Effect of Cold Rolling on the Hydrogen Desorption Behavior of Binary Metal Hydride Powders under Microwave Irradiation
Abstract
:1. Introduction
2. Experimental Section

3. Results and Discussion
3.1. Electron Microscopy

3.2. X-ray Diffraction: Cold Rolling Effects
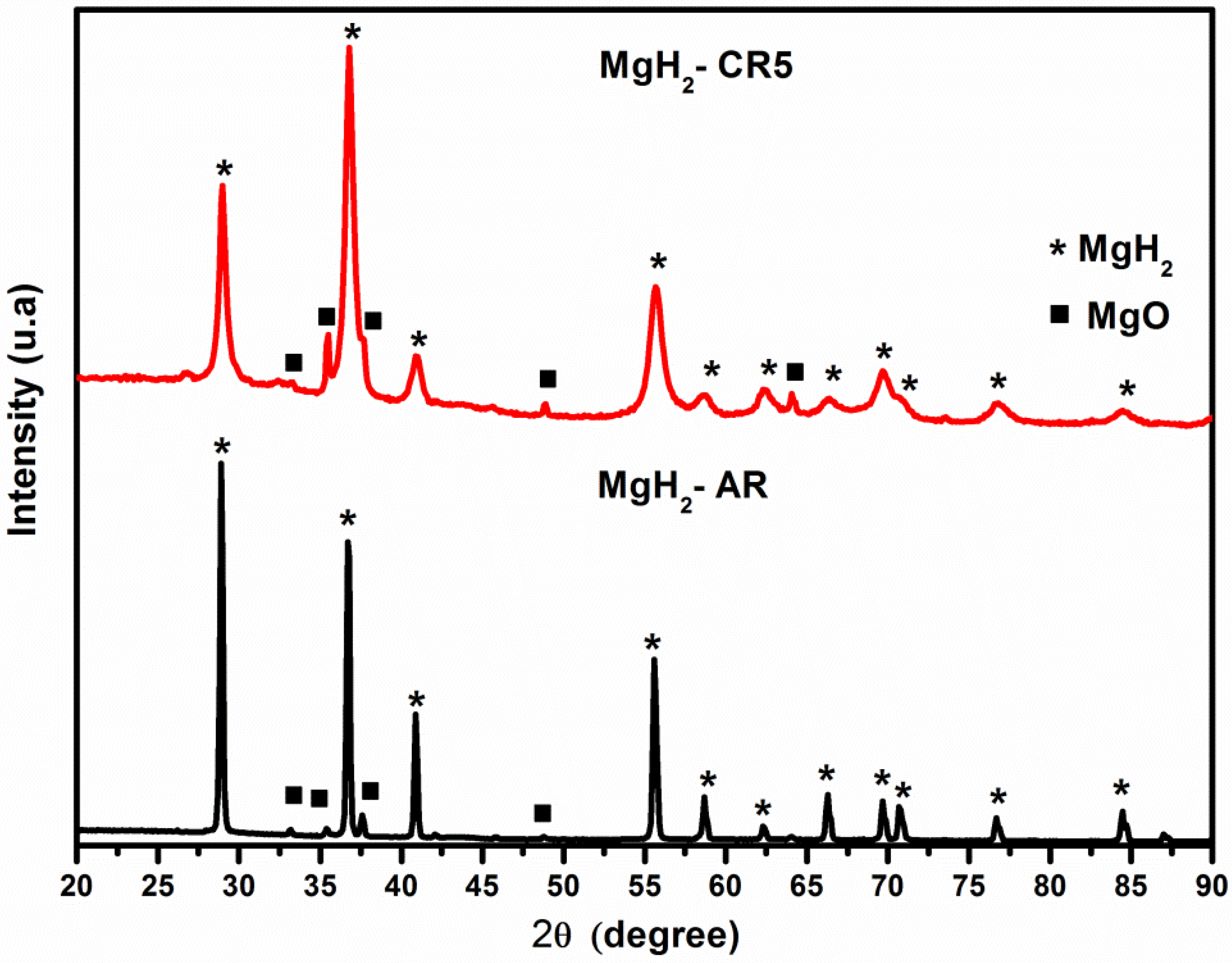
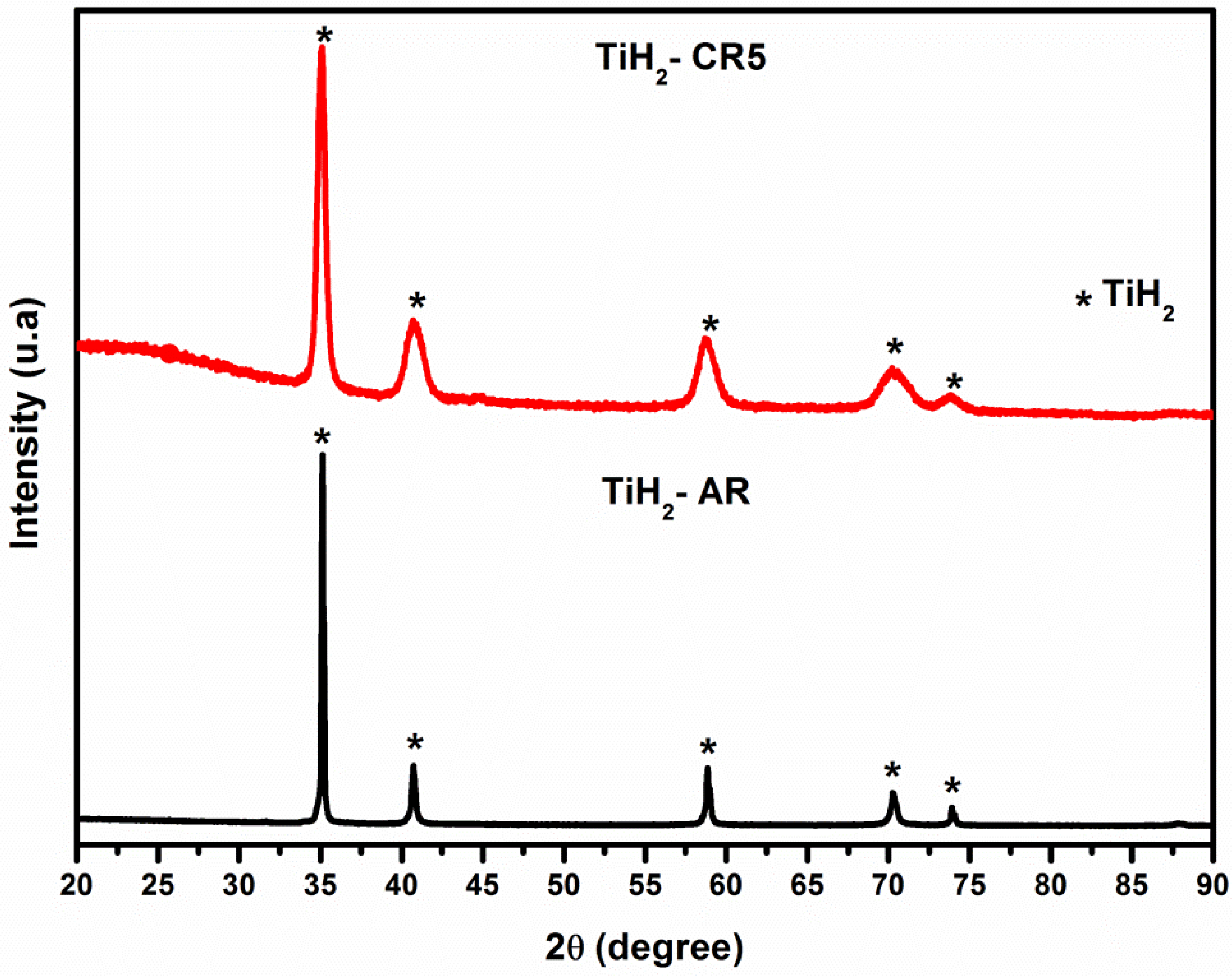
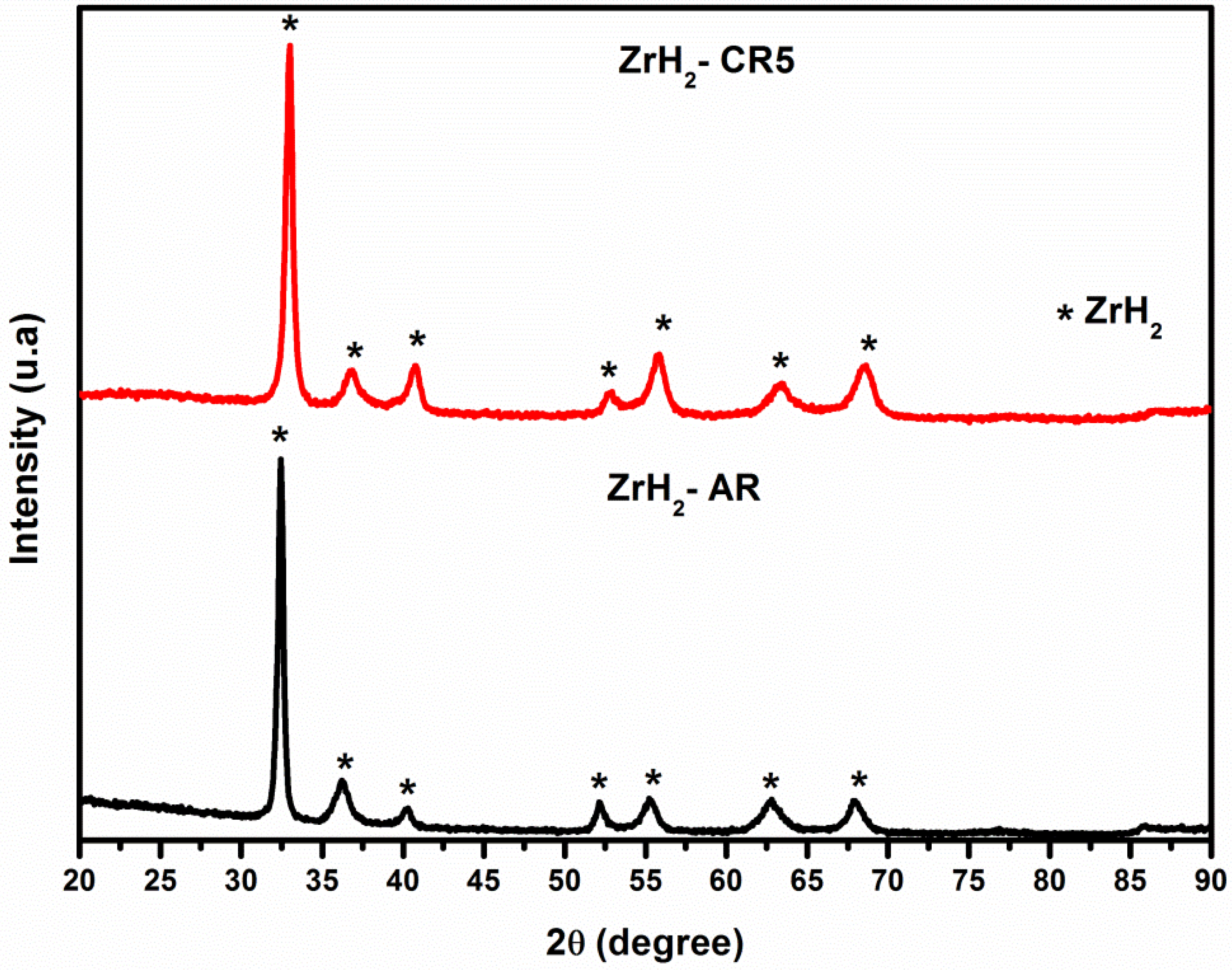
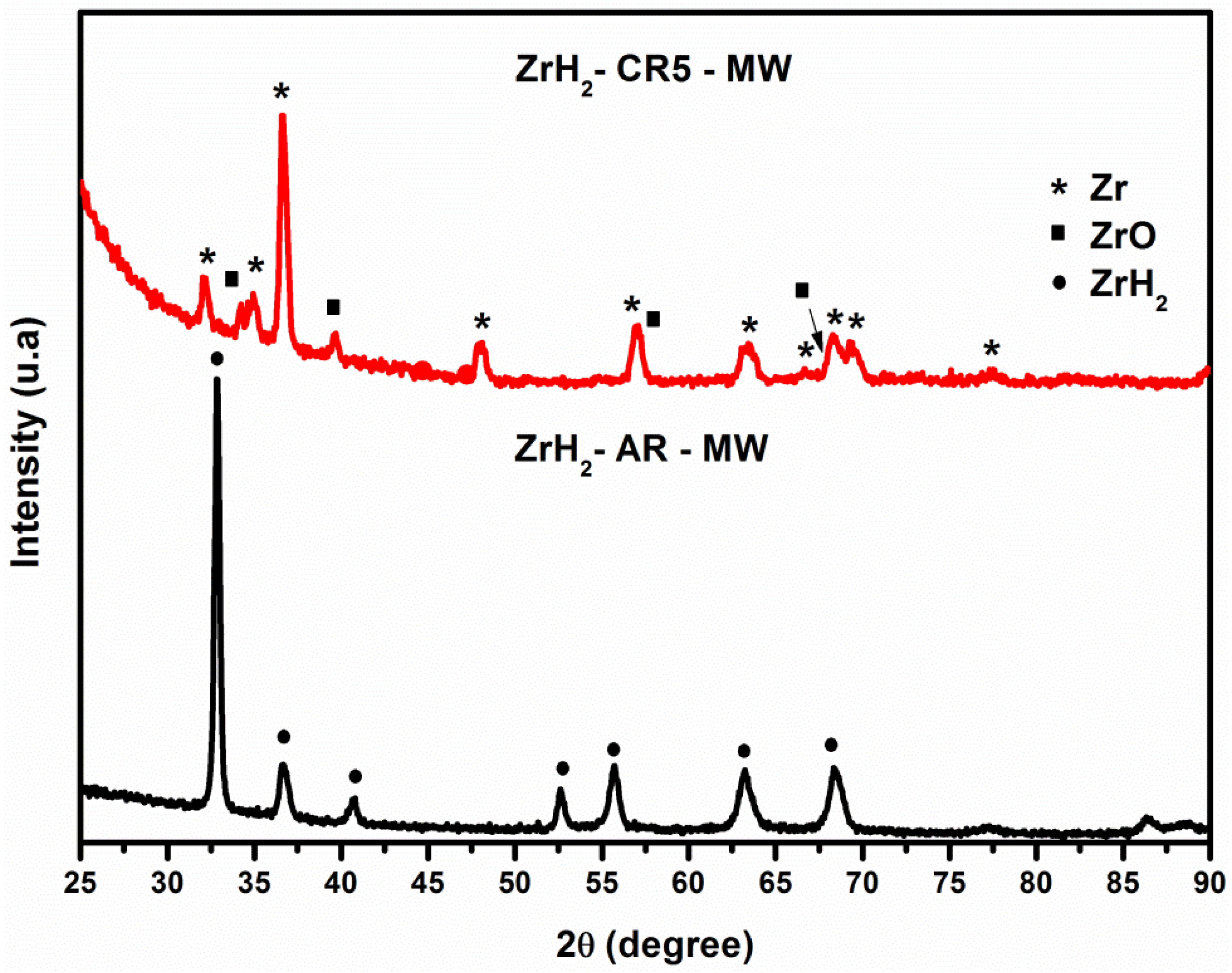
3.3. X-ray Diffraction: Microwave Irradiation Effects

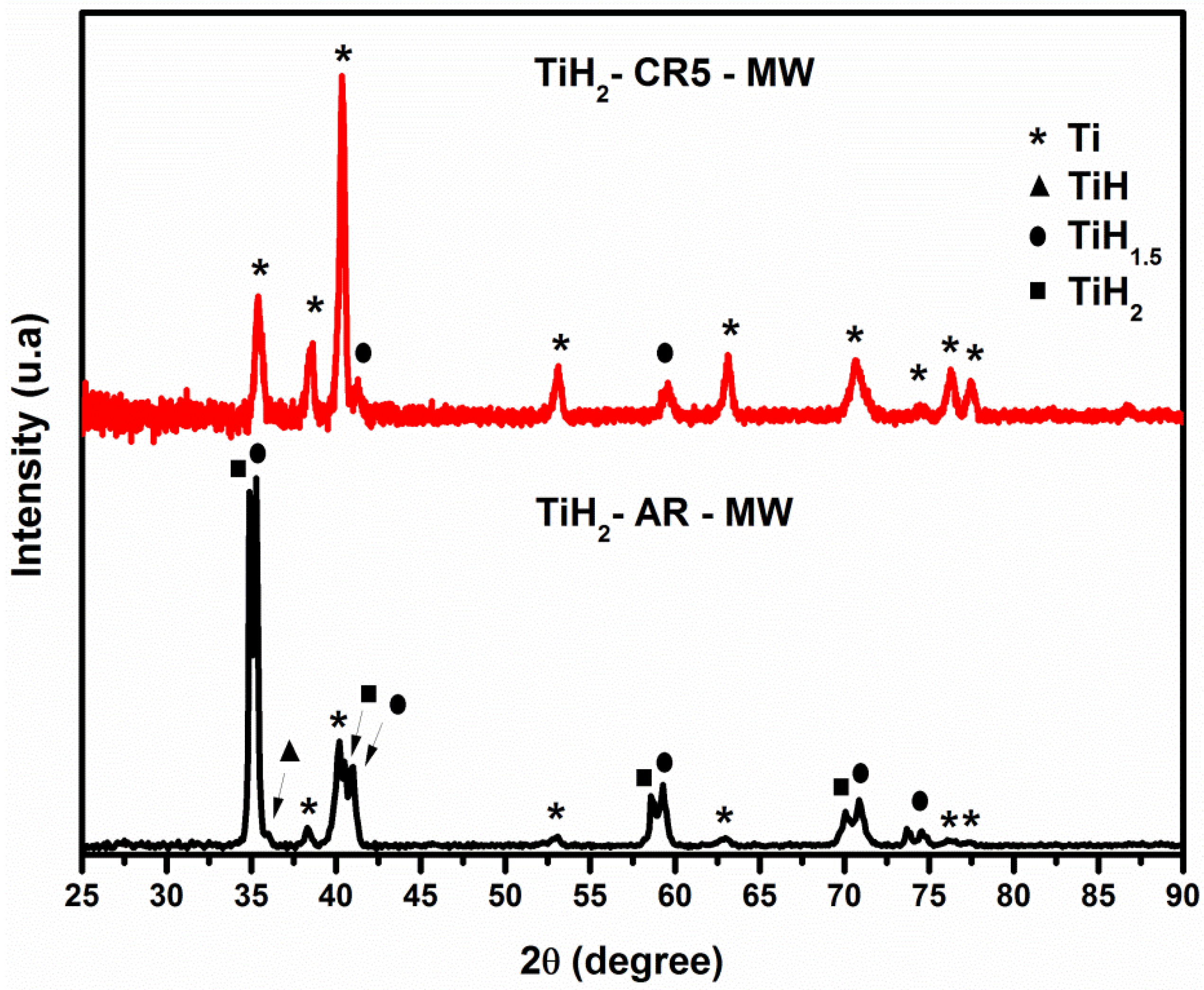
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Das, S.; Mukhopadhyay, A.K.; Datta, S.; Basu, D. Prospects of microwave processing: An overview. Bull. Mater. Sci. 2009, 32, 1–13. [Google Scholar] [CrossRef]
- Alsharaeh, E.H.; Othman, A.A.; Aldosari, M.A. Microwave irradiation effect on the dispersion and thermal stability of RGO nanosheets within a polystyrene matrix. Materials 2014, 7, 5212–5224. [Google Scholar] [CrossRef]
- Tapia-Ruiz, N.; Sorbie, N.; Vaché, N.; Hoang, T.K.A.; Gregory, D.H. Rapid microwave synthesis, characterization and reactivity of litium nitride hydride, Li4NH. Materials 2013, 6, 5410–5426. [Google Scholar] [CrossRef]
- Inamoto, M.; Kurihara, H.; Yajima, T. Vanadium pentoxide-based composite synthesized using microwave water plasma for cathode material in rechargeable magnesium batteries. Materials 2013, 6, 4514–4522. [Google Scholar] [CrossRef]
