Porous Titanium for Dental Implant Applications
Abstract
:1. Introduction
2. Titanium and Its Alloys as Implant Materials
3. Characteristic Features of Porous Metal

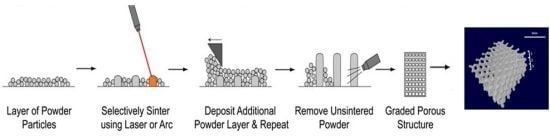
4. Fabrication Methods and Mechanical Evaluation of Porous Titanium Dental Implants
| Fabrication Methods | References |
|---|---|
| Plasma spraying with different powder particles such as: | [14] |
| -titanium oxide | |
| -calcium phosphate | |
| -hydroxyapatite | |
| Sand blasted with stiff particles such as: | [4] |
| -alumina | |
| -TiO2 | |
| -ceramic | |
| Laser micro-machining technique | [15] |
| Anodization TiO2 nanotube | [16] |
| Electron-discharge compaction | [71] |
| One-step microwave processing method | [62] |
| Powder metallurgy | [19,62] |
| -sintering hollow spheres | |
| -thermal decomposition | |
| -sintering of powders, compressing and sintering of titanium beads or fibers | |
| Removable space holder and titanium metal powder particles: | [63,64,65,66,67,68,69] |
| -saccharose crystals | |
| -NaF | |
| -NaCl | |
| -polymer granules | |
| -Magnesium | |
| -ammonium hydrogen carbonate | |
| Additive manufacturing technology: | [60,66,74] |
| -selective laser sintering | |
| -selective laser melting | |
| -electron beam melting |
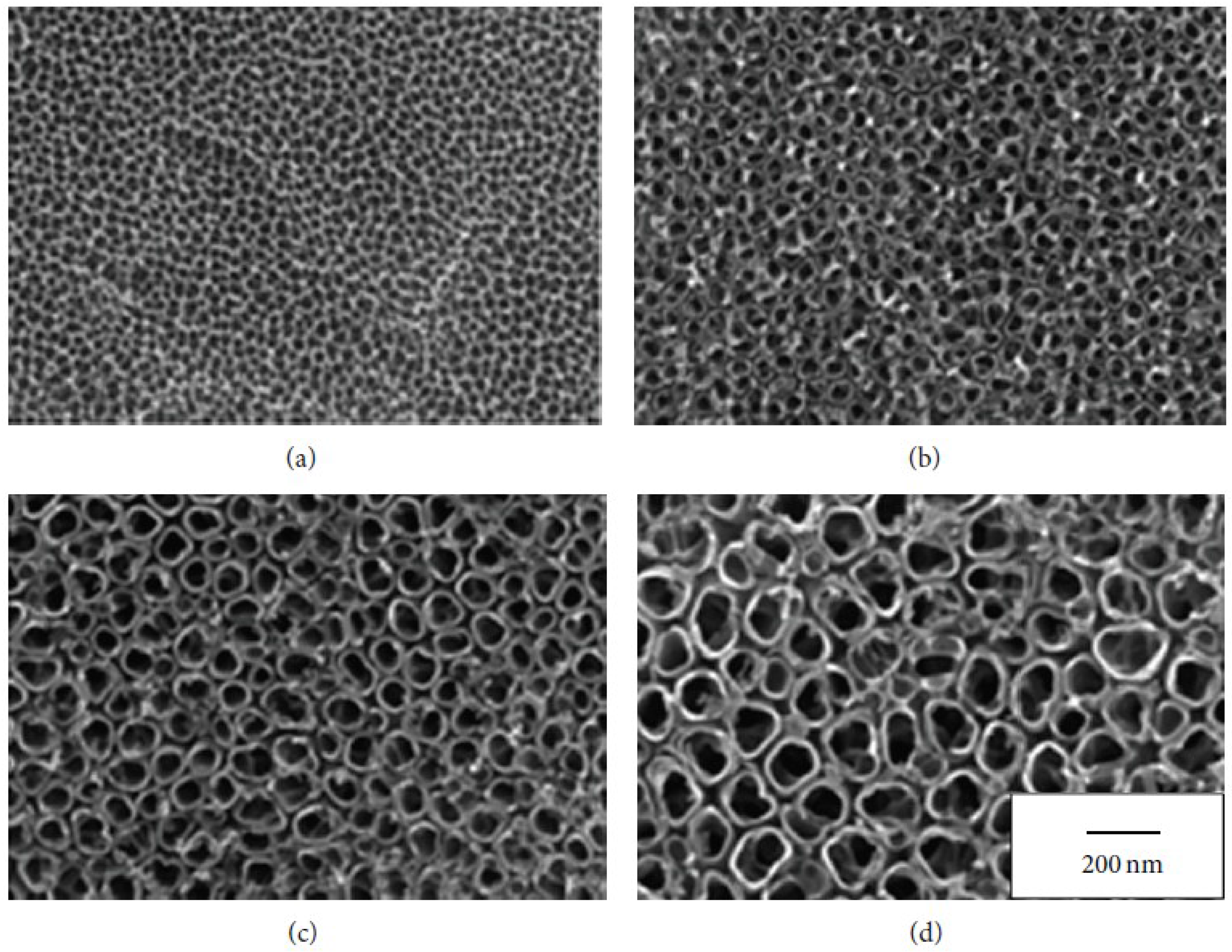
5. Biological Interaction and Porous Surface Geometry
6. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Elias, C.N. Factors Affecting the Success of Dental Implants; Turkyilmaz, I., Ed.; InTech: New York, NY, USA, 2011. [Google Scholar]
- Esposito, M.; Hirsch, J.; Lekholm, U.; Thomsen, P. Differential Diagnosis and Treatment Strategies for Biologic Complications and Failing Oral Implants: A Review of the Literature. Int. J. Oral Maxillofac. Implant. 1999, 14, 473–490. [Google Scholar]
- Cheng, A.; Humayun, A.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Additively manufactured 3D porous Ti–6Al–4V constructs mimic trabecular bone structure and regulate osteoblast proliferation, differentiation and local factor production in a porosity and surface roughness dependent manner. Biofabrication 2014. [Google Scholar] [CrossRef] [PubMed]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Boerrigter, E.M.; Geertman, M.E.; van Oort, R.P.; Bouma, J.; Raghoebar, G.M.; van Waas, M.A.J.; van’t Hof, M.A.; Boering, G.; Kalk, W. Patient satisfaction with implant-retained mandibular overdentures. A comparison with new complete dentures not retained by implants—A multicentre randomized clinical trial. Br. J. Oral Maxillofac. Surg. 1995, 33, 282–288. [Google Scholar] [CrossRef]
- Teixeira, L.N.; Crippa, G.E.; Lefebvre, L.-P.; de Oliveira, P.T.; Rosa, A.L.; Beloti, M.M. The influence of pore size on osteoblast phenotype expression in cultures grown on porous titanium. Int. J. Oral Maxillofac. Surg. 2012, 41, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.; Hämmerle, C. Titanium as a Reconstruction and Implant Material in Dentistry: Advantages and Pitfalls. Materials 2012, 5, 1528–1545. [Google Scholar] [CrossRef]
