Trajectory Analysis of Copper and Glass Particles in Electrostatic Separation for the Recycling of ASR
Abstract
:1. Introduction
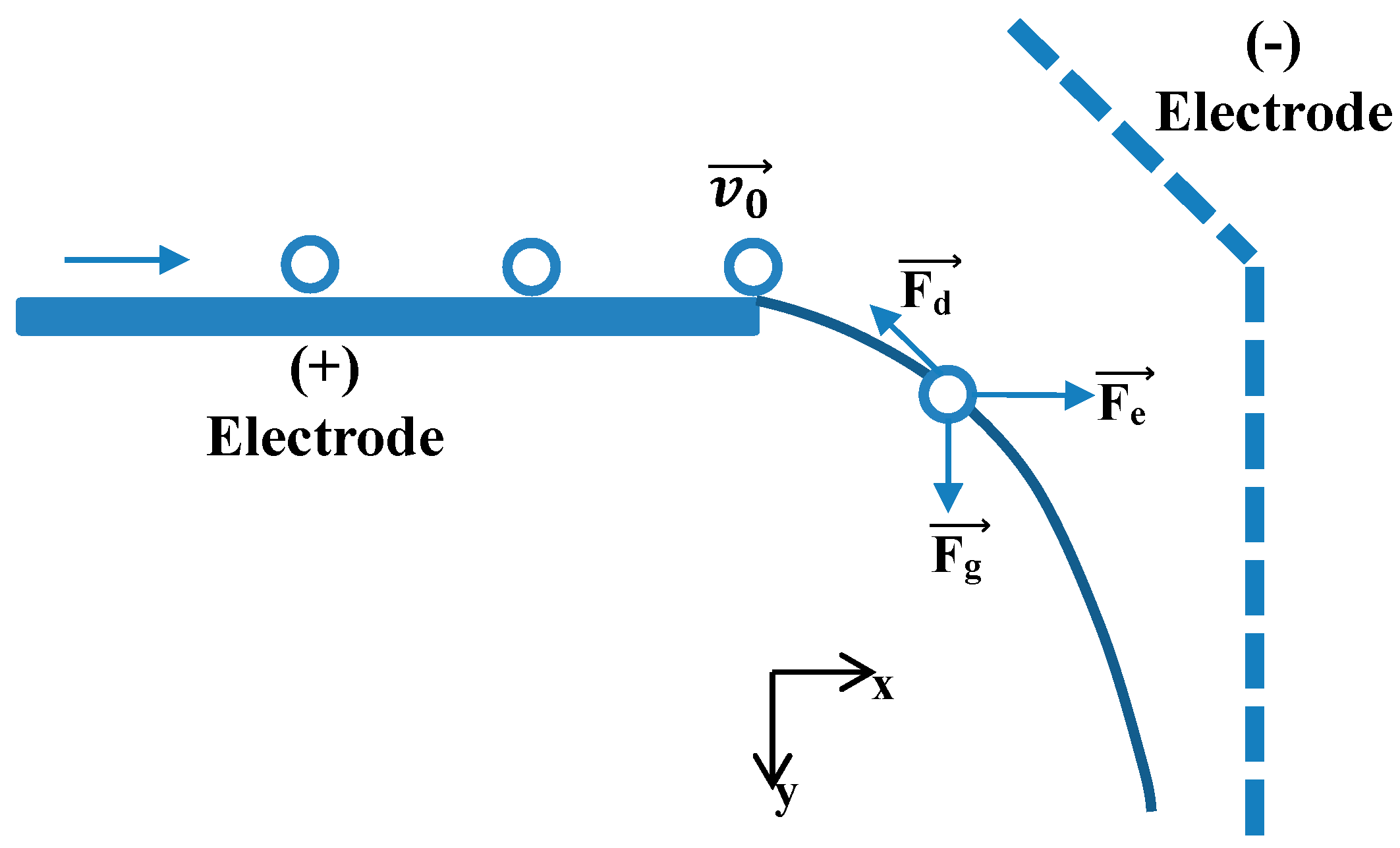
2. Theoretical Aspects
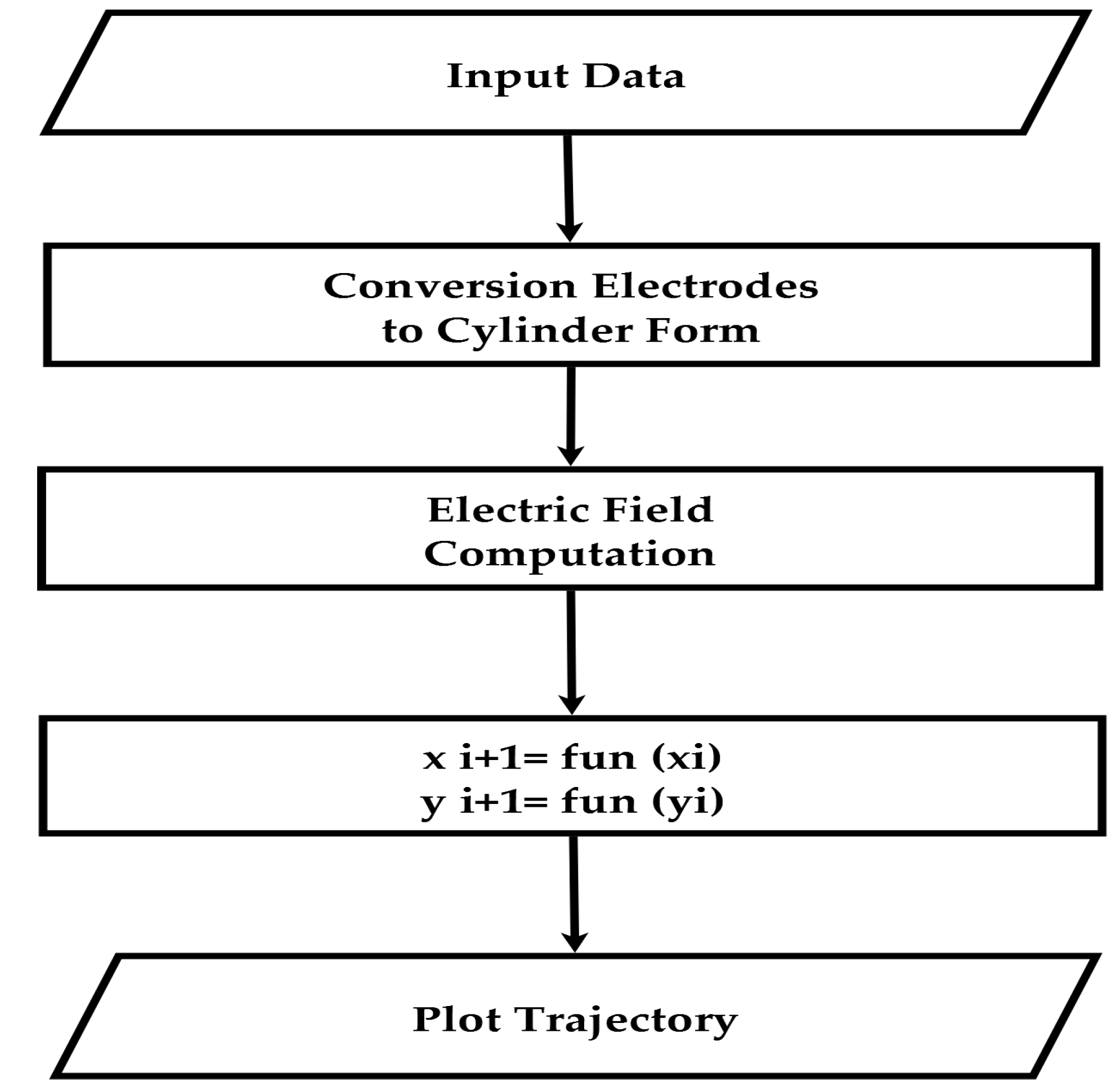
3. Computation Method
3.1. Calculation of Electric Field Strength
3.2. Particle Trajectory Computation
- The two electrodes are interpreted as being cylindrical.
- Particle shapes were a perfect cylinder or sphere model with a radius of () and a density of (ρ).
- Particles were instantly charged using electrostatic induction with saturation charge.
- Depending on the particle shape, the saturation charge was calculated using Félici’s formula [23].
- The intervals between particles were large enough that the particles did not affect one another.
- The electric force before detachment is expressed as shown in Equation (5).
- The electric force after detachment is expressed as shown in Equation (6).
- The tribocharging effects between particles or between particle and electrode are ignored.
4. Materials and Methods