- Seetharaman, S.; Subramanian, J.; Tun, K.S.; Hamouda, A.S.; Gupta, M. Synthesis and characterization of nano boron nitride reinforced magnesium composites produced by the microwave sintering method. Materials 2013, 6, 1940–1955. [Google Scholar] [CrossRef]
- Yu, P.; Stephani, G.; Luo, S.D.; Goehler, H.; Qian, M. Microwave-assisted fabrication of titanium hollow spheres with tailored shell structures for varios potential applications. Mater. Lett. 2012, 86, 84–87. [Google Scholar] [CrossRef]
- Mondal, A.; Agrawal, D.; Upadhyaya, A. Microwave sintering of refractory metals/alloys: W, Mo, W-Cu, W-Ni-Cu and W-Ni-Fe alloys. J. Microw. Power Electromagn. Energy 2010, 44, 28–44. [Google Scholar] [PubMed]
- Yoshikawa, N. Fundamentals and applications of microwave heating of metals. J. Microw. Power Electromagn. Energy 2010, 44, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Agrawal, D.K.; Chang, J.P.; Gedevanishvili, S. Full sintering of powdered metals using microwaves. Nature 1999, 399, 668–670. [Google Scholar]
- Mondal, A.; Upadhyaya, A.; Agrawal, D. Microwave sintering of W-18Cu and W-7Ni3Cu alloys. J. Microw. Power Electromagn. Energy 2009, 43, 11–16. [Google Scholar] [PubMed]
