Application of Co and Mn for a Co-Mn-Br or Co-Mn-C2H3O2 Petroleum Liquid Catalyst from the Cathode Material of Spent Lithium Ion Batteries by a Hydrometallurgical Route
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solvent Extraction for Cobalt-Manganese-Bromide (CMB) and Cobalt-Manganese-Acetate (CMA) Aqueous Catalysts
3. Results and Discussion
3.1. Physical Separation of Cathodic Material Scrap
3.2. Leaching and Removal of Al
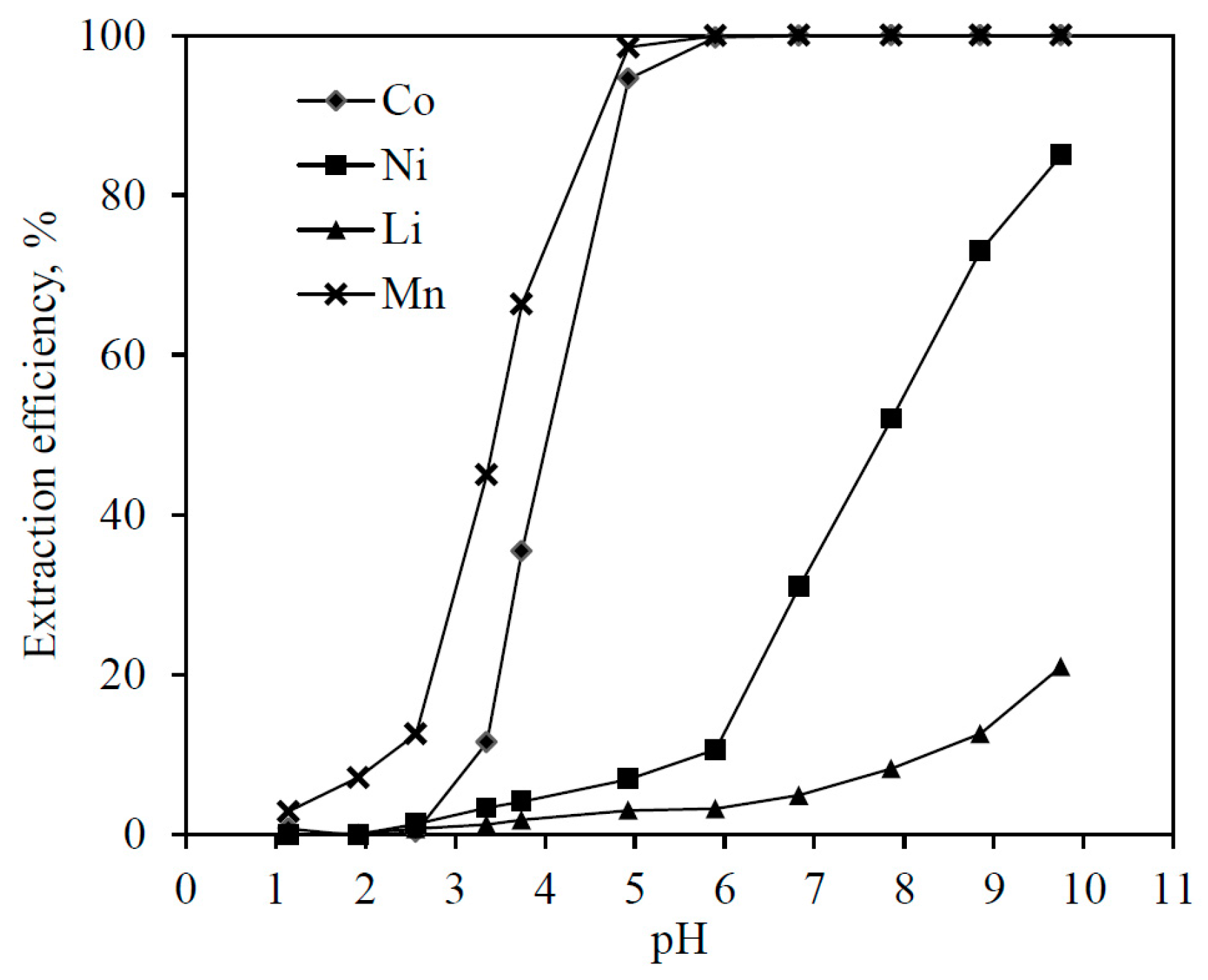
