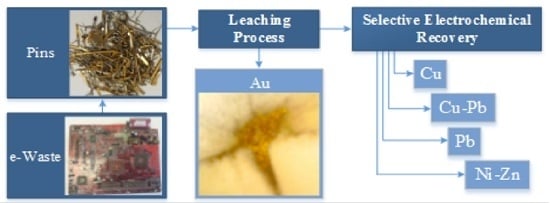
Urban Mining and Electrochemistry: Cyclic Voltammetry Study of Acidic Solutions from Electronic Wastes (Printed Circuit Boards) for Recovery of Cu, Zn, and Ni
Abstract
:1. Introduction
2. Materials and Methods
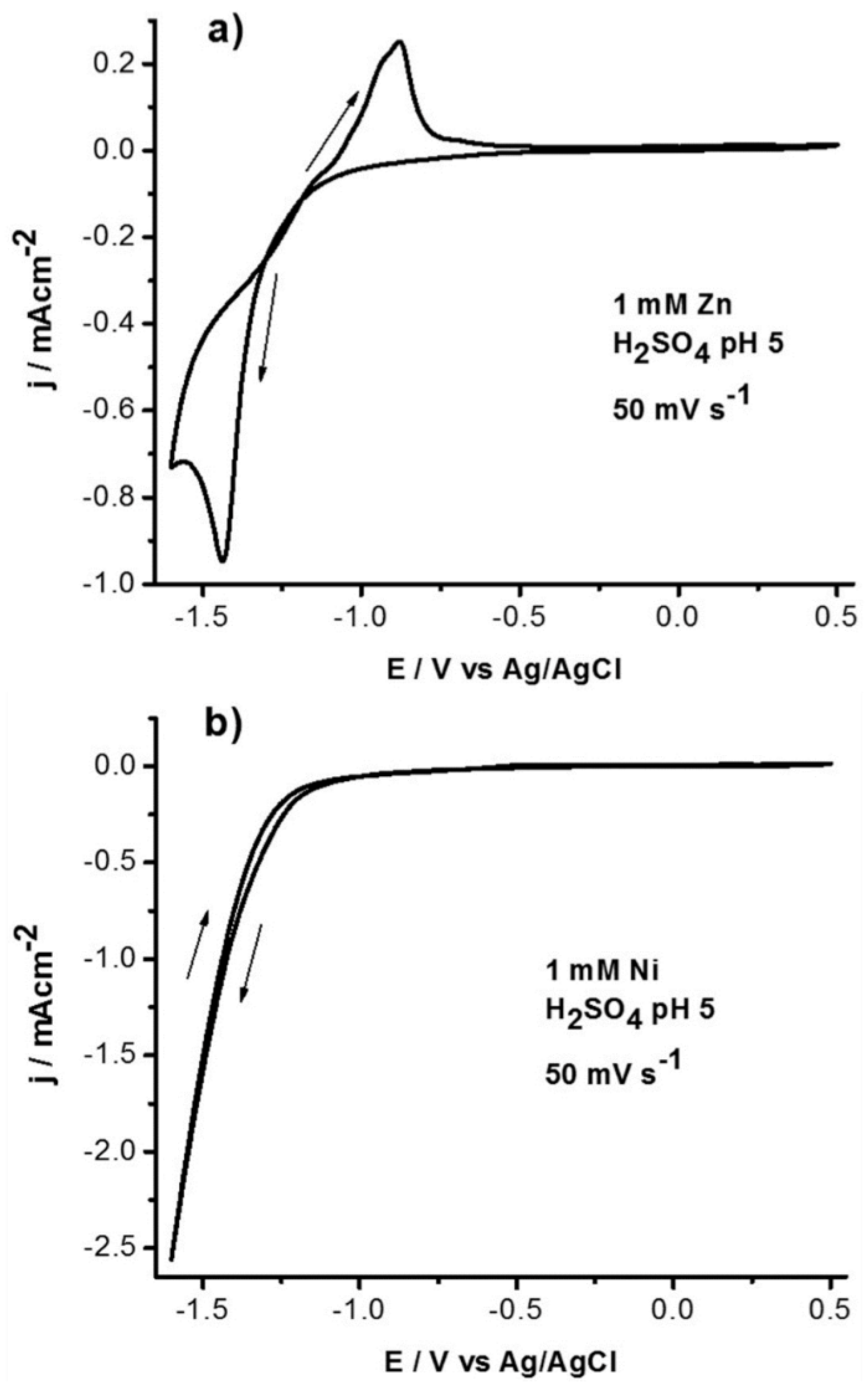
3. Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brunner, P.H. Urban mining—A contribution to reindustrializing the city. J. Ind. Ecol. 2011, 15, 339–341. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, L.; Vegliò, F.; Kopacek, B.; Beolchini, F. Environmental impact assessment of hydrometallurgical processes for metal recovery from residues using a portable prototype plant. Environ. Sci. Technol. 2013, 47, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Schoenung, J.M. Electronic waste recycling: A review of US infrastructure and technology options. Resour. Conserv. Recycl. 2005, 45, 368–400. [Google Scholar] [CrossRef]
- LaDou, J.; Lovegrove, S. Export of electronics waste. Int. J. Occup. Environ. Health 2008, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.W. Unfair trade: E-waste in Africa. Environ. Health Perspect. 2006, 114, A232–A235. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tanaka, M.; Matsui, Y. Generation amount prediction and material flow analysis of economic waste: A case study in Beijing, China. Waste Manag. Res. 2006, 24, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Morf, L.S.; Tremp, J.; Gloor, R.; Schuppisser, F.; Stengele, M.; Taverna, R. Metals, non-metals and PCB in electrical and electronic waste—Actual levels in Switzerland. Waste Manag. 2007, 27, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.H. E-waste: An assessment of global production and environmental impacts. Sci. Total Environ. 2009, 408, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Xiu, F.R.; Qi, Y.; Zhang, F.S. Co-treatment of waste printed circuit boards and polyvinyl chloride by subcritical water oxidation: Removal of brominated flame retardants and recovery of Cu and Pb. Chem. Eng. J. 2014, 237, 242–249. [Google Scholar] [CrossRef]
- Reyes, M.I.; Rivera, I.; Patiño, F.; Flores, M.U.; Reyes, M. Total recovery of gold contained in computer circuit boards. Leaching kinetics of Cu, Zn and Ni. J. Mex. Chem. Soc. 2012, 56, 144–148. [Google Scholar]