- Takayama, S.; Link, G.; Miksch, M.; Sato, M.; Ichikawa, J.; Thumm, M. Millimetre wave effects on sintering behavior of metal powder compacts. Powder Metall. 2006, 48, 274–280. [Google Scholar] [CrossRef]
- Rybakov, K.I.; Semenov, V.E.; Egorov, S.V.; Eremeev, A.G.; Plotnikov, I.V.; Bykov, Y.V. Microwave heating of conductive powder materials. J. Appl. Phys. 2006. [Google Scholar] [CrossRef]
- Jayalakshmi, S.; Sahu, S.; Sankaranarayanan, S.; Gupta, S.; Gupta, M. Development of novel Mg-Ni60Nb40 amorphous particle reinforced composites with enhanced hardness and compressive response. Mater. Des. 2014, 53, 849–855. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Huot, J. Metal hydrides. In Handbook of Hydrogen Storage: New Materials for Future Energy Storage, 1st ed.; Hirscher, M., Ed.; Willey—VCH: Darmstadt, Germany, 2010; pp. 81–116. [Google Scholar]
- Zaluska, A.; Zaluski, L.; Strom-Olsen, J.O. Nanocrystalline magnesium for hydrogen storage. J. Alloys Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- Chen, F.; Tao, Z.; Liang, J.; Chen, J. Efficient hydrogen storage with the combination of lightweight Mg/MgH2 and nanostructures. Chem. Commun. 2012, 48, 7334–7343. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.F.; Ishikawa, T.T.; Botta, W.J.; Huot, J. MgH2 + FeNb nanocomposites for hydrogen storage. Mater. Chem. Phys. 2014, 147, 557–562. [Google Scholar] [CrossRef]