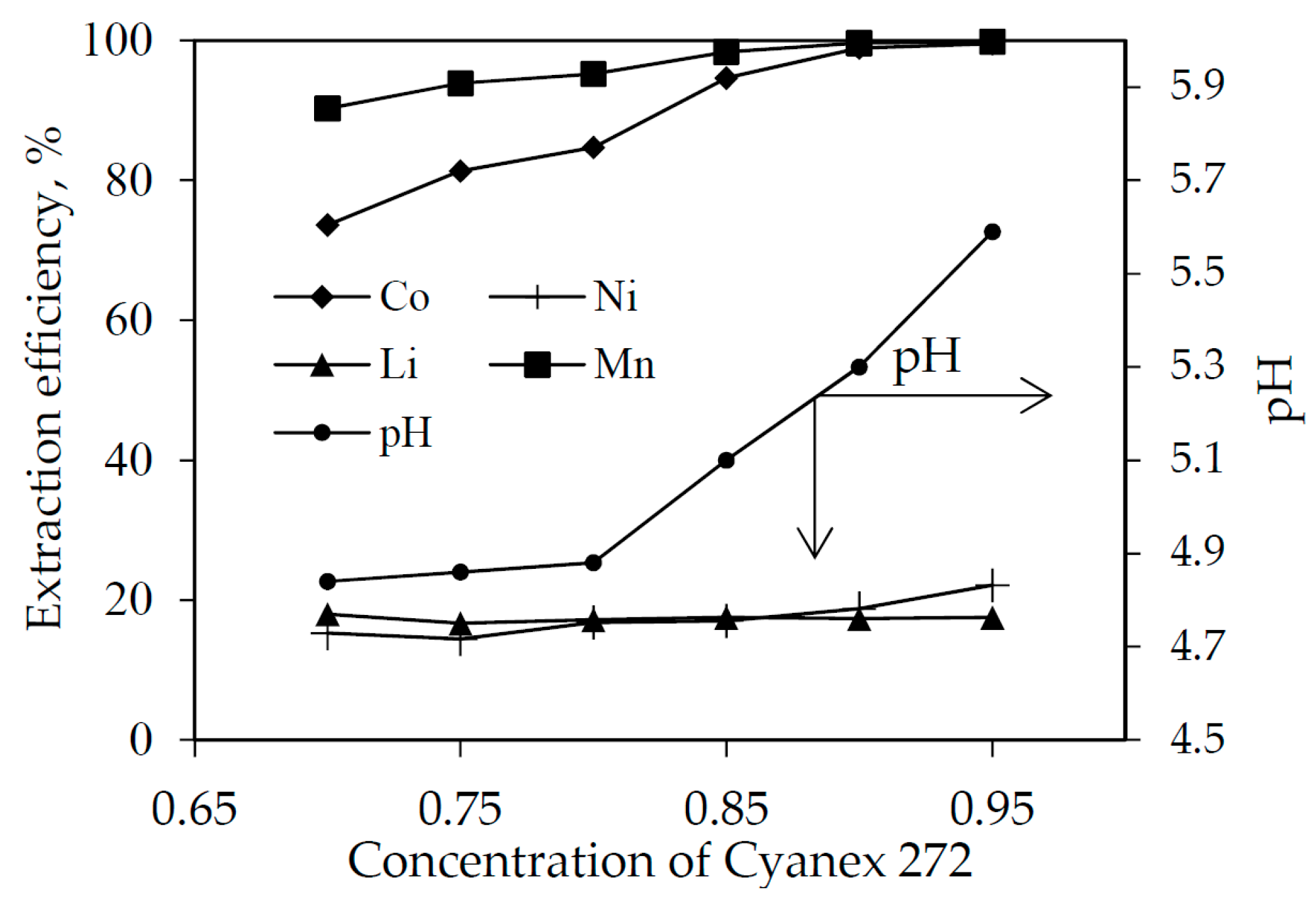
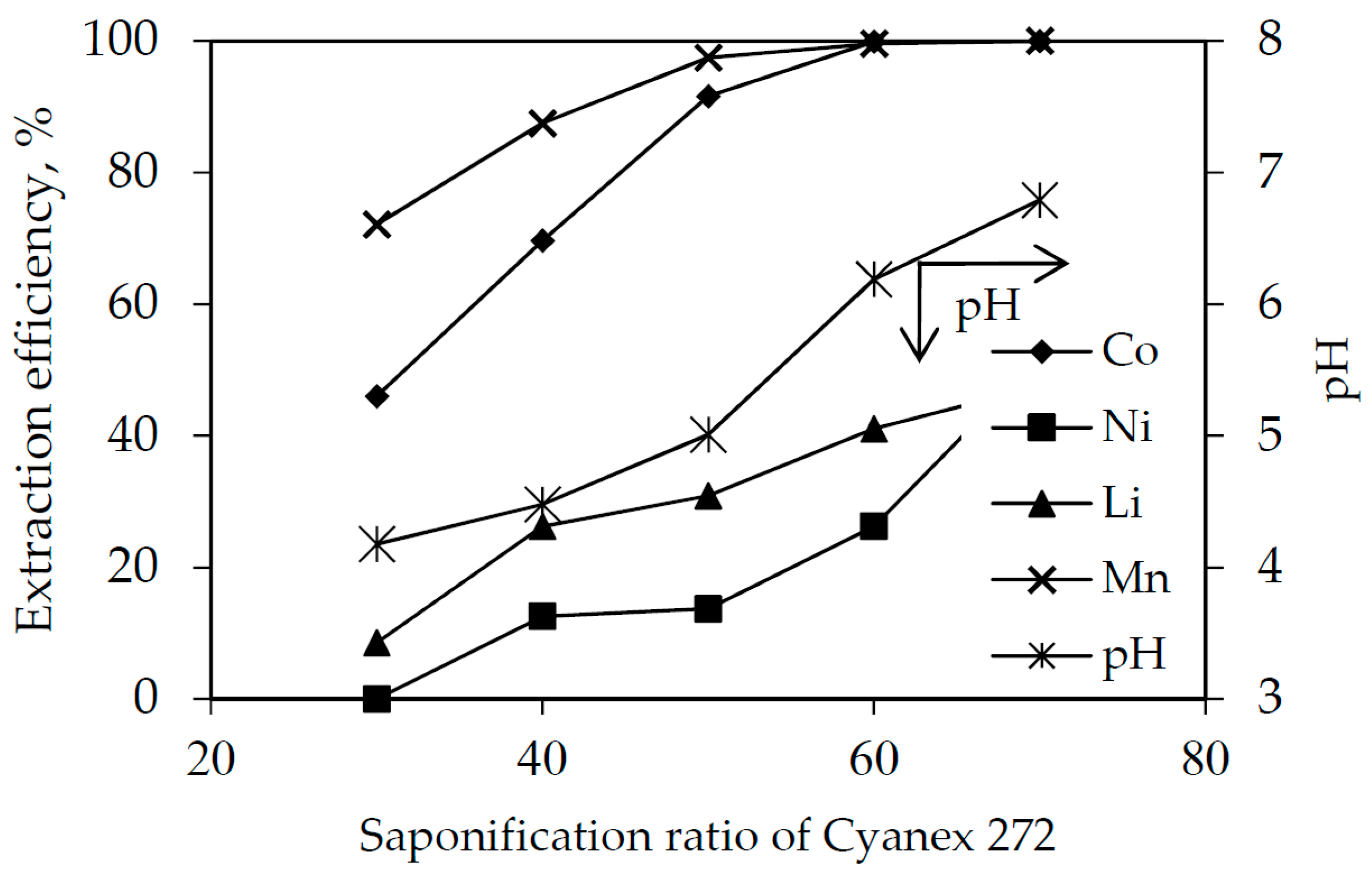
3.3. Solvent Extraction with Saponified Cyanex 272
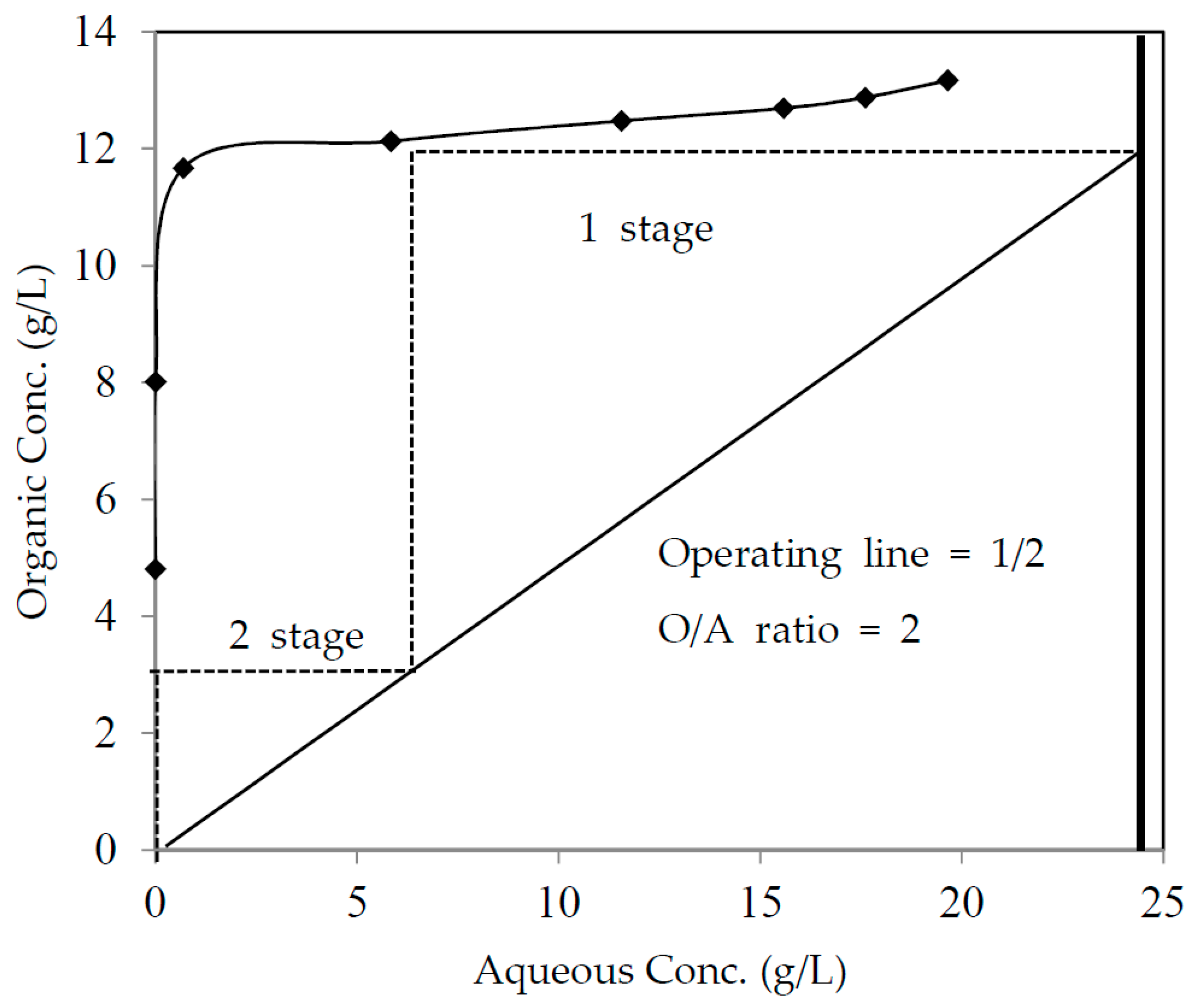
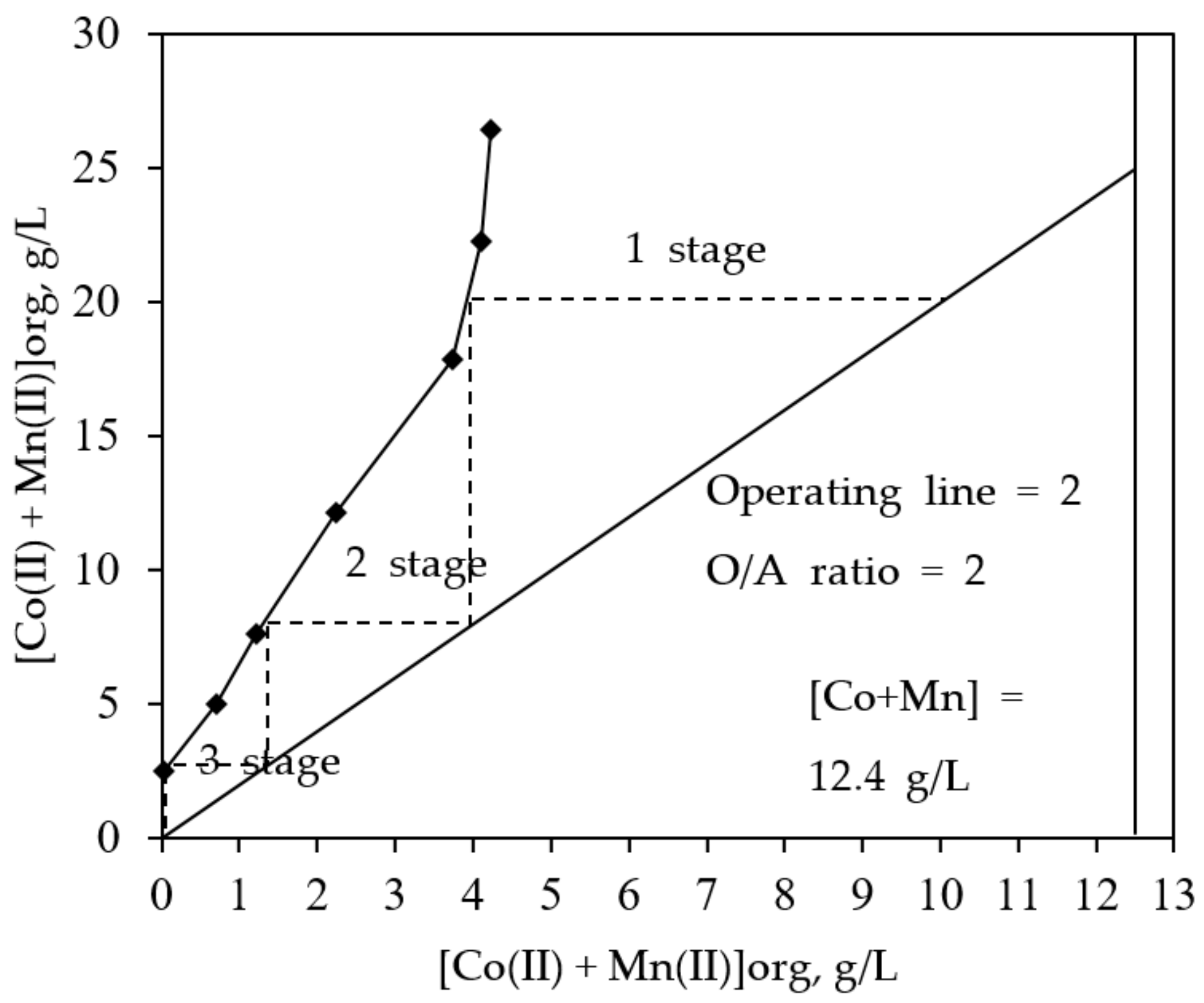
3.4. McCabe-Thiele Extraction Isotherm Study
3.5. Two Stages Counter-Current Extraction Batch Simulation
3.6. Stripping Test for Preparing Aqueous CMB and CMA Catalyst
3.6.1. Preparation of CMB Liquid Catalyst by Stripping with HBr
3.6.2. Preparation of CMA Catalyst by Stripping with C2H4O2
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- The Cobalt Development Institute. Cobalt Factor. 2015. Available online: http://www.thecdi.com/cdi/images/documents/facts/Cobalt%20Facts%20%20Supply%20%20Demand%20-%2015.pdf (accessed on 18 March 2016).
