Microstructural Evolution during Pressureless Sintering of Blended Elemental Ti-Al-V-Fe Titanium Alloys from Fine Hydrogenated-Dehydrogenated Titanium Powder
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials
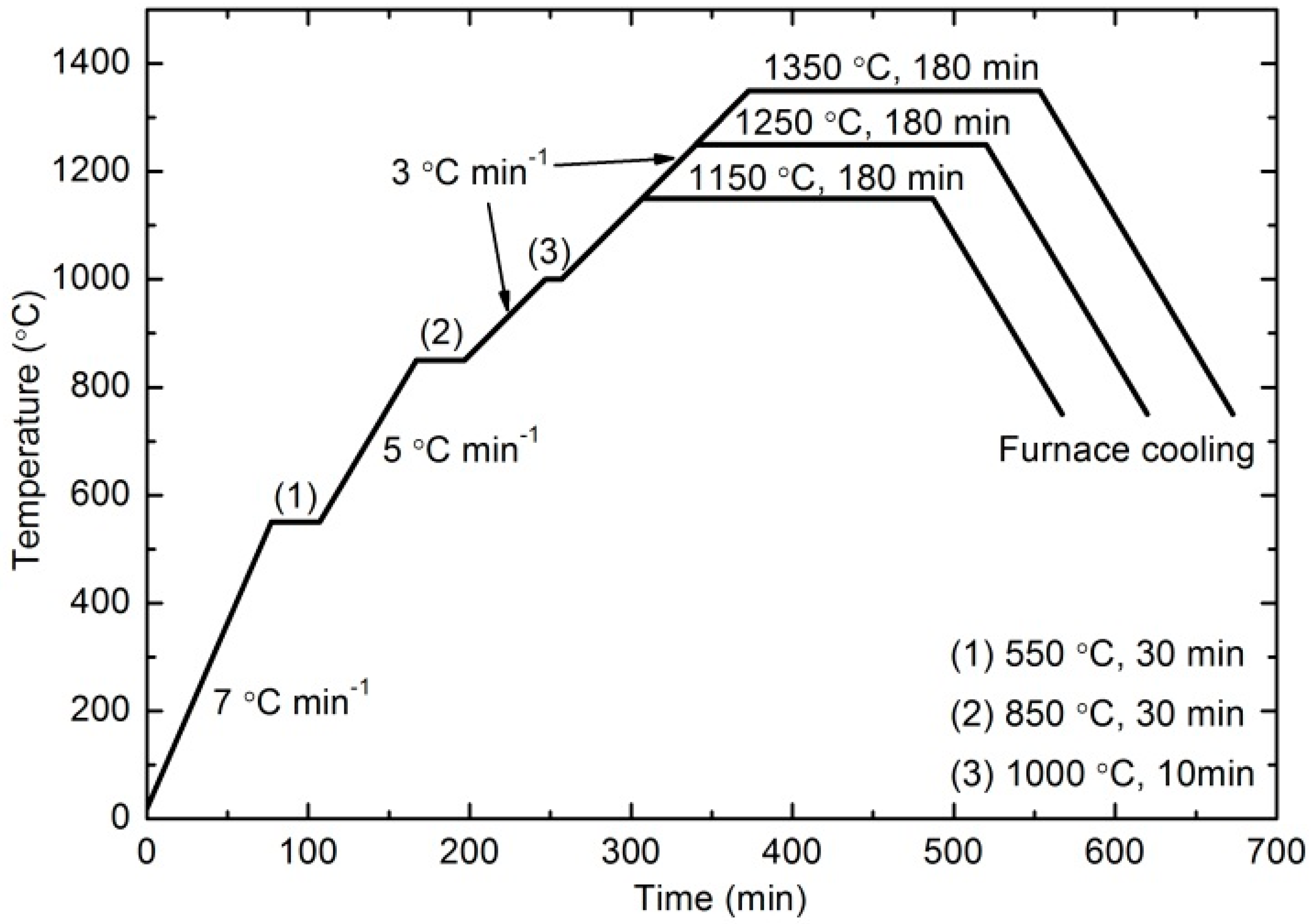
2.2. Press-and-Sinter
2.3. Characterization and Mechanical Testing
3. Results
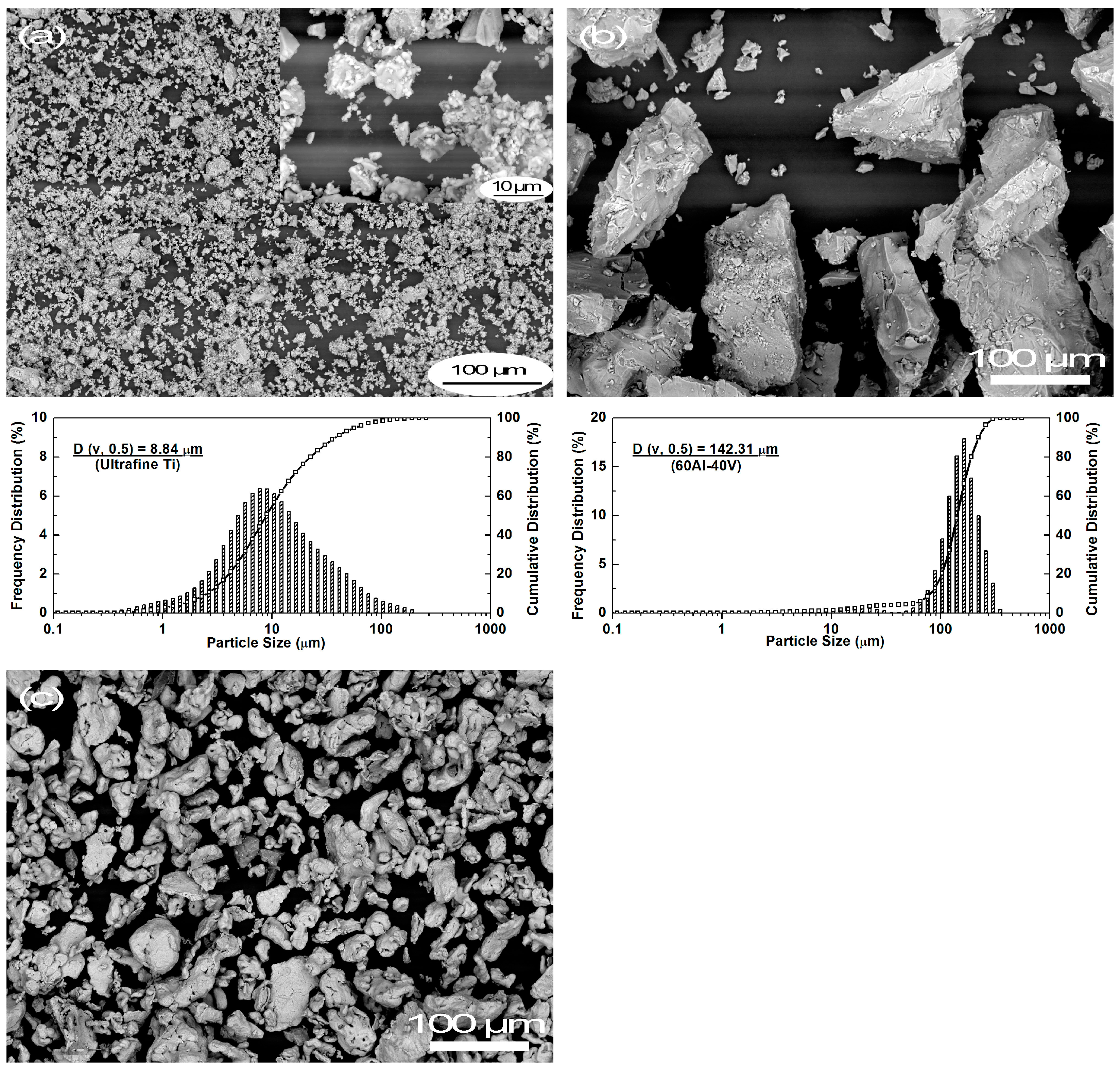
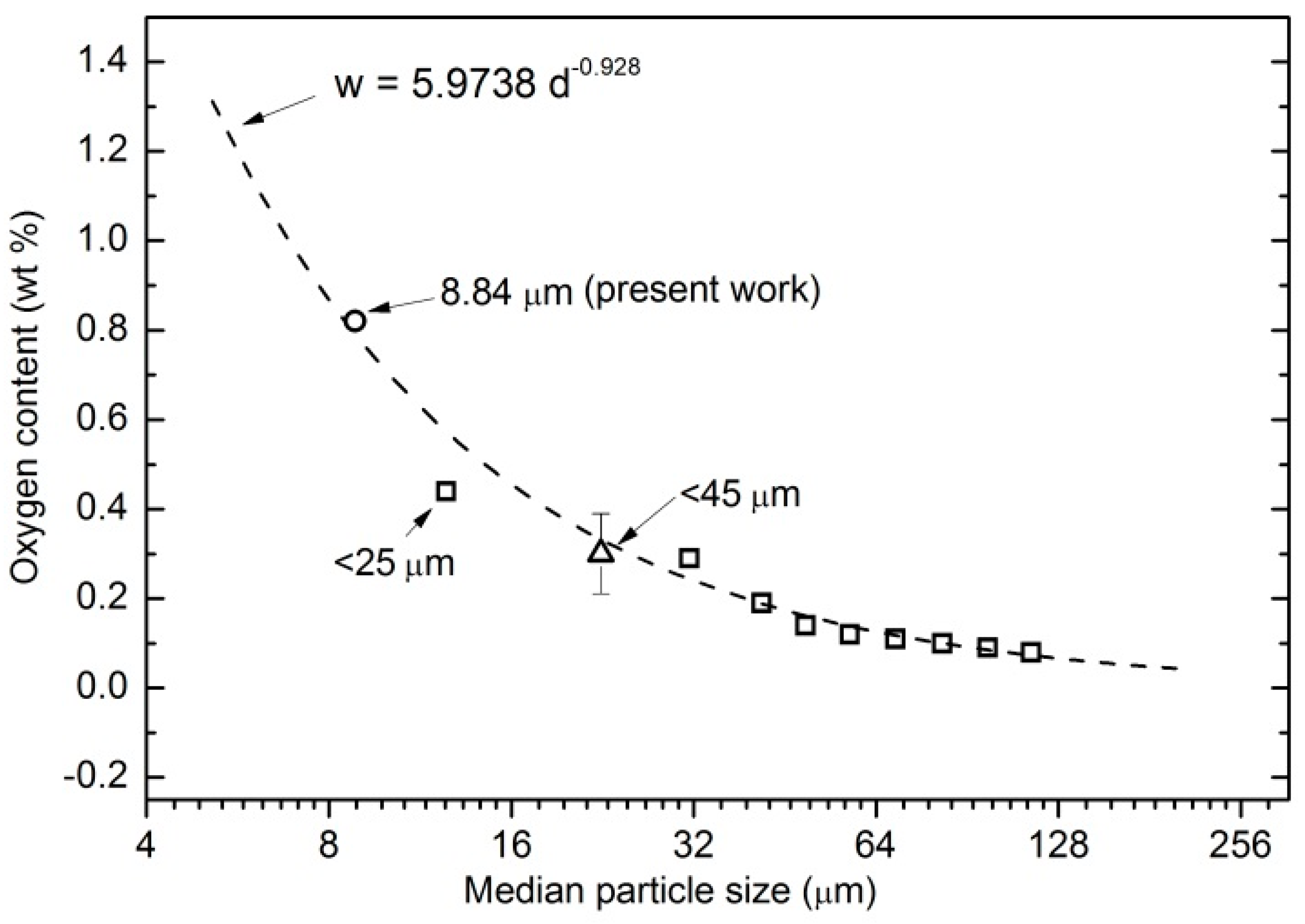
3.1. Characteristics of As-Received Powders
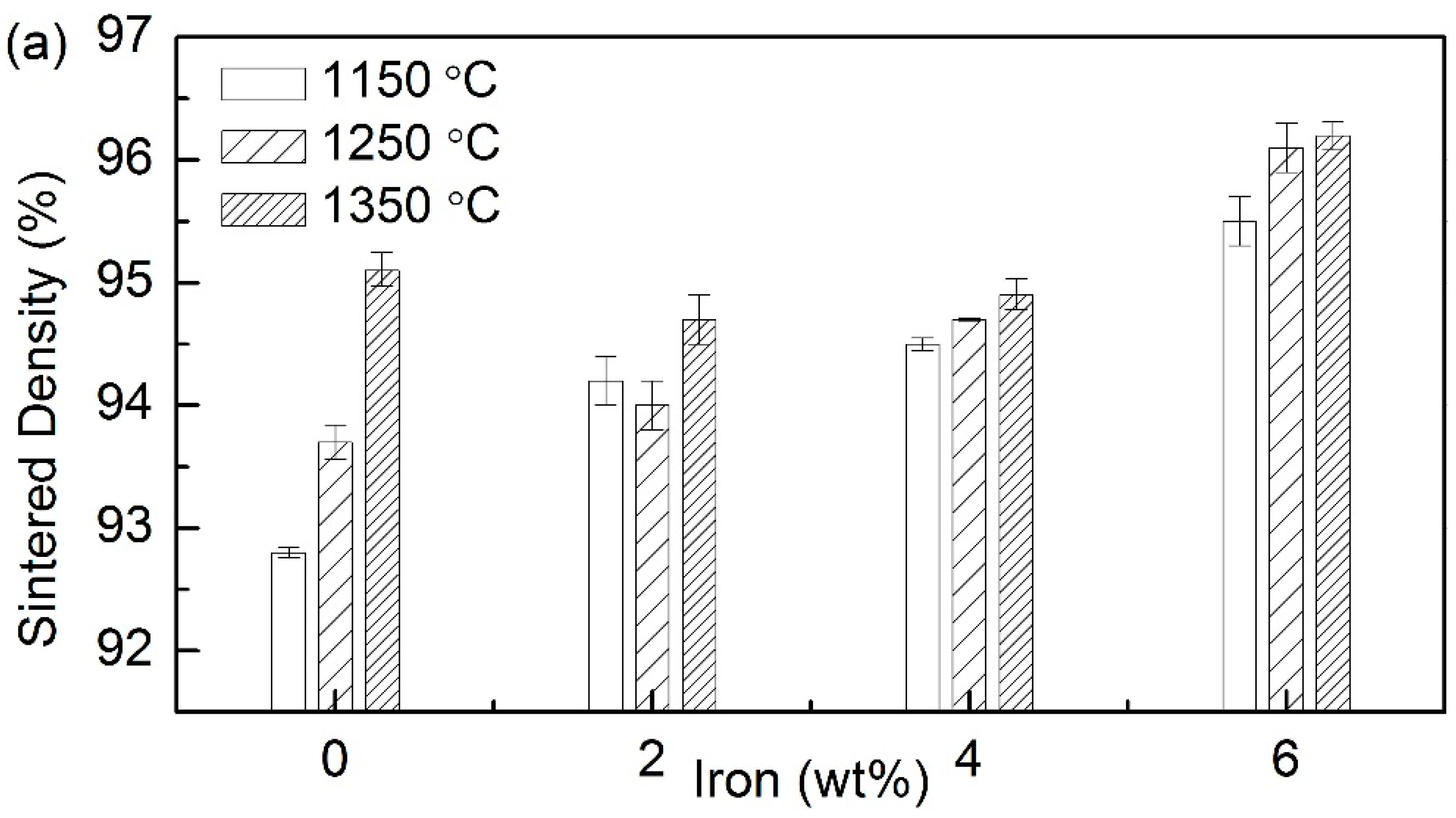
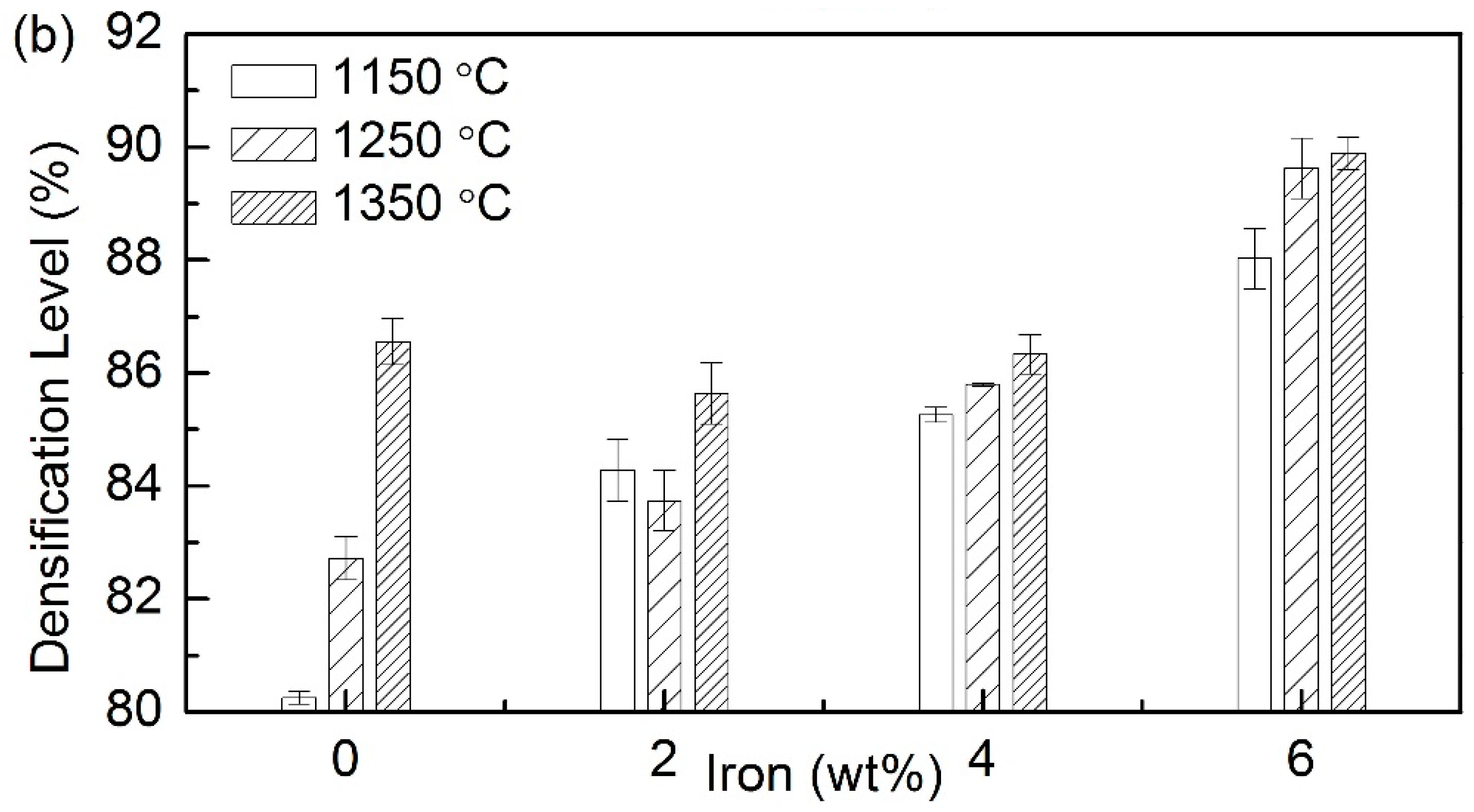
3.2. Densification
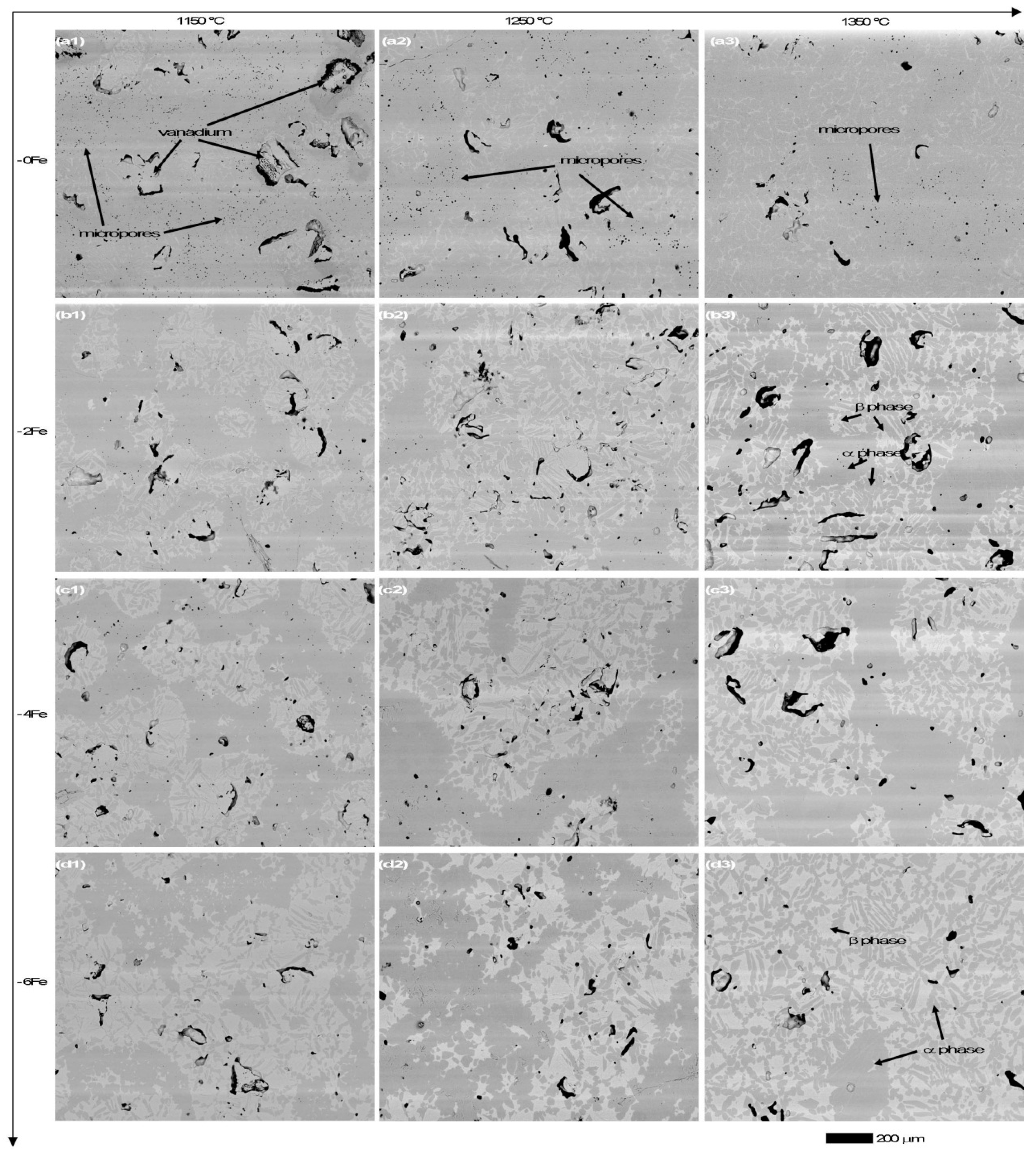
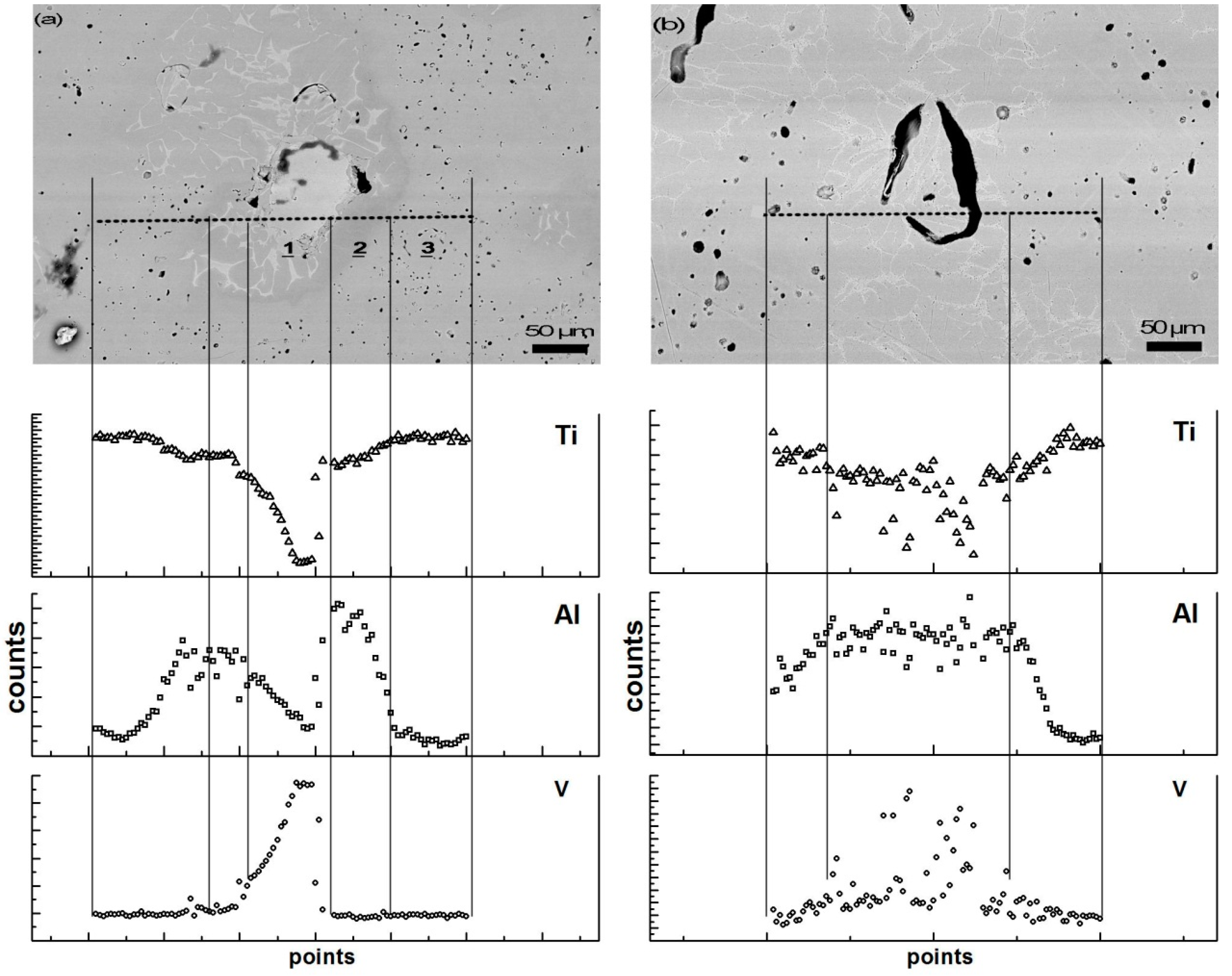
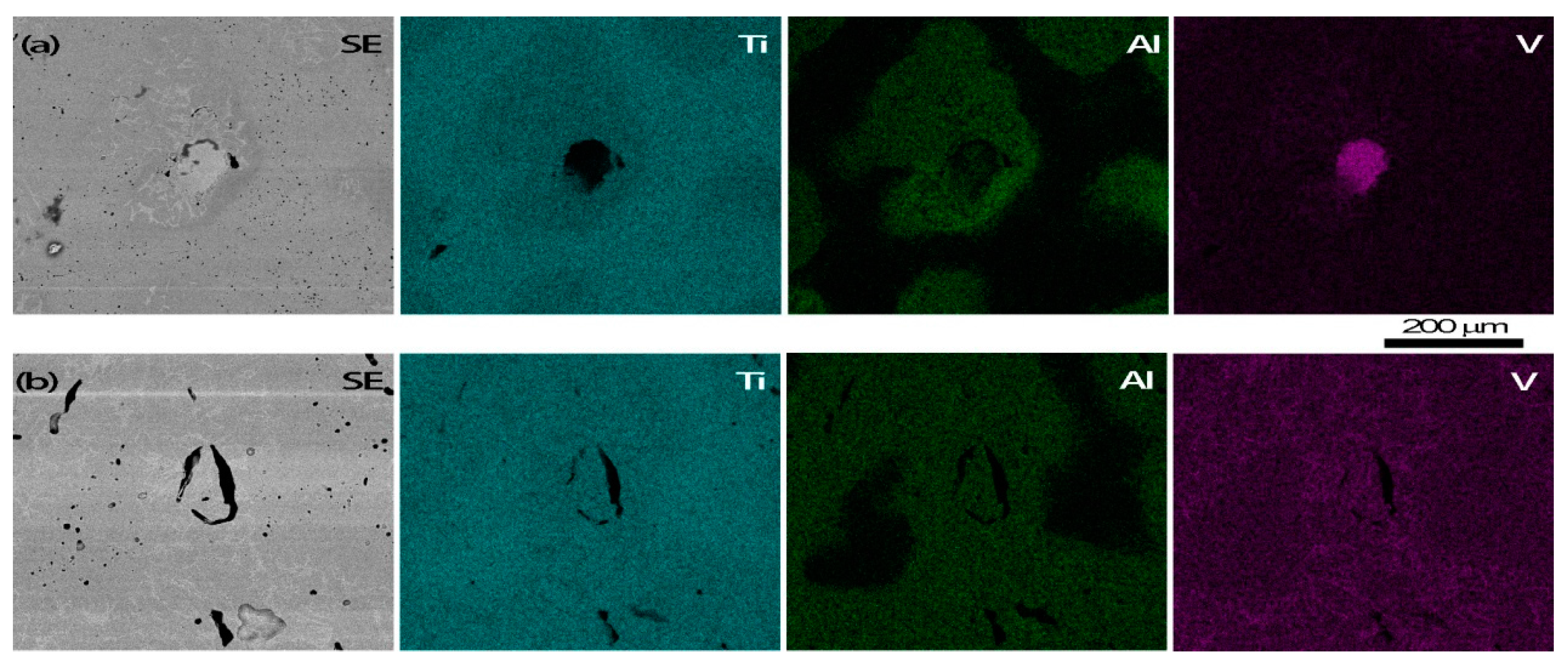
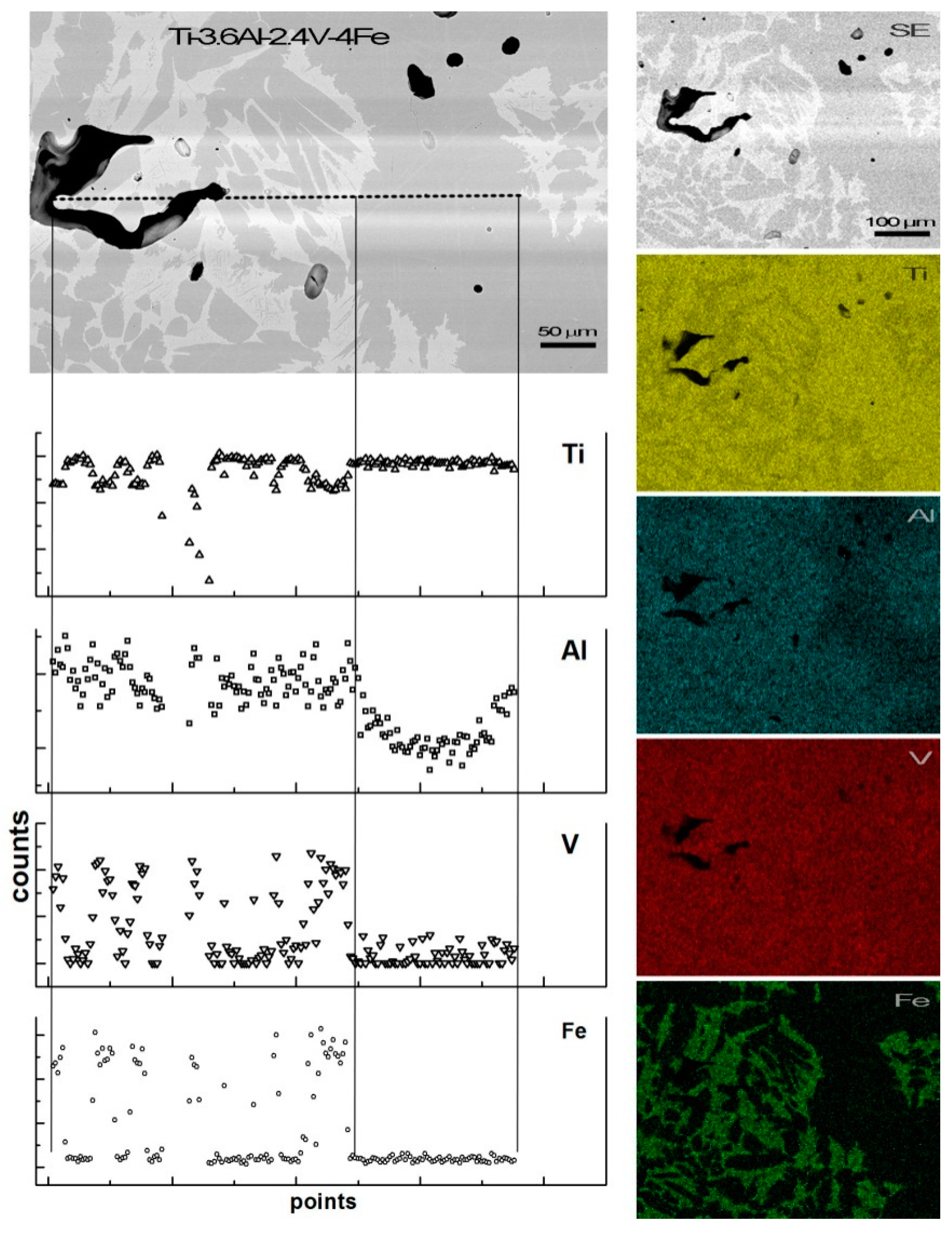
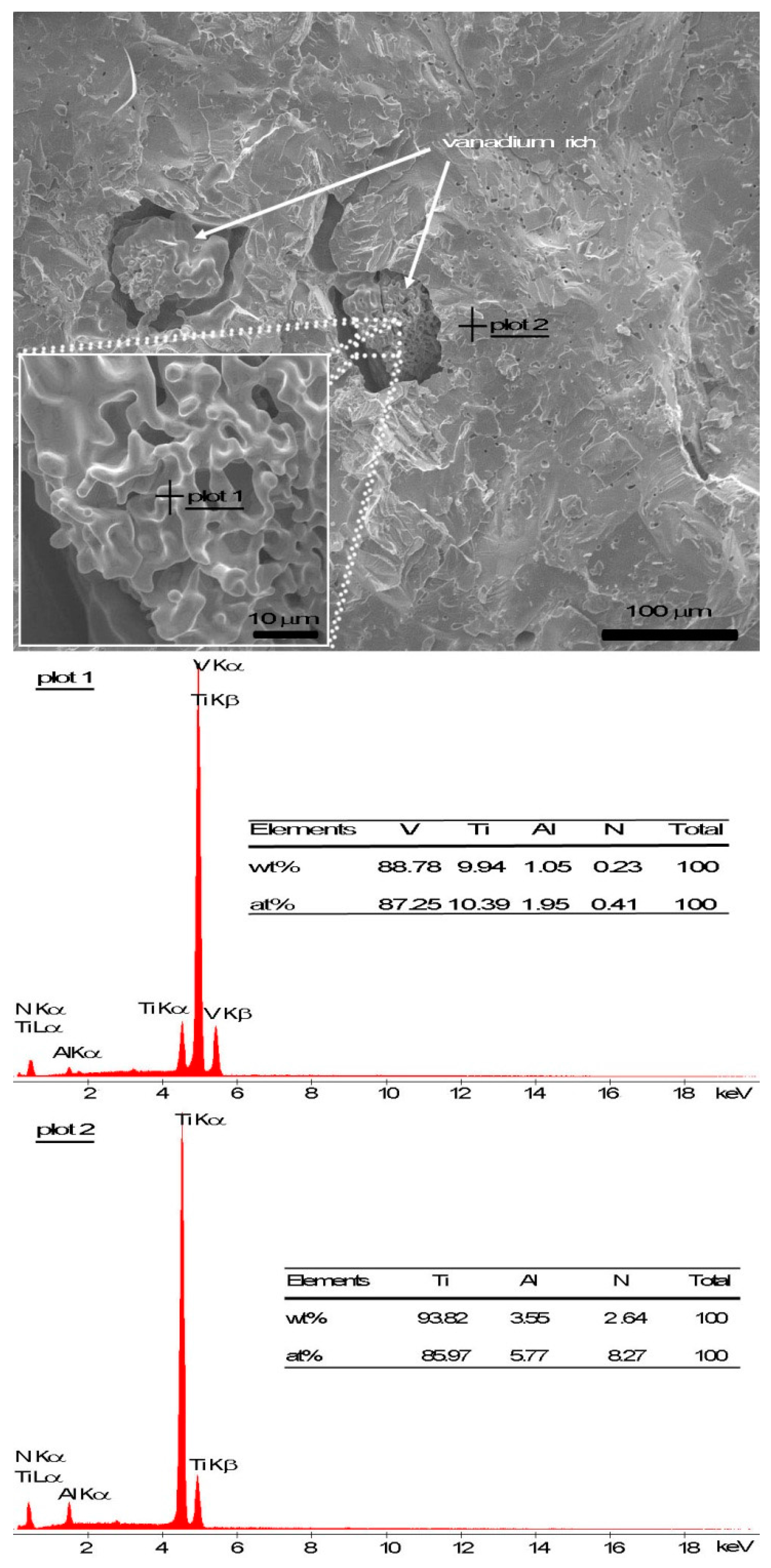
3.3. Microstructure Observation and Compositional Analysis
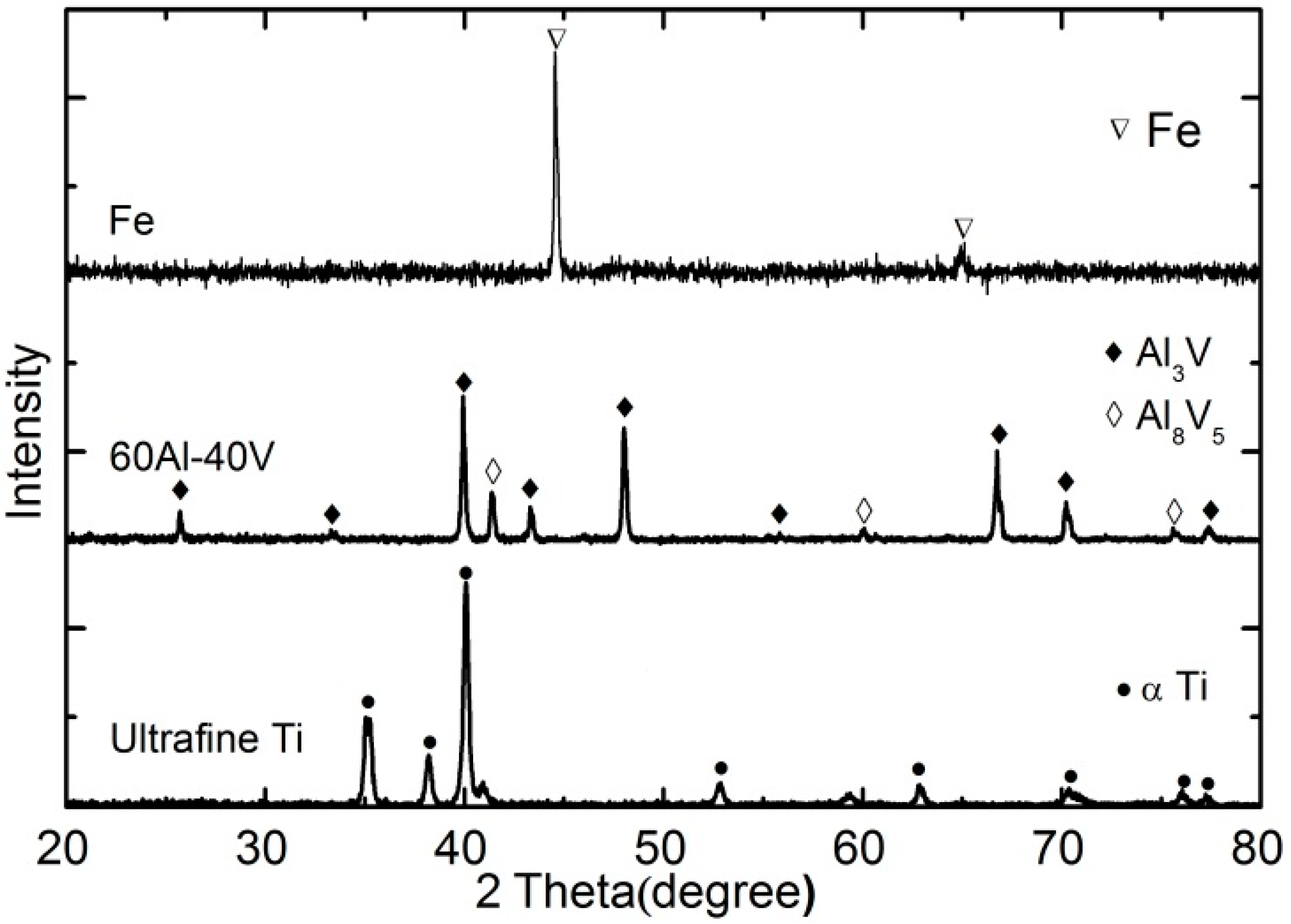
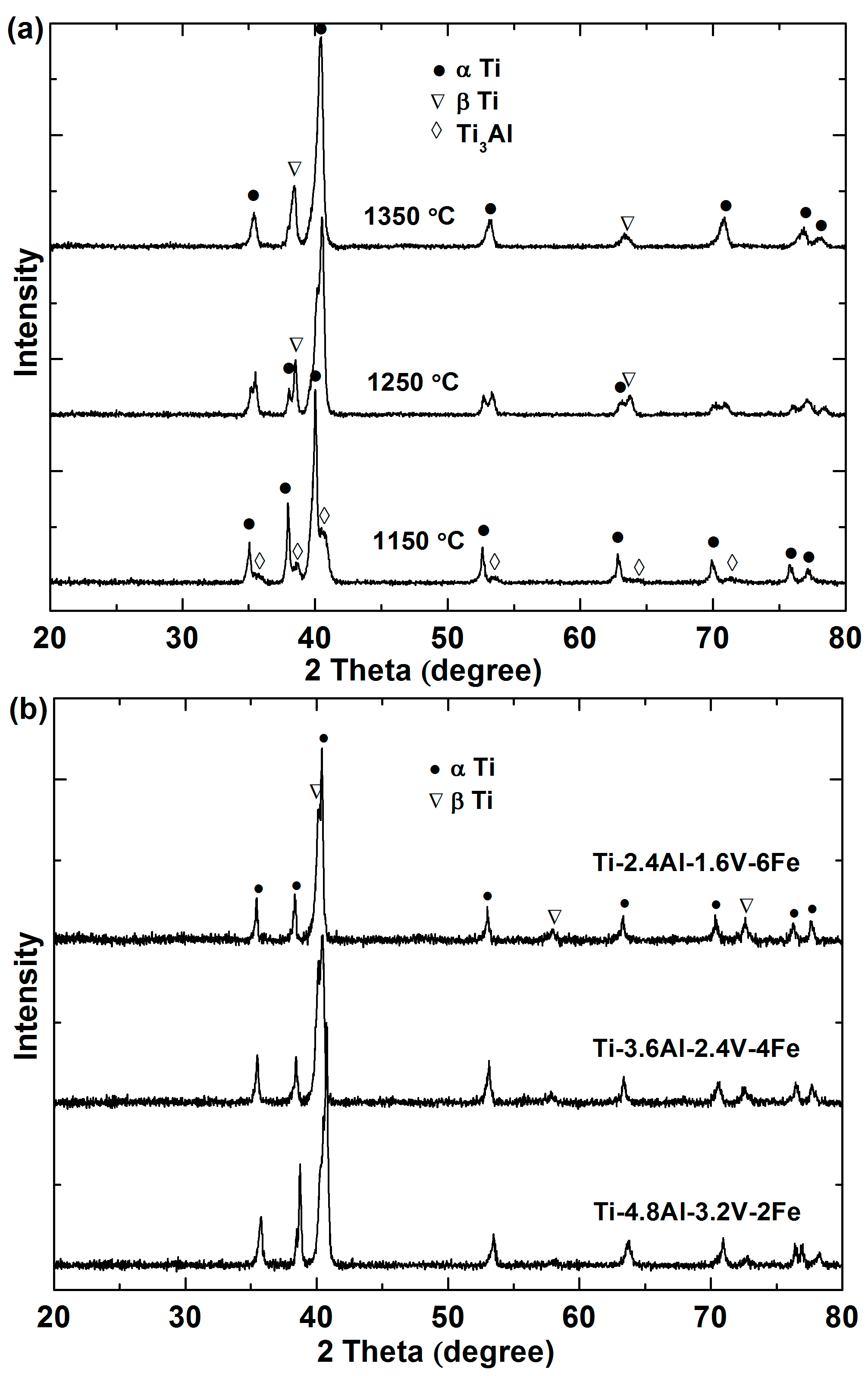
3.4. Phase Determination
4. Discussion
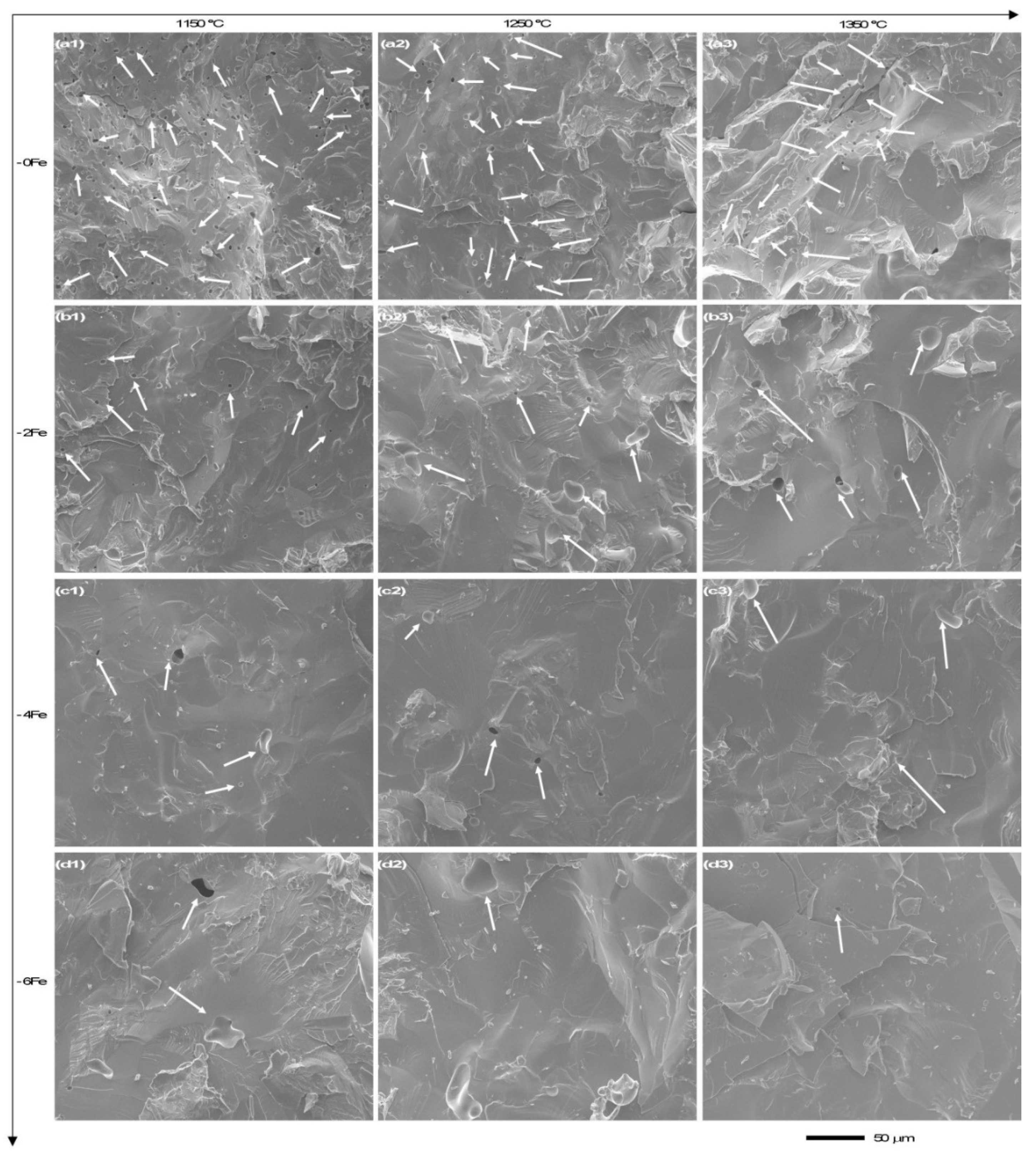
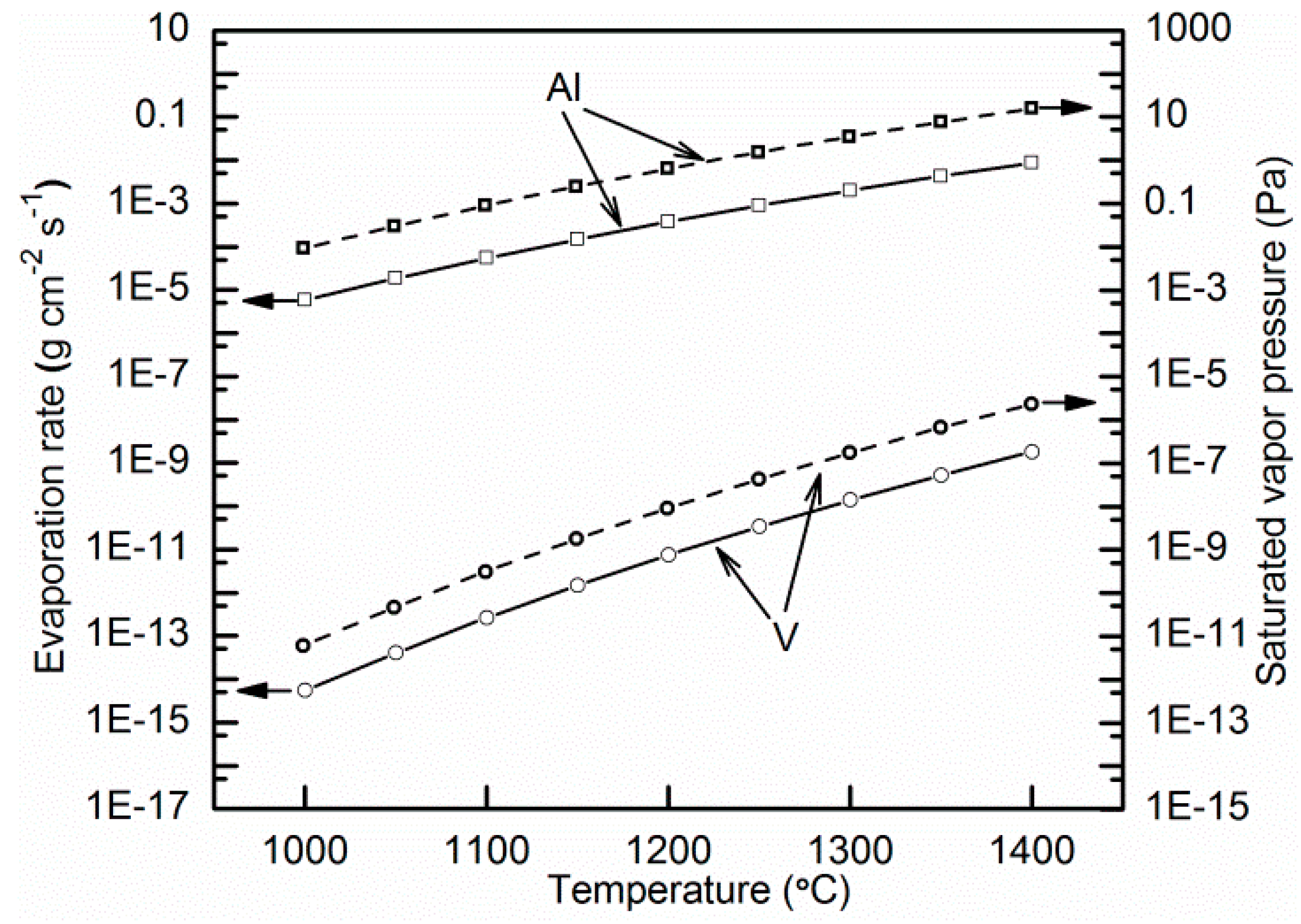
4.1. Microporosity Formation
4.2. Macroporosity Formation
4.3. Microstructural Evaluation and Phase Transformation
4.4. Mechanical Properties
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- German, R.M. Powder Metallurgy and Particulate Materials Processing; Metal Powder Industries Federation: Princeton, NJ, USA, 2005. [Google Scholar]