- Huot, J.; Ravnsbaek, D.B.; Zhang, J.; Cuevas, F.; Latroche, M.; Jensen, T.R. Mechanochemical synthesis of hydrogen storage materials. Prog. Mater. Sci. 2013, 58, 30–75. [Google Scholar] [CrossRef]
- Santos, S.F.; Huot, J. Hydrogen storage in Ti-Mn-(FeV) BCC alloys. J. Alloys Compd. 2009, 480, 5–9. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Emami, H.; Edalati, K.; Matsuda, J.; Akiba, E.; Horita, Z. Hydrogen storage performance of TiFe after processing by ball milling. Acta Mater. 2015, 88, 190–195. [Google Scholar] [CrossRef]
- Shao, H.; Xin, G.; Zheng, J.; Li, X.; Akiba, E. Nanotechnology in Mg-based materials for hydrogen storage. Nano Energy 2012, 1, 590–601. [Google Scholar] [CrossRef]
- Jeon, K.-J.; Moon, H.R.; Ruminski, A.M.; Jiang, B.; Kisielowski, C.; Bardhan, R.; Urban, J.J. Air-stable magnesium nanocomposites provide rapid and high-capacity hydrogen storage without using heavy-metal catalysts. Nat. Mater. 2011, 10, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.D.; Yang, Y.F.; Schaffer, G.B.; Qian, M. Novel fabrication of titanium by pure microwave radiation of titanium hydride powder. Scr. Mater. 2013, 69, 69–72. [Google Scholar] [CrossRef]