- Leonardou, S.A.; Tsakiridis, P.E.; Oustadakis, P.; Karidakis, T.; Katsiapi, A. Hydrometallurgical process for the separation and recovery of nickel from sulphate heap leach liquor of nickeliferrous laterite ores. Miner. Eng. 2009, 22, 1181–1192. [Google Scholar] [CrossRef]
- Katsiapi, A.; Tsakiridis, P.E.; Oustadakis, P.; Leonardou, S.A. Cobalt recovery from mixed Co-Mn hydroxide precipitates by ammonia-ammonium carbonate leaching. Miner. Eng. 2010, 23, 643–651. [Google Scholar] [CrossRef]
- Zhang, W.; Singh, P.; Muir, D. Oxidative precipitation of manganese with SO2/O2 and separation from cobalt and nickel. Hydrometallurgy 2002, 6, 3127–3135. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C. Hydrometallurgical process for recovery of cobalt from zinc plant residue. Hydrometallurgy 2002, 63, 225–234. [Google Scholar] [CrossRef]
- Joo, S.H.; Kang, J.G.; Kwon, W.; Shin, S.M. Production of chemical manganese dioxide from lithium ion battery ternary cathodic material by selective oxidative precipitation of manganese. Mater. Trans. 2003, 54, 844–849. [Google Scholar] [CrossRef]
- Alguacil, F.J. Facilitated transport and separation of manganese and cobalt by a supported liquid membrane using DP-8R as a mobile carrier. Hydrometallurgy 2002, 65, 9–14. [Google Scholar] [CrossRef]
- Hoh, Y.C.; Chuang, W.S.; Lee, B.D.; Chang, C.C. The separation of manganese from cobalt by D2EHPA. Hydrometallurgy 1984, 12, 375–386. [Google Scholar] [CrossRef]
- Cheng, C.Y. Purification of synthetic laterite leach solution by solvent extraction using D2EHPA. Hydrometallurgy 2000, 56, 369–386. [Google Scholar] [CrossRef]
- Devi, N.B.; Nathsarma, K.C.; Chakravortty, V. Separation of divalent manganese and cobalt ions from sulphate solutions using sodium salts of D2EHPA, PC 88A and Cyanex 272. Hydrometallurgy 2000, 54, 117–131. [Google Scholar] [CrossRef]
- Hossain, M.R.; Nash, S.; Rose, G.; Alam, S. Cobalt loaded D2EHPA for selective separation of manganese from cobalt electrolyte solution. Hydrometallurgy 2001, 107, 137–140. [Google Scholar] [CrossRef]
- Wang, F.; He, F.; Zhao, J.; Sui, N.; Xu, L.; Liu, H. Extraction and separation of cobalt (II), copper (II) and manganese (II) by Cyanex272, PC-88A and their mixtures. Sep. Purif. Technol. 2012, 93, 8–14. [Google Scholar] [CrossRef]
- Mishra, R.K.; Rout, P.C.; Sarangi, K.; Nathsarma, K.C. Solvent extraction of zinc, manganese, cobalt and nickel from nickel laterite bacterial leach liquor using sodium salts of TOPS-99 and Cyanex 272. Trans. Nonferr. Met. Soc. China 2016, 26, 301–309. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Urbani, M.D.; Davies, M.G.; Pranolo, Y.; Zhu, Z. Recovery of nickel and cobalt from leach solutions of nickel laterites using a synergistic system consisting of Versatic 10 and Acorga CLX 50. Miner. Eng. 2015, 77, 17–24. [Google Scholar] [CrossRef]
