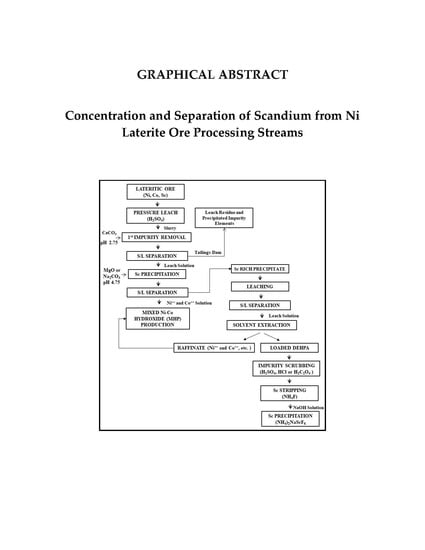
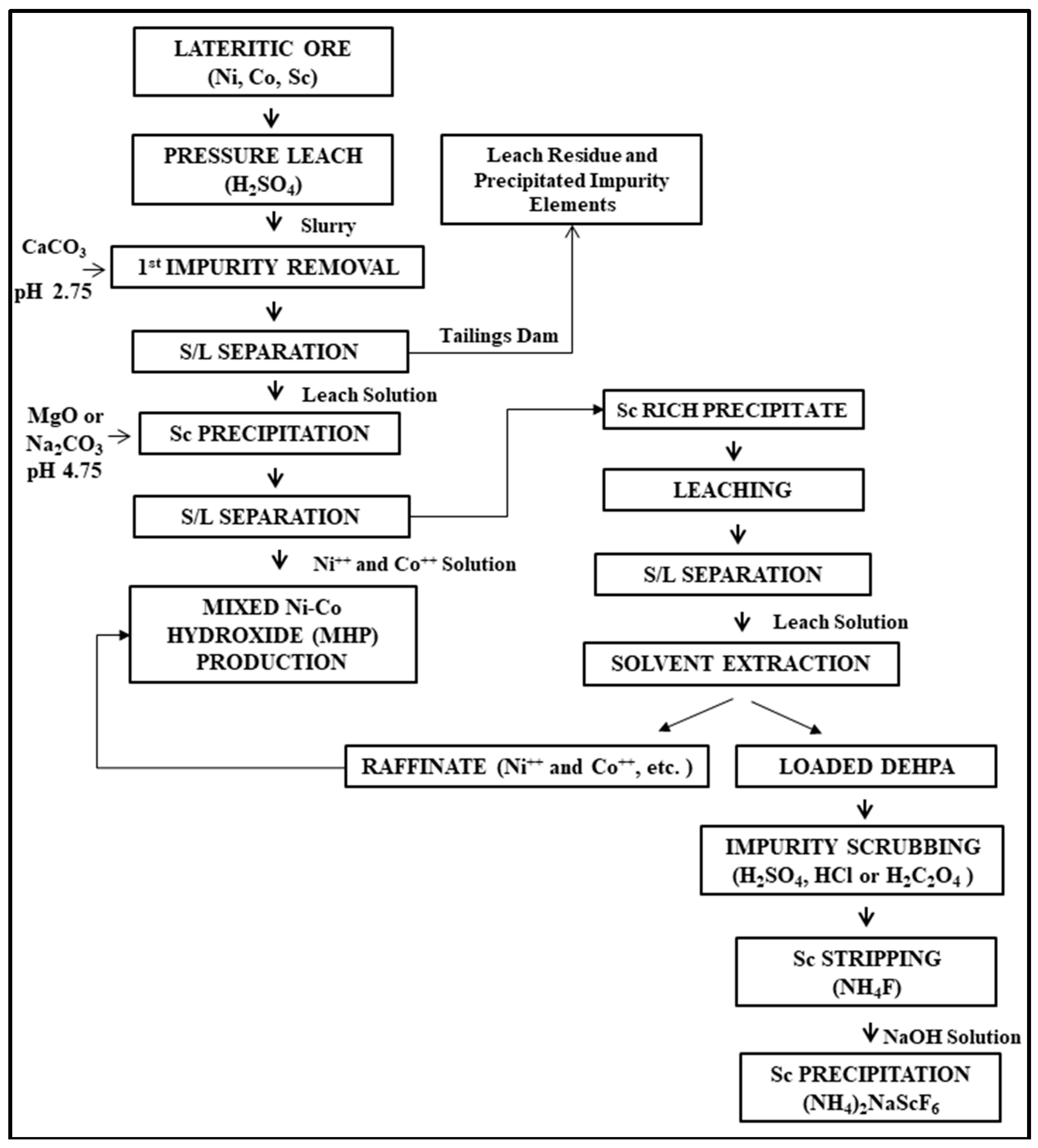
Concentration and Separation of Scandium from Ni Laterite Ore Processing Streams
Abstract
:1. Introduction
2. Previous Studies and State of the Art
3. Materials and Methods
4. Results and Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Riggall, S. Australian scandium supply—A paradigm shift for a strategic metal. In Proceedings of the Latest Word on Aerospace Materials, Long Beach, CA, USA, 11–14 May 2015. [Google Scholar]
- The Critical Metals Report. Richard Karn: Australian Scandium Could Create New Market. Streetwise Reports. Available online: https://www.streetwisereports.com/pub/na/richard-karn-australian-scandium-could-create-new-market (accessed on 8 September 2011).