- Li, Q.; Pan, Y.; Leng, H.; Chou, K. Structure and properties of Mg-La-Ni ternary hydrogen storage alloys by microwave-assisted activation synthesis. Int. J. Hydrog. Energy 2014, 39, 14247–14254. [Google Scholar] [CrossRef]
- Nakamori, Y.; Matsuo, M.; Yamada, K.; Tsutaoka, T.; Orimo, S. Effect of microwave irradiation on metal hydrides and complex hydrides. J. Alloys Compd. 2007, 446–447, 698–702. [Google Scholar] [CrossRef]
- Krishnan, R.; Agrawal, D.; Dobbins, T. Microwave irradiation effects on reversible hydrogen desorption in sodium aluminum hydrides (NaAlH4). J. Alloys Compd. 2009, 470, 250–255. [Google Scholar] [CrossRef]
- Zhang, H.; Geerlings, H.; Lin, J.; Chin, W.S. Rapid microwave hydrogen release from MgH2 and other hydrides. Int. J. Hydrog. Energy 2011, 36, 7580–7586. [Google Scholar] [CrossRef]
- Awad, A.S.; Tayed, T.; Nakl, M.; Zakhour, M.; Ourane, B.; Troedec, M.L.; Bobet, J.-L. Effect of carbon types (graphite, CFs and diamond) on the hydrogen desorption of Mg-C powder mixtures under microwave irradiation. J. Alloys Compd. 2014, 607, 223–229. [Google Scholar] [CrossRef]
- Dupim, I.S.; Moreira, J.M.L.; Huot, J.; Santos, S.F. Effect of cold rolling on the hydrogen absorption and desorption kinetics of Zircaloy-4. Mater. Chem. Phys. 2015, 155, 241–245. [Google Scholar] [CrossRef]