- Cheng, C.Y. Solvent extraction of nickel and cobalt with synergistic systems consisting of carboxylic acid and aliphatic hydroxyoxime. Hydrometallurgy 2006, 84, 109–117. [Google Scholar] [CrossRef]
- Joo, S.H.; Shin, D.J.; Oh, C.H.; Wang, J.P.; Shin, S.M. Extraction of manganese by alkyl monocarboxylic acid in a mixed extractant from a leaching solution of spent lithium ion battery ternary cathodic material. J. Power Sources 2016, 305, 175–181. [Google Scholar] [CrossRef]
- Joo, S.H.; Shin, D.J.; Oh, C.H.; Wang, J.P.; Shin, S.M. Extractive separation studies of manganese from spent lithium battery leachate using mixture of PC88A and Versatic 10 acid in kerosene. Hydrometallurgy 2015, 156, 136–141. [Google Scholar] [CrossRef]
- Joo, S.H.; Shin, D.J.; Oh, C.H.; Wang, J.P.; Senanayake, G.; Shin, S.M. Selective extraction and separation of nickel from cobalt, manganese and lithium in pre-treated leach liquors of ternary cathode material of spent lithium-ion batteries using synergism caused by Versatic 10 acid and LIX 84-I. Hydrometallurgy 2016, 159, 65–74. [Google Scholar] [CrossRef]
- Trade Data International Pty Ltd. An Analysis of Global Trade in Terephthalic Acid and Its Salts. Available online: http://www.tradedata.net/wp-content/uploads/2014/12/Global-Analysis-Template-terephthalic-Acid_nolinks.pdf (accessed on 22 December 2014).
- Sarangi, K.; Reddy, B.R.; Das, R.P. Extraction studies of cobalt (II) and nickel (II) from chloride solutions using Na-Cyanex 272.: Sepration of Co (II)/Ni (II) by the sodium salts of D2EHPA, PC88A and Cyanex 272 and their mixtures. Hydrometallurgy 1999, 52, 253–265. [Google Scholar] [CrossRef]
- Parhi, P.K.; Padhan, E.; Palai, A.K.; Sarangi, K.; Nathsarma, K.C.; Park, K.H. Separation of Co (II) and Ni (II) from the mixed sulphate/chloride solution using NaPC-88A. Desalination 2011, 267, 201–208. [Google Scholar] [CrossRef]
- Kang, J.G.; Senanayake, G.; Sohn, J.S.; Shin, S.M. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex-272. Hydrometallurgy 2010, 100, 168–171. [Google Scholar] [CrossRef]
- Ritcey, G.M.; Ashbrook, A.W. Solvent Extraction—Principles and Applications to Process Metallurgy; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1984; Volume 1, ISBN 0-444-41770-2. [Google Scholar]

| Elements | Co | Mn | Ni | Li | Al |
|---|---|---|---|---|---|
| Content | 18.2 | 17.3 | 19.1 | 6.8 | 11.1 |
| Division (mm) | Co | Li | Ni | Mn | Al | Mass (%) |