- Nomura, N.; Kohama, T.; Oh, I.H.; Hanada, S.; Chiba, A.; Kanehira, M.; Sasaki, K. Mechanical properties of porous Ti–15Mo–5Zr–3Al compacts prepared by powder sintering. Mater. Sci. Eng. C 2005, 25, 330–335. [Google Scholar]
- Krishna, B.V.; Bose, S.; Bandyopadhyay, A. Low stiffness porous Ti structures for load-bearing implants. Acta Biomater. 2007, 3, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Schiefer, H.; Bram, M.; Buchkremer, H.P.; Stöver, D. Mechanical examinations on dental implants with porous titanium coating. J. Mater. Sci. Mater. Med. 2009, 20, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Isidor, F. Influence of forces on peri-implant bone. Clin. Oral Implants Res. 2006, 17, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.; Chambrone, L.; van Noort, R.; Miller, C.; Hatton, P.; Mangano, C. Direct Metal Laser Sintering Titanium Dental Implants. Int. J. Biomater. 2014. [Google Scholar] [CrossRef] [PubMed]
- Junker, R.; Dimakis, A.; Thoneick, M.; Jansen, J.A. Effects of implant surface coatings and composition on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 185–206. [Google Scholar]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50–60. [Google Scholar] [PubMed]
- Çelen, S.; Özden, H. Laser-induced novel patterns: As smart strain actuators for new-age dental implant surfaces. Appl. Surf. Sci. 2012, 263, 579–585. [Google Scholar] [CrossRef]
- Yi, Y.; Park, Y.; Choi, H.; Lee, K.; Kim, S.; Kim, K.; Oh, S.; Shim, J. The Evaluation of Osseointegration of Dental Implant Surface with Different Size of TiO2 Nanotube in Rats. J. Nanomater. 2015. [Google Scholar] [CrossRef]
- Bencharit, S.; Byrd, W.C.; Altarawneh, S.; Hosseini, B.; Leong, A.; Reside, G.; Morelli, T.; Offenbacher, S. Development and Applications of Porous Tantalum Trabecular Metal-Enhanced Titanium Dental Implants. Clin. Implant Dent. Relat. Res. 2013, 16, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, G.; Oskolkov, N.; McMahon, M.T.; Walczak, P.; Janowski, M. Porous tantalum and tantalum oxide nanoparticles for regenerative medicine. Acta Neurobiol. Exp. 2014, 74, 188–196. [Google Scholar]
- Mour, M.; Das, D.; Winkler, T.; Hoenig, E.; Mielke, G.; Morlock, M.M.; Schilling, A.F. Advances in Porous Biomaterials for Dental and Orthopaedic Applications. Materials 2010, 3, 2947–2974. [Google Scholar] [CrossRef]
- Dabrowski, B.; Swieszkowski, W.; Godlinski, D.; Kurzydlowski, K.J. Highly porous titanium scaffolds for orthopaedic applications. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 95, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; de Wijn, J.R.; van Blitterswijk, C.A.; de Groot, K. Porous Ti6Al4V scaffolds directly fabricated by 3D fibre deposition technique: Effect of nozzle diameter. J. Mater. Sci. Mater. Med. 2005, 16, 1159–1163. [Google Scholar] [PubMed]
- Van Noort, R. Titanium the implant material of today. Mater. Sci. 1987, 22, 3801–3811. [Google Scholar] [CrossRef]
- Nouri, A.; Hodgson, P.D.; Wen, C. Biomimetic Porous Titanium Scaffolds for Orthopedic and Dental Applications; InTech: New York, NY, USA, 2010; pp. 415–451. [Google Scholar]