- Amira, S.; Santos, S.F.; Huot, J. Hydrogen sorption properties of Ti-Cr alloys synthesized by ball milling and cold rolling. Intermetallics 2010, 18, 140–144. [Google Scholar] [CrossRef]
- Huot, J.; Skryabina, N.Y.; Fruchart, D. Application of severe plastic deformation techniques to magnesium for enhanced hydrogen sorption properties. Metals 2012, 2, 329–343. [Google Scholar] [CrossRef]
- Huot, J. Nanocrystalline metal hydrides obtained by severe plastic deformations. Metals 2012, 2, 22–40. [Google Scholar] [CrossRef]
- Jain, P.; Lang, J.; Skryabina, N.; Fruchart, D.; Santos, S.F.; Binder, K.; Klassen, T.; Huot, J. MgH2 as dopant for improved activation of commercial Mg ingot. J. Alloys Compd. 2013, 575, 364–369. [Google Scholar] [CrossRef]
- Zhang, L.T.; Ito, K.; Vasudevan, V.K.; Yamaguchi, M. The effect of cold-rolling on the hydrogen absorption/desorption behavior of Ti-22Al-27Nb alloys. Mater. Sci. Eng. A 2002, 329–331, 362–366. [Google Scholar] [CrossRef]
- Bruker AXS. TOPAS V4: General profile and structure analysis software for powder diffraction data; Bruker AXS: Karlsruhe, Germany, 2008. [Google Scholar]
- Mondal, A.; Shukla, A.; Upadhyaya, A.; Agrawal, D. Effect of porosity and particle size on microwave heating of copper. Sci. Sinter. 2010, 42, 169–182. [Google Scholar] [CrossRef]
