Abstract
The preparation of highly pure vanadyl sulfate from sulfate solutions containing impurities of iron and aluminumwas investigated by solvent extraction with 2-ethylhexyl phosphoric acid mono-2-ethylhexyl ester (EHEHPA) and tri-n-butyl phosphate (TBP) as the phase modifier. The extraction and stripping conditions of vanadium (IV) and its separation from iron and aluminum were optimized. Under the optimal extraction conditions, the extraction of vanadium (IV) and iron were 68% and 53%, respectively, while only 2% aluminum was extracted in a single contact, suggesting good separation of vanadium (IV) from aluminum. Sulfuric acid solution was used for the stripping. Nearly 100% vanadium (IV) and 95% aluminum were stripped, while only 10% iron was stripped under the optimal stripping conditions in a single contact, suggesting good separation of vanadium (IV) from iron. After five stages of extraction and stripping, highly pure vanadyl sulfate containing 76.5 g/L V (IV) with the impurities of 12 mg/L Fe and 10 mg/L Al was obtained, which is suitable for the electrolyte of a vanadium redox flow battery. Organic solution was well regenerated after stripping by oxalic acid solution to remove the remaining iron. The mechanism of vanadium (IV) extraction using EHEHPA was also discussed based on the Fourier transform infrared spectroscopy (FT-IR) analysis.
1. Introduction
Vanadium has found a variety of applications in alloys, catalysts, advanced materials, batteries, etc. [1,2,3]. The application in vanadium redox flow battery (VRFB) is particularly important due to itsadvantages over similar technologies, such as high energy efficiency, short response time, low self-discharge, and a long lifetime [4]. Moreover, VRFB systems are more favorable for peak shaving, uninterruptible power supplies, and stabilizing wind turbine output than other energy backup systems [5].
Vanadium based electrolytes in sulfuric acid solutions are a crucial component of VRFB systems, and, hence, their preparation has attracted much attention. V2O5 and vanadium-bearing solutions are conventionally used as the raw materials for the preparation of vanadyl sulfate electrolytes. For example, V2O5 was dissolved in dilute sulfuric acid and subsequently reduced with a reducing agent to obtain highly concentrated vanadyl sulfate electrolytes by Zhong et al. [6]. The detailed dissolution properties of V2O5 using concentrated sulfuric acid were investigated by Mao et al. [7]. The resulting solutions were then diluted to a desired concentration by the addition of deionized water and filtered to remove the undissolved solids. Following that, the above-mentioned solutions were served as the catholyte for the preparation of vanadyl sulfate by electrolysis. Xu et al. [8] developed a preparation method involving two solvent extraction circuits. In the first circuit, pentavalent vanadium in the solutions was extracted by N,N-didodecyl-1-dodecanamine (Alamine 304) and followed by vanadium stripping with ammonium chloride. Hydrazine hydrate was used to reduce the resulting ammonium metavanadate. Tetravalent vanadium-bearing solutions were then extracted by di-(2-ethylhexyl) phosphoric acid (D2EHPA) and stripped by a sulfuric acid solution in the second circuit. Finally, activated carbon was employed to remove entrained organic contaminations to obtain the vanadium electrolytes. All these methods involved complex processes and required expensive raw materials, resulting in high costs. Furthermore, the final products were always of low quality with high concentrations of impurities.
Solvent extraction is an effective and well-established method for metal recovery and separation due to its high selectivity, steady operations, and cost effectiveness [9]. A number of extractants have been investigated for the extraction of vanadium (IV) and vanadium (V) from their solutions, including acidic extractants such as di-(2-ethylhexyl) phosphoric acid (D2EHPA), 2-ethylhexyl phosphoric acid mono-2-ethylhexyl ester (EHEHPA), and bis(2,4,4-trimethylpentyl)-phosphinic acid (Cyanex272) [10]; basic extractants such as primary aliphatic amine Primene JM-T [11], trialkylamine (N235) [12,13], and methyltrialkyl ammonium chloride (N263) [14]; neutral extractants such as tri-n-butylphosphate (TBP) [15], trioctyl-phosphine oxide (TOPO) [16], and tri-n-octyl phosphine oxide (Cyanex 923) [17]; and chelating extractants like 5,8-diethyl-7-hydroxy-6-dodecanone oxime (LIX63) [18]. Among them, EHEHPA has been extensively studied for separating vanadium from other metal ions. The co-extraction and separation of vanadium (IV) and molybdenum (VI) from sulfate solutions by selective stripping using EHEHPA was studied by Li et al. [19]. The effect of various factors on the extraction and stripping was optimized in detail. It was proved that the extraction of vanadium (V) from sulfate solutions in the initial pH values of 1.2–6 with EHEHPA was via a cationic exchange mechanism by Nguyen and Lee [20] using a slope analysis method. Cai et al. [21] developed a novel technology, which was via solvent extraction for the selective separation and extraction of vanadium (IV) and manganese (II) from co-leaching solutions of roasted stone coal and pyrolusite. However, no detailed investigation was found for the preparation of highly pure vanadyl sulfate by means of solvent extraction and selective stripping of vanadium (IV) with EHEHPA. In addition, no effective method was available to separate vanadium (IV) from the impurities of iron and aluminum in sulfate solutions, particularly when the concentration of vanadium (IV) is high. According to the requirements for manufacturing VRFB in China, highly pure vanadyl sulfate electrolytes must contain at least 76.5g/L V (IV), with the main impurities of iron and aluminum being less than 100 and 50 mg/L, respectively. With such a high requirement, it is very difficult to achieve the accepted product using conventional separation methods. In this work, a new method of vanadium purification from a vanadium sulfate solution by solvent extraction using EHEHPA was studied.
2. Materials and Methods
2.1. Materials and Reagents
EHEHPA supplied by Qinshi Technology Co., Ltd. (Henan, China) was used as the extractant. TBP was supplied by Xilong Chemical Co., Ltd. (Guangdong, China) and used as a phase modifier. In addition, n-heptane, supplied by Guoyao Chemistry Co., Ltd. (Beijing, China), was used as a diluent. All these reagents were of analytical grade. Sulfuric acid of a reagent grade was used for metal stripping. The feed solution, with a pH of about 0.8, was obtained from a previous process in which vanadium-bearing titanomagnetite was firstly reduced by hydrogen and then leached by diluted hydrochloric acid generating leaching solution and titanium concentrated residue. In the leaching solution, vanadium and some impurities were extracted by EHEHPA and then stripped by sulfuric acid, generating a loaded strip solution, which was used as the feed solution for the present study. It contains 11.4 g/L V (IV) and the main impurities of 0.8 g/L Fe and 1.3 g/L Al in sulfate solution. Deionised water was employed for all tests.
2.2. Procedures and Apparatus
Extraction and stripping experiments were carried out in a flask (500 mL). TBP (5%, v/v) was added to the organic phase as a phase modifier to improve the phase separation. The aqueous and oil phases were mixed by mechanical shaking at a frequency of 300 r/min and separated using separating funnels. After phase separation, aqueous phases were sampled and metal concentrations were measured by inductively coupled plasma atomic emission spectroscopy (ICP-OES, Optima 5300DV, PerkinElmer, Waltham, MA, USA). Metal concentrations in organic phases were calculated by mass balance based on the concentration in the feed solutions and in the corresponding aqueous phase. Fourier transform infrared spectroscopy (FT-IR) measurements were performed using a Spectrum GX spectrometer (PerkinElmer, Waltham, MA, USA).
2.3. Data Treatment
The extraction (%E), stripping (%S), distribution ratio (D), and separation factor (β) were calculated based on Equations (1)–(4), respectively.
In the above equations, CF, CR, and CS represent the concentrations of metals in the feed solution, raffinate, and stripping solution, respectively. VF, VR, VS, and VOrg are the volumes of feed solution, raffinate, stripping solution, and organic phase, respectively. βM1/M2 represents the separation factor of any metal (metal-1) over another (metal-2). The standard deviations affecting the extraction and stripping percentages were determined to be in the range of ±5%.