- Robertson, I.M.; Schaffer, G.B. Some effects of particle size on the sintering of titanium and a master sintering curve model. Metall. Mater. Trans. A 2009, 40, 1968–1979. [Google Scholar] [CrossRef]
- Luo, S.D.; Yan, M.; Schaffer, G.B.; Qian, M. Sintering of Titanium in Vacuum by Microwave Radiation. Metall. Mater. Trans. A 2011, 42, 2466–2474. [Google Scholar] [CrossRef]
- Robertson, I.M.; Schaffer, G.B. Review of densification of titanium based powder systems in press and sinter processing. Powder Metall. 2010, 53, 146–162. [Google Scholar] [CrossRef]
- Qian, M. Cold compaction and sintering of titanium and its alloys for near-net-shape or preform fabrication. Int. J. Powder Metall. 2010, 46, 29–44. [Google Scholar]
- He, W.; Weng, Q.G.; He, Y.H.; Jiang, Y. Preparation of ultrafine Ti powder by inhibitor coated/HDH combined method. Powder Metall. 2013, 56, 239–244. [Google Scholar] [CrossRef]
- Wang, H.T.; Fang, Z.Z.; Sun, P. A critical review of mechanical properties of powder metallurgy titanium. Int. J. Powder Metall. 2010, 46, 45–57. [Google Scholar]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Ivasishin, O.M.; Savvakin, D.G.; Froes, F.; Mokson, V.C.; Bondareva, K.A. Synthesis of alloy Ti-6Al-4V with low residual porosity by a powder metallurgy method. Powder Metall. Met. Ceram. 2002, 41, 382–390. [Google Scholar] [CrossRef]