- Xu, S.; Li, S. Review of the extractive metallurgy of scandium in China. Hydrometallurgy 1996, 42, 337–343. [Google Scholar]
- Ricketts, N.; Duyvesteyn, W. The current status of scandium supply projects and their technical challenges. In Proceedings of the ALTA’17 Nickel-Cobalt-Copper Conference, Perth, Australia, 20–27 May 2017. [Google Scholar]
- Chasse, M.; Griffin, W.L.; O’Reilly, S.Y.; Calas, G. Scandium speciation in a world-class lateritic deposit. Geochem. Perspect. Lett. 2017, 105–114. [Google Scholar] [CrossRef]
- Kaya, Ş.; Topkaya, Y.A. Extraction behaviour of scandium from a refractory nickel laterite ore during the pressure acid leaching process. In Rare Earths Industry: Technological, Economic and Environmental Implications; de Lima, I.B., Filho, W.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 171–181. [Google Scholar]
- Kaya, Ş.; Topkaya, Y.A.; Dittrich, C. Hydrometallurgical Extraction of Scandium from Lateritic Nickel Ores. In Proceedings of the Bauxite Residue Valorisation and Best Practices Conference BR 2015, Leuven, Belgium, 5–7 October 2015; pp. 347–354. [Google Scholar]
| Element | Ni | Co | Sc | Fe | Al | Cr | Mn | Mg | Cu | Zn | Ca |
| Initial Leach Solution (mg/L) | 5827 | 371 | 30 | 1814 | 4317 | 150 | 2056 | 1369 | 29 | 74 | - |
| After First Impurity Removal (mg/L) | 4650 | 268 | 23 | 227 | 3599 | 98 | 1905 | 1211 | 28 | 67 | 570 |
| Prepared Synthetic Solution (mg/L) | 4919 | 266 | 20 | 102 | 3279 | 55 | 2244 | 3277 | 141 | 76 | 487 |
| Experiment | Analysis of the Initial Solution at pH 2.75 | |||||||||||
| Precipitation Reagent | Fe | Al | Cr | Ni | Co | Ca | Mg | Na | Sc | Cu | Zn | Mn |
| mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | |
| - | 102 | 3279 | 55 | 4919 | 266 | 487 | 3277 | 88 | 20 | 141 | 76 | 2242 |
| Experiment | Analysis of the Solution after Precipitation at pH 4.75 | |||||||||||
| Precipitation Reagent | Fe | Al | Cr | Ni | Co | Ca | Mg | Na | Sc | Cu | Zn | Mn |
| mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | |
| CaCO3 | <1 | <1 | <1 | 3863 | 208 | 596 | 2880 | 78 | <1 | 1 | 23 | 1870 |
| Na2CO3 | <1 | <1 | <1 | 3840 | 209 | 420 | 2800 | 8602 | <1 | 4 | 34 | 1865 |
| MgO | <1 | <1 | <1 | 4040 | 218 | 486 | 7642 | 93 | <1 | 6 | 34 | 1952 |
| NaOH | <1 | <1 | 0 | 3102 | 166 | 348 | 2460 | 7043 | <1 | 2 | 20 | 1635 |
| Experiment | Analysis of the Obtained Precipitate at pH 4.75 | |||||||||||
| Precipitation Reagent | Fe | Al | Cr | Ni | Co | Ca | Mg | Na | Sc | Cu | Zn | Mn |
| wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | |
| CaCO3 | 0.43 | 0.71 | 0.11 | 1.74 | 0.06 | 15.6 | 0.05 | <0.02 | 0.04 | 0.22 | 0.08 | 0.03 |
| Na2CO3 | 1.01 | 2.06 | 0.29 | 4.48 | 0.15 | 0.04 | 0.26 | 0.63 | 0.10 | 0.63 | 0.11 | 0.20 |
| MgO | 0.91 | 1.85 | 0.22 | 3.39 | 0.12 | 0.03 | 1.55 | <0.02 | 0.09 | 0.57 | 0.14 | 0.22 |
| NaOH | 0.73 | 1.78 | 0.28 | 6.84 | 0.28 | 0.11 | 0.82 | 1.80 | 0.09 | 0.59 | 0.20 | 0.68 |
| Reagent Used | Fe | Al | Cr | Ni | Co | Ca | Mg | Na | Sc | Cu | Zn | Mn | Reagent Consumed | Precipitate Obtained |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | % | % | % | % | % | % | g/L soln. | g/L soln. | |
| CaCO3 | 100 | 100 | 100 | 6.5 | 6.9 | - | - | - | 100 | 99.1 | 64.3 | 0.7 | 23.7 | 57.7 |
| Na2CO3 | 100 | 100 | 100 | 8.7 | 8.1 | - | - | - | 100 | 97.1 | 48.3 | 1.7 | 21.7 | 20.4 |
| MgO | 100 | 100 | 100 | 2.3 | 2.5 | - | - | - | 100 | 94.8 | 46.6 | 1.6 | 11.6 | 25.2 |
| NaOH | 100 | 100 | 100 | 11.7 | 12.6 | - | - | - | 100 | 98.5 | 62.6 | 2.1 | 17.0 | 23.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, Ş.; Dittrich, C.; Stopic, S.; Friedrich, B. Concentration and Separation of Scandium from Ni Laterite Ore Processing Streams. Metals 2017, 7, 557. https://doi.org/10.3390/met7120557
Kaya Ş, Dittrich C, Stopic S, Friedrich B. Concentration and Separation of Scandium from Ni Laterite Ore Processing Streams. Metals. 2017; 7(12):557. https://doi.org/10.3390/met7120557
Chicago/Turabian StyleKaya, Şerif, Carsten Dittrich, Srecko Stopic, and Bernd Friedrich. 2017. "Concentration and Separation of Scandium from Ni Laterite Ore Processing Streams" Metals 7, no. 12: 557. https://doi.org/10.3390/met7120557
APA StyleKaya, Ş., Dittrich, C., Stopic, S., & Friedrich, B. (2017). Concentration and Separation of Scandium from Ni Laterite Ore Processing Streams. Metals, 7(12), 557. https://doi.org/10.3390/met7120557