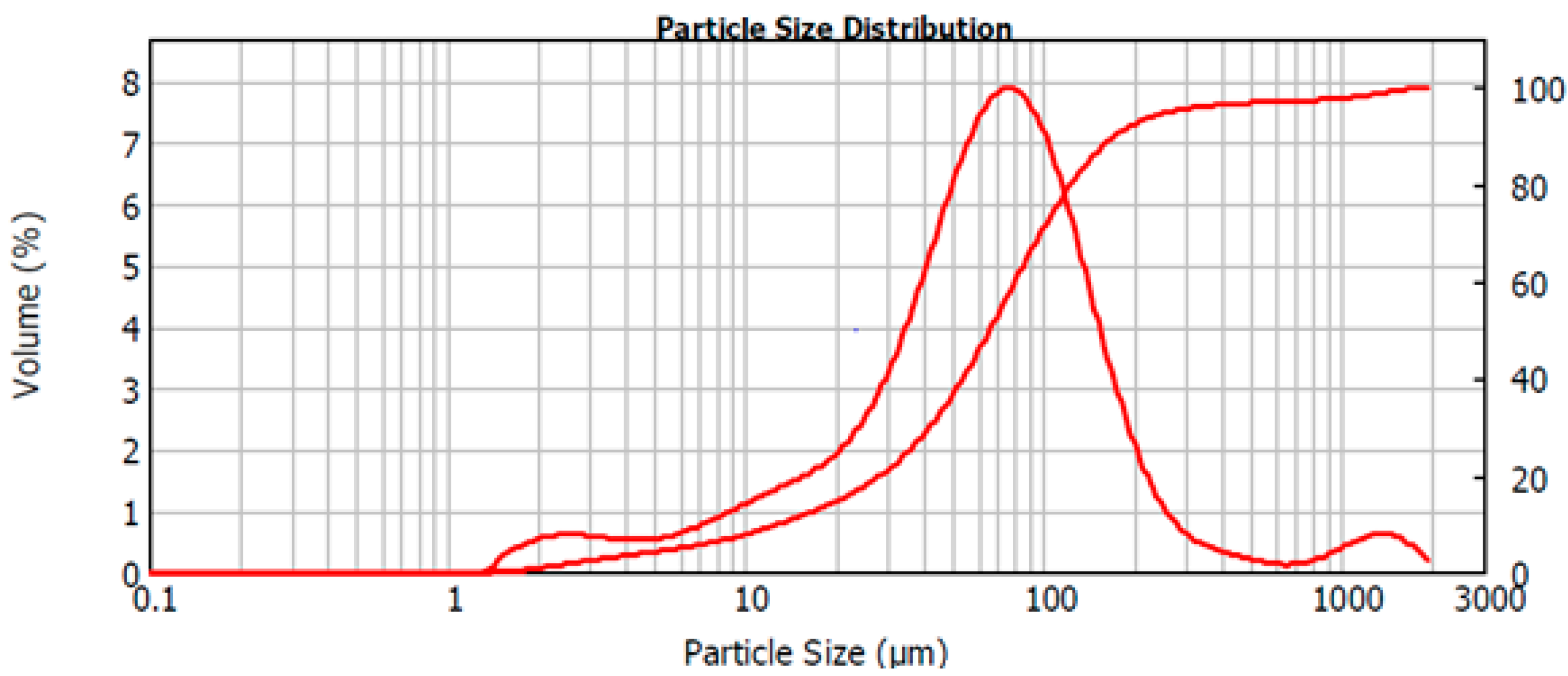
The particle size of the crushed intermediate raw material was analyzed by a Mastersizer 2000 laser diffraction particle size analyzer with a Scirocco 2000 Dry Powder Feeder, both manufactured by Malvern Instruments (UK). Dispersion pressure was varied from 2.0 to 3.0 bar, vibration feed rate was 50% and measurement time was varied from 12 to 30 s. Fraunhofer diffraction model was used as an optical model. The particle size distribution of the homogenized raw material is demonstrated in the volume versus particle size diagram, Figure 1. The size distribution was observed to extend from 1.4 μm to 1905 μm. The cumulative particle size distribution revealed D80 value of 123 µm. The mean particle size D10 = 13 µm, the surface weighted mean was D32 = 25 μm, and the volume weighted mean D43 = 114 μm.
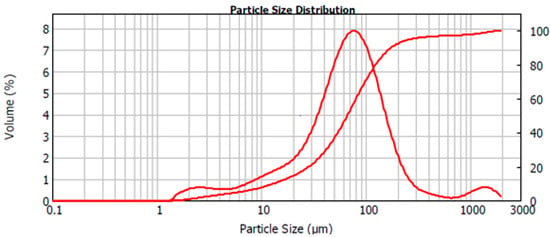
Figure 1.
The observed particle size distribution of the homogenized raw material.
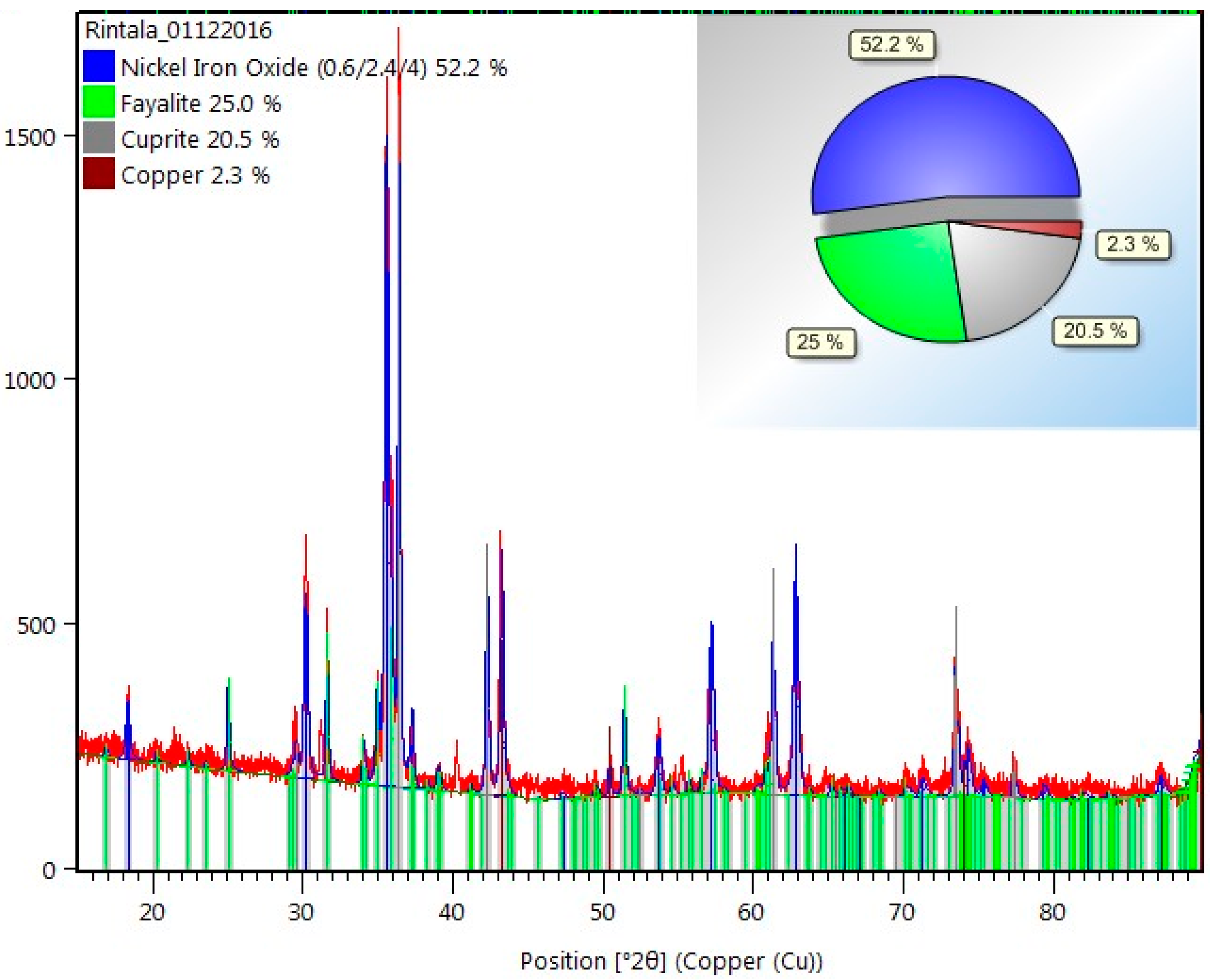
An X’Pert PRO-PAN Analytical X-ray diffractometer, operating at an anode current of 40 mA at 45 kV with a Cukɑ, by Rietveld refinement method [27] using HighScore Plus software (PANalytical), performed mineralogical analysis of the sample. Fixed Divergence Slit (FDS) 1/2° was fitted in the incident beam path to control the equatorial divergence of the incident beam and fixed incident beam. A copper mask of 15 mm was fitted in the incident beam path to control the axial width of the incident beam. Fixed Anti-Scatter Slit (FASS) 1° was used to reduce background signal. The XRD analysis of the raw material by Rietveld refinement suggested a composition of 52.2 wt. % NiFe2O4, 25.0 wt. % Fe2SiO4 (fayalite), 20.5 wt. % of Cu2O (cuprite), and 2.3 wt. % of metallic Cu, Figure 2.
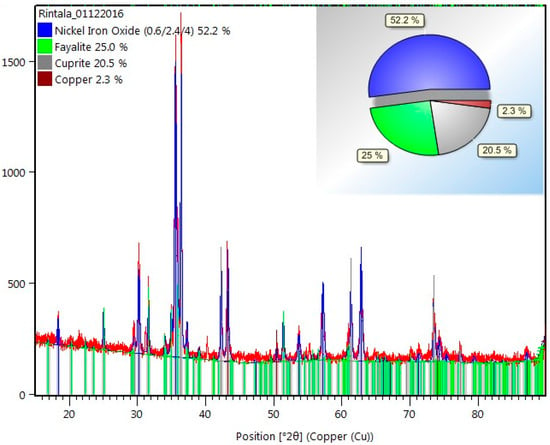
Figure 2.
