Effect of Hydrogen and Strain-Induced Martensite on Mechanical Properties of AISI 304 Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Hydrogen Charging
2.2. Determination of the Amount of Strain-Induced Martensite (SIM) and Tensile Testing Procedure
3. Results and Discussion
3.1. Hydrogen Diffusion in AISI 304
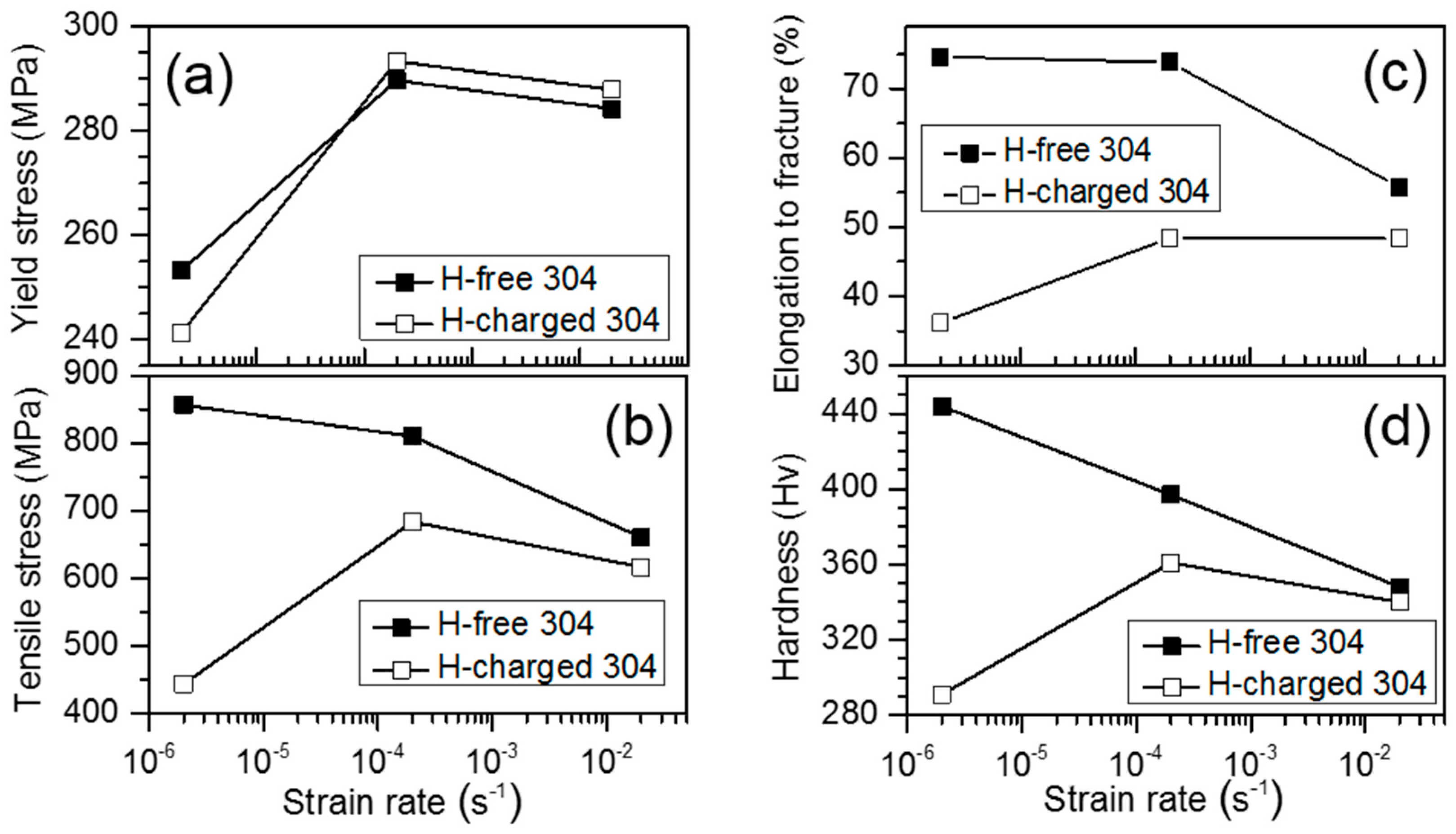
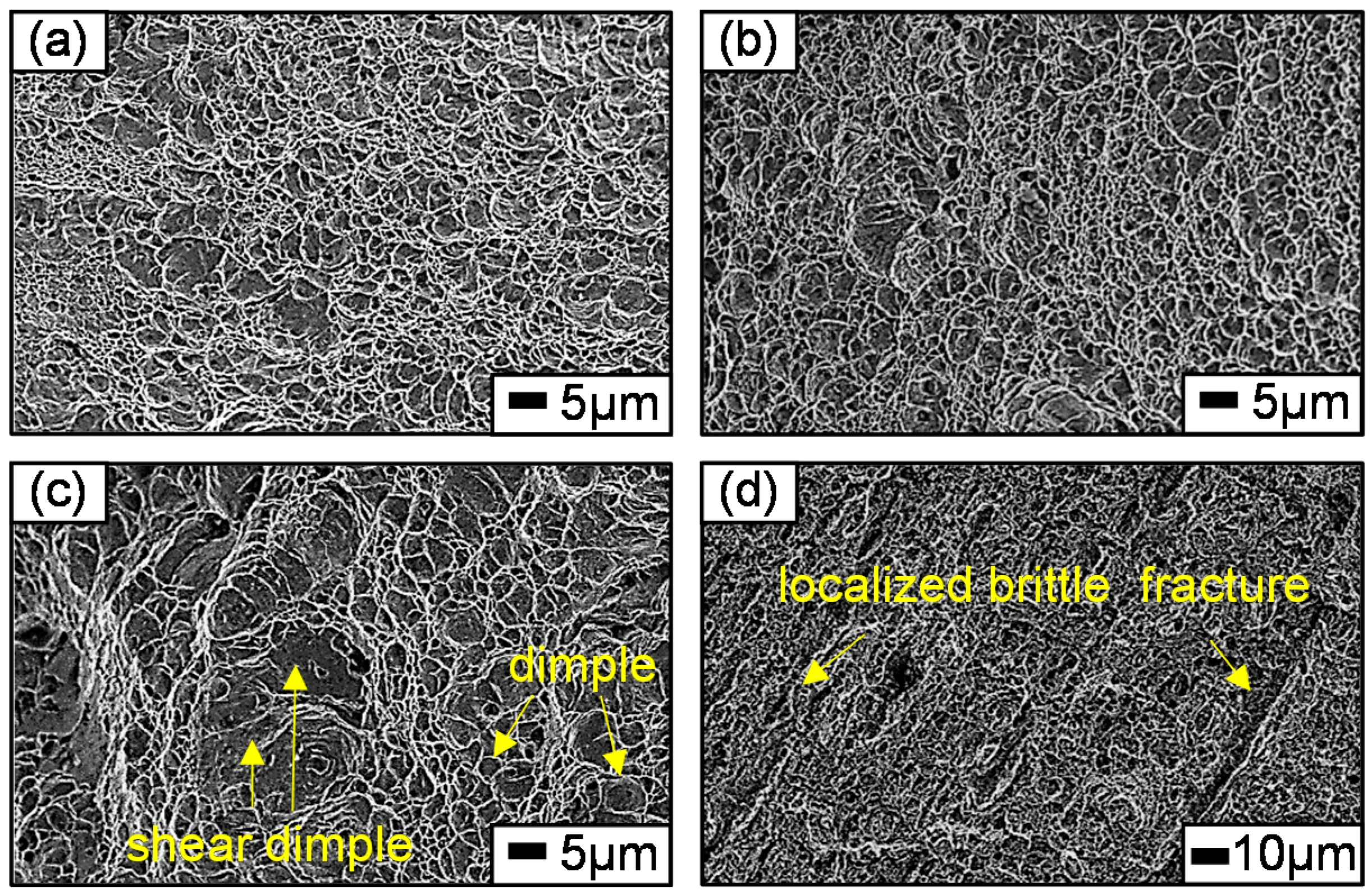
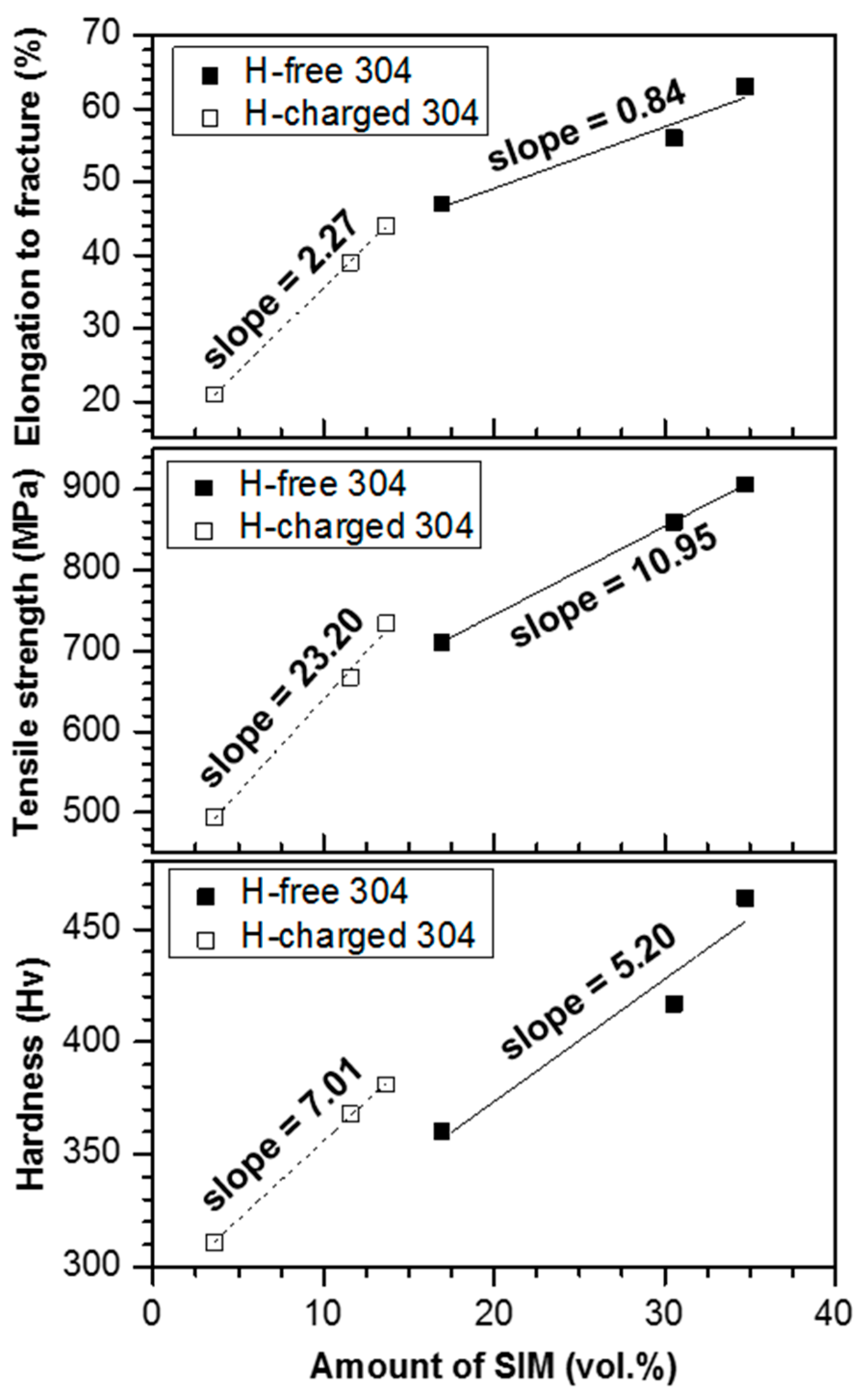
3.2. Hydrogen Effect on Mechanical Property
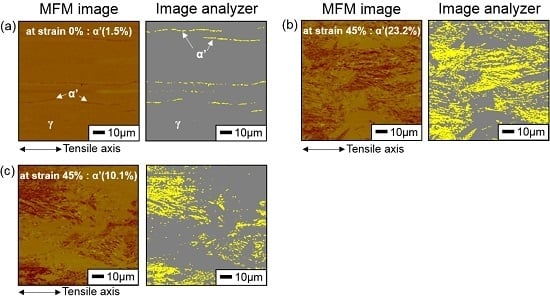
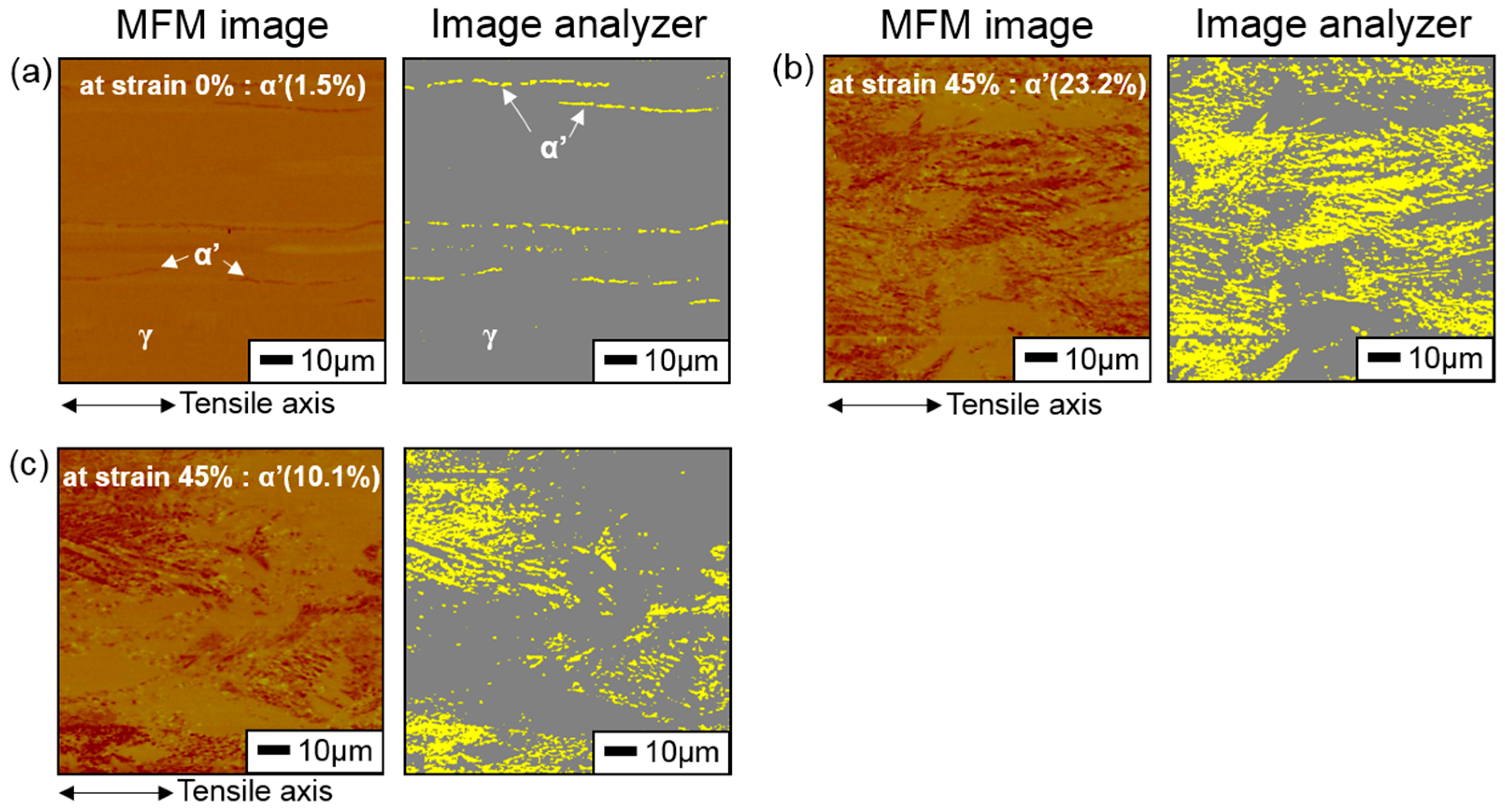
3.3. Hydrogen Effect on Formation of SIM
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Antolovich, S.D.; Fahr, D. An experimental investigation of the fracture characteristics of TRIP alloys. Eng. Fract. Mech. 1972, 4, 133–144. [Google Scholar] [CrossRef]
- Perdahcıoğlu, E.S.; Geijselaers, H.J.M.; Groen, M. Influence of plastic strain on deformation-induced martensitic transformations. Scr. Mater. 2008, 58, 947–950. [Google Scholar] [CrossRef]
- Hwang, J.; Son, I.; Yoo, J.; Zargaran, A.; Kim, N. Effect of reduction of area on microstructure and mechanical properties of twinning-induced plasticity steel during wire drawing. Met. Mater. Int. 2015, 21, 815–822. [Google Scholar] [CrossRef]
