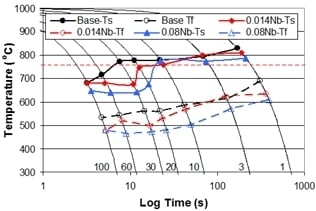
The Effect of Nb on the Continuous Cooling Transformation Curves of Ultra-Thin Strip CASTRIP© Steels
Abstract
:1. Introduction
2. Experimental Section
| (wt%) | Base | 0.014% Nb | 0.024% Nb | 0.04% Nb | 0.06% Nb | 0.08% Nb |
|---|---|---|---|---|---|---|
| C | 0.027 | 0.032 | 0.029 | 0.036 | 0.031 | 0.031 |
| P | 0.009 | 0.012 | 0.013 | 0.013 | 0.014 | 0.015 |
| Mn | 0.693 | 0.686 | 0.730 | 0.870 | 0.738 | 0.830 |
| Si | 0.189 | 0.180 | 0.175 | 0.210 | 0.159 | 0.200 |
| S | 0.002 | 0.003 | 0.002 | 0.002 | 0.003 | 0.002 |
| Ni | 0.010 | 0.028 | 0.036 | 0.039 | 0.047 | 0.029 |
| Cr | 0.027 | 0.041 | 0.045 | 0.043 | 0.057 | 0.037 |
| Mo | 0.006 | 0.010 | 0.014 | 0.011 | 0.017 | 0.008 |
| Cu | 0.040 | 0.070 | 0.084 | 0.083 | 0.095 | 0.054 |
| Nb | 0.000 | 0.014 | 0.024 | 0.040 | 0.06 | 0.080 |
| N | 0.004 | 0.006 | 0.004 | 0.007 | 0.005 | 0.006 |
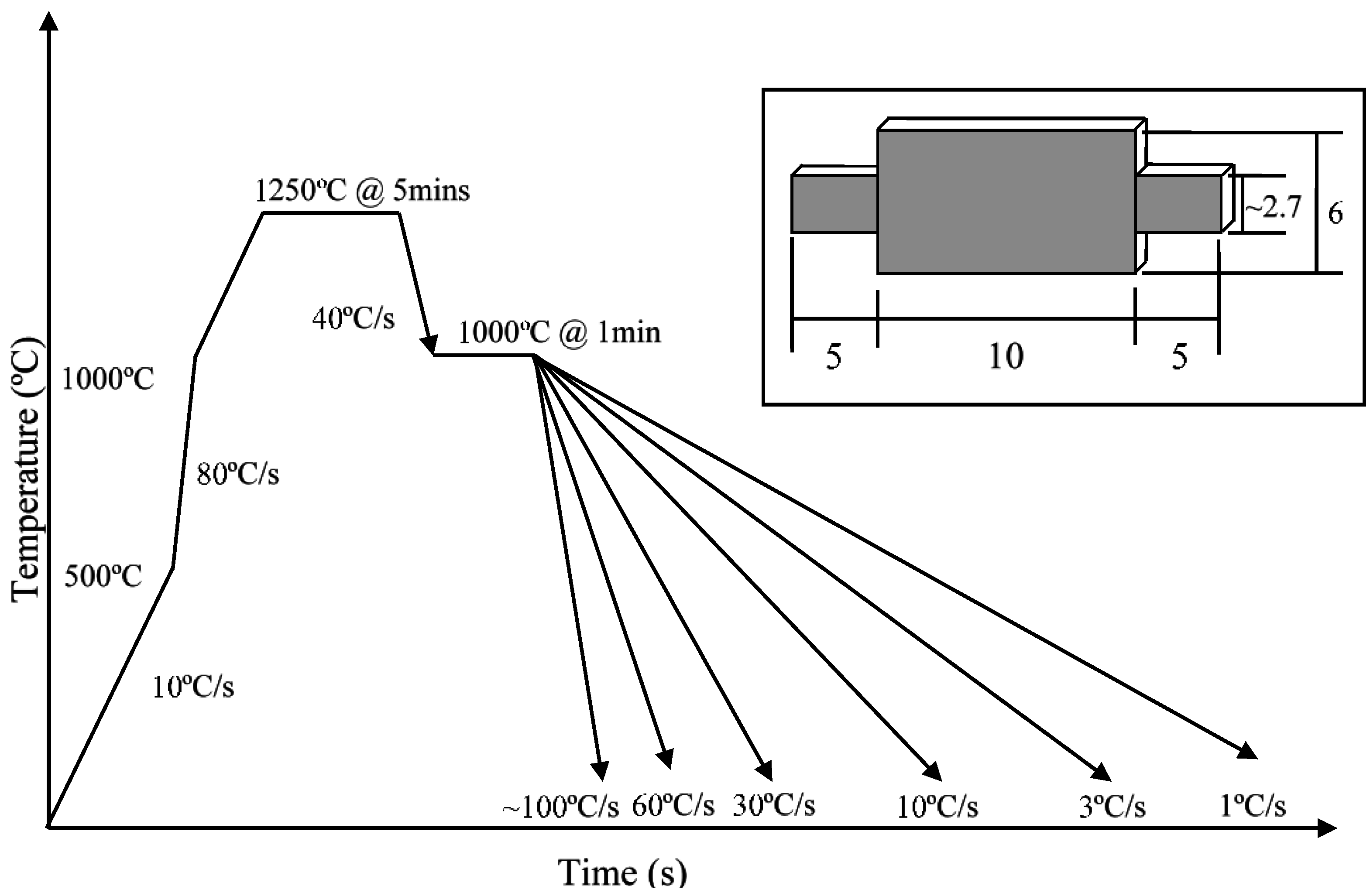
3. Results and Discussion