- Peter, W.; Chen, W.; Yamamoto, Y.; Dehoff, R.; Muth, T.; Nunn, S.; Kiggans, J.; Clark, M.; Sabau, A.; Gorti, S.; et al. Current Status of Ti PM: Progress, Opportunities and Challenges. Key Eng. Mater. 2012, 520, 1–7. [Google Scholar] [CrossRef]
- Bolzoni, L.; Ruiz-Navas, E.M.; Gordo, E. Understanding the properties of low-cost iron-containing powder metallurgy titanium alloys. Mater. Des. 2016, 110, 317–323. [Google Scholar] [CrossRef]
- Bolzoni, L.; Ruiz-Navas, E.M.; Gordo, E. Quantifying the properties of low-cost powder metallurgy titanium alloys. Mater. Sci. Eng. A 2017, 687, 47–53. [Google Scholar] [CrossRef]
- Wei, W.; Liu, Y.; Zhou, K.; Huang, B. Effect of Fe addition on sintering behaviour of titanium powder. Powder Metall. 2003, 46, 246–250. [Google Scholar] [CrossRef]
- Savvakin, D.G.; Carman, A.; Ivasishin, O.M.; Matviychuk, M.V.; Gazder, A.A.; Pereloma, E.V. Effect of Iron Content on Sintering Behavior of Ti-V-Fe-Al Near-beta Titanium Alloy. Metall. Mater. Trans. A 2012, 43, 716–723. [Google Scholar] [CrossRef]
- Qian, M.; Yang, Y.F.; Yan, M.; Luo, S.D. Design of low cost high performance powder metallurgy titanium alloys: Some basic considerations. Key Eng. Mater. 2012, 520, 24–29. [Google Scholar] [CrossRef]