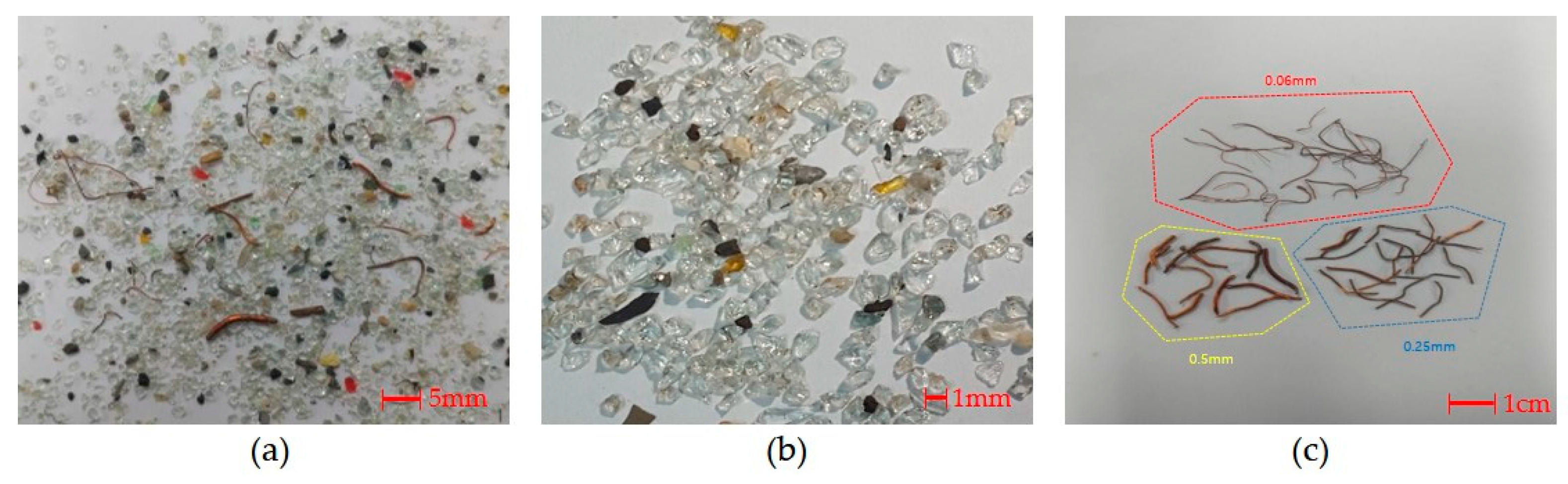
4.1. Materials
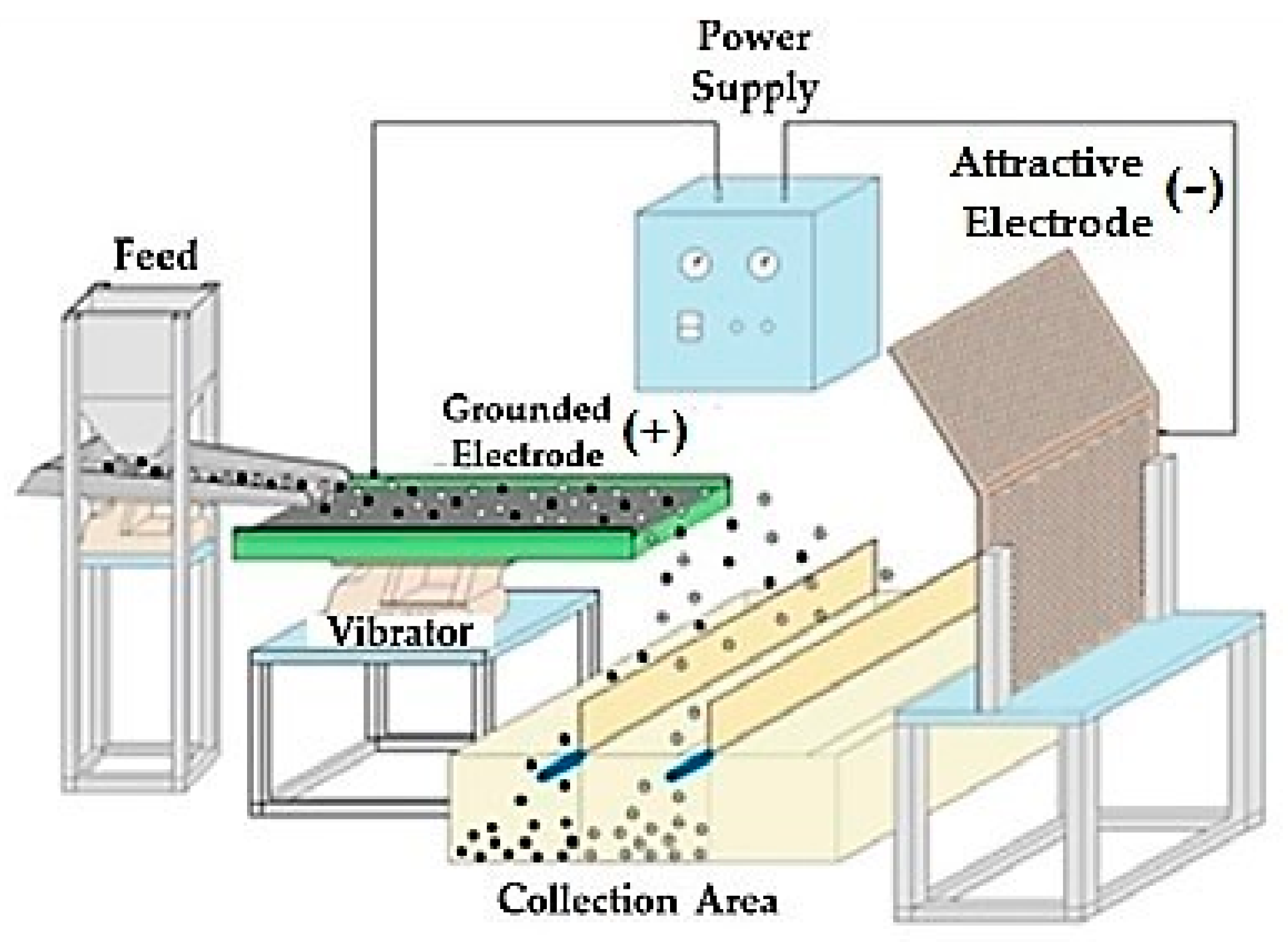
4.2. Methods
5. Results and Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| Electric field strength | |
| Horizontal Electric field strength | |
| Vertical Electric field strength | |
| Air drag force | |
| Electric force | |
| Gravity force | |
| Resultant force | |
| Acceleration due to gravitation | |
| The distance between two electrodes | |
| Length of section of attractive cylindrical electrode | |
| Length of section of grounded electrode | |
| Length of the particle | |
| Mass of the particle | |
| Quantity of electricity | |
| Radius of imagined attractive cylindrical electrode | |
| Radius of imagined grounded roll electrode | |
| Radius of the particle | |
| Applied high voltage | |
| Velocity of particle | |
| Initial velocity of the particle | |
| Greek letters | |
| Included angle of horizontal line and electrodes shortest line (°) | |
| Relative dielectric constant of air (1.00059) | |
| Dielectric constant of vacuum | |
| Air drag coefficient | |
| Mass density of the particle | |
References
- Cossu, R.; Lai, T. Automotive shredder residue (ASR) management: An overview. Waste Manag. 2015, 45, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Passarini, F.; Ciacci, L.; Santini, A.; Vassura, I.; Morselli, L. Aluminium flows in vehicles: Enhancing the recovery at end-of-life. J. Mater. Cycles Waste Manag. 2014, 16, 39–45. [Google Scholar] [CrossRef]
- Santini, A.; Passarini, F.; Vassura, I.; Serrano, D.; Dufour, J.; Morselli, L. Auto shredder residue recycling: Mechanical separation and pyrolysis. Waste Manag. 2012, 32, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.; Soulliere, K.; Sawyer-Beaulieu, S.; Tseng, S.; Tam, E. Challenges and alternatives to plastics recycling in the automotive sector. Materials 2014, 7, 5883–5902. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Envrionment of Korea. Available online: http://www.law.go.kr/lsInfoP.do?lsiSeq=188594&urlMode=engLsInfoR&viewCls=engLsInfoR#0000 (accessed on 30 August 2017).