- Niinomi, M.; Hattori, T.; Niwa, S. Material characteristics and biocompatibility of low ridgidity titanium alloys for biomedical applications. In Biomaterials in Orthopedics; Yaszemski, M.J., Trantolo, D.J., Lewandrowski, K.-U., Hasirci, V., Altobelli, D.E., Wise, D.L., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 41–62. [Google Scholar]
- Gong, C.W.; Wang, Y.N.; Yang, D.Z. Phase transformation and second phases in ternary Ni–Ti–Ta shape memory alloys. Mater. Chem. Phys. 2006, 96, 183–187. [Google Scholar] [CrossRef]
- Shishkovsky, I.V. Shape Memory Effect in Porous Volume NiTi Articles Fabricated by Selective Laser Sintering. Tech. Phys. Lett. 2005, 31, 186–188. [Google Scholar] [CrossRef]
- Nouri, A.; Hodgson, P.D.; Wen, C.E. Effect of process control agent on the porous structure and mechanical properties of a biomedical Ti–Sn–Nb alloy produced by powder metallurgy. Acta Biomater. 2010, 6, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Li, S.H.; van Blitterswijk, C.A.; de Groot, K. A novel porous Ti6Al4V: Characterization and cell attachment. J. Biomed. Mater. Res. A 2005, 73, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Bahraminasab, M.; Sahari, B.B. NiTi Shape Memory Alloys, Promising Materials in Orthopedic Applications. In Shape Memory Alloys—Processing, Characterization and Applications; InTech: New York, NY, USA, 2013; pp. 261–278. [Google Scholar]
- Seah, K.H.W.; Thampuran, R.; Teoh, S.H. The influence of pore morphology on corrosion. Corros. Sci. 1998, 40, 547–556. [Google Scholar]
- Arifvianto, B.; Zhou, J. Fabrication of Metallic Biomedical Scaffolds with the Space Holder Method: A Review. Materials 2014, 7, 3588–3622. [Google Scholar]
- Andani, M.T.; Moghaddam, N.S.; Haberland, C.; Dean, D.; Miller, M.J.; Elahinia, M. Metals for bone implants. Part 1. Powder metallurgy and implant rendering. Acta Biomater. 2014, 10, 4058–4070. [Google Scholar] [CrossRef] [PubMed]
- Rack, H.J.; Qazi, J.I. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, D.; Niinomi, M.; Morinaga, M.; Kato, Y.; Yashiro, T. Design and mechanical properties of new β type titanium alloys for implant materials. Mater. Sci. Eng. A 1998, 243, 244–249. [Google Scholar] [CrossRef]
- Matsumoto, H.; Watanabe, S.; Hanada, S. β TiNbSn Alloys with Low Young’s Modulus and High Strength. Mater. Trans. 2005, 46, 1070–1078. [Google Scholar] [CrossRef]
- Nomura, N.; Sakamoto, K.; Takahashi, K.; Kato, S.; Abe, Y.; Doi, H.; Tsutsumi, Y.; Kobayashi, M.; Kobayashi, E.; Kim, W.-J.; et al. Fabrication and Mechanical Properties of Porous Ti/HA Composites for Bone Fixation Devices. Mater. Trans. 2010, 51, 1449–1454. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2000, 22, 87–96. [Google Scholar] [CrossRef]
- Thiyagasundaram, P.; Sankar, B.V.; Arakere, N.K. Elastic Properties of Open-Cell Foams with Tetrakaidecahedral Cells Using Finite Element Analysis. AIAA J. 2010, 48, 818–828. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Campoli, G.; Yavari, S.A.; Sajadi, B.; Wauthle, R.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Mechanical behavior of regular open-cell porous biomaterials made of diamond lattice unit cells. J. Mech. Behav. Biomed. Mater. 2014, 34, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Goodall, R.; Mortensen, A. Porous Metals. In Physical Metallurgy, 5th ed.; Laughlin, D., Hono, K.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Li, D.S.; Zhang, Y.P.; Eggeler, G.; Zhang, X.P. High porosity and high-strength porous NiTi shape memory alloys with controllable pore characteristics. J. Alloy. Compd. 2009, 470, L1–L5. [Google Scholar] [CrossRef]