- Montiel, J.F.; Reyes, M.I.; Rivera, I.; Patiño, F.; Hernández, J. Caracterización de circuitos impresos vía SEM-EDS y su lixiviación en el sistema O2-H2SO4. Bol. Soc. Quim. Mex. 2012, 6, 21–23. [Google Scholar]
- Montiel, J.F.; Reyes, M.I.; Rivera, I.; Patiño, F.; Hernández, J. Recuperación de Au, Cu, Ni y Zn contenidos en desechos electrónicos. Bol. Soc. Quim. Mex. 2013, 7, 120. [Google Scholar]
- Hall, W.J.; Williams, P.T. Separation and recovery of materials from scrap printed circuit boards. Resour. Conserv. Recycl. 2007, 51, 691–709. [Google Scholar] [CrossRef]
- Li, N.; Lu, X.; Zhang, S. A novel reuse method for waste printed circuit boards as catalyst for wastewater bearing pyridine degradation. Chem. Eng. J. 2014, 257, 253–261. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Xie, H.; Zeng, X.; Li, J. Current status on leaching precious metals from waste printed circuit boards. Proced. Environ. Sci. 2012, 16, 560–568. [Google Scholar] [CrossRef]
- Xing, M.; Zhang, F.S. Degradation of brominated epoxy resin and metal recovery from waste printed circuits board through batch sub/supercritical water treatments. Chem. Eng. J. 2013, 219, 131–136. [Google Scholar] [CrossRef]
- Awal, M.R. New type mesoporous conjugate material for selective optical copper (II) ions monitoring & removal from polluted waters. Chem. Eng. J. 2017, 307, 85–94. [Google Scholar]
- Awal, M.R.; Hasan, M.M. Colorimetric detection and removal of copper (II) ions from wastewater samples using tailor-made composites adsorbent. Sens. Actuators B Chem. 2015, 2016, 692–700. [Google Scholar] [CrossRef]
- Awual, M.R. A novel facial composite adsorbent for enhanced copper (II) detection and removal from wastewater. Chem. Eng. J. 2015, 266, 368–375. [Google Scholar] [CrossRef]
- Awal, M.R.; Hasan, M.M.; Khaleque, M.A.; Sheikh, M.C. Treatment of copper (II) containing wastewater by a newly developed ligand based facial conjugate materials. Chem. Eng. J. 2016, 288, 368–376. [Google Scholar] [CrossRef]
- Liu, W.Q.; Shang, T.M.; Lei, W.N.; Zhou, Q.F. Progress in the study of recycling and innocuous treatment of waste printed circuit boards. Huanjing Kexue yu Jishu. 2011, 34, 48–54. [Google Scholar]
- Ningtao, Y.; Zhanxu, T.; Fahui, W. The Methods for Recycling of Waste Printed Circuits Boards. China Resour. Compr. Util. 2011, 7, 024. [Google Scholar]
- Yao, Y.Q.; Xu, X.P.; Liu, Y.; Qiu, X.Y. Review on the comprehensive recovery technologies of waste printed circuits boards. Mater. Res. Appl. 2011, 5, 17–20. [Google Scholar]