- Tripathy, S.K.; Ramamurthy, Y.; Kumar, C.R. Modeling of high-tension roll separator for separation of titanium bearing minerals. Powder Technol. 2010, 201, 181–186. [Google Scholar] [CrossRef]
- Tilmatine, A.; Medles, K.; Bendimerad, S.-E.; Boukholda, F.; Dascalescu, L. Electrostatic separators of particles: Application to plastic/metal, metal/metal and plastic/plastic mixtures. Waste Manag. 2009, 29, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, J.; Xu, Z. Electrostatic separation for recovering metals and nonmetals from waste printed circuit board: Problems and improvements. Environ. Sci. Technol. 2008, 42, 5272–5276. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Jeon, H.S.; Yu, H.S.; Han, O.H.; Park, J.K. Application of electrostatic separation to the recycling of plastic wastes: Separation of PVC, PET, and ABS. Environ. Sci. Technol. 2007, 42, 249–255. [Google Scholar] [CrossRef]
- Dascalescu, L.; Mizuno, A.; Tobazeon, R.; Atten, P.; Morar, R.; Iuga, A.; Mihailescu, M.; Samuila, A. Charges and forces on conductive particles in roll-type corona-electrostatic separators. IEEE Trans. Ind. Appl. 1995, 31, 947–956. [Google Scholar] [CrossRef]
- Vlad, S.; Iuga, A.; Dascalescu, L. Modelling of conducting particle behaviour in plate-type electrostatic separators. J. Phys. D Appl. Phys. 2000, 33, 127. [Google Scholar] [CrossRef]
- Vlad, S.; Mihailescu, M.; Rafiroiu, D.; Iuga, A.; Dascalescu, L. Numerical analysis of the electric field in plate-type electrostatic separators. J. Electrost. 2000, 48, 217–229. [Google Scholar] [CrossRef]