- Kutty, M.G.; Bhaduri, S.; Jokisaari, J.R.; Bhaduri, S.B. Development of gradient porosities in Ti dental implant. In 25th Annual Conference on Composites, Advanced Ceramics, Materials, and Structures: B: Ceramic Engineering and Science Proceedings; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; Volume 22, pp. 587–592. [Google Scholar]
- Aly, M.S.; Bleck, W.; Scholz, P.-F. How metal foams behave if the temperature rises. Met. Powder Rep. 2005, 60, 38–45. [Google Scholar] [CrossRef]
- Murray, G.; Semple, J. Transfer of tensile loads from a prosthesis to bone using porous titanium. J. Bone Joint Surg. 1981, 63, 138–141. [Google Scholar]
- Li, X.; Wang, C.; Zhang, W.; Li, Y. Fabrication and compressive properties of Ti6Al4V implant with honeycomb-like structure for biomedical applications. Rapid Prototyp. J. 2010, 16, 44–49. [Google Scholar] [CrossRef]
- Parthasarathy, J.; Starly, B.; Raman, S.; Christensen, A. Mechanical evaluation of porous titanium (Ti6Al4V) structures with electron beam melting (EBM). J. Mech. Behav. Biomed. Mater. 2010, 3, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Harrysson, O.L.; Cansizoglu, O.; Marcellin-Little, D.J.; Cormier, D.R.; West, H. Direct metal fabrication of titanium implants with tailored materials and mechanical properties using electron beam melting technology. Mater. Sci. Eng. C 2008, 28, 366–373. [Google Scholar] [CrossRef]
- Campoli, G.; Borleffs, M.S.; Yavari, S.A.; Wauthle, R.; Weinans, H.; Zadpoor, A.A. Mechanical properties of open-cell metallic biomaterials manufactured using additive manufacturing. Mater. Des. 2013, 49, 957–965. [Google Scholar] [CrossRef]
- Heinl, P.; Körner, C.; Singer, R.F. Selective Electron Beam Melting of Cellular Titanium: Mechanical Properties. Adv. Eng. Mater. 2008, 10, 882–888. [Google Scholar] [CrossRef]
- Van Grunsven, W. Porous Metal Implants for Enhanced Bone Ingrowth and Stability. Ph.D. Thesis, University of Sheffield, Sheffield, UK, September 2014. [Google Scholar]
- Heinl, P.; Müller, L.; Körner, C.; Singer, R.F.; Müller, F.A. Cellular Ti–6Al–4V structures with interconnected macro porosity for bone implants fabricated by selective electron beam melting. Acta Biomater. 2008, 4, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Mabuchi, M.; Yamada, Y.; Shimojima, K.; Chino, Y.; Asahina, T. Processing of biocompatible porous Ti and Mg. Scr. Mater. 2001, 45, 1147–1153. [Google Scholar] [CrossRef]