- Oliver, E.C.; Withers, P.J.; Daymond, M.R.; Ueta, S.; Mori, T. Neutron-diffraction study of stress-induced martensitic transformation in TRIP steel. Appl. Phys. A 2002, 74, S1143–S1145. [Google Scholar] [CrossRef]
- Tamura, I. Deformation-induced martensitic transformation and transformation-induced plasticity in steels. Met. Sci. 1982, 16, 245–253. [Google Scholar] [CrossRef]
- Briant, C.L. Hydrogen assisted cracking of type 304 stainless steel. Metall. Trans. A 1979, 10, 181–189. [Google Scholar] [CrossRef]
- Hanninen, H.; Hakkarainen, H. On the effects of α′ martensite in hydrogen embrittlement of a cathodically charged AISI type 304 austenitic stainless steel. Corrosion 1980, 36, 47–51. [Google Scholar] [CrossRef]
- Zapffe, C.A.; Sims, C.E. Hydrogen embrittlement internal stress and defects in steels. Trans. Am. Inst. Min. Eng. 1941, 145, 225–259. [Google Scholar]
- Garofalo, F.; Chou, Y.T.; Ambegaokar, V. Effect of hydrogen on stability of micro cracks in iron and steel. Acta Metall. 1960, 8, 504–512. [Google Scholar] [CrossRef]