- Vlad, S.; Urs, A.; Iuga, A.; Dascalescu, L. Premises for the numerical computation of conducting particle trajectories in plate-type electrostatic separators. J. Electrost. 2001, 51, 259–265. [Google Scholar] [CrossRef]
- Vlad, S.; Iuga, A.; Dascalescu, L. Numerical computation of conducting particle trajectories in plate-type electrostatic separators. IEEE Trans. Ind. Appl. 2003, 39, 66–71. [Google Scholar] [CrossRef]
- Caron, A.; Dascalescu, L. Numerical modeling of combined corona-electrostatic fields. J. Electrost. 2004, 61, 43–55. [Google Scholar] [CrossRef]
- Labair, H.; Touhami, S.; Tilmatine, A.; Hadjeri, S.; Medles, K.; Dascalescu, L. Study of charged particles trajectories in free-fall electrostatic separators. J. Electrost. 2017, 88, 10–14. [Google Scholar] [CrossRef]
- Richard, G.; Salama, A.R.; Medles, K.; Lubat, C.; Touhami, S.; Dascalescu, L. Experimental and numerical study of the electrostatic separation of two types of copper wires from electric cable wastes. IEEE Trans. Ind. Appl. 2017, 53, 3960–3969. [Google Scholar] [CrossRef]
- Richard, G.; Touhami, S.; Zeghloul, T.; Dascalescu, L. Optimization of metals and plastics recovery from electric cable wastes using a plate-type electrostatic separator. Waste Manag. 2017, 60, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, H.; Xu, Z.; Zhou, Y. A model for computing the trajectories of the conducting particles from waste printed circuit boards in corona electrostatic separators. J. Hazard. Mater. 