The obtained X-ray diffraction (XRD) pattern of the raw material.
SEM-EDS analysis for two raw material samples was performed with a LEO 1450 VP (Carl Zeiss, Oberkochen, Germany) scanning electron microscope (SEM) and a X-MAX-50 mm2 energy dispersive X-ray spectrometer (EDS) with INCA Software (Oxford Instruments, Abingdon, UK). Tungsten filament was used as a cathode and the acceleration voltage used was 15 kV.
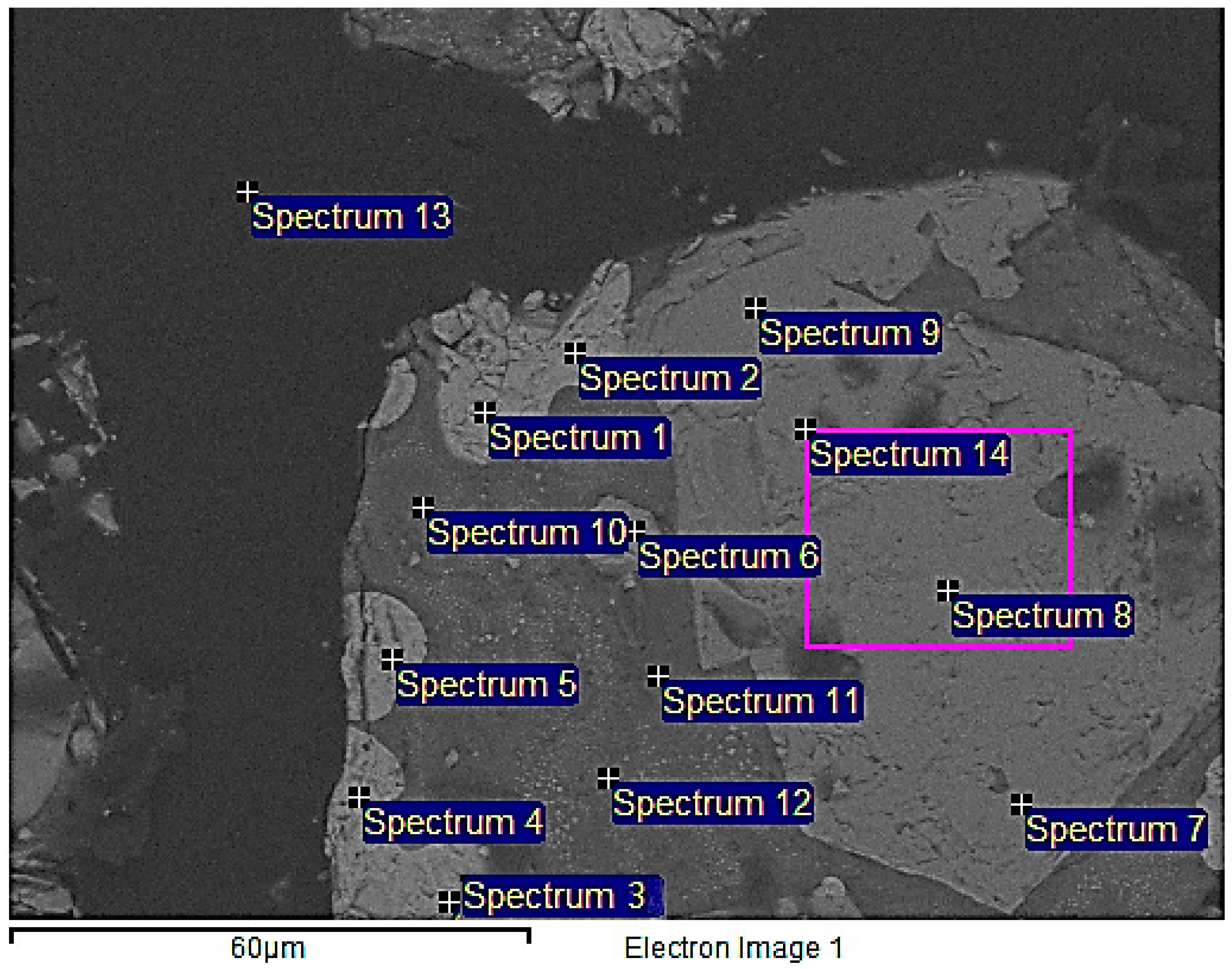
The raw material samples were cast in epoxy and treated in vacuum, for eliminating gas bubbles attached into the particles, and prepared for SEM-EDS examination polished sections using standard wet methods. It can be discerned from Figure 3 that a larger particle of size around 500 µm is encompassed by smaller particles of particle size ranging 2–50 µm in the raw material. Three phases could be observed, one of the larger particles and two phases in the smaller particles. The average weight percentages of the elements detected in spectrum 1–14 in Figure 3 are presented in Table 2. The lightest color in the back scattered electron (BSE) image corresponds to the phase of the larger particle. It consisted of an average of 88.6 wt. % Cu, 1.9 wt. % Fe, 8.8 wt. % O, and 0.6 wt. % Si (Spectra 1–5), suggesting the presence of Cu and Cu2O (cuprite), as analyzed oxygen eventually is trace from a surface contamination. The light-gray areas in spectra 6, 9, and 12 correspond to an average of 2.2 wt. % Cu, 52.4 wt. % Fe, 14.7 wt. % Ni, 23.8 wt. % O, and 0.6 wt. % Si (Spectra 6–9, 14), indicating the three main phases, namely Fe2SiO4, possibly NiFe2O4 and Cu2O. Nevertheless, the dark-gray region represented by Spectra 10–12, consisted of an average of 3.2 wt. % Cu, 3.2 wt. % Fe, 1.1 wt. % Ni, 45.8 wt. % O, and 32.3 wt. % Si, corresponding to the presence of almost pure SiO2. Spectra 13 corresponds to epoxy, where samples were casted.