- Esteban, P.G.; Ruiz-Navas, E.M.; Bolzoni, L.; Gordo, E. Low-cost titanium alloys? Iron may hold the answers. Met. Powder Rep. 2008, 63, 24–27. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.F.; Tang, H.P.; Liu, C.T.; Liu, B.; Huang, B.Y. Design of powder metallurgy titanium alloys and composites. Mater. Sci. Eng. A 2006, 418, 25–35. [Google Scholar] [CrossRef]
- Carman, A.; Zhang, L.C.; Ivasishin, O.M.; Savvakin, D.G.; Matviychuk, M.V.; Pereloma, E.V. Role of alloying elements in microstructure evolution and alloying elements behaviour during sintering of a near-beta titanium alloy. Mater. Sci. Eng. A 2011, 528, 1686–1693. [Google Scholar] [CrossRef]
- Yang, Y.F.; Luo, S.D.; Schaffer, G.B.; Qian, M. Sintering of Ti-10V-2Fe-3Al and mechanical properties. Mater. Sci. Eng. A 2011, 528, 6719–6726. [Google Scholar] [CrossRef]
- Esteban, P.G.; Ruiz-Navas, E.M.; Gordo, E. Influence of Fe content and particle size the on the processing and mechanical properties of low-cost Ti-xFe alloys. Mater. Sci. Eng. A 2010, 527, 5664–5669. [Google Scholar] [CrossRef]
- Chen, B.Y.; Hwang, K.S.; Ng, K.L. Effect of cooling process on the alpha phase formation and mechanical properties of sintered Ti-Fe alloys. Mater. Sci. Eng. A 2011, 528, 4556–4563. [Google Scholar] [CrossRef]