2008, 151, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Z.; Zhou, Y. Theoretic model and computer simulation of separating mixture metal particles from waste printed circuit board by electrostatic separator. J. Hazard. Mater. 2008, 153, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, J.; Guo, J.; Xu, Z. Movement behavior in electrostatic separation: Recycling of metal materials from waste printed circuit board. J. Mater. Process. Technol. 2008, 197, 101–108. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Xu, Z. An improved model for computing the trajectories of conductive particles in roll-type electrostatic separator for recycling metals from weee. J. Hazard. Mater. 2009, 167, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Félici, N.J. Forces et charges de petits objets en contact avec une électrode affectée ďun champ électrique. Rev. Gén. Elec. 1966, 75, 1145–1160. [Google Scholar]
- Angelov, A.; Vereshyagin, I.; Yershov, V. Physical Basis at Electrostatic Separation; Moscow Geology Press: Moscow, Russia, 1983. [Google Scholar]
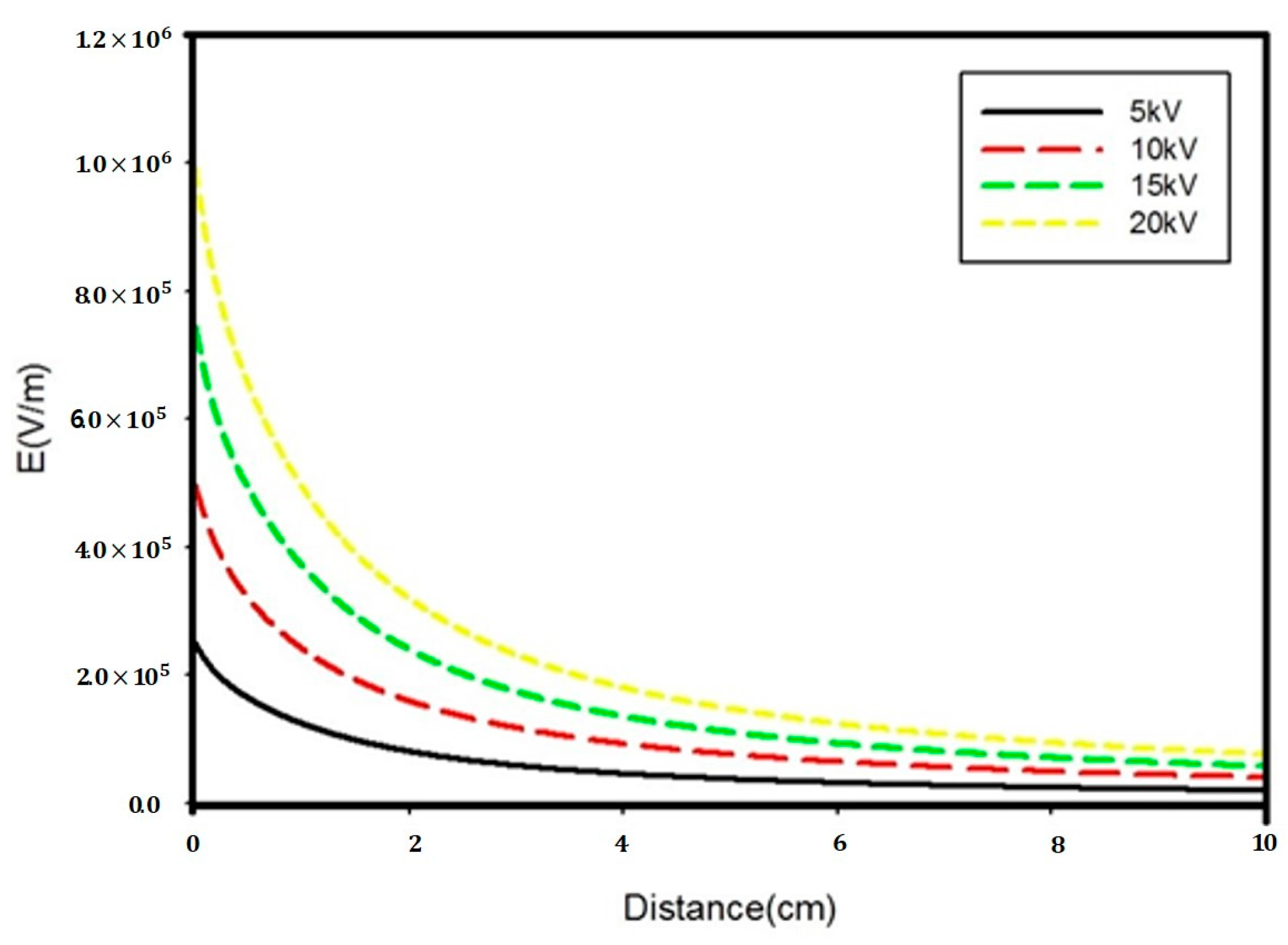

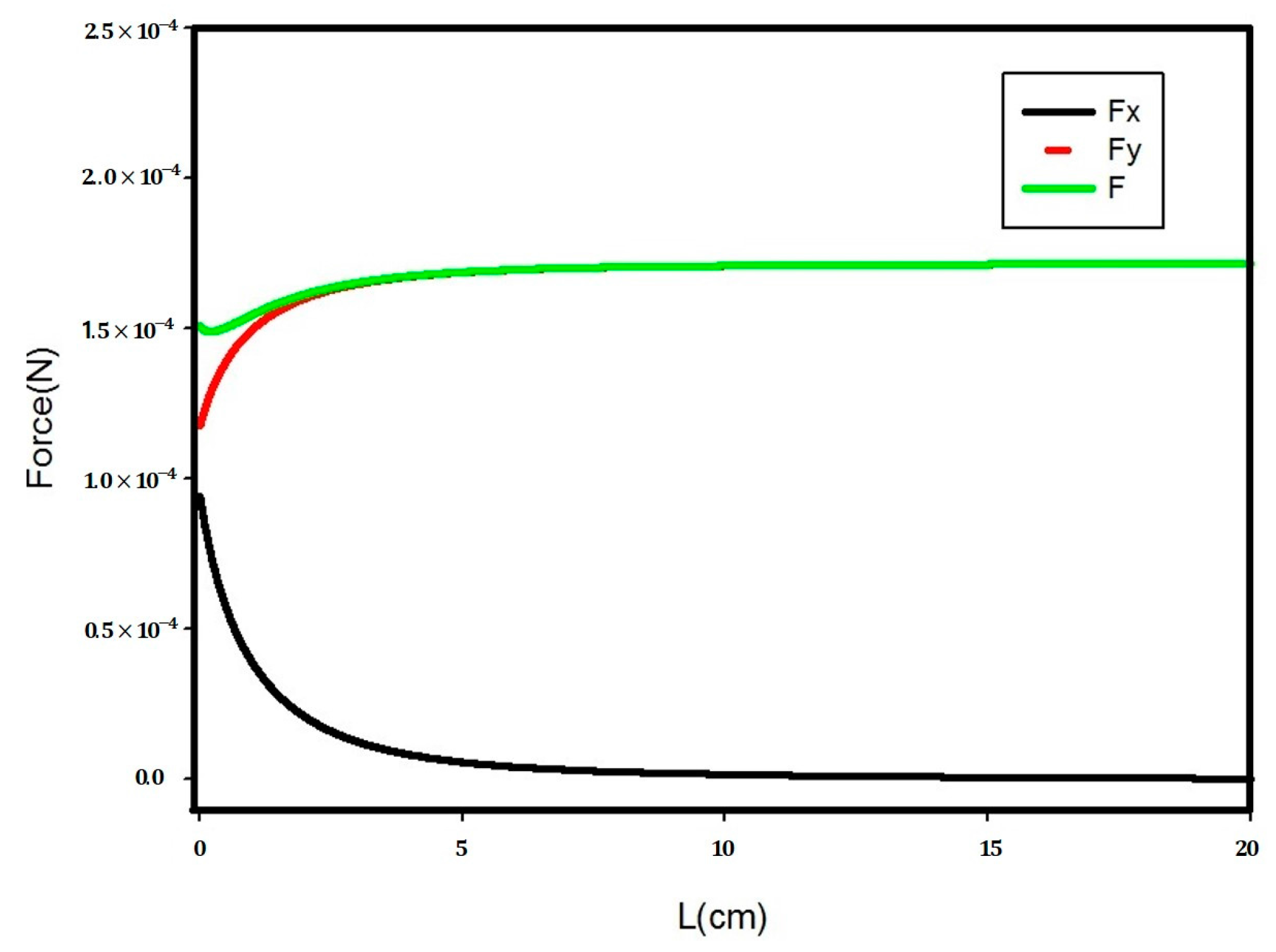
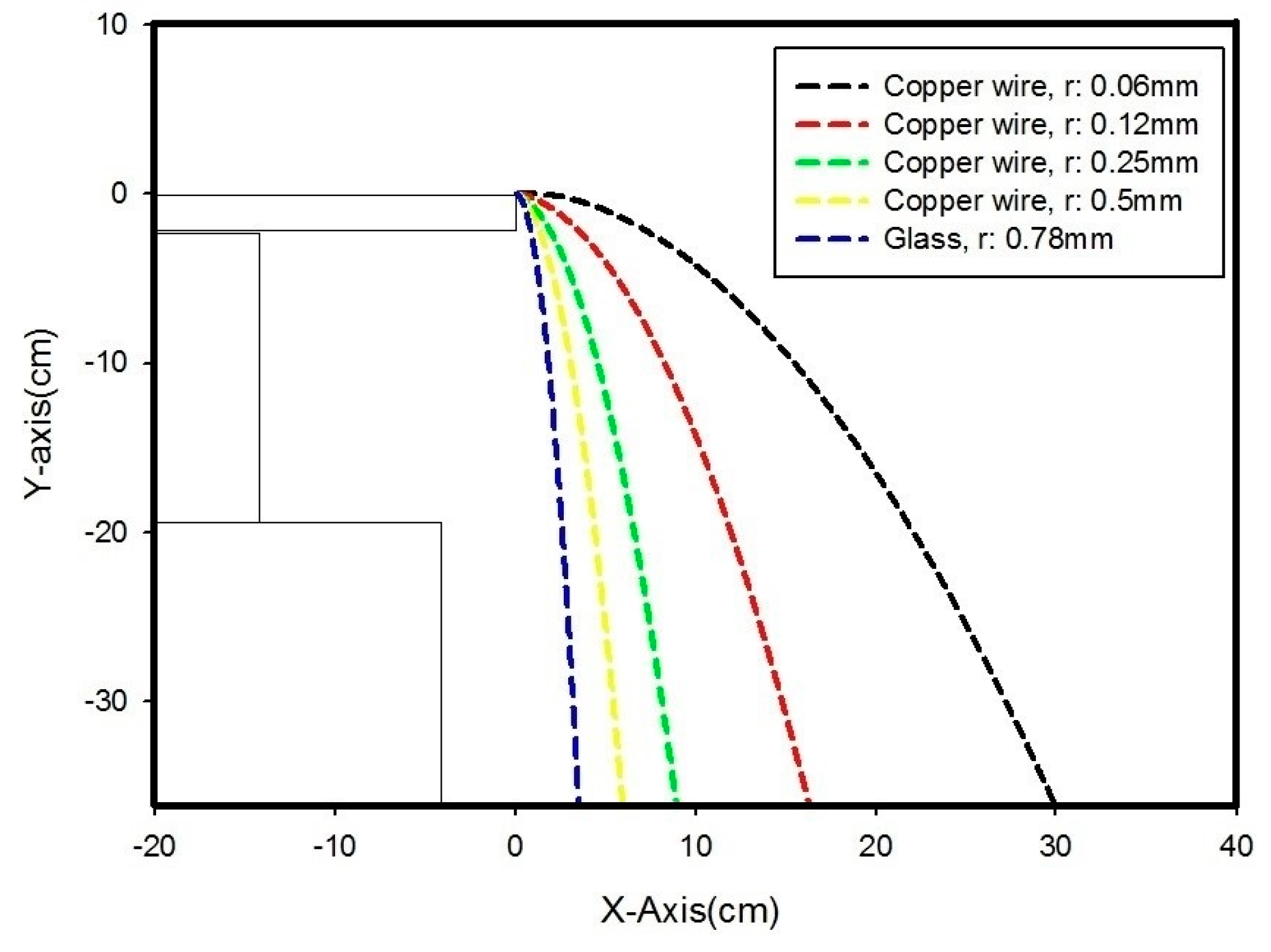
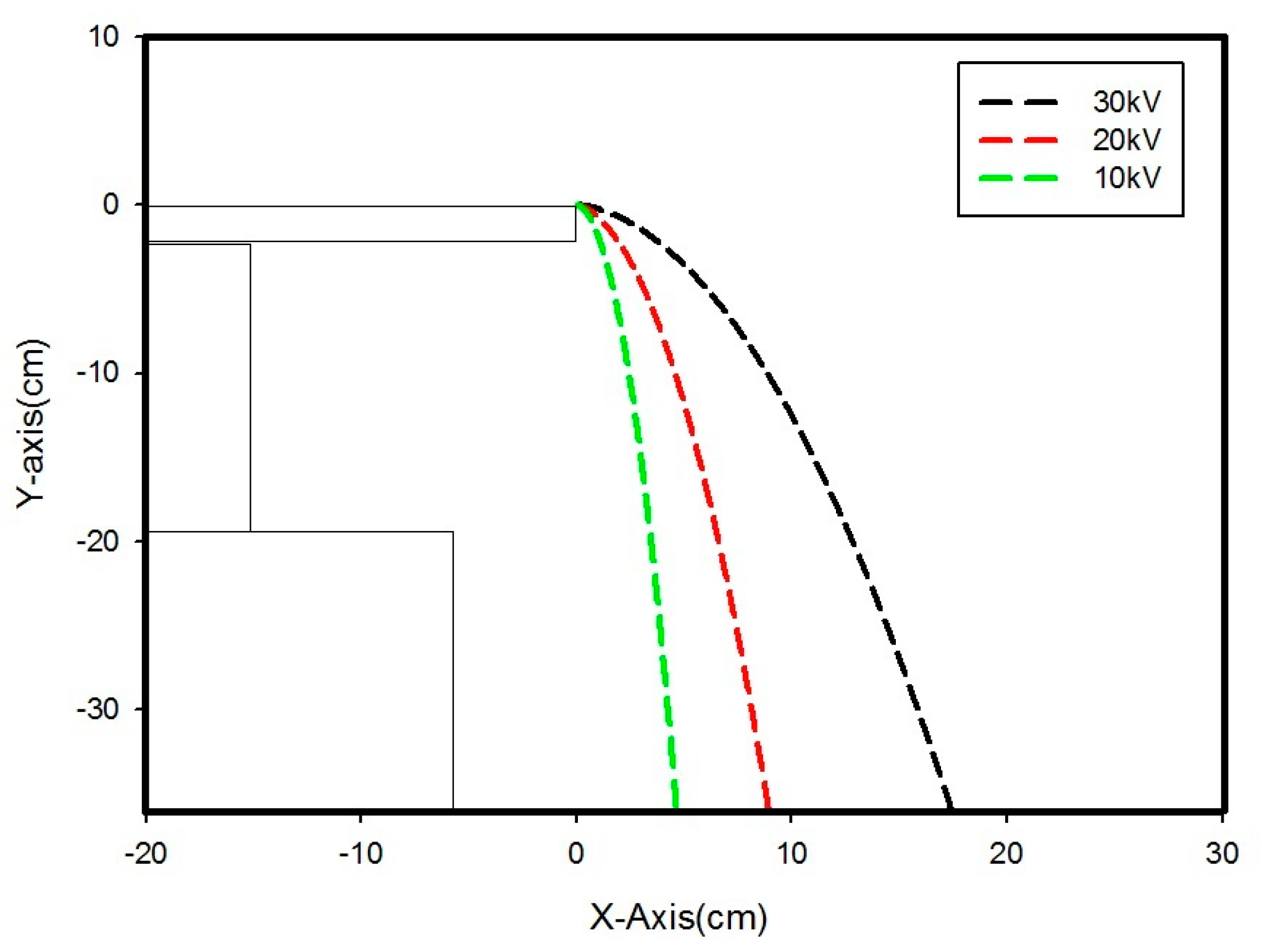

| Author | Sample | Type of Separator | Year |
|---|---|---|---|
| L. Dascalescu et al. [10] | Artificial Sample | Roll-type Corona Electrostatic Separator | 1995 |
| S. Vlad et al. [13] | none | Plate-type Electrostatic Separator | 2001 |