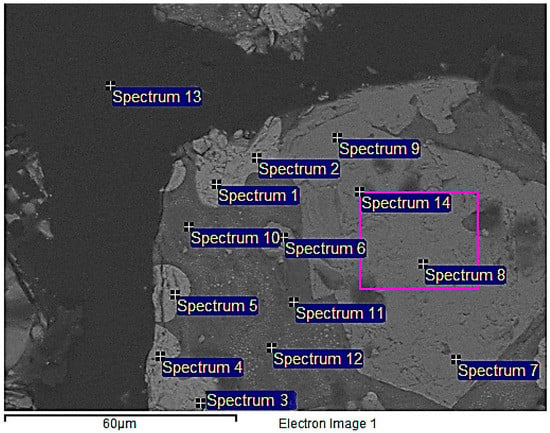
Figure 3.
Back scattered Scanning Electron Microscopy (SEM) micrograph of the overall raw material. Spectra 1–5 (Cu2O phase), Spectra 6–9, 14 (NiFe2O4 phase).

Table 2.
SEM-EDS point analysis of the particles presented in Figure 3.
2.2. Leaching Experiments
In order to investigate the extraction without external heating, leaching was conducted at ambient temperature (25 °C) for 48 h in several solutions (Table 3). Leaching experiments were conducted in an Erlenmeyer flasks and the solutions were mixed by an IKA RO 10 Multi Station Digital Magnetic Stirrer at 300 RPM. The used S/L ratio was 0.025 (5 g solids/200 mL solution). To evaluate the leaching efficiency of Ni, Zn, Cr, Pb, Cu, Fe, and Al, the solution was filtered after the leaching step and the filtrate was analysed by AAS (atomic absorption spectrophotometer), using a Varian AA240 (Varian, Palo Alto, CA, USA), and ICP-OES [28,29].

Table 3.
Solutions used in the leaching tests.
No external oxidation by gas bubbling was used in the experiments. Redox potential was measured by a Fluke 115 True RMS Multimeter using platinum wire and Saturated Calomel Electrode (SCE). Mettler Toledo Seven (Easy pH meter) was used for pH measurements, except in the NaOH solutions, where Hanna Instruments Edge pH meter was employed.
3. Results and Discussion
Leaching was performed on the raw material to get an insight into the dissolution phenomena related to Cu, Ni, Zn, and Fe in various lixiviants. Also leaching of trace metals, such as Cr, Pb, and Al, was explored. The aim was to find a selective, low temperature, and low chemical consumption leaching procedure for copper present in the raw material. Furthermore, the target was to leave nickel in the leach residue in the leaching stage.
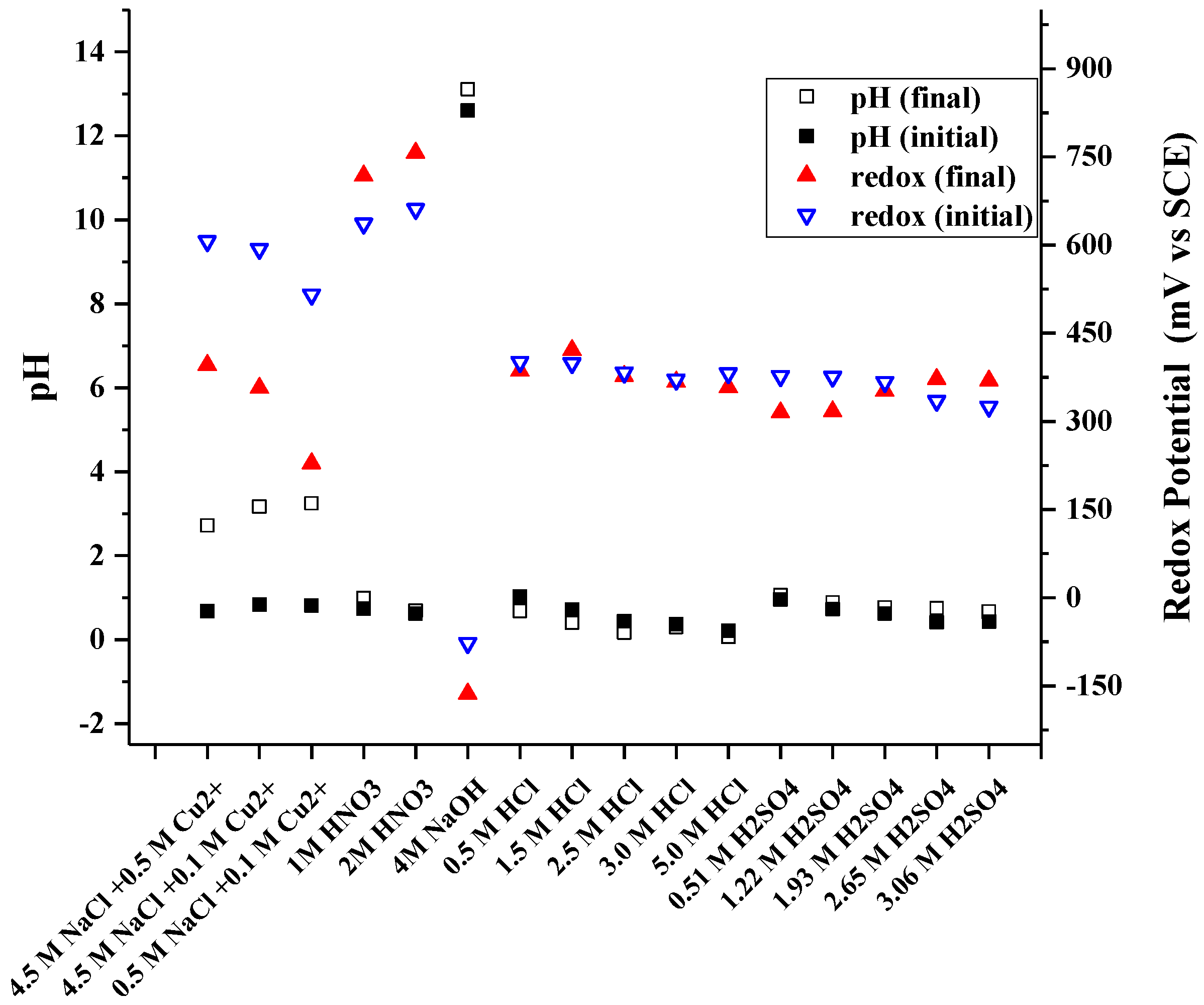
Table 4 presents the metal yields to the solution in all 16 investigated media. The corresponding redox potentials, as well as pHs before and after the experiment are presented in Figure 4. It can be seen that there is some variety in the recovery percentage—this is most likely attributed to the heterogeneous nature of the investigated raw material with big particle size and wide particle size range combined with small solid/liquid ratio in the leaching experiments. This leads in to some variation in the representativeness of each sample, thus also resulting some error in the recovery calculations.