- Yang, Y.F.; Luo, S.D.; Schaffer, G.B.; Qian, M. The Sintering, Sintered Microstructure and Mechanical Properties of Ti-Fe-Si Alloys. Metall. Mater. Trans. A 2012, 43A, 4896–4906. [Google Scholar] [CrossRef]
- Chen, G.; Cao, P.; He, Y.H.; Shen, P.Z.; Gao, H.Y. Effect of aluminium evaporation loss on pore characteristics of porous FeAl alloys produced by vacuum sintering. J. Mater. Sci. 2012, 47, 1244–1250. [Google Scholar] [CrossRef]
- Kang, K.S.; Kim, C.H.; Cho, W.C.; Bae, K.K.; Woo, S.W.; Park, C.S. Reduction characteristics of CuFe2O4 and Fe3O4 by methane; CuFe2O4 as an oxidant for two-step thermochemical methane reforming. Int. J. Hydrogen Energy 2008, 33, 4560–4568. [Google Scholar] [CrossRef]
- Haase, C.; Lapovok, R.; Ng, H.P.; Estrin, Y. Production of Ti-6Al-4V billet through compaction of blended elemental powders by equal-channel angular pressing. Mater. Sci. Eng. A 2012, 550, 263–272. [Google Scholar] [CrossRef]
- Bolzoni, L.; Esteban, P.G.; Ruiz-Navas, E.M.; Gordo, E. Mechanical behaviour of pressed and sintered titanium alloys obtained from prealloyed and blended elemental powders. J. Mech. Behav. Biomed. Mater. 2012, 14, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Tuijn, C. Self-Diffusion and Impurity Diffusion in Pure Metals: Handbook of Experimental Data; Elservier: Amsterdam, The Netherlands, 2008; Volume 14, pp. 1–349. [Google Scholar]