| S. Vlad et al. [14] | Artificial Sample | Plate-type Electrostatic Separator | 2003 |
| H. Labair et al. [16] | ABS 1, PVC 2 | Free-Fall Electrostatic Separator | 2017 |
| G. Richard et al. [17] | Electric Cable Waste | Roll-type Electrostatic Separator | 2017 |
| G. Richard et al. [18] | Electric Cable Waste | Plate-type Electrostatic Separator | 2017 |
| J. Li [19] | PCB 3 | Roll-type Electrostatic Separator | 2008 |
| H. Lu et al. [21] | PCB | Roll-type Electrostatic Separator | 2008 |
| J. Wu et al. [22] | PCB | Roll-type Electrostatic Separator | 2009 |
| Experimental Conditions | |||
|---|---|---|---|
| Feed rate (g·min−1) | 50 | ||
| Temperature (°C) | 29 | ||
| Humidity (%) | 35–45 | ||
| Initial Speed (m·s−1) | 0.13 | ||
| High Voltage (kV) | 5, 10, 15, 20 | ||
| Distance between Two Electrodes (m) | 0.6 | ||
| Particle Size | Copper | Diameter (m) | 0.00012, 0.0005, 0.001 |
| Length(m) | 0.01 | ||
| Glass | Diameter(m) | 0.00014 | |
| Material | R (mm) | U (kV) | Experimental Observation (cm) | Computation (cm) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | Ave. | ||||
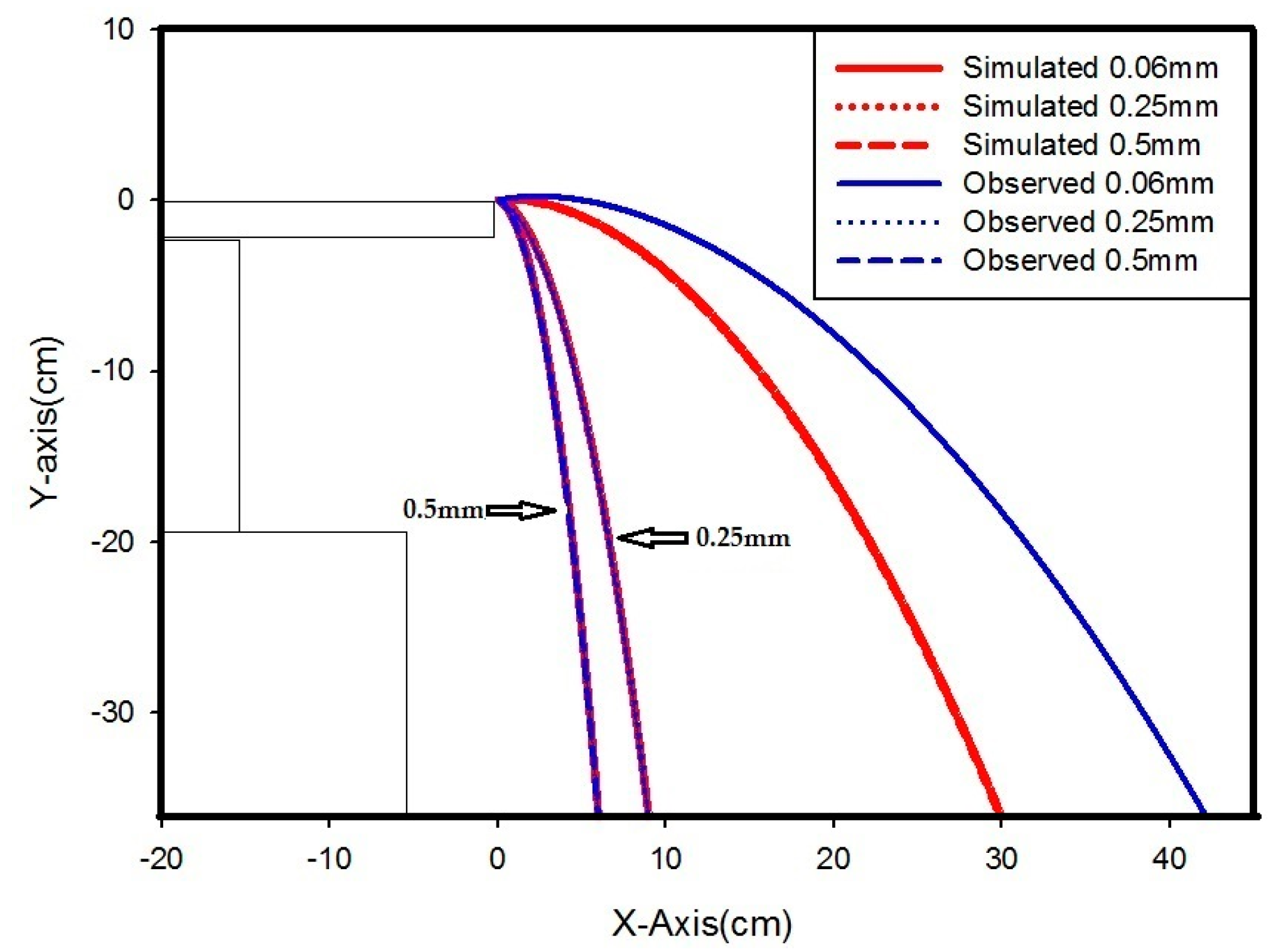
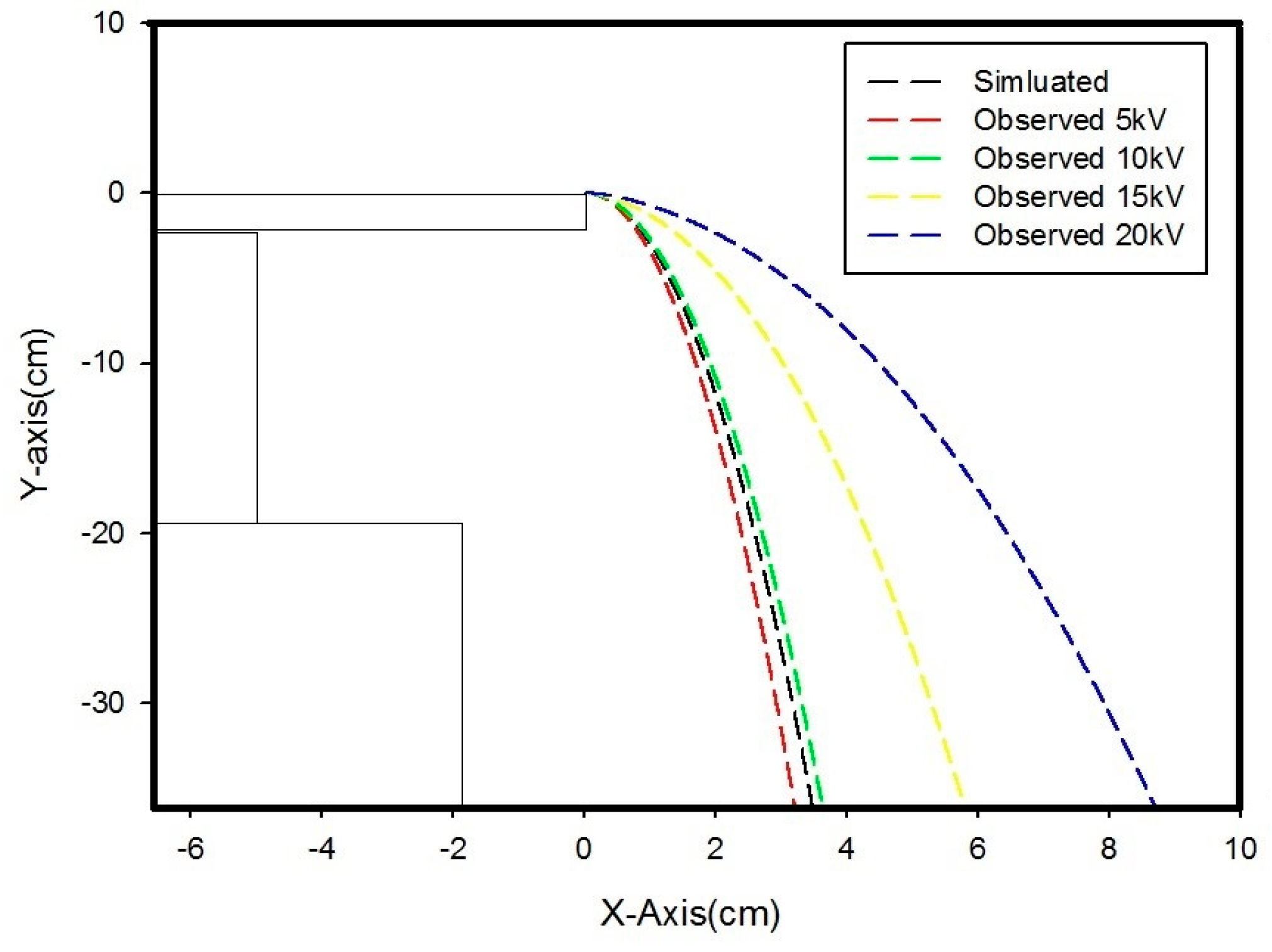
| Copper | 0.06 | 5 | 4.3 | 4.7 | 4.8 | 4.9 | 5.7 | 4.88 | 4.72 |
| 10 | 13.8 | 15.3 | 16.5 | 17.7 | 18.4 | 16.34 | 9.20 | ||
| 15 | 30 | 34 | 35 | 40 | 40.5 | 35.9 | 18.06 | ||
| 20 | 38.3 | 42 | 42.1 | 44 | 46 | 42.48 | 29.88 | ||
| 0.25 | 5 | 3.9 | 4 | 3.9 | 4.1 | 4.3 | 4.04 | 3.80 | |
| 10 | 4.4 | 5 | 5.1 | 5.1 | 5.5 | 5.02 | 4.67 | ||