Table 4.
Extraction of investigated metals from the raw material (%).
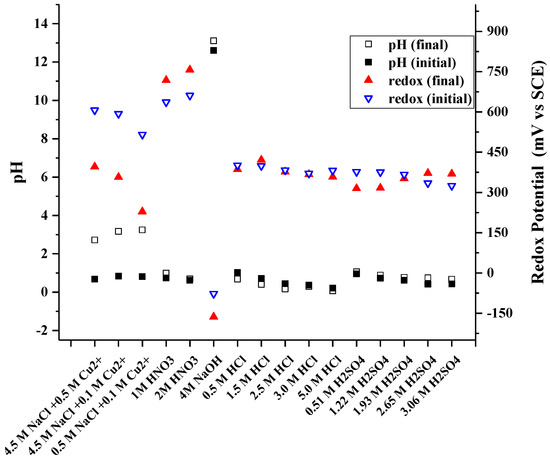
Figure 4.
Measured redox potentials and pH during leaching in 16 investigated leaching media.
3.1. Leaching of Copper
Table 4 shows that copper was dissolved well into most lixiviants investigated. The highest extraction of Cu was achieved with 1.5 M HCl. Also 4 M NaOH, 0.5 M HCl, 2.5 M HCl, and 2 M HNO3 resulted in yields higher than 93%, and 1 M HNO3,1.22 M H2SO4, and 1.93 M H2SO4 showed >75% extraction. The chloride leaching experiments (0.1 and 0.5 M of copper (II) as oxidant along with 4.5 M NaCl) showed only minor Cu dissolution (≤5%), most likely due to a final pH close to 3 (see Figure 4), indicating copper precipitation as atacamite [30]. Sulfuric acid concentration increase was shown to increase Cu extraction up to 80% at 1.93 M, however at higher concentrations the extraction was decreased, being 65% at 3.0 M H2SO4. The extraction efficiency of copper was found to be comparatively lower in H2SO4 than in HCl and HNO3 medium (Table 4). Habashi et al. [31] have suggested that since HCl and HNO3 generate 1 mole of H+ ions when dissolved in water, they produce similar dissolution efficiency compared to H2SO4, which produces 2 moles of H+ ions. Also, the extraction efficiency of Cu was higher in 2 M HNO3 than in 1 M HNO3 (Table 4) as the oxidizing potential of NO3− ions has been reported to increase with increase in solution acidity [32].
In chloride media, it is suggested that cuprous chloride complexes CuCl32− and CuCl43− will be produced sequentially from CuCl2− with chloride concentration above 1 M [33]. Chloride ions complexes can stabilize Cu(I) ions thereby increasing copper solubility. The complexation also increases the redox potential of Cu(II)/Cu(I) thereby enhancing the oxidative power of the solution. Copper is also known to be dissolvable at high pHs such as in 4 M NaOH media. The pH values measured in NaOH leaching (Figure 4) suggest the prevailing species as Cu(OH)3− [34].
The suggested reactions acid/basic leaching reactions in HCl (3), sulfuric acid (4), and basic NaOH (5) for Cu2O, are presented below with their standard Gibbs energies of the reactions at 25 °C from HSC Chemistry database [35]:
Cu2O + 8HCl(a) = 2CuCl43−(a) + 6H+(a) + H2O(a), ∆G° = −121.28 kcal/mol
Cu2O + H2SO4(l) = Cu + CuSO4(ia) + H2O(a), ∆G° = −14.71 kcal/mol
Cu2O + 4NaOH(a) + H2O(a) = 2Cu(OH)3−(a) + 4Na+(a) + 2e−, ∆G° = −28.13 kcal/mol
The species (a), (ia) and (l) refers to aqueous, neutral aqueous and liquid phase.
3.2. Ni Leaching and Selectivity between Copper and Nickel
According to the mineralogy, the prevailing nickel phase in the raw material investigated is nickel ferrite NiFe2O4. Ferrites are known to be refractory in leaching. This is confirmed by the results which showed that the maximum Ni extraction (97%) was observed in aggressive concentrated leaching media (3–5 M HCl). The suggested leaching reactions in HCl are presented in (6) and (7). From the speciation diagram of nickel containing NiCl2 and HCl [36], most nickel is suggested to exist as Ni2+ up to 5 M HCl. However, the concentration of NiCl+ gradually increases with increases in HCl. Nickel dissolution did not show any selectivity versus iron in any of the leaching media investigated. This is due to the dominating Ni phase NiFe2O4 resulting in a simultaneous Ni and Fe dissolution. Also, in the absence of neutralization, no back precipitation was observed.