- Hadi, P.; Ning, C.; Ouyang, W.; Lin, C.S.K.; Hui, C.W.; McKay, G. Conversion of an aluminosilicate-based waste material to high-value efficient adsorbent. Chem. Eng. J. 2014, 256, 415–420. [Google Scholar] [CrossRef]
- Habbache, N.; Alane, N.; Djerad, S.; Tifouti, L. Leaching of copper oxide with different acid solutions. Chem. Eng. J. 2009, 152, 503–508. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, M.S.; Lee, J.C.; Pandey, B.D. Selective recovery of gold from waste mobile phone PCBs by hydrometallurgical process. J. Hazard. Mater. 2011, 198, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Kaminari, N.; Schultz, D.; Ponte, M.; Ponte, H.; Marino, C.; Neto, A. Heavy metals recovery from industrial wastewater using Taguchi method. Chem. Eng. J. 2007, 126, 139–146. [Google Scholar] [CrossRef]
- Lapicque, F.; Storck, A.; Wragg, A.A. Electrochemical Engineering and Energy; Plenum Press: New York, NY, USA, 2008; Volume 1. [Google Scholar]
- Kammel, R.; Lieber, H.W. Possibilities of treatment electroplating effluents without sludge formation. Pt. 3. metal recovery instead of sludge disposal. Galvanotechnik 1977, 68, 413–418. [Google Scholar]
- Kreysa, G. Festbettelektrolyse-ein Verfahren zur Reinigung metaahaltiger Abwässer. Chem. Ing. Tech. 1978, 50, 332–337. [Google Scholar] [CrossRef]
- Scott, K. Metal recovery using a moving-bed electrode. J. Appl. Electrochem. 1981, 11, 339–346. [Google Scholar] [CrossRef]
- Gao, J.; Liu, F.; Ling, P.; Lei, J.; Li, L.; Li, C.; Li, A. High efficient removal of Cu(II) by a chelating resin from strong acidic solutions: Complex formation and DFT certification. Chem. Eng. J. 2013, 222, 240–247. [Google Scholar] [CrossRef]
- Wong, E.T.; Chan, K.H.; Idris, A. Kinetic and equilibrium investigation of Cu (II) removal by Co(II)-doped iron oxide nanoparticle-immobilized in PVA-alginate recyclable adsorbent under dark and photo condition. Chem. Eng. J. 2015, 268, 311–324. [Google Scholar] [CrossRef]
- Espinoza, E.; Escudero, R.; Tavera, F.J. Waste water treatment by precipitating copper, lead and nickel species. Res. J. Recent Sci. 2012, 1, 1–6. [Google Scholar]
- Granados, M.N.; Huizar, L.H.M.; Rios-Reyes, C.H. Electrochemical study about zinc electrodeposition onto GCE and HOPG substrates. Quím. Nova 2011, 34, 439–443. [Google Scholar] [CrossRef]