|---|---|---|---|---|---|---|
| +2.38 | 0.29 | 0.0 | 0.34 | 0.14 | 8.33 | 18.72 |
| −2.38 + 1.0 | 0.08 | 0.3 | 0.08 | 0.13 | 0.54 | 1.49 |
| −1.0 + 0.42 | 0.02 | 0.10 | 0.22 | 0.22 | 1.88 | 4.63 |
| −0.42 + 0.25 | 2.81 | 0.30 | 1.99 | 2.43 | 0.25 | 12.18 |
| −0.25 | 15.0 | 6.10 | 16.47 | 14.38 | 0.10 | 61.78 |
| Total | 18.2 | 6.80 | 19.1 | 17.30 | 11.10 | 98.80 (1.2) 1 |
| Element | Ni | Co | Mn | Li | Al | pH |
|---|---|---|---|---|---|---|
| Leach liquor | 18,860 | 17,740 | 16,700 | 7020 | 830 | 0.97 |
| pH 5.5 solution | 17,530 | 16,400 | 16,380 | 6912 | - | 5.5 |
| After dilution prior to solvent extraction | 12,910 | 12,800 | 12,130 | 4320 | - | 5.7 |
| No. of Counter-Current Extraction Stages | O: Organic Phase A: Aqueous Phase | Co | Mn | Ni | Li | |
|---|---|---|---|---|---|---|
| 2 | 1 | O1 | 6.4 | 6.0 | 0.0006 | 0.0004 |
| A1 | 7.7 | 2.7 | 13.8 | 4.8 | ||
| 2 | O2 | 3.7 | 1.3 | 0.45 | 0.25 | |
| A2 | 0.005 | 0.002 | 12.9 | 4.3 | ||
| Type | Experimental Condition | Co | Mn | Br | C2H4O2 | Ni | Li |
|---|---|---|---|---|---|---|---|
| CMB specification | - | 30 | 60 | 153 | - | <10 mg/L | <10 mg/L |
| CMA specification | - | 22 | 11 | - | 37 | <10 mg/L | <10 mg/L |
| Re-manufactured CMB | Stripping ratio: O/A = 5 | 33.1 | 29.8 | 168 | - | 7.2 | 4.1 |
| Re-manufactured CMA | Stripping ratio: O/A = 2 | 12.67 | 12.0 | - | 511 | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, S.-H.; Shin, D.J.; Oh, C.H.; Wang, J.-P.; Park, J.T.; Shin, S.M. Application of Co and Mn for a Co-Mn-Br or Co-Mn-C2H3O2 Petroleum Liquid Catalyst from the Cathode Material of Spent Lithium Ion Batteries by a Hydrometallurgical Route. Metals 2017, 7, 439. https://doi.org/10.3390/met7100439
Joo S-H, Shin DJ, Oh CH, Wang J-P, Park JT, Shin SM. Application of Co and Mn for a Co-Mn-Br or Co-Mn-C2H3O2 Petroleum Liquid Catalyst from the Cathode Material of Spent Lithium Ion Batteries by a Hydrometallurgical Route. Metals. 2017; 7(10):439. https://doi.org/10.3390/met7100439
Chicago/Turabian StyleJoo, Sung-Ho, Dong Ju Shin, Chang Hyun Oh, Je-Pil Wang, Jin Tae Park, and Shun Myung Shin. 2017. "Application of Co and Mn for a Co-Mn-Br or Co-Mn-C2H3O2 Petroleum Liquid Catalyst from the Cathode Material of Spent Lithium Ion Batteries by a Hydrometallurgical Route" Metals 7, no. 10: 439. https://doi.org/10.3390/met7100439
APA StyleJoo, S.-H., Shin, D. J., Oh, C. H., Wang, J.-P., Park, J. T., & Shin, S. M. (2017). Application of Co and Mn for a Co-Mn-Br or Co-Mn-C2H3O2 Petroleum Liquid Catalyst from the Cathode Material of Spent Lithium Ion Batteries by a Hydrometallurgical Route. Metals, 7(10), 439. https://doi.org/10.3390/met7100439