- Bilby, B.A.; Hewitt, J. Hydrogen in steel—The stability of micro-cracks. Acta Metall. 1962, 10, 587–600. [Google Scholar] [CrossRef]
- Escobar, D.P.; Miñambres, C.; Duprez, L.; Verbeken, K.; Verhaege, M. Internal and surface damage of multiphase steels and pure iron after electrochemical hydrogen charging. Corros. Sci. 2011, 53, 3166–3176. [Google Scholar] [CrossRef]
- Simmons, G.W.; Pao, P.S.; Wei, R.P. Fracture mechanics and surface chemistry studies of subcritical crack growth in AISI 4340 steel. Metall. Trans. A 1978, 9, 1147–1158. [Google Scholar] [CrossRef]
- Takano, N. First principles calculation of hydrogen embrittlement in iron. Key Eng. Mater. 2010, 417–418, 285–288. [Google Scholar] [CrossRef]
- Birnbaum, H.K.; Sofronis, P. Hydrogen-enhanced localized plasticity—A mechanism for hydrogen-related fracture. Mater. Sci. Eng. A 1994, 176, 191–202. [Google Scholar] [CrossRef]
- Takai, K.; Shoda, H.; Suzuki, H.; Nagumo, M. Lattice defects dominating hydrogen-related failure of metals. Acta Mater. 2008, 56, 5158–5167. [Google Scholar] [CrossRef]
- Nagumo, M. Hydrogen related failure of steels—A new aspect. Mater. Sci. Technol. 2004, 20, 940–950. [Google Scholar] [CrossRef]