NiFe2O4 + 8HCl(a) = Ni+2(a) + 2FeCl+2(a) + 4H2O(a) + 6Cl−(a), ∆G° = −127.78 kcal/mol
NiFe2O4 + 8HCl(a) = NiCl+(a) + 2FeCl2+(a) + 4H2O(a) + 3Cl−(a), ∆G° = −161.17 kcal/mol
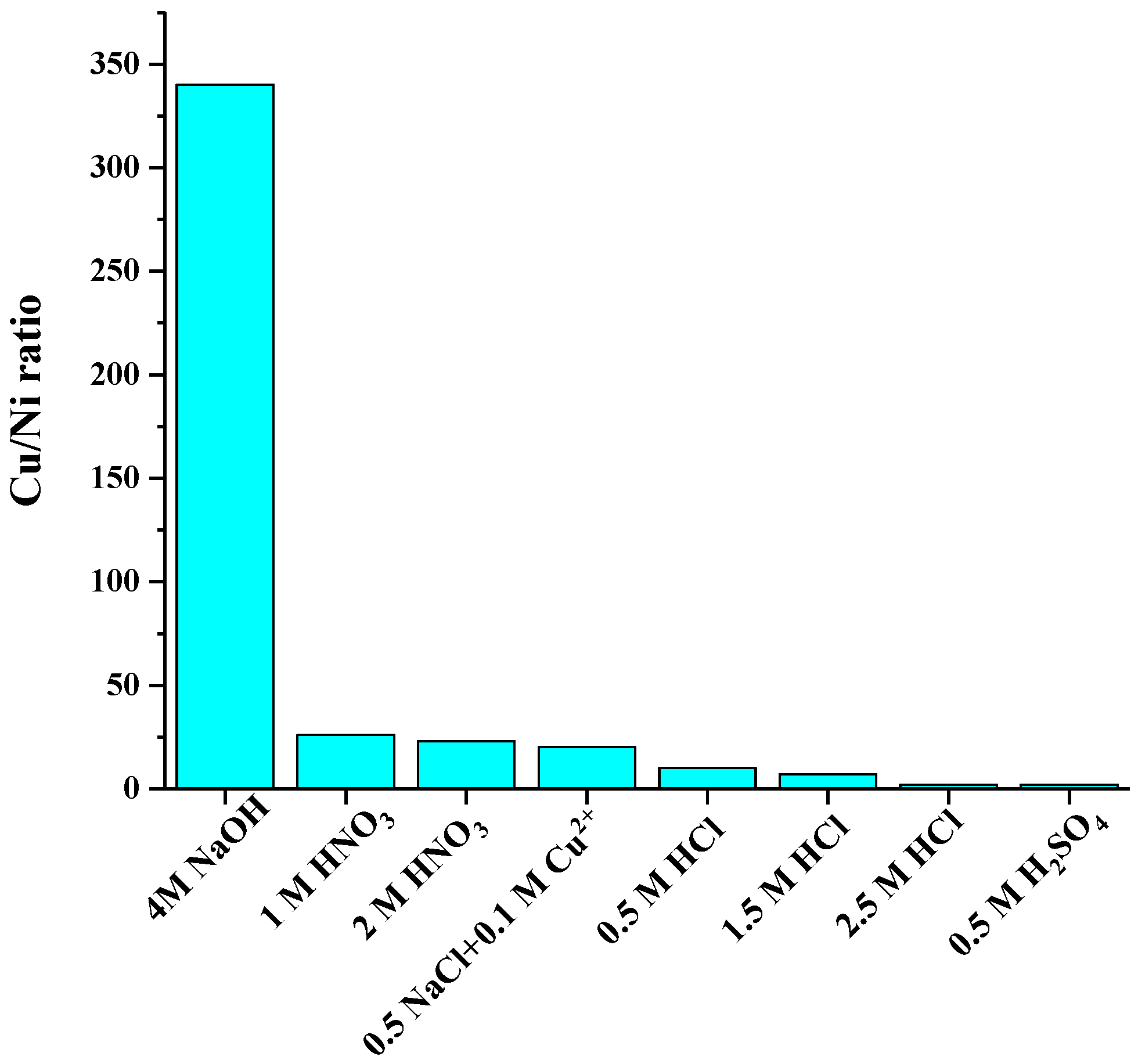
The current study aims to selectively dissolve Cu versus nickel. Figure 5 presents the dissolved Cu/Ni ratio in solution with eight of the most selective lixiviants. It can be seen that the highest selectivity was achieved with 4 M NaOH (w(Cu):w(Ni) = 340 in solution). Also 1 and 2 M HNO3 (w(Cu)/w(Ni) = 26 and 23) provided excellent selectivity as well as 0.5 M HCl solution (w(Cu)/w(Ni) = 10).
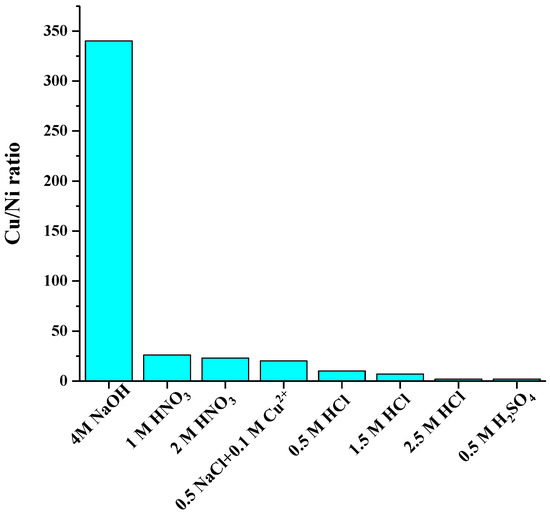
Figure 5.
Cu selectivity against Ni in the best eight selective leaching media.
The lowest Ni dissolution (≤4%) was observed with 4 M NaOH, 1–2 M HNO3, and all the investigated NaCl solutions. Dilute HCl (0.5 M) dissolved only 10% of nickel.
3.3. Leaching of Iron
Iron originated from the two main minerals of the raw material, NiFe2O4 and Fe2SiO4. Most notable extractions of Fe (81–82%) was observed in 1.93 M H2SO4 and 2.5 M HCl. Furthermore, the Fe extraction was high (>50%) in all hydrochloric and sulphuric acid media. Generally, no selectivity between iron and nickel or copper was found. However, minor selectivity between Cu and Fe was observed in 0.5 M and 1.5 M HCl (Cu/Fe = 1.9 and 1.5, respectively).
In chloride media, Fe(III) forms FeCl2+ and Fe3+ at lower Cl− concentrations, whereas FeCl2+ is formed at higher chloride concentration [37,38]. The suggested reactions for NiFe2O4 are presented earlier in (6) and (7), in addition fayalite is suggested to leach according to reactions (8) and (9):
Fe2SiO4 + 2H2SO4 (l) = 2FeSO4 (ia) + H4SiO4(a), ∆G° = −51.67 kcal/mol
H4SiO4 (colloid) = SiO2·2H2O
It is clear that iron dissolution is strongly related to the solution pH. At pHs < 2 iron is known to remain soluble [39]. This can be taken as an advantage in the leaching, as pH adjustment can significantly improve the selectivity between Cu and Fe.