- Yang, Y.F.; Luo, S.D.; Bettles, C.J.; Schaffer, G.B.; Qian, M. The effect of Si additions on the sintering and sintered microstructure and mechanical properties of Ti-3Ni alloy. Mater. Sci. Eng. A 2011, 528, 7381–7387. [Google Scholar] [CrossRef]
- Su, Y.Q.; Guo, J.J.; Jia, J.; Liu, G.Z.; Liu, Y.A. Composition control of a TiAl melt during the induction skull melting (ISM) process. J. Alloys Compd. 2002, 334, 261–266. [Google Scholar]
- Guo, J.J.; Liu, Y.; Su, Y.Q.; Ding, H.S.; Liu, G.Z.; Jia, J. Evaporation behavior of aluminum during the cold crucible induction skull melting of titanium aluminum alloys. Metall. Mater. Trans. B 2000, 31B, 837–844. [Google Scholar] [CrossRef]
- Xu, Z.; Hodgson, M.A.; Chang, K.; Chen, G.; Yuan, X.; Cao, P. Effect of Sintering Time on the Densification, Microstructure, Weight Loss and Tensile Properties of a Powder Metallurgical Fe-Mn-Si Alloy. Metals 2017, 7, 81. [Google Scholar] [CrossRef]
- Langmuir, I. The Vapor Pressure of Metallic Tungsten. Phys. Rev. 1913, 2, 329–342. [Google Scholar] [CrossRef]
- Alcock, C.B. Vapor pressure of the metallic elements—Equations. In CRC Handbook of Chemistry and Physics (Internet Version 2013), 93rd ed.; Haynes, W.M., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 125–126. [Google Scholar]