- Zhang, L.; An, B.; Fukuyama, S.; Iijima, T.; Yokogawa, K. Characterization of hydrogen-induced crack initiation in metastable austenitic stainless steels during deformation. J. Appl. Phys. 2010, 108, 0635226. [Google Scholar] [CrossRef]
- Miller, A.; Estrin, Y.; Hu, X.Z. Magnetic force microscopy of fatigue crack tip region in a 316L austenitic stainless steel. Scr. Mater. 2002, 47, 441–446. [Google Scholar] [CrossRef]
- Sort, J.; Concustell, A.; Menéndez, E.; Suriñach, S.; Baró, M.D.; Farran, J.; Nogués, J. Selective generation of local ferromagnetism in austenitic stainless steel using nanoindentation. Appl. Phys. Lett. 2006, 89, 032509. [Google Scholar] [CrossRef]
- Minkovitz, E.; Eliezer, D. Grain-size and heat-treatment effects in hydrogen-assisted cracking of austenitic stainless steels. J. Mater. Sci. 1982, 17, 3165–3172. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of grain size on corrosion: A review. Corrosion 2006, 66, 075005. [Google Scholar] [CrossRef]
- Pan, C.; Chu, W.Y.; Li, Z.B.; Liang, D.T.; Su, Y.J.; Gao, K.W.; Qiao, L.J. Hydrogen embrittlement induced by atomic hydrogen and hydrogen-induced martensites in type 304L stainless steel. Mater. Sci. Eng. A 2003, 351, 293–298. [Google Scholar] [CrossRef]