- Lang, J.; Huot, J. A new approach to the processing of metal hydrides. J. Alloys Compd. 2011, 509, L18–L22. [Google Scholar] [CrossRef]
- Lang, J.; Eagles, M.; Conradi, M.S.; Huot, J. Hydrogenation rate limiting step, diffusion and thermal conductivity in cold rolled magnesium hydride. J. Alloys Compd. 2014, 583, 116–120. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Silva Dupim, I.; Ferreira Santos, S.; Huot, J. Effect of Cold Rolling on the Hydrogen Desorption Behavior of Binary Metal Hydride Powders under Microwave Irradiation. Metals 2015, 5, 2021-2033. https://doi.org/10.3390/met5042021
Da Silva Dupim I, Ferreira Santos S, Huot J. Effect of Cold Rolling on the Hydrogen Desorption Behavior of Binary Metal Hydride Powders under Microwave Irradiation. Metals. 2015; 5(4):2021-2033. https://doi.org/10.3390/met5042021
Chicago/Turabian StyleDa Silva Dupim, Ivaldete, Sydney Ferreira Santos, and Jacques Huot. 2015. "Effect of Cold Rolling on the Hydrogen Desorption Behavior of Binary Metal Hydride Powders under Microwave Irradiation" Metals 5, no. 4: 2021-2033. https://doi.org/10.3390/met5042021
APA StyleDa Silva Dupim, I., Ferreira Santos, S., & Huot, J. (2015). Effect of Cold Rolling on the Hydrogen Desorption Behavior of Binary Metal Hydride Powders under Microwave Irradiation. Metals, 5(4), 2021-2033. https://doi.org/10.3390/met5042021