3. Results and Discussion
3.1. Extraction Process
3.1.1. Effect of Initial pH
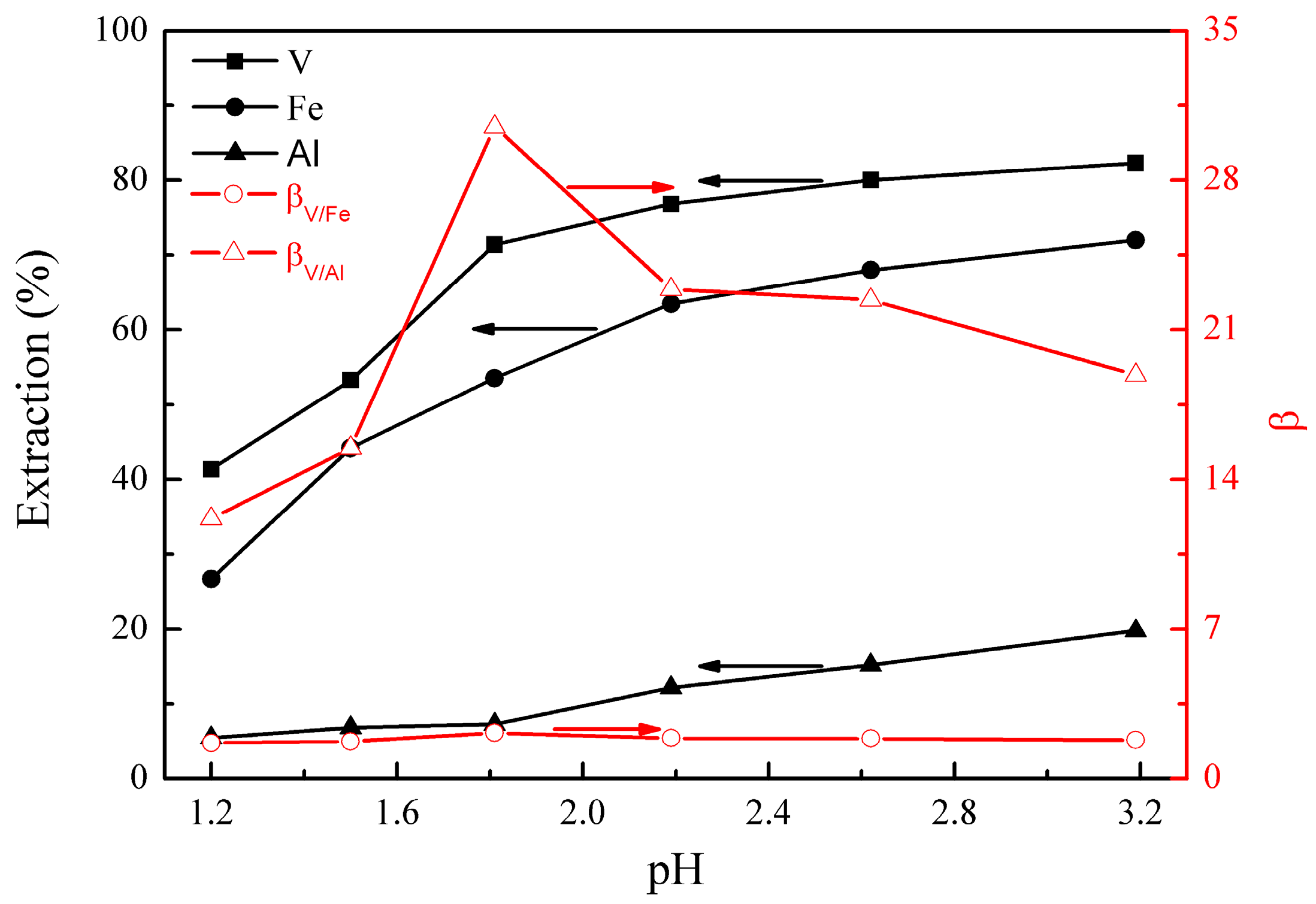
The effect of initial pH on the metal extraction and separation was studied under the following conditions; extraction temperature of 25°C, extraction time of 10 min, EHEHPA concentration (v/v) of 20%, and phase ratio (O/A) of 1:1. The results are presented in Figure 1.
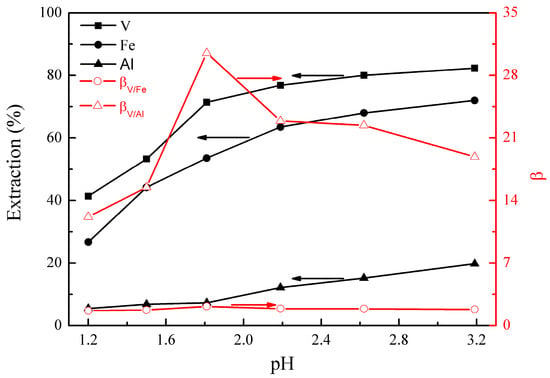
Figure 1.
Effect of initial pH on the extraction percentages of V (IV), Fe, and Al, as well as on the separation factors βV/Fe and βV/Al.
Figure 1 shows that the extractions of vanadium (IV), iron, and aluminum increased from 41% to 82%, 27% to 72%, and 6% to 20%, respectively, as the initial solution pH increased from 1.2 to 3.2. βV/Fe and βV/Al reached maximums of 2.2 and 30.5 at pH 1.8, respectively. The effect of the initial pH on the extraction can be explained by considering the reaction between the extractant (EHEHPA) and the metal ions, shown in Equation (5) [22].
In Equation (5), M represents VO, Fe, or Al, respectively. The values of n and m are equal to the valence of metal ions. The extraction equilibrium will shift to the right side with the pH increase, which promotes the extraction of metal ions. As the pH was higher than 1.8, the extractions of iron and aluminum increased more significantly than that of vanadium (IV), leading to a decrease in βV/Fe and βV/Al. Therefore, the optimal initial pH was selected as 1.8.
3.1.2. Effect of Extraction Temperature
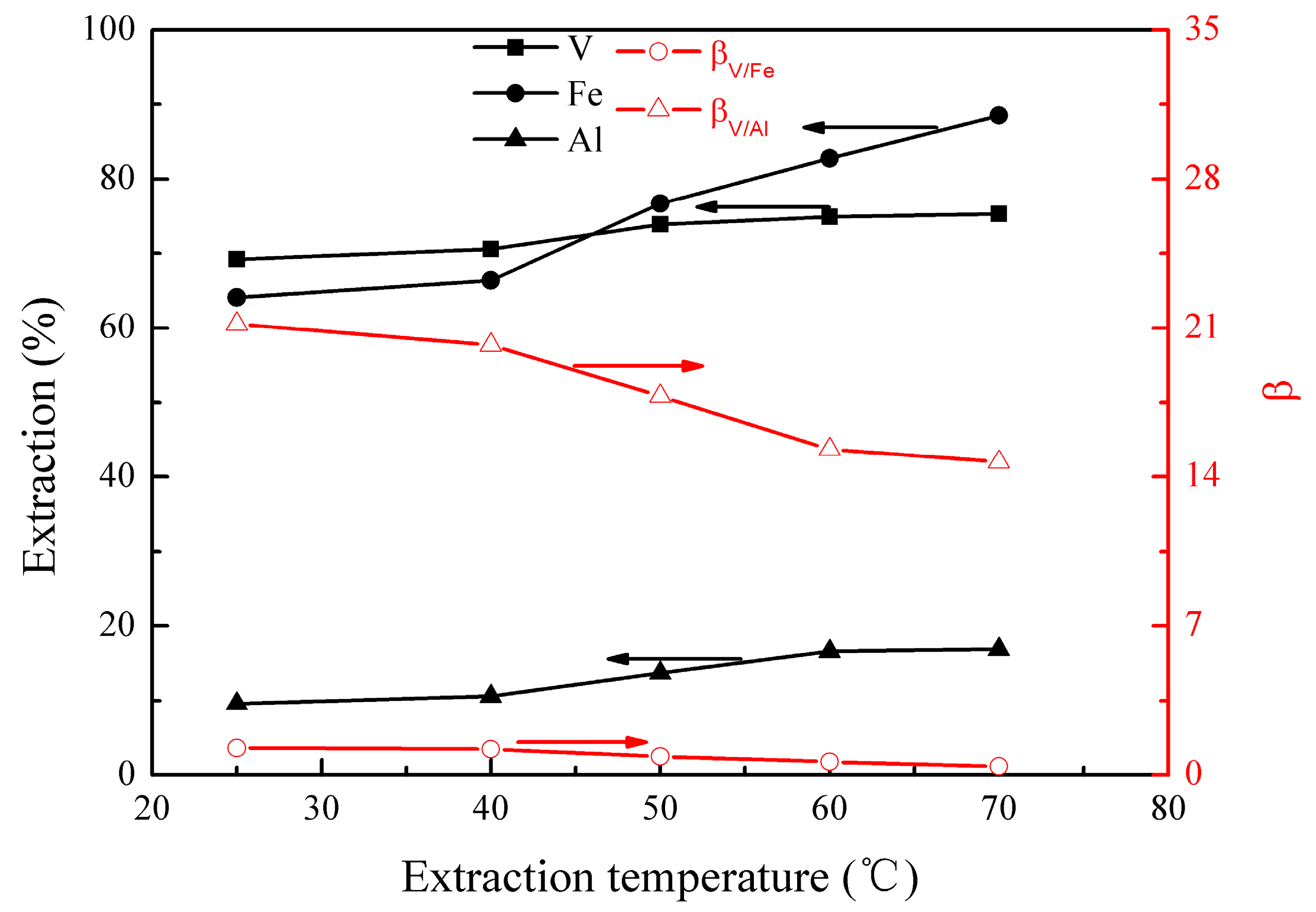
The effect of the extraction temperature on the metal extraction and separation was studied under the following conditions; initial pH of 1.8, extraction time of 10 min, EHEHPA concentration (v/v) of 20%, and phase ratio (O/A) of 1:1. The results are presented in Figure 2.
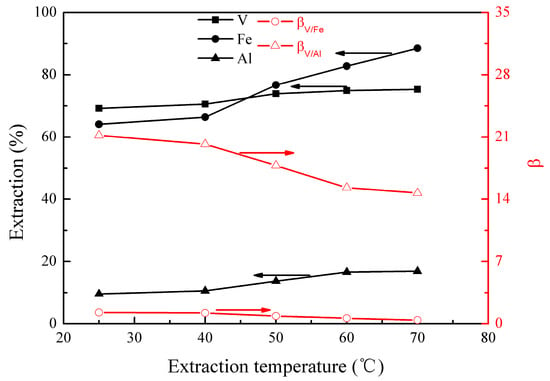
Figure 2.
Effect of extraction temperature on the extraction percentages of V (IV), Fe, and Al, as well as on the separation factors βV/Fe and βV/Al.
Figure 2 shows that all extractions of vanadium (IV), iron, and aluminum increased from 69% to 75%, 64% to 88%, and 10% to 17%, respectively, as the extraction temperature increased from 25 to 70 °C. The results could probably be interpreted by the dissociation of the internal hydrogen bond of EHEHPA at an elevated temperature, which promotes the metal extraction. It can be also seen that the separation factors of βV/Fe and βV/Al decreased from 1.3 to 0.4 and 21.2 to 14.7, respectively. This is due to the effect of temperature on the extraction of iron and aluminum being more significantly than that of vanadium (IV). When the temperature was below 40°C, the extractions of vanadium (IV), iron, and aluminum did not change significantly. Hence, an optimal extraction temperature range of 25–40°C was selected.