- Otsuki, B.; Takemoto, M.; Fujibayashi, S.; Neo, M.; Kokubo, T.; Nakamura, T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: Three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials 2006, 27, 5892–5900. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Hong, Y.; Liu, X.; Fan, H.; Zhang, X. A hierarchically graded bioactive scaffold bonded to titanium substrates for attachment to bone. Biomaterials 2011, 32, 7333–7346. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, C.; Zhu, X.; Zhang, K.; Fan, Y.; Zhang, X. Fabrication of porous titanium scaffolds by stack sintering of microporous titanium spheres produced with centrifugal granulation technology. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 43, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.; Bormann, T.; Rossi, A.; Müller, B.; Schumacher, R.; Martin, I.; de Wild, M.; Wendt, D. Rapid prototyped porous nickel-titanium scaffolds as bone substitutes. J. Tissue Eng. 2014. [Google Scholar] [CrossRef] [PubMed]
- Sidambe, A. Biocompatibility of Advanced Manufactured Titanium Implants—A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef]
- Tolochko, N.K.; Savich, V.V.; Laoui, T.; Froyen, L.; Onofrio, G.; Signorelli, E.; Titov, V.I. Dental root implants produced by the combined selective laser sintering/melting of titanium powders. J. Mater. Des. Appl. 2002, 216, 267–270. [Google Scholar] [CrossRef]
- Laptev, A.; Bram, M.; Buchkremer, H.P.; Stöver, D. Study of production route for titanium parts combining very high porosity and complex shape. Powder Metall. 2004, 47, 85–92. [Google Scholar] [CrossRef]
- Kutty, M.G.; Bhaduri, S.; Bhaduri, S.B. Gradient surface porosity in titanium dental implants: Relation between processing parameters and microstructure. J. Mater. Sci. Mater. Med. 2004, 15, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, J.; Adamek, G.; Dewidar, M. Titanium foam made with saccharose as a space holder. J. Porous Mater. 2013, 20, 1137–1141. [Google Scholar] [CrossRef]
- Bansiddhi, A.; Dunand, D.C. Shape-memory NiTi foams produced by solid-state replication with NaF. Intermetallics 2007, 15, 1612–1622. [Google Scholar] [CrossRef]
- Bansiddhi, A.; Dunand, D.C. Shape-memory NiTi foams produced by replication of NaCl space-holders. Acta Biomater. 2008, 4, 1996–2007. [Google Scholar] [CrossRef] [PubMed]
- Van Grunsven, W.; Goodall, R.; Reilly, G.C. Highly Porous Titanium Alloy: Fabrication and Mechanical Properties. J. Biomech. 2012, 45, S339. [Google Scholar] [CrossRef]
- Jee, C.S.Y.; Guo, Z.X.; Evans, J.R.G.; Özgüven, N. Preparation of High Porosity Metal Foams. Metall. Mater. Trans. B 2000, 31, 1345–1352. [Google Scholar] [CrossRef]
- Kim, S.W.; Jung, H.-D.; Kang, M.-H.; Kim, H.-E.; Koh, Y.-H.; Estrin, Y. Fabrication of porous titanium scaffold with controlled porous structure and net-shape using magnesium as spacer. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2808–2815. [Google Scholar] [CrossRef] [PubMed]
- Jurczyk, M.U.; Jurczyk, K.; Niespodziana, K.; Miklaszewski, A.; Jurczyk, M. Titanium-SiO2 nanocomposites and their scaffolds for dental applications. Mater. Charact. 2013, 77, 99–108. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef]
- Lifland, M.I.; Kim, D.K.; Okazaki, K. Mechanical properties of a Ti–6A1–4V dental implant produced by electro-discharge compaction. Clin. Mater. 1993, 14, 13–19. [Google Scholar] [CrossRef]