- Porter, D.A.; Easterling, K.E.; Sherif, M.Y. Phase Transformations in Metals and Alloys, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Miedema, A.R.; de Chatel, P.F.; de Boer, F.R. Cohesion in alloys—Fundamentals of a semi-empirical model. Physica B 1980, 100, 1–28. [Google Scholar] [CrossRef]
- Mishin, Y.; Herzig, C. Diffusion in the Ti-Al system. Acta Mater. 2000, 48, 589–623. [Google Scholar] [CrossRef]
- McCracken, C. Production of Fine Titanium Powders via the Hydrid-Dehydride (HDH) Process. PIM Int. 2008, 2, 55–57. [Google Scholar]
- Massalski, T.D. Binary Alloys Phase Diagams; Okamoto, H., Subramanian, P.R., Kasprzak, L., Eds.; ASM International: Geauga County, OH, USA, 1990. [Google Scholar]
- Yan, M.; Liu, Y.; Liu, Y.B.; Kong, C.; Schaffer, G.B.; Qian, M. Simultaneous gettering of oxygen and chlorine and homogenization of the beta phase by rare earth hydride additions to a powder metallurgy Ti-2.25Mo-1.5Fe alloy. Scr. Mater. 2012, 67, 491–494. [Google Scholar] [CrossRef]
- Luo, S.D.; Yang, Y.F.; Schaffer, G.B.; Qian, M. The effect of a small addition of boron on the sintering densification, microstructure and mechanical properties of powder metallurgy Ti-7Ni alloy. J. Alloys Compd. 2013, 555, 339–346. [Google Scholar] [CrossRef]
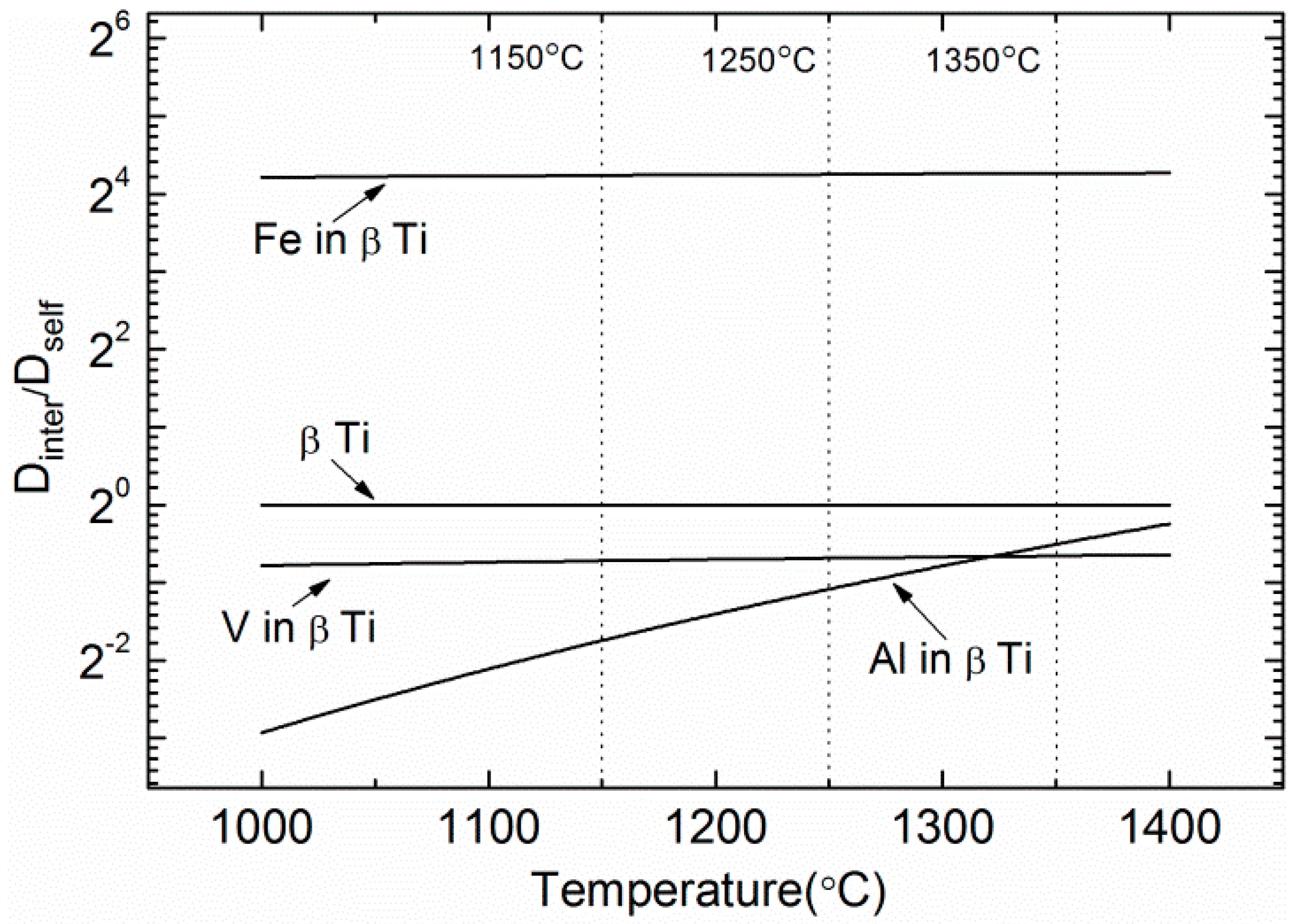
| Specimen | Diffusion Type | Temperature (°C) | D0 (m2·s−1) | Q (kJ·mol−1) |
|---|---|---|---|---|