3.1.3. Effect of Extraction Time
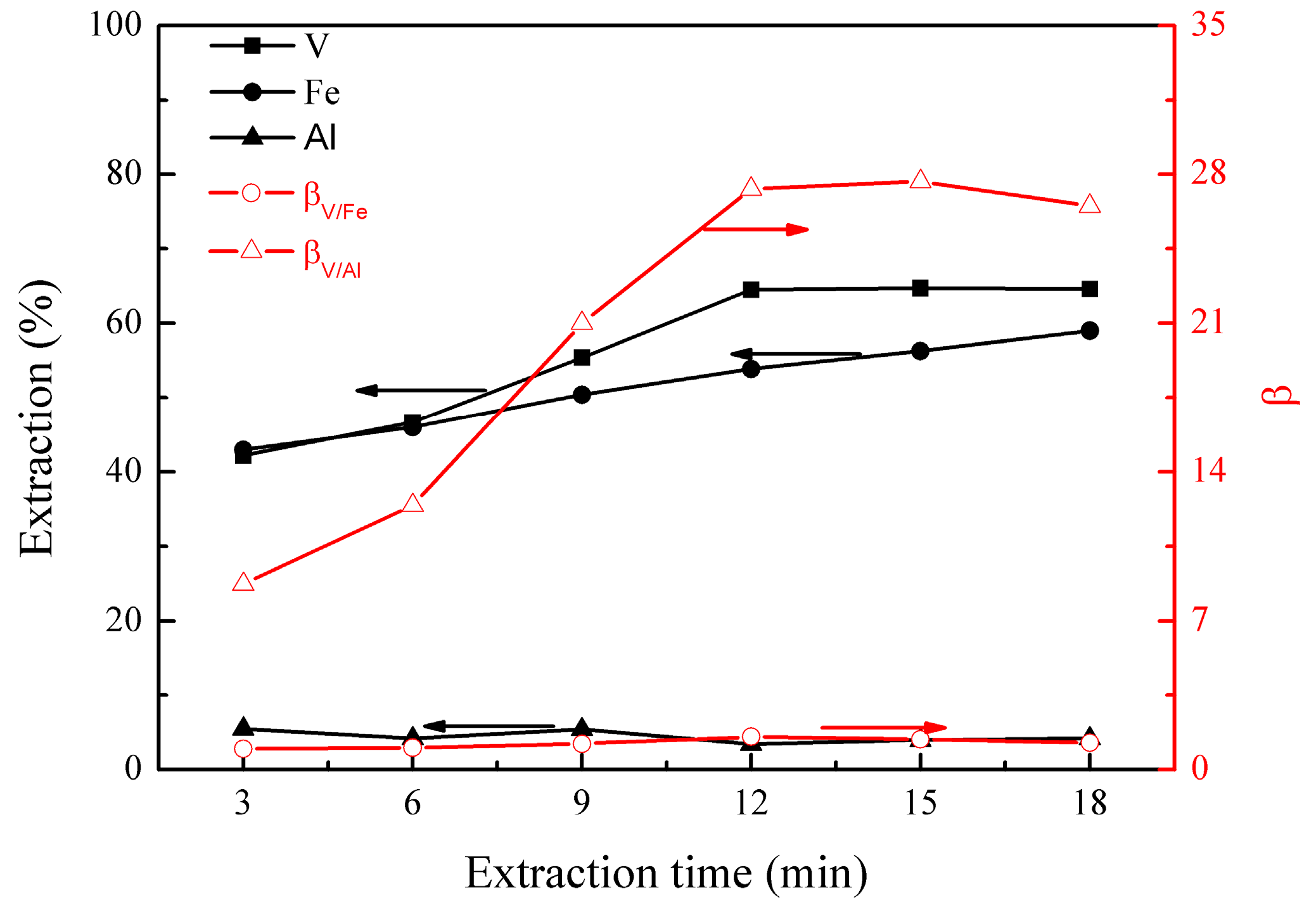
The effect of extraction time on the metal extraction and separation was studied under the following conditions; initial pH of 1.8, extraction temperature of 25 °C, EHEHPA concentration (v/v) of 20%, and phase ratio (O/A) of 1:1. The results are presented in Figure 3.
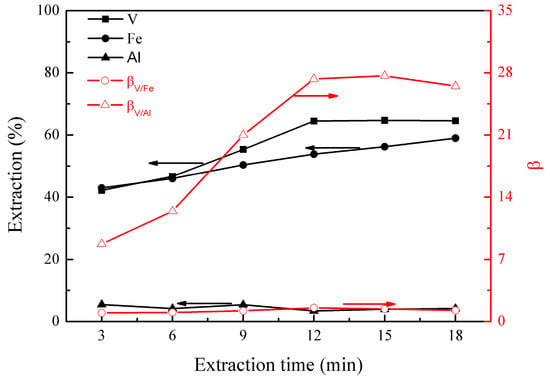
Figure 3.
Effect of extraction time on the extraction percentages of V (IV), Fe, and Al, as well as on the separation factors βV/Fe and βV/Al.
Figure 3 shows that the extraction of vanadium (IV) increased to 64% at 12 min, and no further increase was observed after this, indicating that vanadium (IV) extraction reached equilibrium within 12 min. The extraction of iron increased gradually from 43% to 59% from 3 to 18 min, suggesting that the iron extraction was slow, reaching equilibrium after 18 min. The extraction of aluminum was about 5% between 3 and 18 min, indicating a very fast extraction. In addition, βV/Al reached a maximum of 27.3 at 12 min, showing good vanadium (IV) extraction and its separation from aluminum. Therefore, an optimal extraction time of 12 min was selected.
3.1.4. Effect of Phase Ratio (O/A)
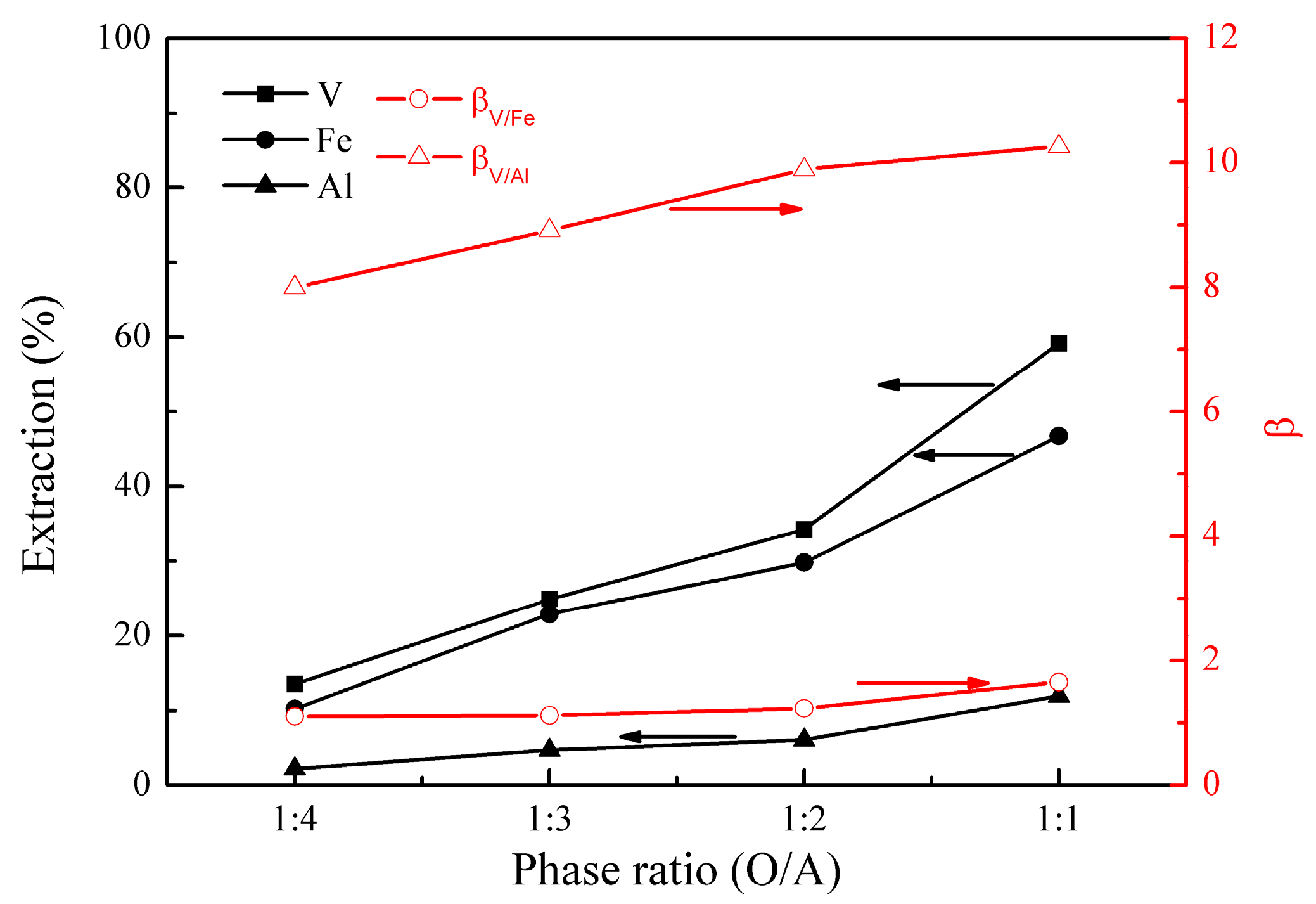
The effect of phase ratio (O/A) on the metal extraction and separation was studied under the following conditions; initial pH of 1.6, extraction temperature of 25 °C, extraction time of 10 min, EHEHPA concentration (v/v) of 20%, and 30 mL aqueous phase. The results are presented in Figure 4.