- Mangano, C.; Mangano, F.G.; Shibli, J.A.; Ricci, M.; Perrotti, V.; d’Avila, S.; Piattelli, A. Immediate loading of mandibular overdentures supported by unsplinted direct laser metal-forming implants: Results from a 1-year prospective study. J. Periodontol. 2012, 83, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Traini, T.; Mangano, C.; Sammons, R.L.; Mangano, F.; Macchi, A.; Piattelli, A. Direct laser metal sintering as a new approach to fabrication of an isoelastic functionally graded material for manufacture of porous titanium dental implants. Direct laser metal sintering as a new approach to fabrication of an isoelastic functionally grad. Dent. Mater. 2008, 24, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Hrabe, N.W.; Heinl, P.; Flinn, B.; Körner, C.; Bordia, R.K. Compression-compression fatigue of selective electron beam melted cellular titanium (Ti–6Al–4V). J. Biomed. Mater. Res. B Appl. Biomater. 2011, 99, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Murr, L.E.; Gaytan, S.M.; Medina, F.; Lopez, H.; Martinez, E.; Machado, B.I.; Hernandez, D.H.; Martinez, L.; Lopez, M.I.; Wicker, R.B.; et al. Next-generation biomedical implants using additive manufacturing of complex, cellular and functional mesh arrays. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 1999–2032. [Google Scholar] [CrossRef] [PubMed]
- Laoui, T.; Santos, E.; Osakada, K.; Shiomi, M.; Morita, M.; Shaik, S.K.; Tolochko, N.K.; Abe, F.; Takahashi, M. Properties of Titanium Dental Implant Models Made by Laser Processing. J. Mech. Eng. Sci. 2006, 220, 857–863. [Google Scholar] [CrossRef]
- Mangano, C.; Raspanti, M.; Traini, T.; Piattelli, A.; Sammons, R. Stereo imaging and cytocompatibility of a model dental implant surface formed by direct laser fabrication. J. Biomed. Mater. Res. A 2009, 88, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.D.; Thongpreda, N.; Anderson, R.C.; Haddad, R.J. The effect of post-sintering heat treatments on the fatigue properties of porous coated Ti–6Al–4V alloy. J. Biomed. Mater. Res. 1988, 22, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.V.; Duan, Y.; Neidigh, J.; Koike, M.; Chahine, G.; Kovacevic, R.; Okabe, T.; Griggs, J.A. Fatigue testing of electron beam-melted Ti–6Al–4V ELI alloy for dental implants. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Van Grunsven, W.; Hernandez-Nava, E.; Reilly, G.; Goodall, R. Fabrication and Mechanical Characterisation of Titanium Lattices with Graded Porosity. Metals 2014, 4, 401–409. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Espana, F.; Balla, V.K.; Bose, S.; Ohgami, Y.; Davies, N.M. Influence of porosity on mechanical properties and in vivo response of Ti6Al4V implants. Acta Biomater. 2010, 6, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Witek, L.; Marin, C.; Granato, R.; Bonfante, E.A.; Campos, F.; Bisinotto, J.; Suzuki, M.; Coelho, P.G. Characterization and in vivo evaluation of laser sintered dental endosseous implants in dogs. J. Biomed. Mater. Res. B. Appl. Biomater. 