- Montiel, J.H.; Reyes, M.I.; Rivera, I.; Rios-Reyes, C.; Rodríguez, V.; Patiño, F.; Reyes-Cruz, V. Thermodynamic study of leached metals (Cu, Zn and Ni) from waste printed circuits by electrochemical method. Adv. Mat. Res. 2014, 1, 86–89. [Google Scholar]
- Hadi, P.; Barford, J.; McKay, G. Synergistic effect in the simultaneous removal of binary cobalt-nickel heavy metals from effluents by a novel e-waste-derived material. Chem. Eng. J. 2013, 228, 140–146. [Google Scholar] [CrossRef]






| Element | Typical E-Waste Concentration (mg/kg) | Annual Global Emission in E-Waste (Tons) |
|---|---|---|
| Cadmium (Cd) | 180 | 3600 |
| Chromium (Cr) | 9900 | 198,000 |
| Copper (Cu) | 41,000 | 820,000 |
| Lead (Pb) | 2900 | 58,000 |
| Mercury (Hg) | 0.68 | 13.6 |
| Nickel (Ni) | 10,300 | 206,000 |
| Tin (Sn) | 2400 | 48,000 |
| Zinc (Zn) | 5100 | 102,000 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes‐Valderrama, M.I.; Salinas‐Rodríguez, E.; Montiel‐Hernández, J.F.; Rivera‐Landero, I.; Cerecedo‐Sáenz, E.; Hernándezvila, J.; Arenas‐Flores, A. Urban Mining and Electrochemistry: Cyclic Voltammetry Study of Acidic Solutions from Electronic Wastes (Printed Circuit Boards) for Recovery of Cu, Zn, and Ni. Metals 2017, 7, 55. https://doi.org/10.3390/met7020055
Reyes‐Valderrama MI, Salinas‐Rodríguez E, Montiel‐Hernández JF, Rivera‐Landero I, Cerecedo‐Sáenz E, Hernándezvila J, Arenas‐Flores A. Urban Mining and Electrochemistry: Cyclic Voltammetry Study of Acidic Solutions from Electronic Wastes (Printed Circuit Boards) for Recovery of Cu, Zn, and Ni. Metals. 2017; 7(2):55. https://doi.org/10.3390/met7020055
Chicago/Turabian StyleReyes‐Valderrama, Ma. Isabel, Eleazar Salinas‐Rodríguez, J. Fabian Montiel‐Hernández, Isauro Rivera‐Landero, Eduardo Cerecedo‐Sáenz, Juan Hernándezvila, and Alberto Arenas‐Flores. 2017. "Urban Mining and Electrochemistry: Cyclic Voltammetry Study of Acidic Solutions from Electronic Wastes (Printed Circuit Boards) for Recovery of Cu, Zn, and Ni" Metals 7, no. 2: 55. https://doi.org/10.3390/met7020055
APA StyleReyes‐Valderrama, M. I., Salinas‐Rodríguez, E., Montiel‐Hernández, J. F., Rivera‐Landero, I., Cerecedo‐Sáenz, E., Hernándezvila, J., & Arenas‐Flores, A. (2017). Urban Mining and Electrochemistry: Cyclic Voltammetry Study of Acidic Solutions from Electronic Wastes (Printed Circuit Boards) for Recovery of Cu, Zn, and Ni. Metals, 7(2), 55. https://doi.org/10.3390/met7020055