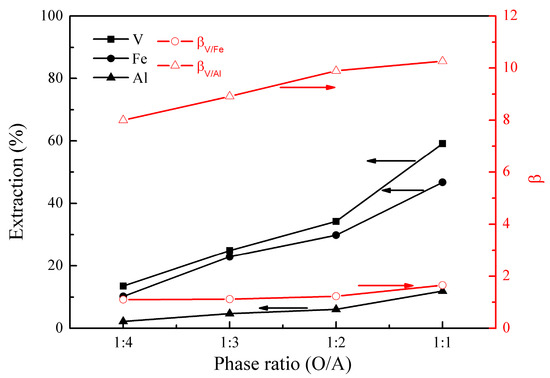
Figure 4.
Effect of phase ratio (O/A) on the extraction percentages of V (IV), Fe, and Al, as well as on the separation factors βV/Fe and βV/Al.
Figure 4 shows that the extractions of vanadium (IV), iron, and aluminum increased from 14% to 59%, 10% to 47%, and 2% to 12%, respectively, as the phase ratio (O/A) increased from 1:4 to 1:1. βV/Fe changed from 1.1 to 1.7, and βV/Al was relatively constant at about 9.0, representing good separation of vanadium (IV) from aluminum, but poor separation of vanadium (IV) from iron. Considering the cost of the extractant and the size of the equipment in practical operations, the phase ratio (O/A) of 1:1 is optimal.
3.1.5. Effect of EHEHPA Concentration
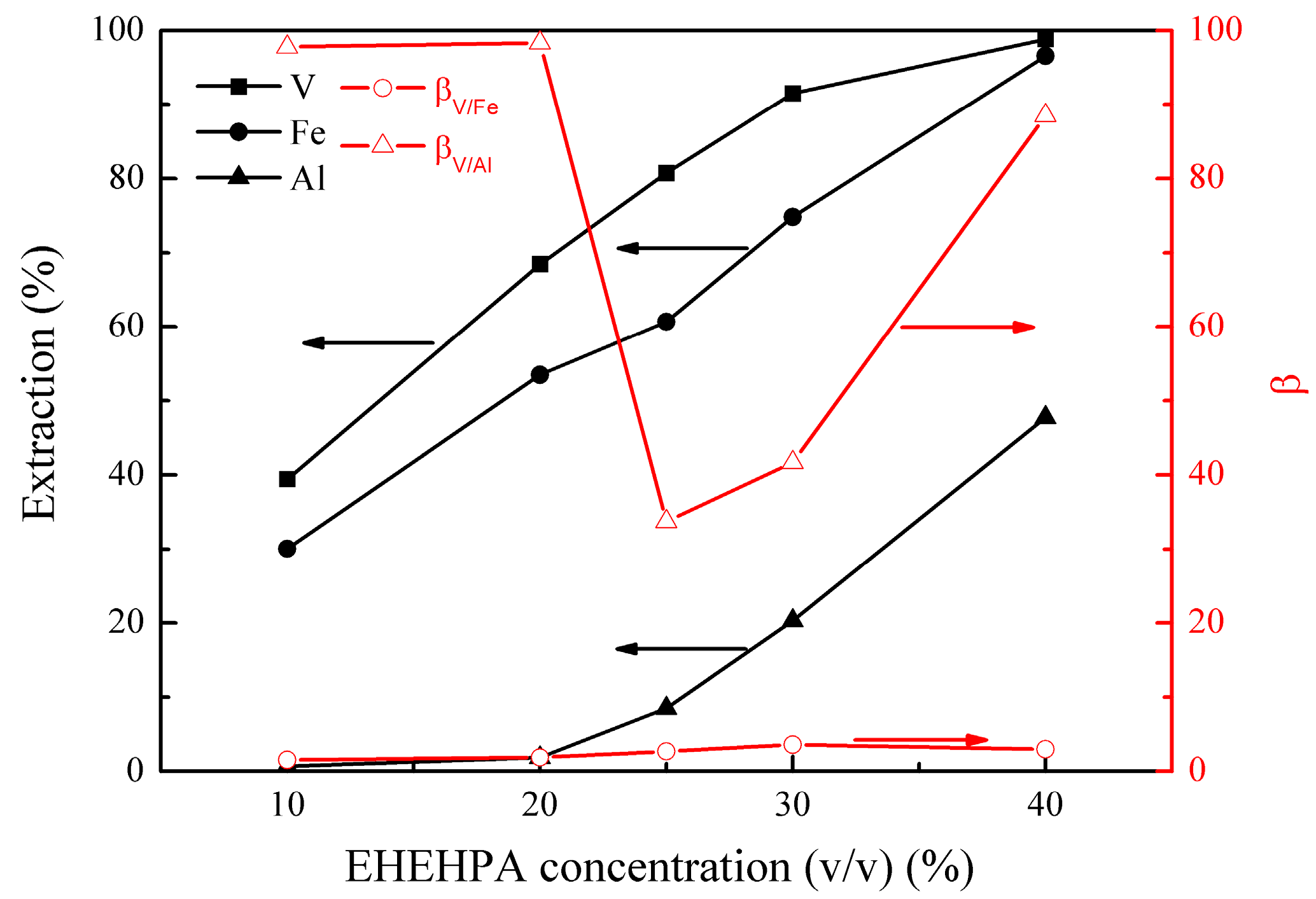
The effect of EHEHPA concentration (v/v) on the metal extraction and separation was studied under the following conditions; initial pH of 1.8, extraction temperature of 25 °C, extraction time of 12 min, and phase ratio (O/A) of 1:1. The results are presented in Figure 5.
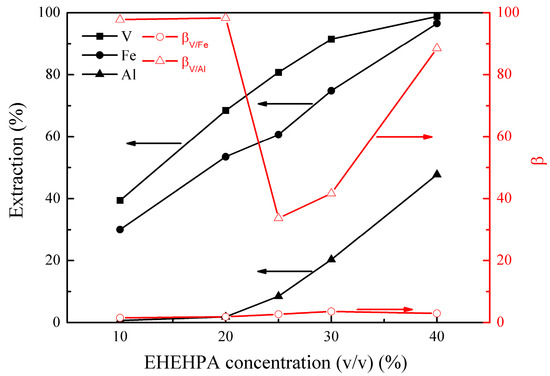
Figure 5.
Effect of 2-ethylhexyl phosphoric acid mono-2-ethylhexyl ester (EHEHPA) concentration (v/v) on the extraction percentages of V (IV), Fe, and Al, as well as on the separation factors βV/Fe and βV/Al.
Figure 5 shows that the extractions of vanadium (IV), iron, and aluminum increased from 39% to 99%, 30% to 97%, and 1% to 48%, respectively, as the EHEHPA concentration (v/v) increased from 10% to 40%. This was due to the increase in the organic extraction capacity with the EHEHPA concentration increasing. However, the extraction of aluminum increased rapidly when the EHEHPA concentration (v/v) was higher than 20%, leading to a decrease in βV/Al from 98.4 to 33.7. Taking into account of vanadium (IV) extraction and its separation from aluminum, an optimal EHEHPA concentration (v/v) of 20% was selected.
3.2. Stripping Process
3.2.1. Effect of Stripping Time
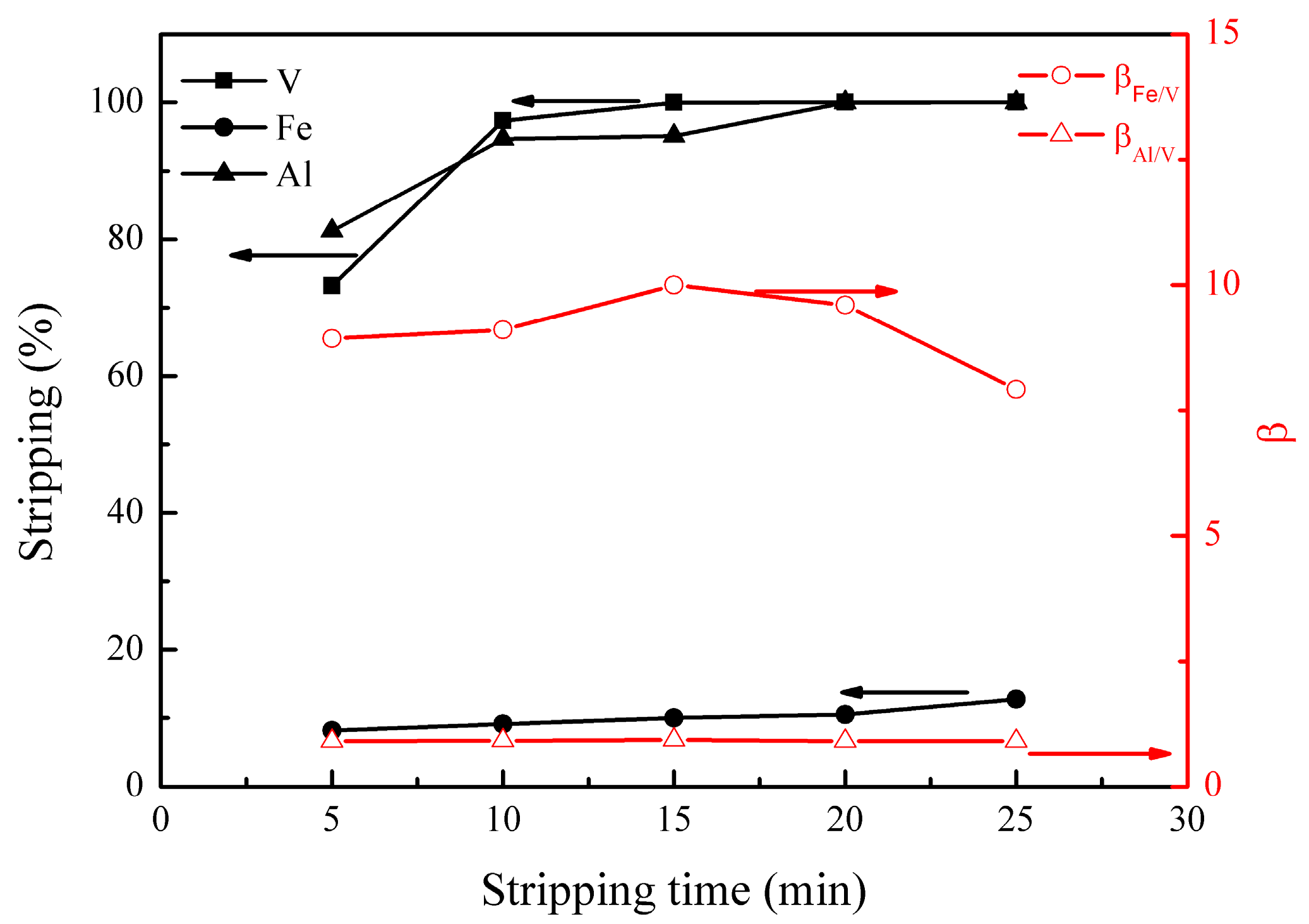
The effect of stripping time on the metal stripping and separation was studied under the following conditions; stripping temperature of 30 °C, H2SO4 concentration of 3.8 mol/L, and phase ratio (O/A) of 3:1. The results are presented in Figure 6.