3.4. Leaching of Zinc
The maximum extraction of Zn (90%) was achieved in 5 M HCl. Several researchers [40,41] have reported 90% recovery of Zn in leaching of zinc ferrite in the concentration range of 0.5–6 M HCl. When Zn(II) is dissolved into chloride media, it is known to form complex such as ZnCl3− [42]. Decrease in the Zn yield from 86% to 40% was noticed in the following order of the lixiviants:
2.65 M H2SO4 > 3.0 M H2SO4 > 1.93 M H2SO4 > 1.23 M H2SO4 > 3 M HCl > 2.5 M HCl > 0.5 M H2SO4 > 0.5 M HCl
However, Zn extraction was ≤30% in 1–2 M HNO3 and 0.5 M NaCl + 0.1 M Cu2+. The alkaline leaching of zinc ferrite (4 M NaOH) was shown to result in lower Zn extraction compared to HCl and H2SO4. Zn extraction was found to be 2% in 4 M NaOH. Thus, zinc ferrite fraction of magnetite was not decomposed even in strong alkaline media. The suggested reactions for Zn dissolution in HCl (10), and sulfuric acid (11) media are:
ZnFe2O4 + 8HCl(a) = ZnCl3−(a) + 2FeCl2+(a) + 4H2O(l) + 3Cl−(a), ∆G° = −124.77 kcal/mol
ZnFe2O4 + H2SO4(l) = ZnSO4 (a) + Fe2O3 + H2O(l), ∆G° = −26.98 kcal/mol
3.5. Leaching of Cr, Pb, and Al
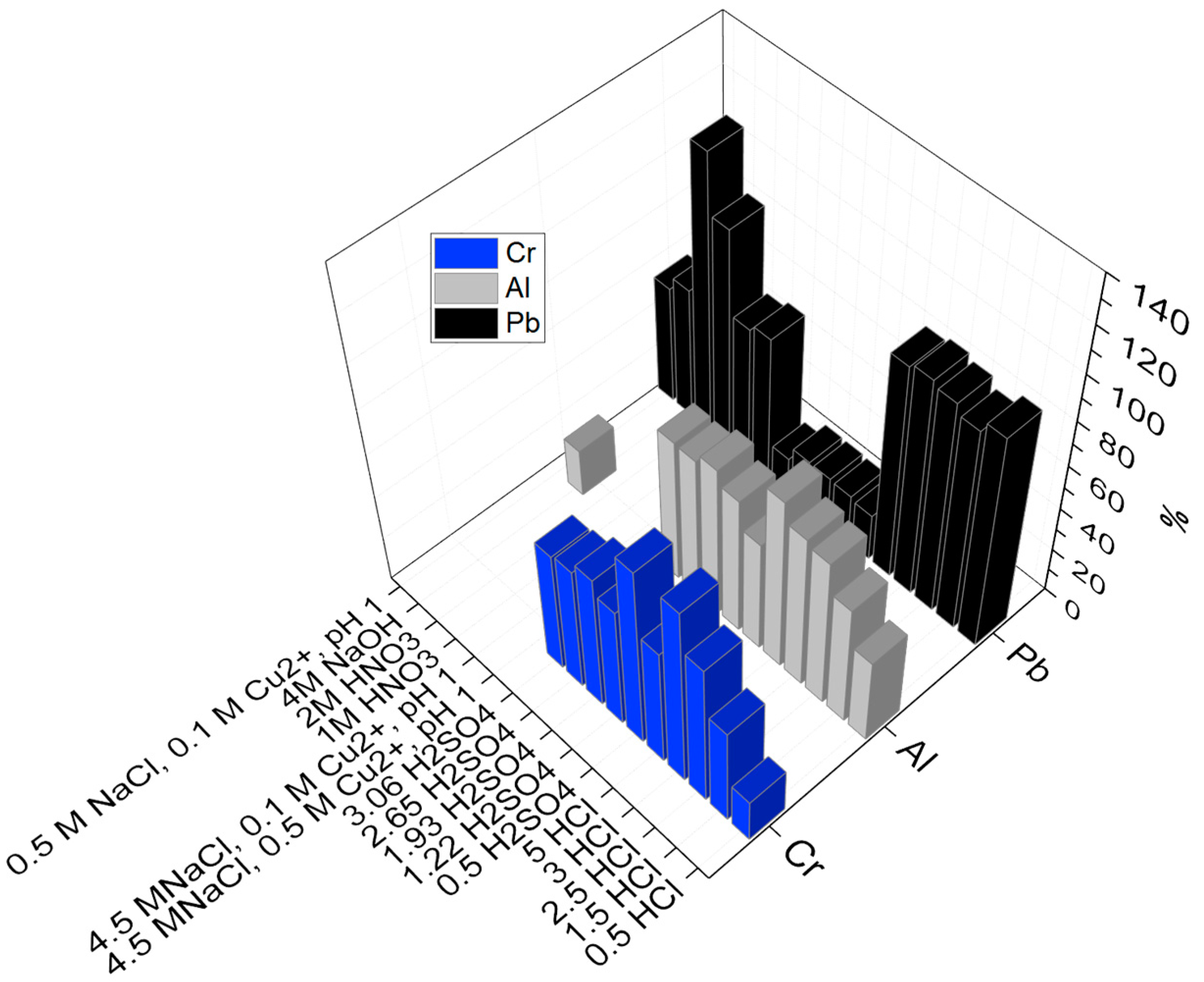
The dissolution of minor elements, such as Cr, Pb, and Al, was observed in the investigated leaching solutions. Figure 6 displays a complete 3D schematic representation of the elements of Al, Pb, and Cr and shows that generally aluminum had a high solubility into sulfuric acid and HCl media. Lead had high tendency towards HCl, NaOH, HNO3, and chloride leaching, but it was dissolved only slightly into sulfuric acid media, evidently due to the low solubility of lead sulfate.
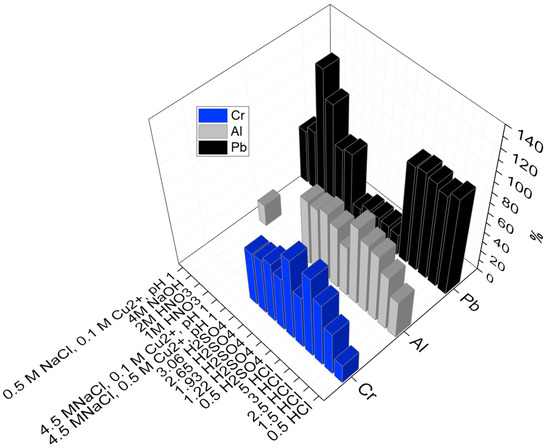
Figure 6.
3D plot displaying the yields of Al, Pb, and Cr obtained from the leaching in various lixiviants.
It was also observed from the above graph (Figure 6) that the maximum Cr yield was 83% in 3 M HCl. Furthermore, the yield of Cr decreased from 66 to 20% in presence of lixiviants in the following order: 2.5 M HCl > 1.93 M H2SO4 > 0.5 M H2SO4 > 2.65 M H2SO4 > 3.06 M H2SO4 > 1.22 M H2SO4 > 5 M HCl > 1.5 M HCl.
Highest aluminum extraction (>70%) was observed in 3 and 5 M HCl solutions. Additionally, all sulphuric acid solutions and HCl solutions ≥1.5 M resulted in higher than 50% leaching. Yield percentage of Pb in all the investigated HCl solutions was high (93–99%), as Pb dissolves forming chloride complexes PbCl+ and PbCl2 at low Cl− concentrations, and forms PbCl3− and PbCl42− at higher concentrations [43].