- Au, M. High temperature electrochemical charging of hydrogen and its application in hydrogen embrittlement research. Mater. Sci. Eng. A 2007, 454–455, 564–569. [Google Scholar] [CrossRef]
- Yao, J.; Cahoon, J.R. Experimental studies of grain boundary diffusion of hydrogen in metals. Acta. Metall. Mater. 1991, 39, 119–126. [Google Scholar] [CrossRef]
- Oudriss, A.; Creus, J.; Bouhattate, J.; Savall, C.; Peraudeau, B.; Feaugas, X. The diffusion and trapping of hydrogen along the grain boundaries in polycrystalline nickel. Scr. Mater. 2012, 66, 37–40. [Google Scholar] [CrossRef]
- Austin, J.H.; Elleman, T.S.; Verghese, K. Surface effects on the diffusion of tritium in 304-stainless steel and zircaloy-2. J. Nucl. Mater. 1973, 48, 307–316. [Google Scholar] [CrossRef]
- Mine, Y.; Horita, Z.; Murakami, Y. Effect of hydrogen on martensite formation in austenitic stainless steels in high-pressure torsion. Acta Mater. 2009, 57, 2993–3002. [Google Scholar] [CrossRef]
- Spencer, K.; Embury, J.D.; Conlon, K.T.; Veron, M.; Brechet, Y. Strengthening via the formation of strain-induced martensite in stainless steels. Mater. Sci. Eng. A 2004, 387, 873–881. [Google Scholar] [CrossRef]