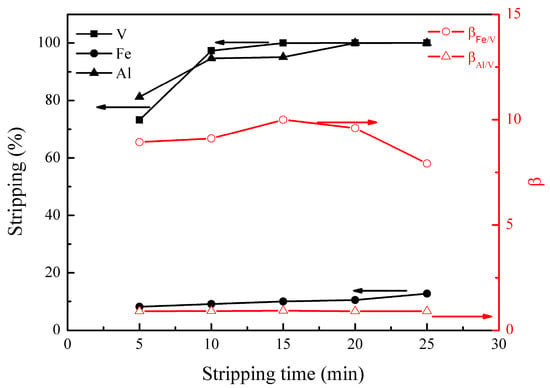
Figure 6.
Effect of stripping time on the stripping percentages of V (IV), Fe, and Al, as well as on the separation factors βFe/V and βAl/V.
Figure 6 shows that the stripping of vanadium (IV) increased to nearly 100% at 15 min. The stripping of iron slowly increased from 8% to 13% with the stripping time varying from 5 to 25 min. The stripping of aluminum increased quickly from 81% to 95% and then slowly to nearly 100%. When the stripping time was 15 min, βFe/V andβAl/V were 10.0 and 0.9, respectively, showing good separation of vanadium from iron and poor separation of vanadium from aluminum during the stripping process. Therefore, an optimal stripping time of 15 min was selected.
3.2.2. Effect of Stripping Temperature
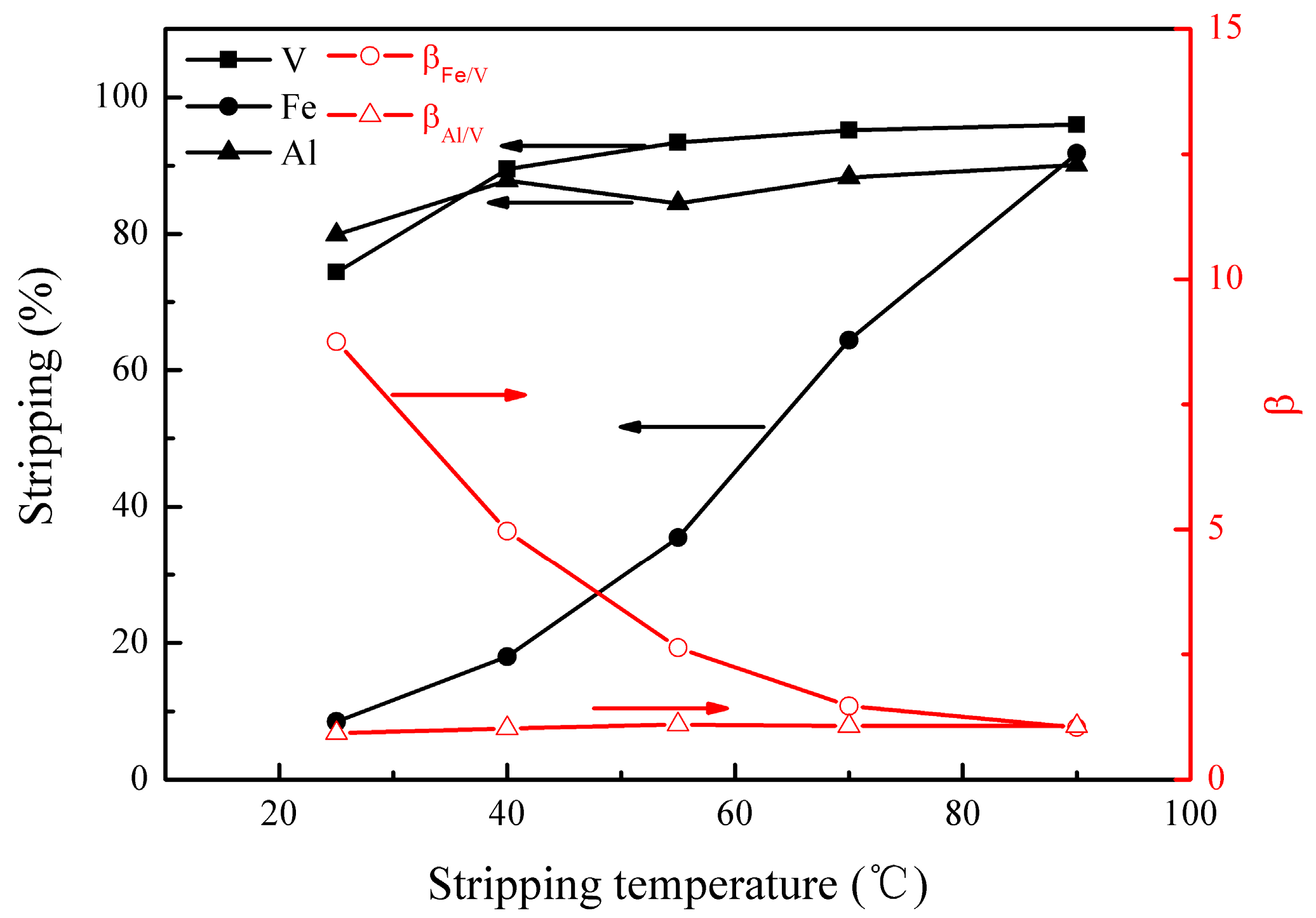
The effect of stripping temperature on the metal stripping and separation was studied under the following conditions; stripping time of 10 min, H2SO4 concentration of 3.8 mol/L, and phase ratio (O/A) of 4:1. The results are presented in Figure 7.
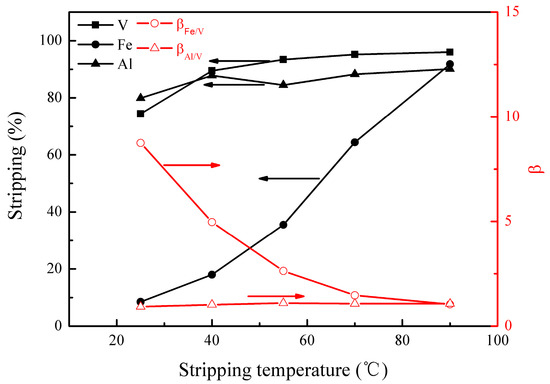
Figure 7.
Effect of stripping temperature on the stripping percentages of V (IV), Fe, and Al, as well as on the separation factors βFe/V and βAl/V.
Figure 7 shows that the stripping of the metals increased as the stripping temperature rose, especially for iron, leading to a substantial decrease in βFe/V. Therefore, an optimal stripping temperature of 25 °C was selected. At this temperature, the stripping of vanadium (IV), iron, and aluminum were 74%, 8%, and 80%, respectively, and βFe/V and βAl/V were 8.5 and 0.9, showing good separation of vanadium (IV) and iron.
3.2.3. Effect of H2SO4 Concentration
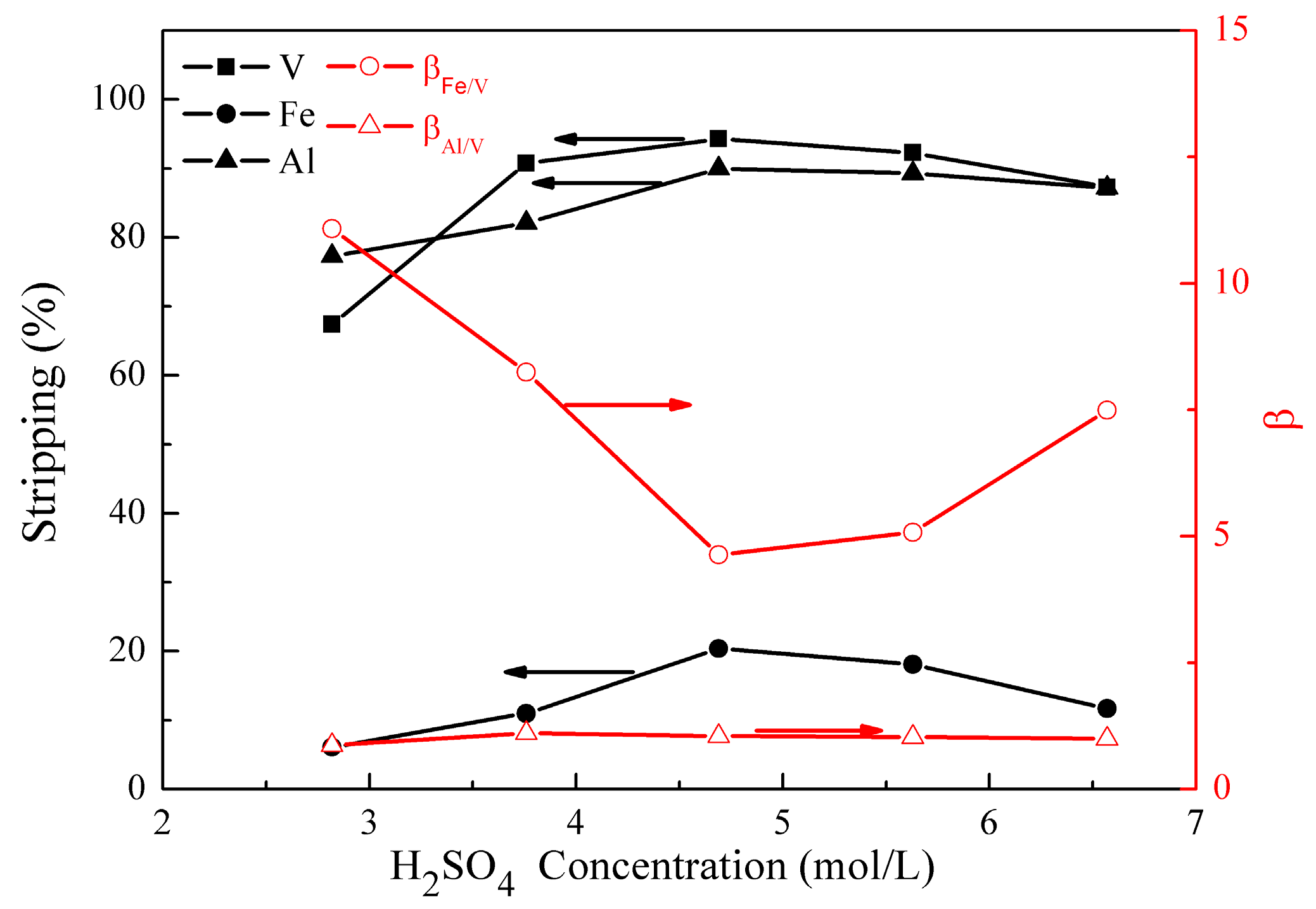
The effect of H2SO4 concentration on the metal stripping and separation was studied under the following conditions; stripping temperature of 30 °C, stripping time of 10 min, and phase ratio (O/A) of 4:1. The results are presented in Figure 8.