3.6. A Comparison of the Used Lixiviants
Table 5 shows a comparison of the best copper lixiviants and the selectivities of copper dissolution for nickel and iron. It can be seen that 4 M NaOH has the highest selectivity in copper extraction. In addition, it has the highest selectivity for Zn as well. The goal of mild, selective, and low temperature leaching of copper is best approached by the 0.5 M HCl media, which was able to dissolve all copper with only 10% nickel extraction, selectivity between copper and nickel concentrations in the solution being 10. Furthermore, 0.5 M HCl was the media showing the highest selectivity towards Fe (Cu/Fe = 1.9) without pH adjustment.

Table 5.
The best lixiviants selected for Cu, Fe, Pb, and Zn extraction.
The calculated composition of the residue after 0.5 M HCl leaching suggests the leach residue composition being 23 mg/g Ni, 106 mg /g Fe, 9 mg/g Zn, 2 mg/g Al, 1.9 mg/g Cu, 0.9 mg/g Cr, and 0.02 mg/g Pb. The advantage of 0.5 M HCl is that it could dissolve also almost all Pb (98%).
4. Conclusions
Along the principles of circular economy, the recovery of metals from industrial side streams, waste, and intermediate fractions is of increasing importance. In the current study, the leaching phenomena and selective leaching of copper was investigated from an intermediate raw material originating from base metal production, with a mineralogy of 52.2% NiFe2O4, 25.0% Fe2SiO4 (fayalite), 20.5% of Cu2O (cuprite), and 2.3% of metallic Cu. In the raw material, the large particles were shown to consist mainly of Cu2O and elemental Cu.
Copper present in the raw material was shown to be easily dissolvable, over 98% Cu could be dissolved with 0.5 M, 1.5 M HCl, and 4 M NaOH. In addition, 0.5 M HCl was shown to provide selectivity towards Ni, with the Cu/Ni concentration ratio in solution being 10. Alkaline leaching in 4 M NaOH resulted in the highest selectivity for copper leaching, with the ratio of dissolved elements of Cu/Ni = 340 and Cu/Zn = 51. Also 1 and 2 M HNO3 provided high selectivity for copper dissolution with a Cu/Ni ratio of 26 and 23, respectively. Aluminum showed high dissolution into sulfuric acid and hydrochloric acid media, the highest Al extraction being 79% in 5 M HCl whereas lead dissolution was strong in HCl, chloride, NaOH, and HNO3. The highest extraction for Ni was obtained in 5 M HCl.
The results indicate that from the 16 investigated leaching media, hydrochloric acid leaching (0.5 M HCl) presents the lowest concentration solution matrix for selective and high copper extraction, even at room temperature.
Acknowledgments
This study is a part of NewEco project of EIT Knowledge and Innovation Community Raw Materials consortium. The authors express their deep gratitude to Petri Latostenmaa from Boliden Harjavalta for providing the raw material. The authors also acknowledge Hannu Revitzer for performing the ICP and chemical analyses and Janne Vuori for performing the particle size analyses. Also METSEK project funded by Association of Finnish Steel and Metal Producers, and Raw MATERS Finland Infrastructure supported by Academy of Finland is greatly acknowledged.
Author Contributions
Mari Lundström and Pekka Taskinen conceived and designed the experiments; Lotta Rintala performed the experiments; Petteri Halli analyzed the data; Udit Surya Mohanty and Mari Lundström wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Avarmaa, K.; O’ Brien, H.; Johto, H.; Taskinen, P. Equilibrium distribution of precious metals between slag and copper matte at 1250–1350 °C. J. Sustain. Metall. 2015, 1, 216–228. [Google Scholar] [CrossRef]
- Avarmaa, K.; Johto, H.; Taskinen, P. Distribution of precious metals (Ag, Au, Pd, Pt, and Rh) between copper matte and iron silicate slag. Metall. Mater. Trans. B 2016, 47, 244–255. [Google Scholar] [CrossRef]
- Avarmaa, K.; O’Brien, H.; Taskinen, P. Equilibria of Gold and Silver between Molten Copper and FeOx-SiO2-Al2O3 Slag in WEEE Smelting at 1300 °C. In Advances in Molten Slags, Fluxes, and Salts: Proceedings of the 10th International Conference on Molten Slags, Fluxes and Salts; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 193–202. [Google Scholar]
- Jyrkonen, S.; Haavanlammi, K.; Luomala, M.; Karonen, J.; Suikkanen, P. Processing of PGM containing Ni/Cu bulk concentrates in a sustainable way by Outotec Direct Nickel Flash Smelting process. In Ni-Co 2013; Springer: Cham, Switzerland, 2013; pp. 325–334. [Google Scholar]
- Taskinen, P. Direct-to-blister smelting of copper concentrates: The slag fluxing chemistry. Miner. Process. Extr. Metall. 2011, 120, 240–246. [Google Scholar] [CrossRef]
- Taskinen, P.; Seppala, K.; Laulumaa, J.; Poijarvi, J. Oxygen pressure in the Outokumpu flash smelting furnace—Part 1: Copper flash smelting settler. Miner. Process. Extr. Metall. 2001, 110, 94–100. [Google Scholar] [CrossRef]
- Davenport, W.G.; King, M.J.; Schlesinger, M.E.; Biswas, A.K. Extractive Metallurgy of Copper, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2002; p. 452. [Google Scholar]
- Taskinen, P.; Dinsdale, A.; Gisby, J. Industrial slag chemistry: A case study of computational thermodynamics. Scand. J. Metall. 2005, 34, 100–107. [Google Scholar] [CrossRef]
- Mihailova, I.; Mehandjiev, D. Characterisation of fayalite from complexes. J. Chem. Technol. Metall. 2010, 45, 317–326. [Google Scholar]
- Deng, T.; Ling, Y.H. Chemical and mineralogical characterisations of a copper converter slag. Rare Met. 2002, 21, 175–178. [Google Scholar]
- Petkov, V.; Jones, P.T.; Boydens, E.; Blanpain, B.; Wollants, P. Chemical corrosion mechanisms of magnesia–chromite and chrome-free refractory bricks by copper metal and anode slag. J. Eur. Ceram. Soc. 2007, 27, 2433–2444. [Google Scholar] [CrossRef]
- Taskinen, P.; Kojo, I. Fluxing options in the direct-to-blister copper smelting. In Proceedings of the Molten 2009 Conference, Santiago, Chile, 18–21 January 2009; pp. 1140–1151. [Google Scholar]