| Fe | Cr | Ni | Mn | Si | Cu | Co | Mo | N | C | S | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bal. | 18.17 | 8.03 | 1.04 | 0.46 | 0.18 | 0.12 | 0.11 | 0.047 | 0.043 | 0.04 | 0.001 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bak, S.H.; Abro, M.A.; Lee, D.B. Effect of Hydrogen and Strain-Induced Martensite on Mechanical Properties of AISI 304 Stainless Steel. Metals 2016, 6, 169. https://doi.org/10.3390/met6070169
Bak SH, Abro MA, Lee DB. Effect of Hydrogen and Strain-Induced Martensite on Mechanical Properties of AISI 304 Stainless Steel. Metals. 2016; 6(7):169. https://doi.org/10.3390/met6070169
Chicago/Turabian StyleBak, Sang Hwan, Muhammad Ali Abro, and Dong Bok Lee. 2016. "Effect of Hydrogen and Strain-Induced Martensite on Mechanical Properties of AISI 304 Stainless Steel" Metals 6, no. 7: 169. https://doi.org/10.3390/met6070169
APA StyleBak, S. H., Abro, M. A., & Lee, D. B. (2016). Effect of Hydrogen and Strain-Induced Martensite on Mechanical Properties of AISI 304 Stainless Steel. Metals, 6(7), 169. https://doi.org/10.3390/met6070169