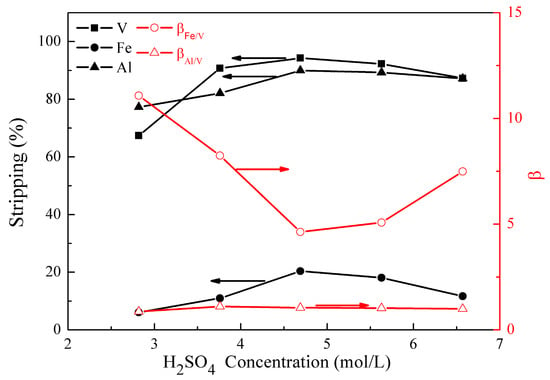
Figure 8.
Effect of H2SO4 concentration on the stripping percentages of V (IV), Fe, and Al, as well as on the separation factors βFe/V and βAl/V.
Figure 8 shows that the stripping of vanadium (IV), iron, and aluminum changed from 67% to 94%, 6% to 20%, and 77% to 90% as the H2SO4 concentration increased from 2.8 to 6.6 mol/L. When the H2SO4 concentration was over 4.7 mol/L, the stripping of vanadium (IV), iron, and aluminum decreased slightly. In consideration of achieving better βAl/V, an optimal H2SO4 concentration of 3.8 mol/L was selected. Under these conditions, βFe/V andβAl/V were 8.2 and 1.1, respectively.
3.2.4. Effect of Phase Ratio (O/A)
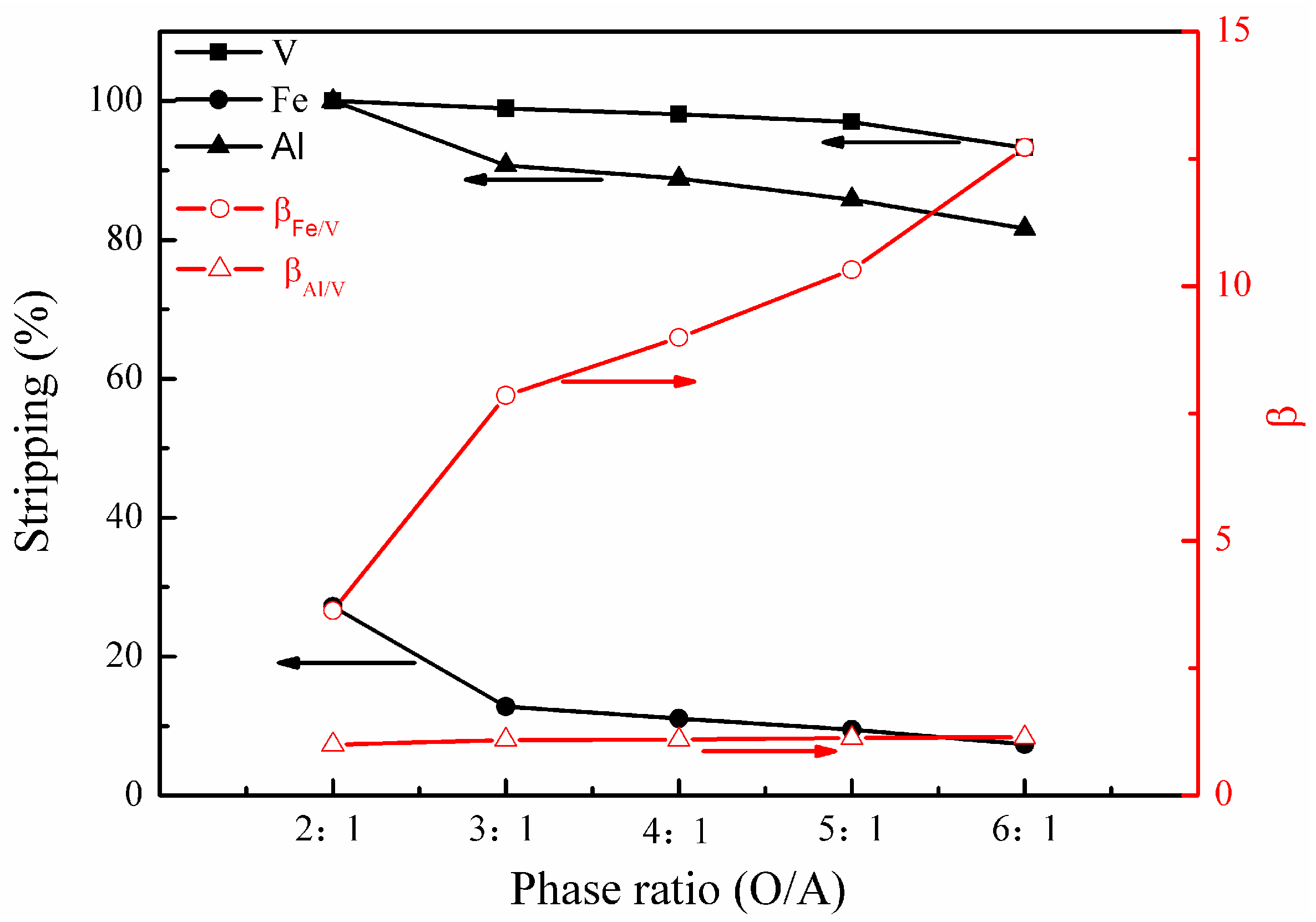
The effect of phase ratio (O/A) on the metal stripping and separation was studied under the following conditions; stripping temperature of 30 °C, stripping time of 15 min, and H2SO4 concentration of 3.8 mol/L. The results are presented in Figure 9.
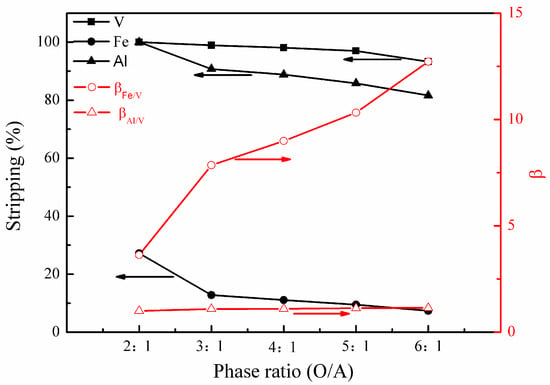
Figure 9.
Effect of phase ratio (O/A) on the stripping percentages of V (IV), Fe, and Al, as well as on the separation factors βFe/V and βAl/V.
Figure 9 shows that phase ratio (O/A) had a slight effect on the stripping of vanadium (IV) in the range of 2:1 to 5:1. In comparison, the effect of the phase ratio (O/A) on the stripping of iron and aluminum is more significant, wherein the metals decreased from 27% to 7% and nearly 100% to 82%, respectively, as the phase ratio (O/A) increased from 2:1 to 6:1. When the phase ratio (O/A) was small, there was more than stoichiometrically required H+ to allow the exchange of metal ions. Thus, a great amount of iron and aluminum was stripped into solution, leading to a decrease in βFe/V andβAl/V. Consequently, the optimal phase ratio (O/A) of 3:1 or 4:1 was selected.
3.3. Contents of Fe and Al by Multiple Stages Extraction and Stripping
From the above discussion, it is known that good separation of vanadium from aluminum could be achieved during the extraction process, while good separation from iron could be achieved during the stripping process. As a result, vanadium purification from iron and aluminum could be achieved by selective extraction and stripping. Since it is difficult to obtain pure vanadium by a single contact of extraction and stripping, multiple stages of extraction and stripping were carried out under the optimal conditions. The contents of the impurities of iron and aluminum after multiple stages of extraction and stripping are shown in Table 1. It can be seen that the contents of iron and aluminum decreased significantly in each contact of extraction and stripping. After five stages of extraction and stripping, highly pure vanadyl sulfate containing 76.5 g/L V (IV) with impurities of 12 mg/L Fe and 10 mg/L Al was achieved, which is suitable for the vanadium electrolyte.