- Li, Y.; Papangelakis, G.V.; Ilya, P. High pressure oxidative acid leaching of nickel smelter slag: Characterization of feed and residue. Hydrometallurgy 2009, 97, 185–193. [Google Scholar] [CrossRef]
- Altundogan, H.S.; Tumen, F. Metal recovery from copper converter slag by roasting with ferric sulphate. Hydrometallurgy 1997, 44, 261–267. [Google Scholar] [CrossRef]
- Sanchez, M.; Parada, F.; Parra, R.; Marquez, F.; Jara, R.; Carrasco, J.; Palcios, J. Management of copper pyrometallurgical slags: Giving additional value to copper mining industry. In Proceedings of the VII International Conference on Molten Slags Fluxes and Salts, Cape Town, South Africa, 25–28 January 2004; pp. 543–550. [Google Scholar]
- Geveci, A.; Topkaya, Y.; Gerceker, E. Recovery of Copper and zinc from copper converter flue dusts. In Proceedings of the 10th International Metallurgy and Material Congress, Istanbul, Turkey, 24–28 May 2000; pp. 59–68. [Google Scholar]
- Yıldız, K.; Alp, A.; Aydın, A.O. Utilization of copper refining slags by a pyro-hydrometallurgical method. In Proceedings of the 10th International Metallurgy and Material Congress, Istanbul, Turkey, 24–28 May 2000; pp. 127–132. [Google Scholar]
- Arslan, F.; Giray, K.; Onal, G.; Gurkan, V. Development of a Flowsheet for Recovering Copper and Tin from Copper Refining Slags. Eur. J. Miner. Process. Environ. Prot. 2002, 2, 94–102. [Google Scholar]
- Anand, S.; Das, R.P.; Jena, P.K. Reduction—Roasting and ferric chloride leaching of copper converter slag for extracting copper, nickel and cobalt. Hydrometallurgy 1981, 7, 243–252. [Google Scholar] [CrossRef]
- Sukla, L.B.; Panda, S.C.; Jean, P.K. Recovery of cobalt, nickel, and copper from converter slag through roasting with ammonium sulphate and sulfuric acid. Hydrometallurgy 1986, 16, 153–165. [Google Scholar] [CrossRef]
- Tumen, F.; Bailey, N.T. Recovery of metal values from copper smelter slags by roasting with pyrite. Hydrometallurgy 1990, 25, 317–328. [Google Scholar] [CrossRef]
- Herreros, O.; Quiroza, R.; Manzanob, E.; Bou, C.; Vinalsb, J. Copper extraction from reverberatory and flash furnace slags by chlorine leaching. Hydrometallurgy 1998, 49, 87–101. [Google Scholar] [CrossRef]
- Tumen, F. Metal recovery from secondary copper slag by roasting with ammonium sulphate. Turkish J. Eng. Environ. Sci. 1994, 18, 1–5. [Google Scholar]
- Anand, S.; Rao, K.S.; Jena, P.K. Pressure leaching of copper converter slag using dilute sulphuric acid for the extraction of cobalt, nickel and copper values. Hydrometallurgy 1983, 10, 305–312. [Google Scholar] [CrossRef]
- Gbor, P.K.; Ahmed, I.B.; Jia, C.Q. Behaviour of Co and Ni during aqueous sulphur dioxide leaching of nickel slag. Hydrometallurgy 2000, 57, 13–22. [Google Scholar] [CrossRef]
- Niemela, A.; Pitkaaho, S.; Ojala, S.; Keiski, R.L.; Peramaki, P. Microwave-assisted aqua regia digestion for determining platinum, palladium, rhodium and lead in catalyst materials. Microchem. J. 2012, 101, 75–79. [Google Scholar] [CrossRef]
- Young, R.A. The Rietveld Method; Oxford University Press: Oxford, UK, 1995; 308p, ISBN 9780198559122. [Google Scholar]
- Cardellicchio, N.; Buccolieri, A.; Di Leo, A.; Spada, L. Heavy metals in marine sediments from the MarPiccolo of Taranto (Ionian Sea, Southern Italy). Ann. Chim. 2006, 96, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Cardellicchio, N.; Buccolieri, A.; Di Leo, A.; Librando, V.; Minniti, Z.; Spada, L. Methodological approach for metal pollution evaluation in sediments collected from the Tarnto Gulf. Toxicol. Environ. Chem. 2009, 91, 1273–1290. [Google Scholar] [CrossRef]
- Lundstrom, M.; Liipo, J.; Karonen, J.; Aromaa, J. Dissolution of six sulfide concentrates in the hydrocopper environment. In Proceedings of the South African Institute of Mining and Metallurgy Base Metals Conference, Kasane, Botswana, 27–31 July 2009; pp. 127–138. [Google Scholar]
- Habbache, N.; Alane, N.; Djerad, S.; Tifouti, L. Leaching of copper oxide with different acid solutions. Chem. Eng. J. 2009, 152, 503–508. [Google Scholar]
- Pacović, N.V. Hydrometallurgy; ŠRIF: Bor, Serbia, 1980. Chapter 3. (In Serbian) [Google Scholar]
- Carneiro, M.F.C.; Leao, V.A. The role of sodium chloride on surface properties of chalcopyrite leached with ferric sulphate. Hydrometallurgy 2007, 87, 73–82. [Google Scholar] [CrossRef]
- Garrels, R.M.; Thompson, M.E. Oxidation of pyrite by iron sulfate solutions. Am. J. Sci. 1960, 258, 57–67. [Google Scholar]
- Roine, A. Sustainable Process Technology and Engineering, A Manual on HSC program, Continuous Research & Development. Outotec Research Centre: Finland, 8 March 2017. [Google Scholar]
- Lee, M.S.; Nam, S.H. Chemical Equilibria of Nickel chloride in HCl solution at 25 °C. Bull. Korean Chem. Soc. 2009, 30, 2203–2207. [Google Scholar]
- Ashurst, K.G. The Thermodynamics of the formation of chlorocomplexes of iron (III), cobalt (II), iron (II), manganese (II) in perchlorate medium. Nat. Inst. Metall. 1976, 1820, 1–43. [Google Scholar]
- Peek, E.M.; Van Weert, G. Chloride Metallurgy. In Proceedings of the 32nd Annual Hydrometallurgy Meeting and International Conference of the Practice and Theory of Chloride/Metal Interaction, Montréal, QC, Canada, 19–23 October 2002; pp. 760–780. [Google Scholar]
- Misawa, T. The thermodynamic consideration for Fe-H2O system at 25 °C. Corros. Sci. 1973, 13, 659–676. [Google Scholar] [CrossRef]
- Langova, S.; Lesko, J.; Matysek, D. Selective leaching of zinc from zinc ferrite with hydrochloric acid. Hydrometallurgy 2009, 95, 179–182. [Google Scholar] [CrossRef]
- Nunez, C.; Vinals, J. Kinetics of leaching of zinc ferrite in aqueous hydrochloric acid solutions. Metall. Mater. Trans. B 1984, 15, 221–228. [Google Scholar] [CrossRef]
- Sato, T.; Nakamura, T. The stability constants of the aqueous chloro complexes of divalent zinc, cadmium and mercury determined by solvent extraction with tri-n octyl phosphine oxide. Hydrometallurgy 1980, 6, 3–12. [Google Scholar] [CrossRef]
- Winand, R. Chloride hydrometallurgy. Hydrometallurgy 1991, 27, 285–316. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).