2012, 100, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Bidan, C.M.; Kommareddy, K.P.; Rumpler, M.; Kollmannsberger, P.; Fratzl, P.; Dunlop, J.W.C. Geometry as a factor for tissue growth: Towards shape optimization of tissue engineering scaffolds. Adv. Healthc. Mater. 2013, 2, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Tison, C.K.; Chatterjee, K.; Pine, P.S.; McDaniel, J.H.; Salit, M.L.; Young, M.F.; Simon, C.G. The determination of stem cell fate by 3D scaffold structures through the control of cell shape. Biomaterials 2011, 32, 9188–9196. [Google Scholar] [CrossRef] [PubMed]
- De Wild, M.; Schumacher, R.; Mayer, K.; Schkommodau, E.; Thoma, D.; Bredell, M.; Gujer, A.K.; Grätz, K.W.; Weber, F.E. Bone regeneration by the osteoconductivity of porous titanium implants manufactured by selective laser melting: A histological and micro computed tomography study in the rabbit. Tissue Eng. Part A 2013, 19, 2645–2654. [Google Scholar] [CrossRef] [PubMed]
- Mangano, C.; Piattelli, A.; d’Avila, S.; Iezzi, G.; Mangano, F.; Onuma, T.; Shibli, J.A. Early human bone response to laser metal sintering surface topography: A histologic report. J. Oral Implantol. 2010, 36, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Hollander, D.A.; von Walter, M.; Wirtz, T.; Sellei, R.; Schmidt-Rohlfing, B.; Paar, O.; Erli, H.-J. Structural, mechanical and in vitro characterization of individually structured Ti–6Al–4V produced by direct laser forming. Biomaterials 2006, 27, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Rumpler, M.; Woesz, A.; Dunlop, J.W.C.; van Dongen, J.T.; Fratzl, P. The effect of geometry on three-dimensional tissue growth. J. R. Soc. Interface 2008, 5, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Markhoff, J.; Wieding, J.; Weissmann, V.; Pasold, J.; Jonitz-Heincke, A.; Bader, R. Influence of Different Three-Dimensional Open Porous Titanium Scaffold Designs on Human Osteoblasts Behavior in Static and Dynamic Cell Investigations. Materials 2015, 8, 5490–5507. [Google Scholar] [CrossRef]
- Shibli, J.A.; Mangano, C.; D’avila, S.; Piattelli, A.; Pecora, G.E.; Mangano, F.; Onuma, T.; Cardoso, L.A.; Ferrari, D.S.; Aguiar, K.C.; et al. Influence of direct laser fabrication implant topography on type IV bone: A histomorphometric study in humans. J. Biomed. Mater. Res. A 2010, 93, 607–614. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wally, Z.J.; Van Grunsven, W.; Claeyssens, F.; Goodall, R.; Reilly, G.C. Porous Titanium for Dental Implant Applications. Metals 2015, 5, 1902-1920. https://doi.org/10.3390/met5041902
Wally ZJ, Van Grunsven W, Claeyssens F, Goodall R, Reilly GC. Porous Titanium for Dental Implant Applications. Metals. 2015; 5(4):1902-1920. https://doi.org/10.3390/met5041902
Chicago/Turabian StyleWally, Zena J., William Van Grunsven, Frederik Claeyssens, Russell Goodall, and Gwendolen C. Reilly. 2015. "Porous Titanium for Dental Implant Applications" Metals 5, no. 4: 1902-1920. https://doi.org/10.3390/met5041902
APA StyleWally, Z. J., Van Grunsven, W., Claeyssens, F., Goodall, R., & Reilly, G. C. (2015). Porous Titanium for Dental Implant Applications. Metals, 5(4), 1902-1920. https://doi.org/10.3390/met5041902