Table 1.
The content of the impurities after multiple stages of different extraction and stripping.
3.4. Organic Regeneration
After vanadium stripping, some iron (about 0.4 g/L) still remained in the organic phase. Therefore, the removal of iron in the organic phase was necessary to regenerate the extractant. An 11 wt % oxalic acid solution was used for the iron removal at the phase ratio (O/A) of 2:1, stripping time of 40 min, and stripping temperature of 40 °C. Nearly 99% Fe was removed from the organic phase in a single contact. The regenerated extractant was reused to extract vanadium (IV) from the feed solutions. The results showed that the extraction of vanadium (IV) was 67%, similar to that obtained using fresh organic solution, proving that the extractant could be well regenerated.
3.5. Metal Extraction Analysis by FT-IR Spectrum
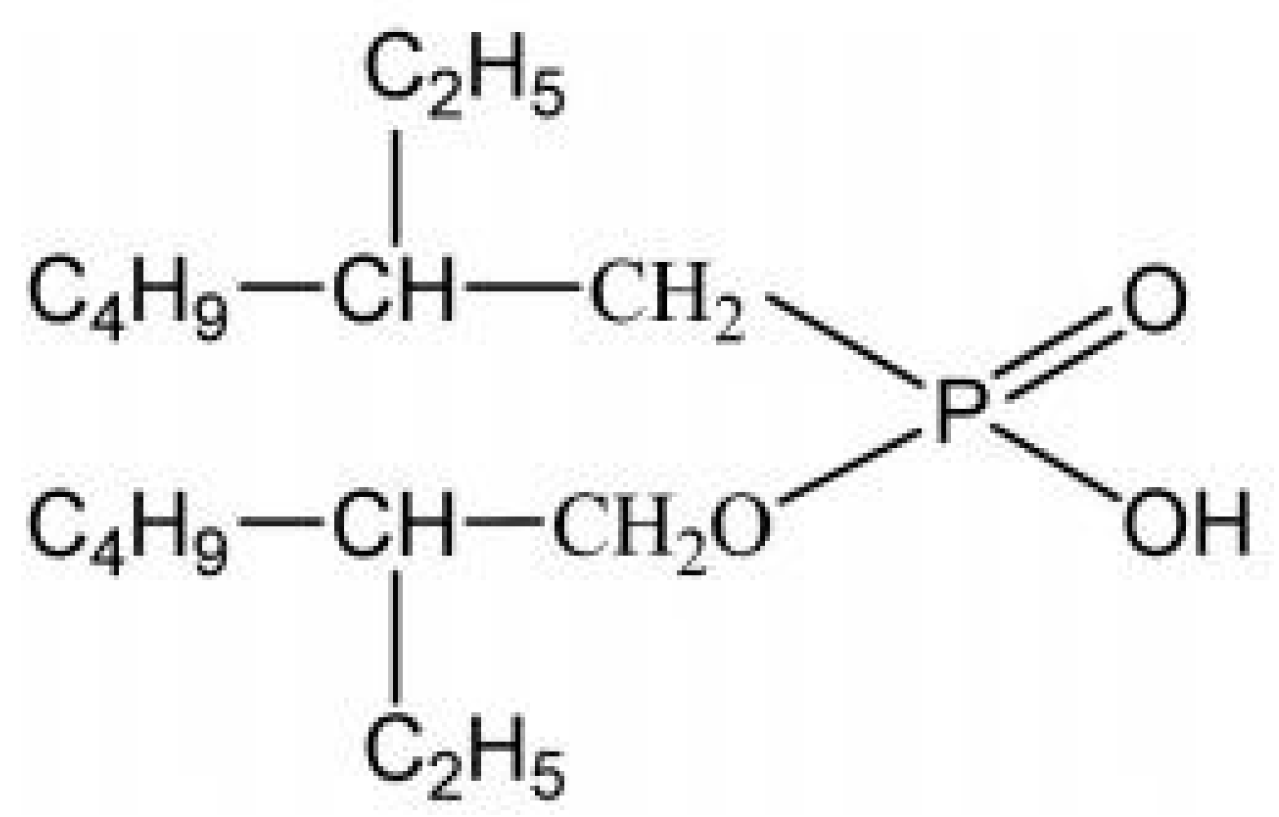
The structural formula of EHEHPA is shown in Figure 10. The FT-IR spectra of EHEHPA and metal ions loaded with EHEHPA were obtained. The results are presented in Figure 11. The major peak changes in the FT-IR spectra are tabulated in Table 2.
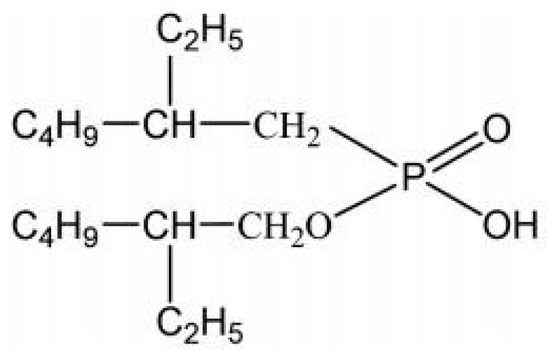
Figure 10.
Structural formula of EHEHPA.
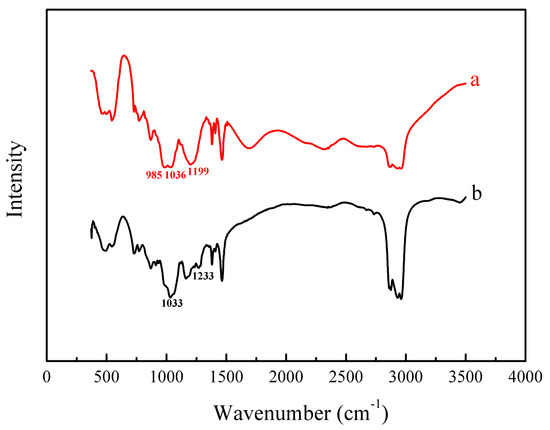
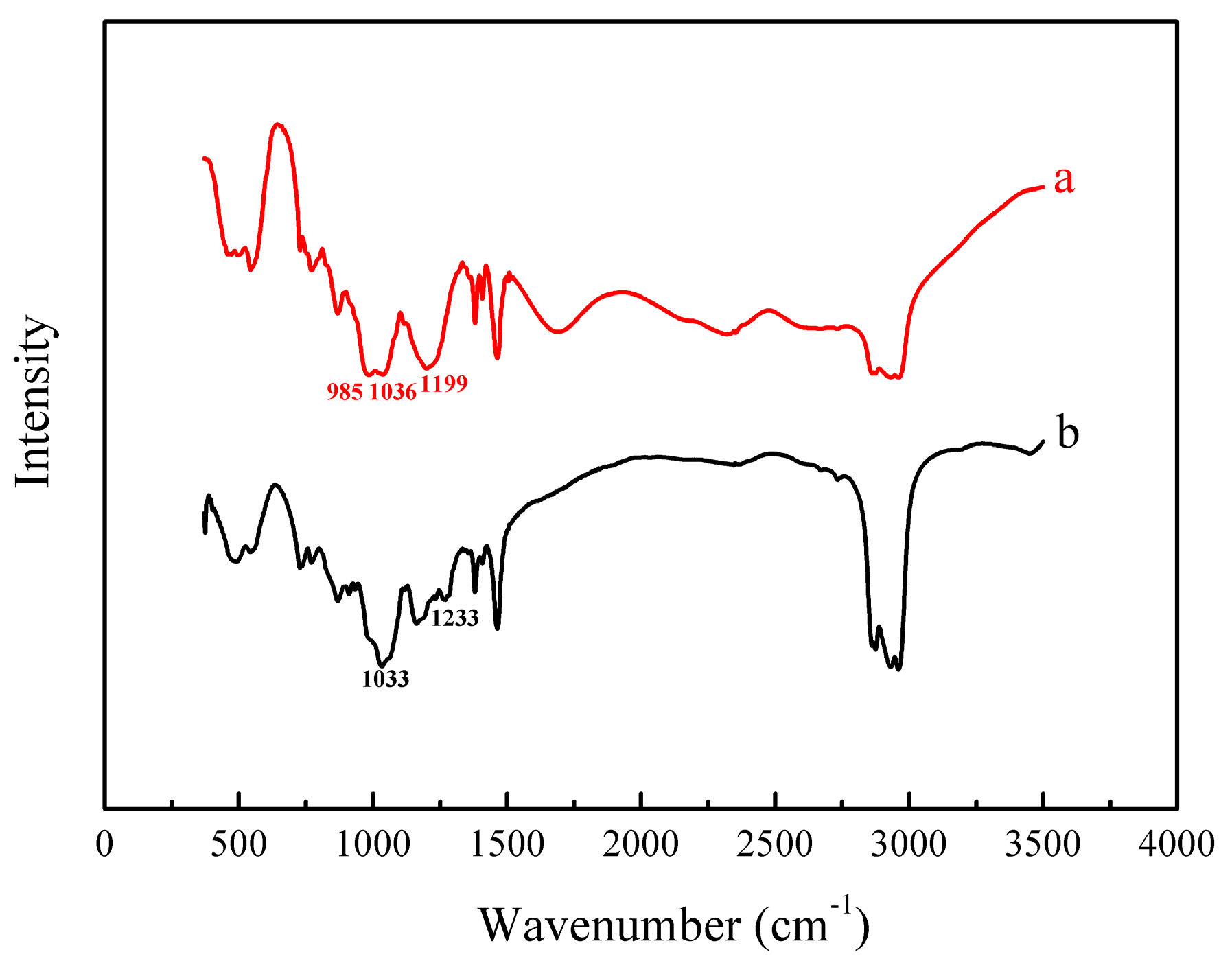
Figure 11.
FT-IR spectra of (a) EHEHPA and (b) metal ions loaded with EHEHPA.

Table 2.