| 15 | 5.7 | 6.2 | 6.1 | 6.3 | 6.8 | 6.22 | 6.31 | ||
| 20 | 8 | 9 | 9 | 9.4 | 9.5 | 8.98 | 8.94 | ||
| 0.5 | 5 | 0.2 | 1 | 2 | 2.6 | 2.5 | 1.66 | 3.66 | |
| 10 | 2.4 | 2.2 | 2.8 | 3.2 | 3 | 2.72 | 4.08 | ||
| 15 | 4.5 | 4.2 | 4.3 | 3.8 | 2.8 | 3.92 | 4.82 | ||
| 20 | 4.4 | 6 | 5 | 5 | 5.2 | 5.12 | 5.97 | ||
| Glass | 0.78 | 5 | 3.6 | 2.4 | 2.4 | 2.3 | 2 | 2.54 | 3.52 |
| 10 | 3.1 | 3.2 | 3.6 | 3.8 | 4.2 | 3.58 | 3.52 | ||
| 15 | 5.8 | 5.9 | 6.4 | 6.5 | 7.4 | 6.4 | 3.52 | ||
| 20 | 8.7 | 9.2 | 10.7 | 11.5 | 12.2 | 10.46 | 3.52 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.-u.; Han, O.-h.; Jeon, H.-s.; Baek, S.-h.; Park, C.-h. Trajectory Analysis of Copper and Glass Particles in Electrostatic Separation for the Recycling of ASR. Metals 2017, 7, 434. https://doi.org/10.3390/met7100434
Kim B-u, Han O-h, Jeon H-s, Baek S-h, Park C-h. Trajectory Analysis of Copper and Glass Particles in Electrostatic Separation for the Recycling of ASR. Metals. 2017; 7(10):434. https://doi.org/10.3390/met7100434
Chicago/Turabian StyleKim, Beom-uk, Oh-hyung Han, Ho-seok Jeon, Sang-ho Baek, and Chul-hyun Park. 2017. "Trajectory Analysis of Copper and Glass Particles in Electrostatic Separation for the Recycling of ASR" Metals 7, no. 10: 434. https://doi.org/10.3390/met7100434
APA StyleKim, B.-u., Han, O.-h., Jeon, H.-s., Baek, S.-h., & Park, C.-h. (2017). Trajectory Analysis of Copper and Glass Particles in Electrostatic Separation for the Recycling of ASR. Metals, 7(10), 434. https://doi.org/10.3390/met7100434