| β-Ti | Self diffusion | 899–1540 | 3.58 × 10−8 | 130.6 |
| Al in β-Ti | Inter-diffusion | 920–1600 | 1.14 × 10−5 | 213.1 |
| V in β-Ti | Inter-diffusion | 902–1543 | 3.1 × 10−8 | 134.8 |
| Fe in β-Ti | Inter-diffusion | 969–1645 | 7.8 × 10−7 | 132.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Cao, P.; Jones, M.I. Microstructural Evolution during Pressureless Sintering of Blended Elemental Ti-Al-V-Fe Titanium Alloys from Fine Hydrogenated-Dehydrogenated Titanium Powder. Metals 2017, 7, 285. https://doi.org/10.3390/met7080285
Yu C, Cao P, Jones MI. Microstructural Evolution during Pressureless Sintering of Blended Elemental Ti-Al-V-Fe Titanium Alloys from Fine Hydrogenated-Dehydrogenated Titanium Powder. Metals. 2017; 7(8):285. https://doi.org/10.3390/met7080285
Chicago/Turabian StyleYu, Changzhou, Peng Cao, and Mark Ian Jones. 2017. "Microstructural Evolution during Pressureless Sintering of Blended Elemental Ti-Al-V-Fe Titanium Alloys from Fine Hydrogenated-Dehydrogenated Titanium Powder" Metals 7, no. 8: 285. https://doi.org/10.3390/met7080285
APA StyleYu, C., Cao, P., & Jones, M. I. (2017). Microstructural Evolution during Pressureless Sintering of Blended Elemental Ti-Al-V-Fe Titanium Alloys from Fine Hydrogenated-Dehydrogenated Titanium Powder. Metals, 7(8), 285. https://doi.org/10.3390/met7080285