Major peak changes of organic solution before and after metal was loaded in the FT-IR spectra.
In Figure 11, the peak at 985 cm−1 was assigned to the P–O–H stretching vibration, while that at 1036 cm−1 was corresponding to the P–O–C stretching vibration [23]. After the metal ions were loaded, the peak at 985 cm−1 became significantly weaker and even disappeared. However, a new peak appeared at 1033 cm−1. In addition, the absorption band at 1199 cm−1, which was due to the dimeric form of the P=O stretching vibration [23], shifted to a higher wavenumber of 1233 cm−1. It is suggested that when the dimeric EHEHPA combined with metal ions, the hydrogen atom represented in the P–O–H peak was replaced by metal ions and that the oxygen atom in the P=O group was also involved in the coordination with the metal ions, which are the same as reported elsewhere [24]. Furthermore, the band might overlap with the P–O–C band (1033 cm−1) of the metal ions loaded with EHEHPA and make it widen [25]. These spectral changes verified again that the mechanism of vanadium (IV) extraction using EHEHPA is cation exchange, as described in previous studies [26].
4. Conclusions
The preparation of highly pure vanadyl sulfate from vanadium sulfate solutions containing impurities of iron and aluminum by solvent extraction was studied. EHEHPA diluted with n-heptane was used as the extractant, and TBP was used as the phase modifier. Vanadium (IV) could be well separated from aluminum in the extraction process, as 68% vanadium (IV) was extracted, while only 2% aluminum was extracted under the optimal conditions. However, vanadium (IV) separation from iron was poor. In the stripping process, good vanadium (IV) separation from iron was obtained, as nearly 100% vanadium (IV) butonly 10% iron was stripped. Therefore, effective vanadium separation from iron and aluminum could be obtained by multiple stages of extraction and stripping. After five stages of extraction and stripping, highly pure vanadyl sulfate containing 76.5 g/L V (IV) with impurities of 12 mg/L Fe and 10 mg/L Al was prepared. The used organic solution could be well regenerated using oxalic acid solution to remove the remaining iron. FT-IR analysis indicated that the mechanism of vanadium (IV) extraction using EHEHPA was cation exchange.
Acknowledgments
This work is financially supported by the National Basic Research Program of China (973 Program, 2013CB632604), the National Nature Science Foundation of China (Grant No. 21506233, No. 21606241, 51504230, No. 51402303, No. 51374191). Thanks to Jiajun Ke and Zhaowu Zhu for the constructive suggestions.
Author Contributions
Dan Li designed and performed the experiments; Desheng Chen, Lina Wang, and Tao Qi supervised the research; and Guozhi Zhang, Hongxin Zhao, Weijing Wang, and Yahui Liu provided valuable scientific advice for the study. Dan Li analyzed data and wrote the paper, while Desheng Chen revised the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, G.; Chen, D.; Zhao, W.; Zhao, H.; Wang, L.; Li, D.; Qi, T. A novel synergistic extraction method for recovering vanadium (V) from high-acidity chloride leaching liquor. Sep. Purif. Technol. 2016, 165, 166–172. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, H.; Hu, G.; Qi, T.; Yu, H.; Zhang, G.; Wang, L.; Wang, W. An extraction process to recover vanadium from low-grade vanadium-bearing titanomagnetite. J. Hazard. Mater. 2015, 294, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Ning, P.; Lin, X.; Wang, X.; Cao, H. High-efficient extraction of vanadium and its application in the utilization of the chromium-bearing vanadium slag. Chem. Eng. J. 2016, 301, 132–138. [Google Scholar] [CrossRef]
- Nam, S.; Lee, D.; Kim, J.; Lee, D.G. Development of a fluoroelastomer/glass fiber composite flow frame for a vanadium redox flow battery (VRFB). Compos. Struct. 2016, 145, 113–118. [Google Scholar] [CrossRef]
- Huang, K.; Li, X.; Liu, S.; Tan, N.; Chen, L. Research progress of vanadium redox flow battery for energy storage in China. Renew. Energy 2008, 33, 186–192. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, S.; Zhang, Q.; Huang, K.; Li, Q.; Qin, D.; Wu, X.; Li, H.; Liu, W. A Method of Preparing Vanadyl Sulfate Applied to the Vanadium Redox Flow Battery. Chinese Patent No.201010140475.2, 26 March 2010. [Google Scholar]
- Mao, F.; Sun, Z.; Li, D.; Peng, H.; Yang, L.; Chen, W.; Cao, M. A Method of Preparing High-Purity Vanadyl Sulfate for the Vanadium Redox Flow Battery Electrolyte. Chinese Patent No.2013103722877.9, 23 August 2013. [Google Scholar]
- Xu, Y.; Wen, Y.; Cheng, J.; Yang, Y.; Cao, G. A Method of Two-Stage Extraction to Prepare High-Purity Vanadyl Sulfate Solution. Chinese Patent No.201210209648.0, 25 June 2010. [Google Scholar]
- Chagnes, A.; Rager, M.-N.; Courtaud, B.; Thiry, J.; Cote, G. Speciation of vanadium (V) extracted from acidic sulfate media by trioctylamine in n-dodecane modified with 1-tridecanol. Hydrometallurgy 2010, 104, 20–24. [Google Scholar] [CrossRef]
- Li, X.; Wei, C.; Wu, J.; Li, C.; Li, M.; Deng, Z.; Xu, H. Thermodynamics and mechanism of vanadium(IV) extraction from sulphate medium with D2EHPA, EHEHPA and Cyanex 272 in kerosene. Trans. Nonferr. Met. Soc. 2012, 22, 461–466. [Google Scholar] [CrossRef]
- Nekovar, P.; Schrotterova, D. Liquid-liquid extraction of Mo(VI) and V (V) by primene JMT. J. Radioanal. Nucl. Chem. 1998, 228, 95–98. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Bao, S.; Shen, C. Separation and recovery of vanadium from a sulfuric-acid leaching solution of stone coal by solvent extraction using trialkylamine. Sep. Purif. Technol. 2016, 164, 49–55. [Google Scholar] [CrossRef]
- Chen, F.; Wang, X.; Liu, W.; Liang, B.; Yue, H.; Li, C. Selective extraction of nitric and acetic acids from etching waste acid using N235 and MIBK mixtures. Sep. Purif. Technol. 2016, 169, 50–58. [Google Scholar] [CrossRef]
- Bal, Y.; Bal, K.E.; Cote, G. Kinetics of the alkaline stripping of vanadium(V) previously extracted by Aliquat® 336. Miner. Eng. 2002, 15, 377–379. [Google Scholar] [CrossRef]
- Cheraghi, A.; Ardakani, M.S.; Keshavarz Alamdari, E.; Haghshenas Fatmesari, D.; Darvishi, D.; Sadrnezhaad, S.K. Thermodynamics of vanadium (V) solvent extraction by mixture of D2EHPAand TBP. Int. J. Miner. Process. 2015, 138, 49–54. [Google Scholar] [CrossRef]
- Sole, K.C.; Feather, A.; O’Connell, J.P. Recovery of titanium from the leach liquors of titaniferous magnetites by solvent extraction: Part 3. Continuous mini-plant trials. Hydrometallurgy 1999, 51, 275–284. [Google Scholar] [CrossRef]
- Narayanan Remya, P.; Lakshmipathy Reddy, M. Solvent extraction separation of titanium(IV), vanadium(V) and iron(III) from simulated waste chloride liquors of titanium minerals processing industry by the trialkylphosphine oxide Cyanex 923. J. Chem. Technol. Biotechnol. 2004, 79, 734–741. [Google Scholar] [CrossRef]
- Zeng, L.; Cheng, C.Y. Recovery of molybdenum and vanadium from synthetic sulphuric acid leach solutions of spent hydrodesulphurisation catalysts using solvent extraction. Hydrometallurgy 2010, 101, 141–147. [Google Scholar] [CrossRef]
- Li, X.; Wei, C.; Wu, J.; Li, M.; Deng, Z.; Li, C.; Xu, H. Co-extraction and selective stripping of vanadium (IV) and molybdenum (VI) from sulphuric acid solution using 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester. Sep. Purif. Technol. 2012, 86, 64–69. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. Solvent extraction of vanadium (V) from sulfate solutions using LIX63 and PC88A. J. Ind. Eng. Chem. 2015, 31, 118–123. [Google Scholar] [CrossRef]
- Cai, Z.; Feng, Y.; Li, H.; Zhou, Y. Selective separation and extraction of vanadium(IV) and manganese(II) from co-leaching solution of roasted stone coal and pyrolusite via solvent extraction. Ind. Eng. Chem. Res. 2013, 52, 13768–13776. [Google Scholar] [CrossRef]
- Tavakoli, M.R.; Dreisinger, D.B. Separation of vanadium from iron by solvent extraction using acidic and neutral organophosporus extractants. Hydrometallurgy 2014, 141, 17–23. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, D.; Zhu, B.; Yang, Y.; Wang, L. Adsorption and selective separation of neodymium with magnetic alginate microcapsules containing the extractant 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester. J.Chem. Eng. Data 2011, 56, 2280–2289. [Google Scholar] [CrossRef]
- Jayadas, S.; Reddy, M.L. Solvent extraction separation of vanadium(V) from multivalent metal chloride solutions using 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester. J. Chem. Technol. Biotechnol. 2002, 77, 1149–1156. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.; Lv, G.; Zhang, G.; Liu, Y.; Zhang, W. Synergistic extraction of vanadium(IV) in sulfuric acid media using amixture of D2EHPA and EHEHPA. Hydrometallurgy 2016, 166, 87–93. [Google Scholar] [CrossRef]
- Li, X.; Wei, C.; Deng, Z.; Li, M.; Li, C.; Fan, G. Selective solvent extraction of vanadium over iron from a stone coal/black shale acid leach solution by D2EHPA/TBP. Hydrometallurgy 2011, 105, 359–363. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).