Effect of Deep Cryogenic Treatment on the Microstructure and the Corrosion Resistance of AZ61 Magnesium Alloy Welded Joint
Abstract
:1. Introduction
2. Materials and Processes
2.1. Test Materials
2.2. Welding Process
2.3. Cryogenic Treatment Process
3. Results and Analysis
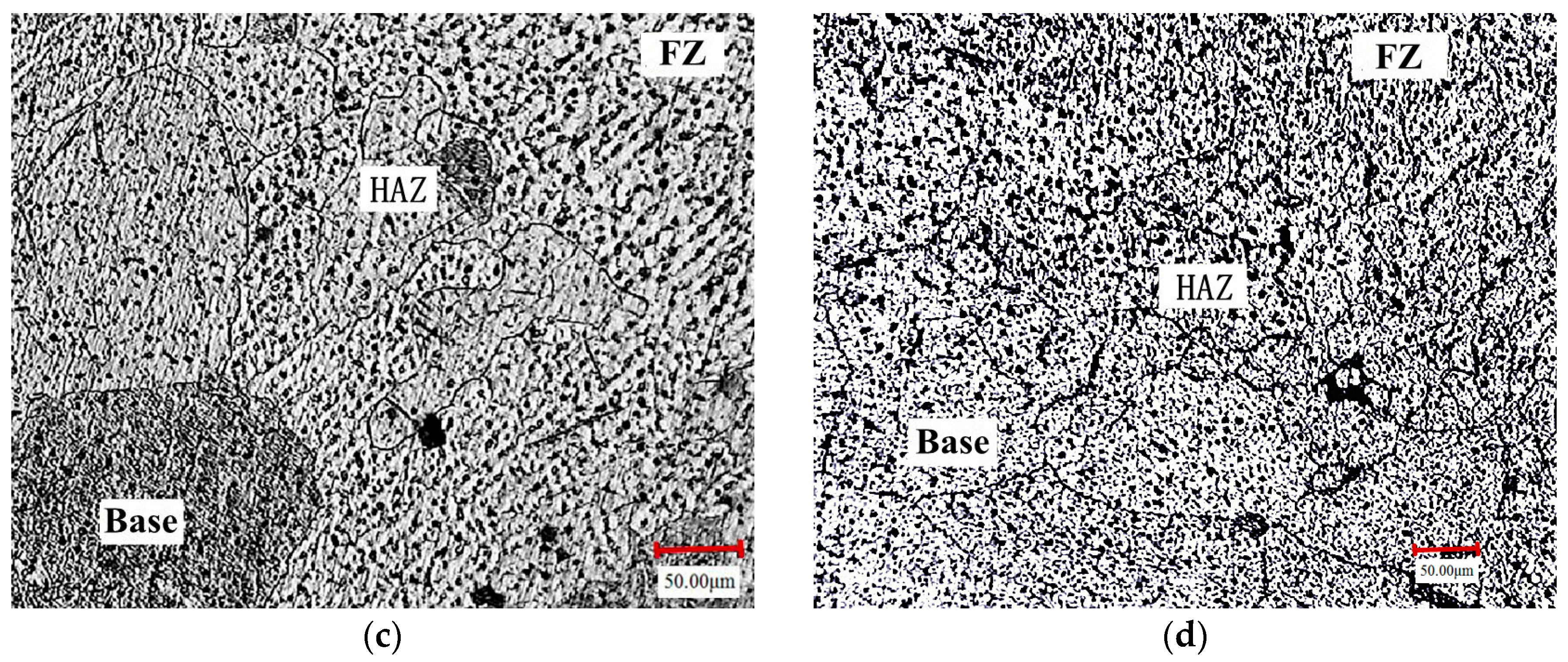
3.1. Microstructure
3.2. Micro-Hardness
3.3. Analysis of X-ray Diffraction Pattern
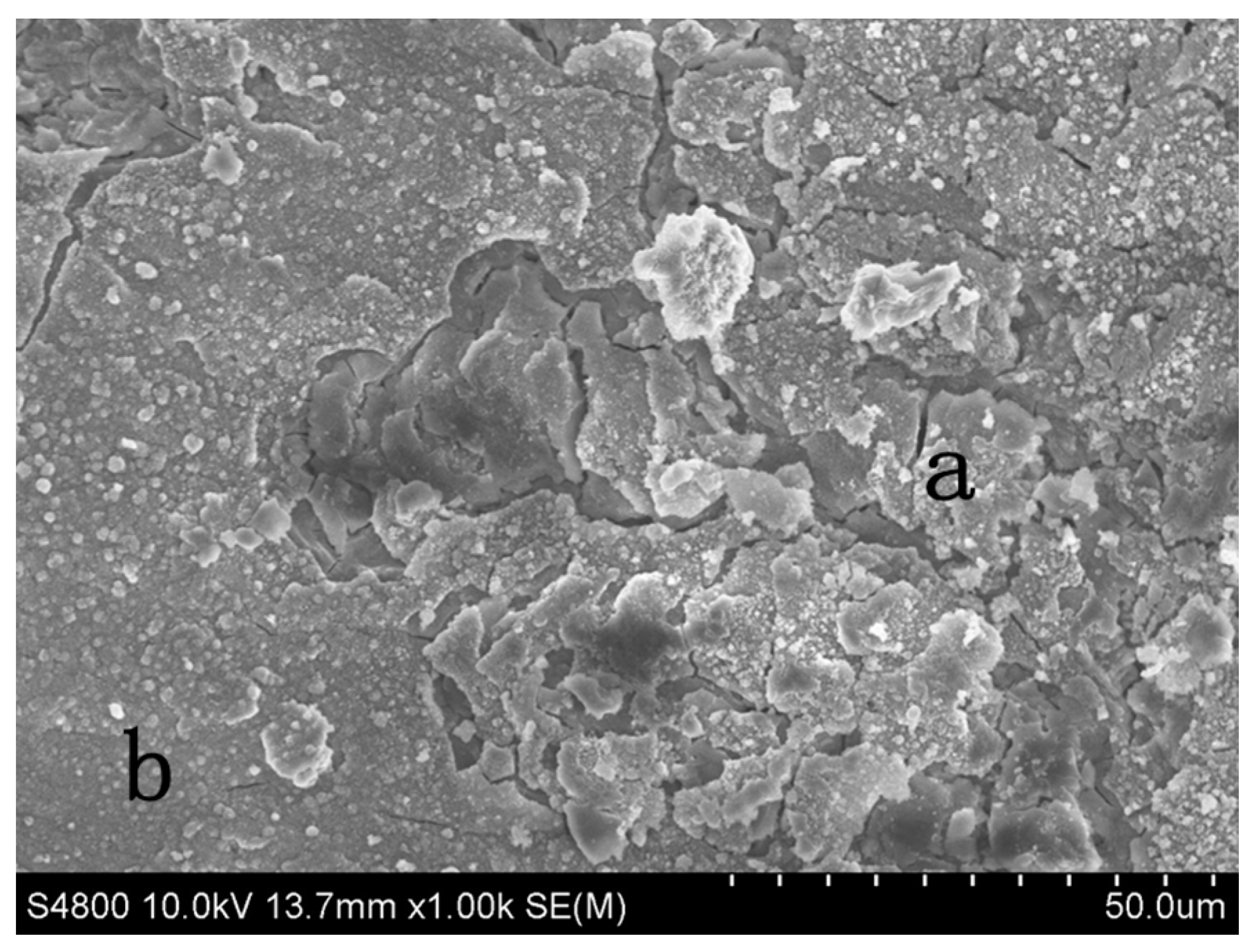
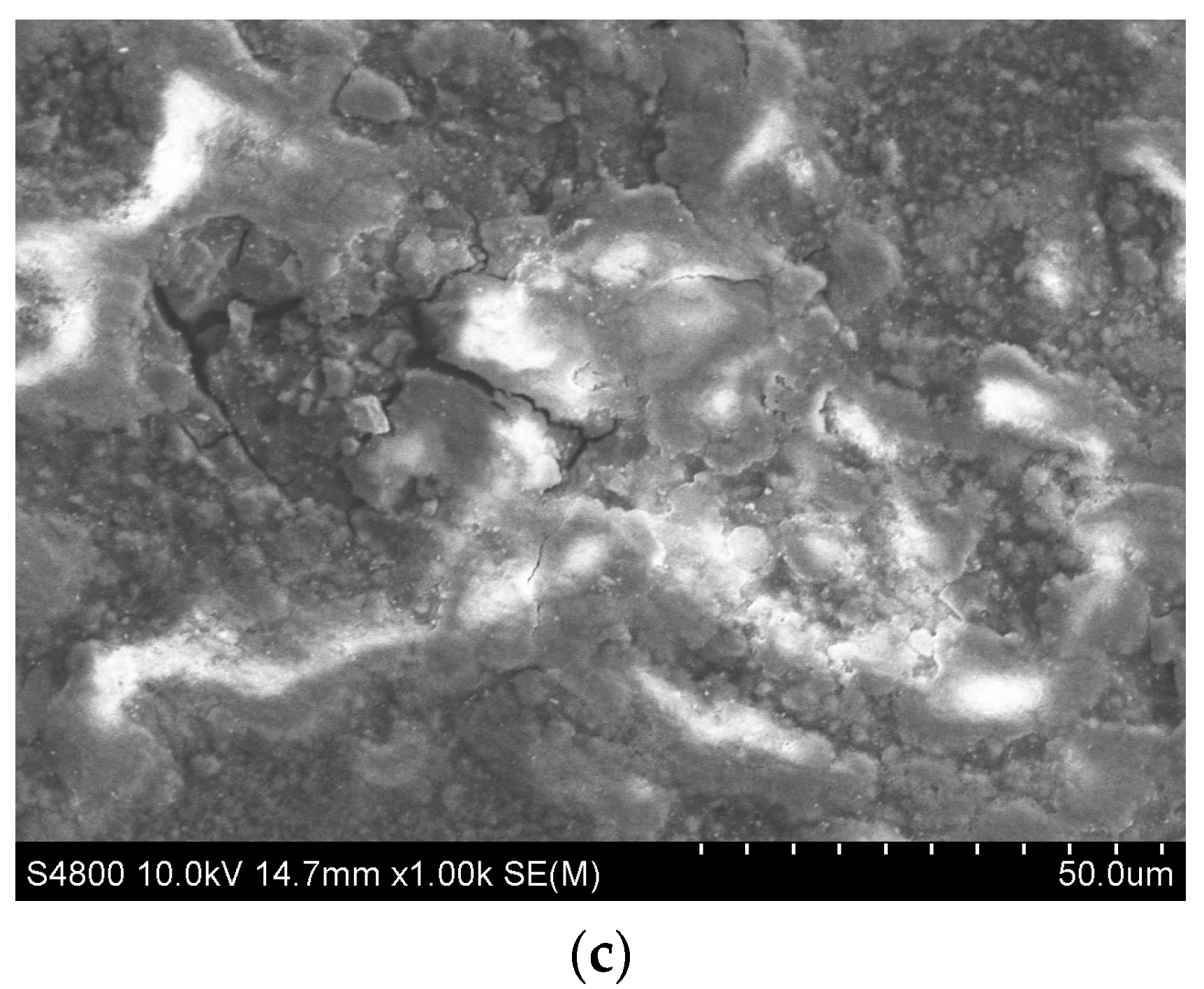
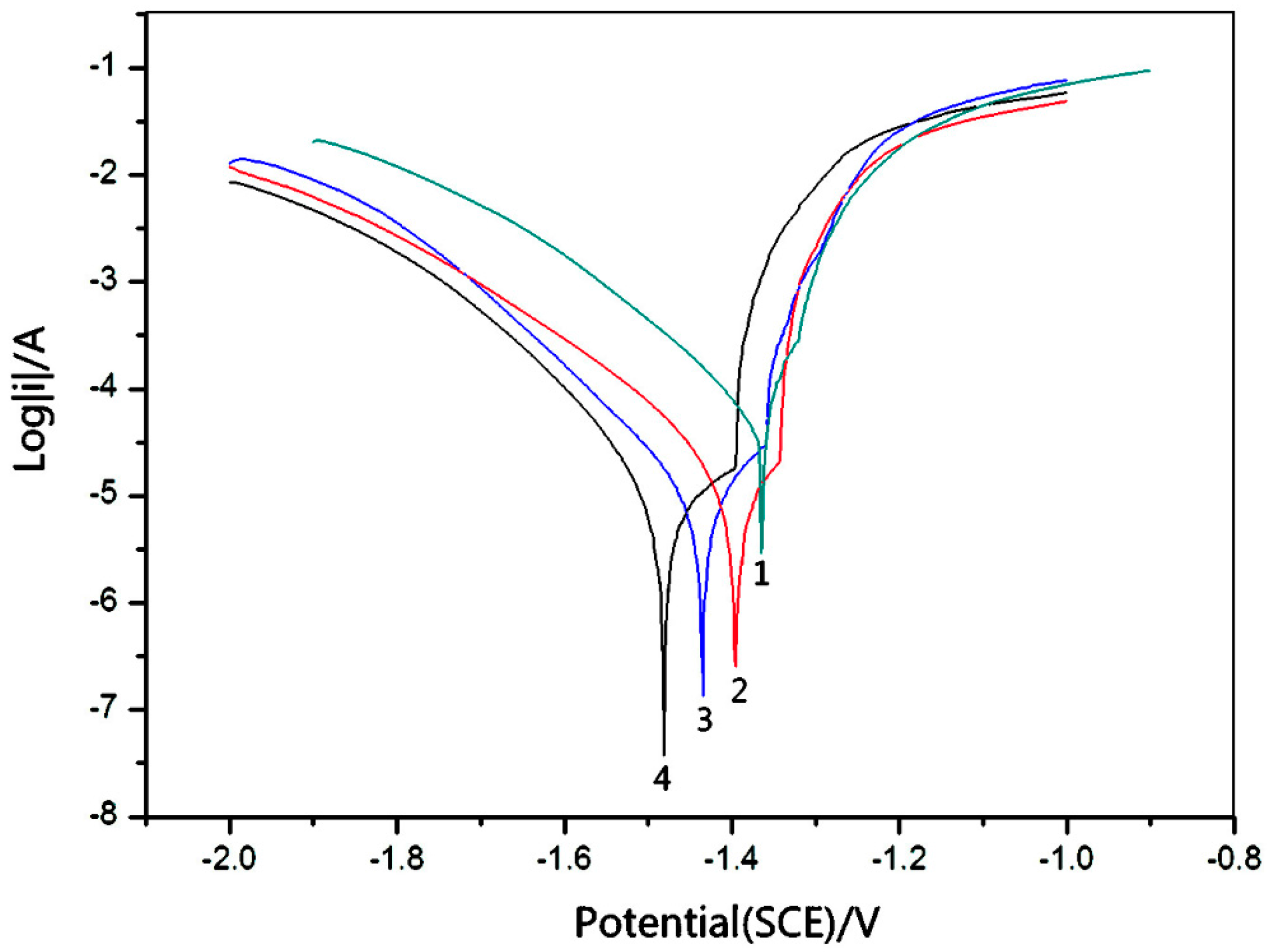
4. Corrosion Studies
NaCl Immersion Test
5. Conclusions
- After the deep cryogenic treatment, there is no phase change in the AZ61 magnesium alloy welded joints, but the organization of α and β phases are refined. The precipitation of β-phase increases significantly and is distributed uniformly; the difference between the three regions of the joint is decreased.
- The microstructural changes have a great influence on the corrosion behavior of the AZ61 magnesium alloy welded joints. The microstructural changes in the joints caused by the deep cryogenic treatment are beneficial to improve the corrosion resistance of the whole joint.
Author Contributions
Conflicts of Interest
References
- Zhang, W.; Bai, P.; Yang, J.; Xu, H.; Dang, J.; Du, Z. Tensile behavior of 3104 aluminum alloy processed by homogenization and cryogenic treatment. Trans. Nonferrous Met. Soc. China. 2014, 24, 2453–2458. [Google Scholar] [CrossRef]
- Niaki, K.S.; Vahdat, S.E. Fatigue Scatter of 1.2542 Tool Steel after Deep Cryogenic Treatment. Mater. Today Proc. 2015, 2, 1210–1215. [Google Scholar] [CrossRef]
- Amini, K.; Akhbarizadeh, A.; Javadpour, S. Investigating the effect of quench environment and deep cryogenic treatment on the wear behavior of AZ91. Mater. Des. 2014, 54, 154–160. [Google Scholar] [CrossRef]
- Idayan, A.; Gnanavelbabu, A.; Rajkumar, K. Influence of Deep Cryogenic Treatment on the Mechanical Properties of AISI 440C Bearing Steel. Proced. Eng. 2014, 97, 1683–1691. [Google Scholar] [CrossRef]
- IShak, M.; Yamasaki, K.; Maekawa, K. Microstructure and Corrosion Behavior of Laser Welded Magnesium Alloys with Silver Nanoparticles. World Acad. Sci., Eng. Technol. 2010, 70, 354–359. [Google Scholar]
- Cao, F.; Song, G.-L.; Atrens, A. Corrosion and passivation of magnesium alloys. Corros. Sci. 2016, 111, 835–845. [Google Scholar] [CrossRef]
- Mónica, P.; Bravo, P.M.; Cárdenas, D. Deep cryogenic treatment of HPDC AZ91 magnesium alloys prior to aging and its influence on alloy microstructure and mechanical properties. J. Mater. Proc. Technol. 2017, 239, 297–302. [Google Scholar] [CrossRef]
- Pu, Z.; Dillon, O.W., Jr.; Puelo, D.A.; Jawahir, I.S. Cryogenic machining and burnishing of magnesium alloys to improve in vivo corrosion resistance. Surf. Modif. Magnes. Alloy. Biomed. Appl. 2015, 2, 103–133. [Google Scholar]
- Templemana, Y.; Hamub, G.B.; Meshia, L. Friction stir welded AM50 and AZ31 Mg alloys: Microstructural evolution and improved corrosion resistance. Mater. Charact. 2017, 126, 86–95. [Google Scholar] [CrossRef]
- Liao, J.; Hotta, M. Corrosion products of field-exposed Mg-Al series magnesium alloys. Corros. Sci. 2016, 112, 276–288. [Google Scholar] [CrossRef]





| Al | Mn | Zn | Cu | Si | Ni | Fe | Impurities | Mg |
|---|---|---|---|---|---|---|---|---|
| 5.8–7.2 | 0.15 min | 0.4–1.5 | 0.05 max | 0.30 max | 0.005 max | 0.005 max | 0.30 max | other |
| Process Parameters | Wire Feed Speed (m/min) | Current (A) | Voltage (V) | Welding Speed (m/min) |
|---|---|---|---|---|
| The first pass | 9.2 | 123 | 9.6 | 0.7 |
| Second pass | 13 | 165 | 11 | 0.35 |
| Swing settings | Pendulum length: 3.5 mm, swing: 4 mm, jagged side swing | |||
| Nozzle diameter | 15 mm | |||
| Argon flow | 14 L/min | |||
| Wire diameter | 1.6 mm | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, X.; Wu, Z.; Zhao, F. Effect of Deep Cryogenic Treatment on the Microstructure and the Corrosion Resistance of AZ61 Magnesium Alloy Welded Joint. Metals 2017, 7, 179. https://doi.org/10.3390/met7050179
Gong X, Wu Z, Zhao F. Effect of Deep Cryogenic Treatment on the Microstructure and the Corrosion Resistance of AZ61 Magnesium Alloy Welded Joint. Metals. 2017; 7(5):179. https://doi.org/10.3390/met7050179
Chicago/Turabian StyleGong, Xiaoyuan, Zhisheng Wu, and Fei Zhao. 2017. "Effect of Deep Cryogenic Treatment on the Microstructure and the Corrosion Resistance of AZ61 Magnesium Alloy Welded Joint" Metals 7, no. 5: 179. https://doi.org/10.3390/met7050179
APA StyleGong, X., Wu, Z., & Zhao, F. (2017). Effect of Deep Cryogenic Treatment on the Microstructure and the Corrosion Resistance of AZ61 Magnesium Alloy Welded Joint. Metals, 7(5), 179. https://doi.org/10.3390/met7050179