3.1. Phase Nomenclature for Low Carbon Steels
| Symbol | Nomenclature | Characteristics—Major Matrix phases |
|---|---|---|
| αp | Polygonal Ferrite | Equiaxed, polyhedral shaped, mostly recrystallized |
| αq | Quasi-polygonal α | Irregular changeful shape, formed at lower temperature crossing over γ-grain boundary, mostly recovered |
| αw | Widmanstätten α | Characteristic lath/plate-like shape; not usual in very low-C steels, mostly recovered |
| αB (Zw) | (Granular bainitic) α | Granular bainitic ferritic Zw structure; dislocated substructure but fairly recovered like “lath-less” |
| αB° | Bainitic Ferrite | Sheaf-like with laths but no carbide; conserving the prior γ-grain boundary, generally enough dislocated |
| Minor Secondary Phases | C-enriched and scattered in Islands or films | |
| P | Pearlite | Eutectoidal reaction product |
3.2. Prior Austenite Grain Size
| Grade | Base | 0.04% Nb | 0.08% Nb |
|---|---|---|---|
| PAGS (µm) | 124 | 81 | 74 |

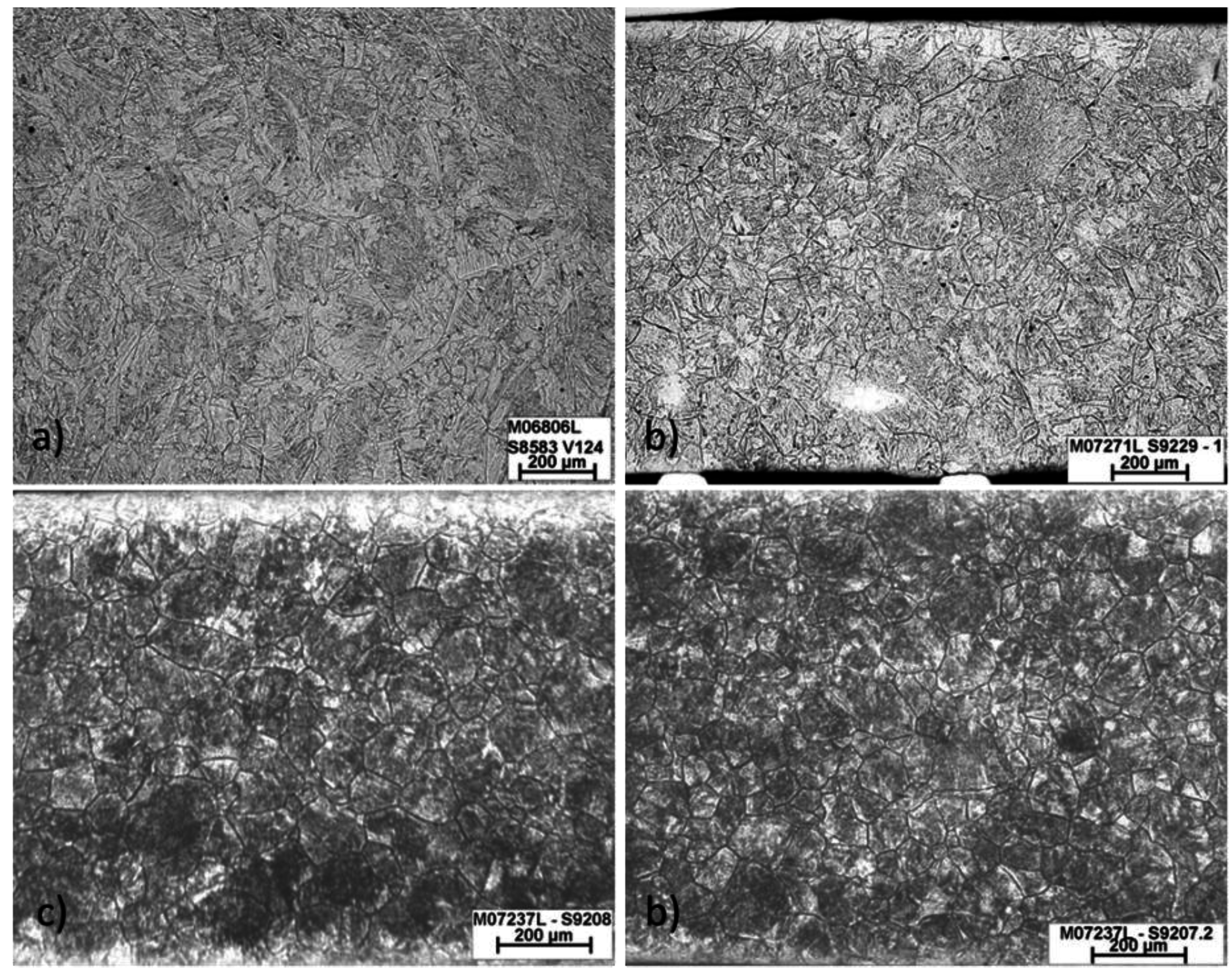
3.3. The Effect of Cooling Rate on Transformed Microstructures

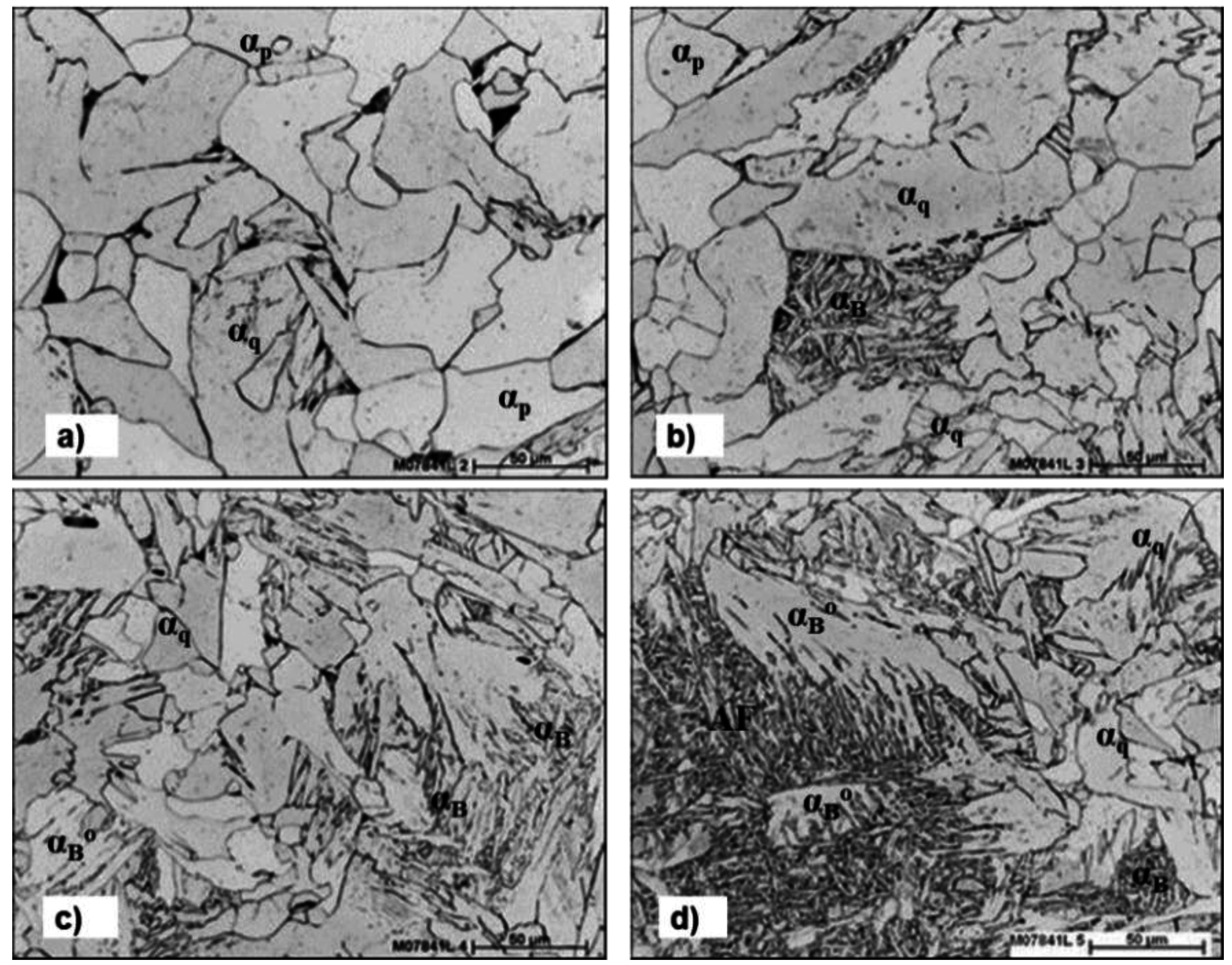
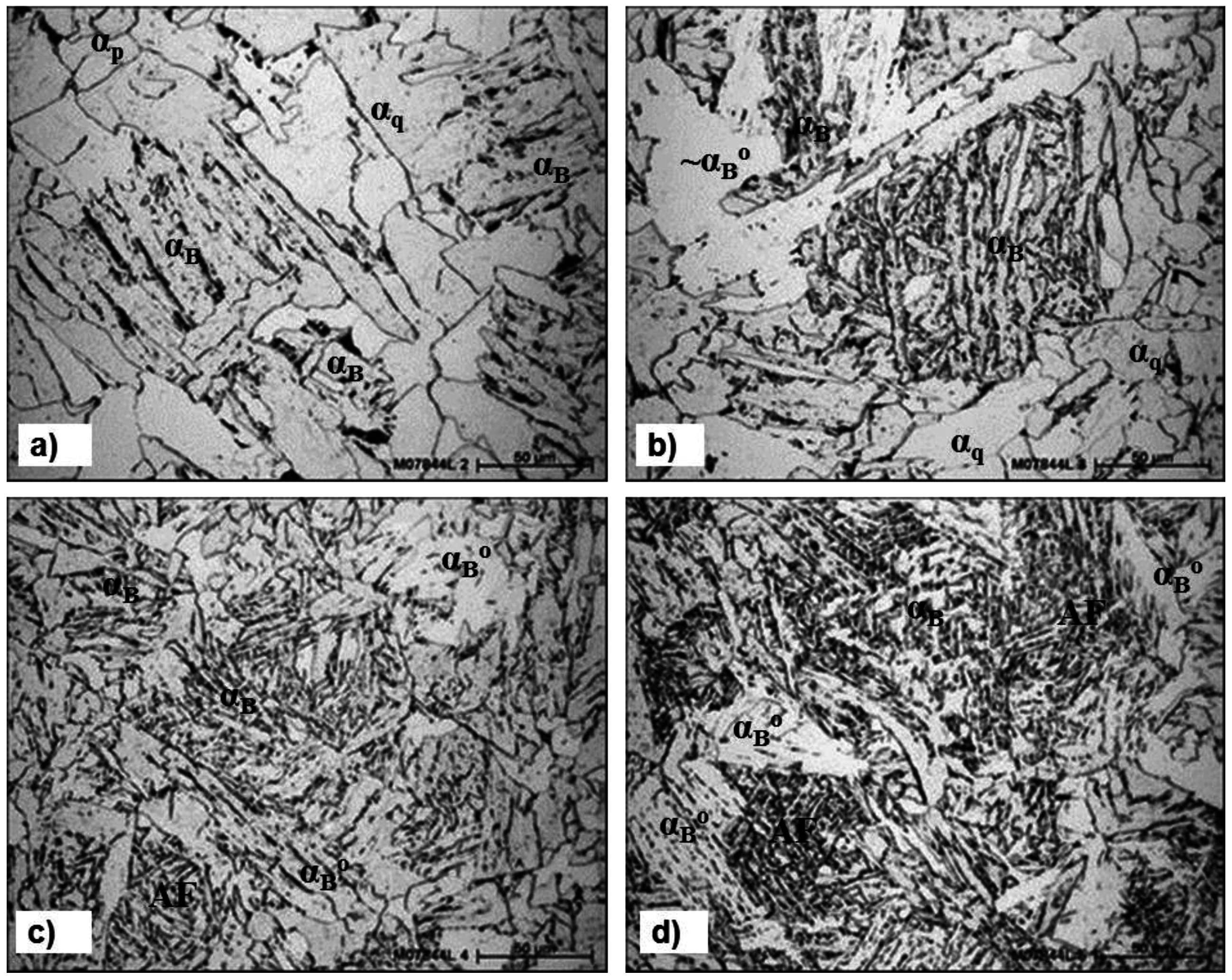
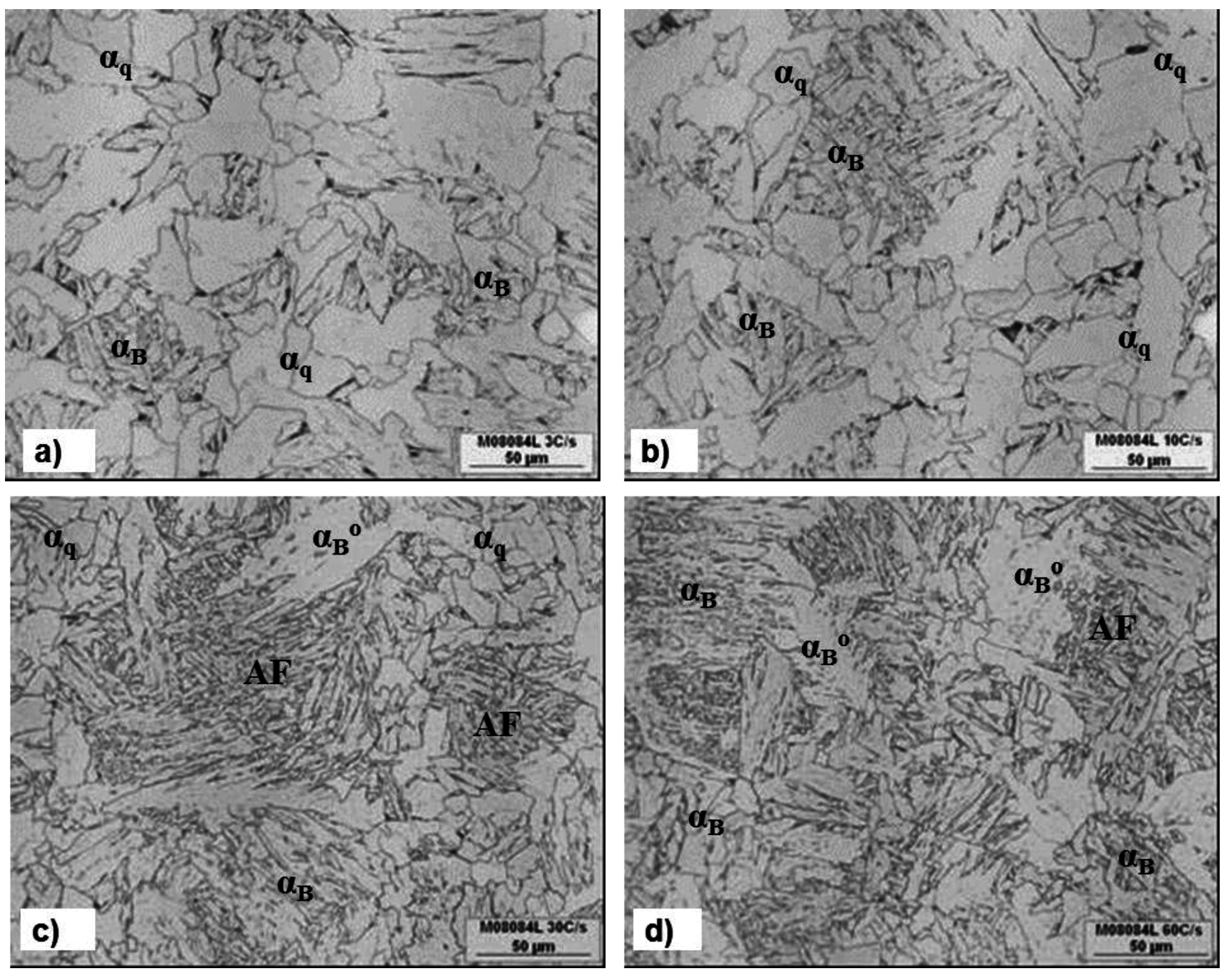
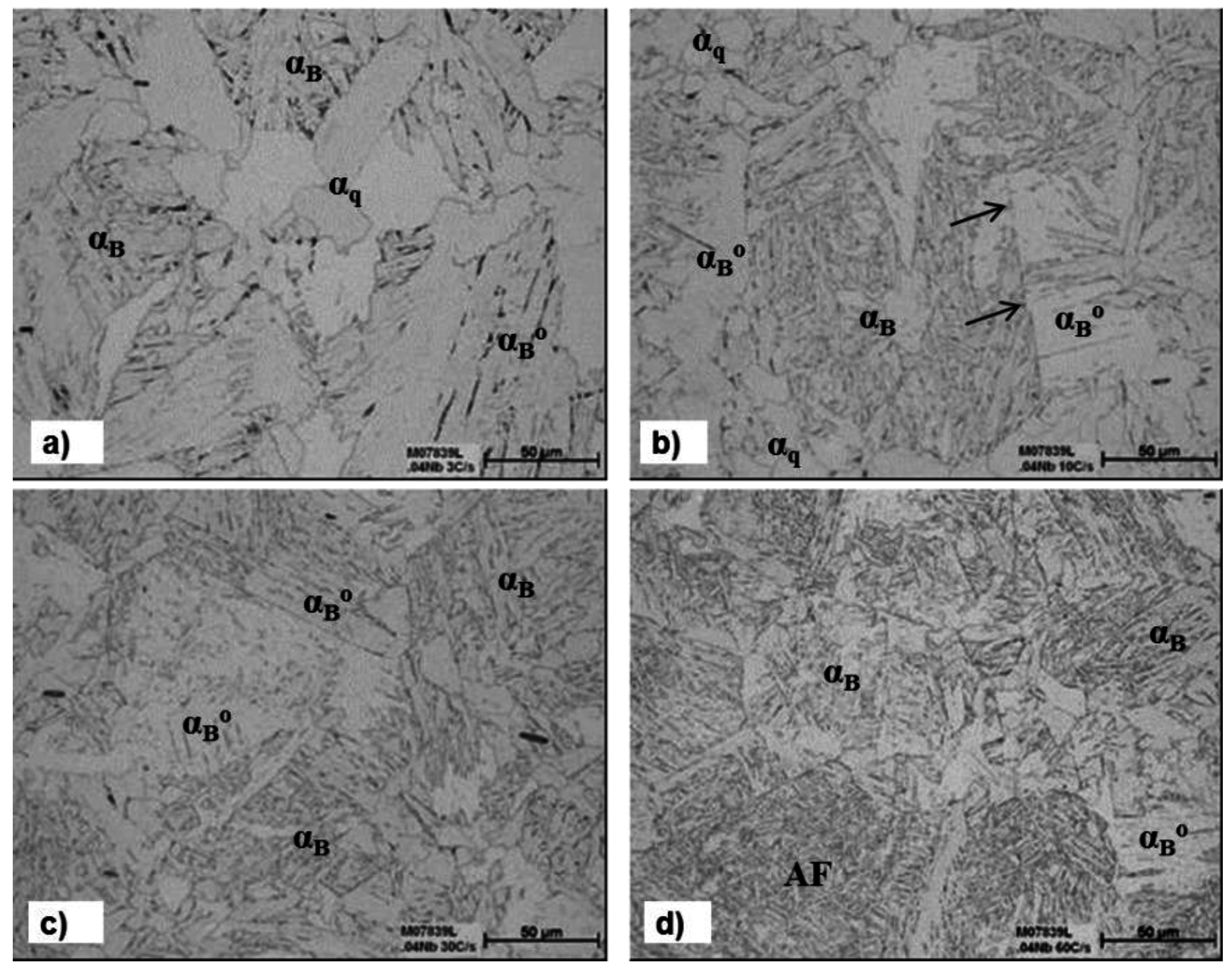
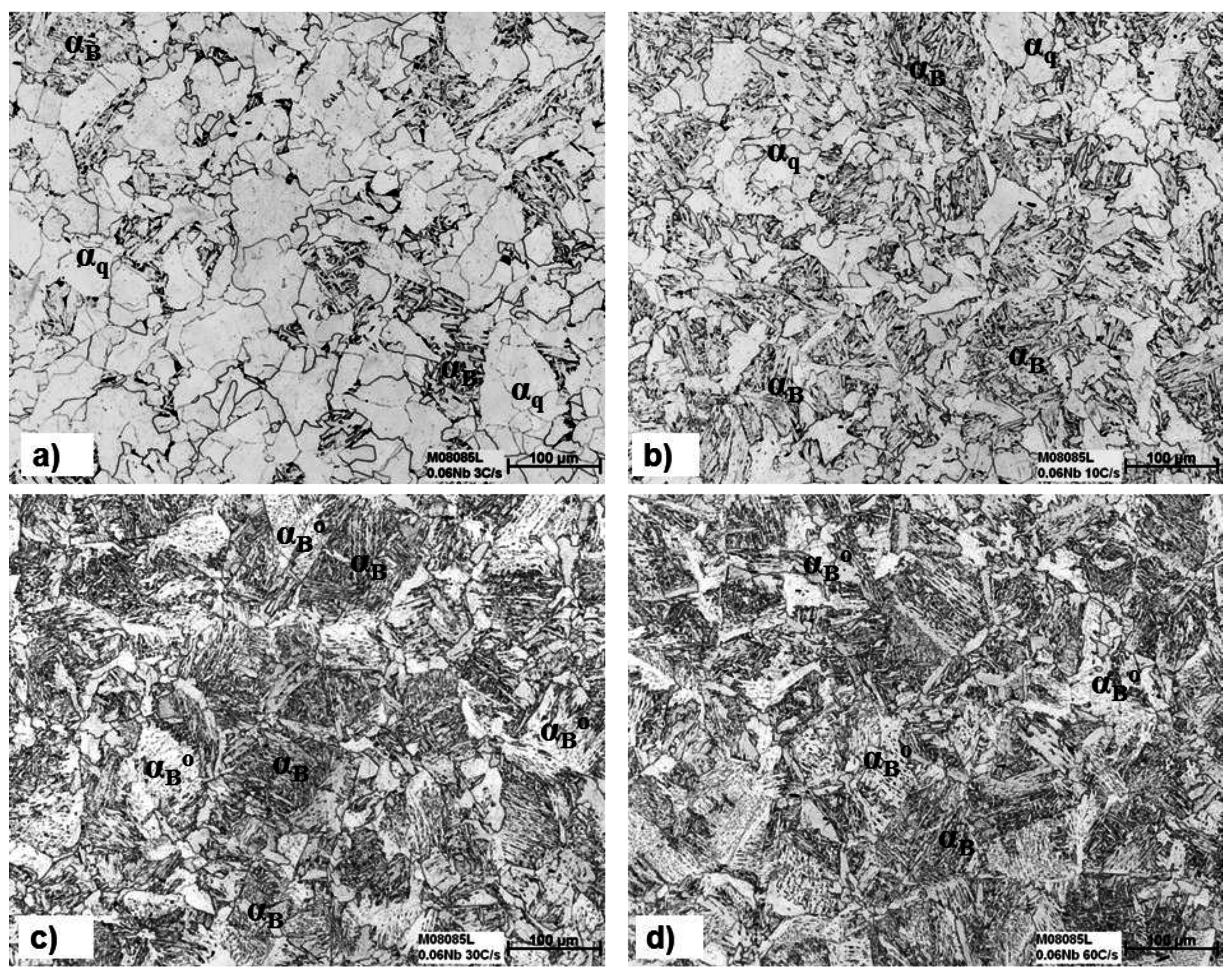
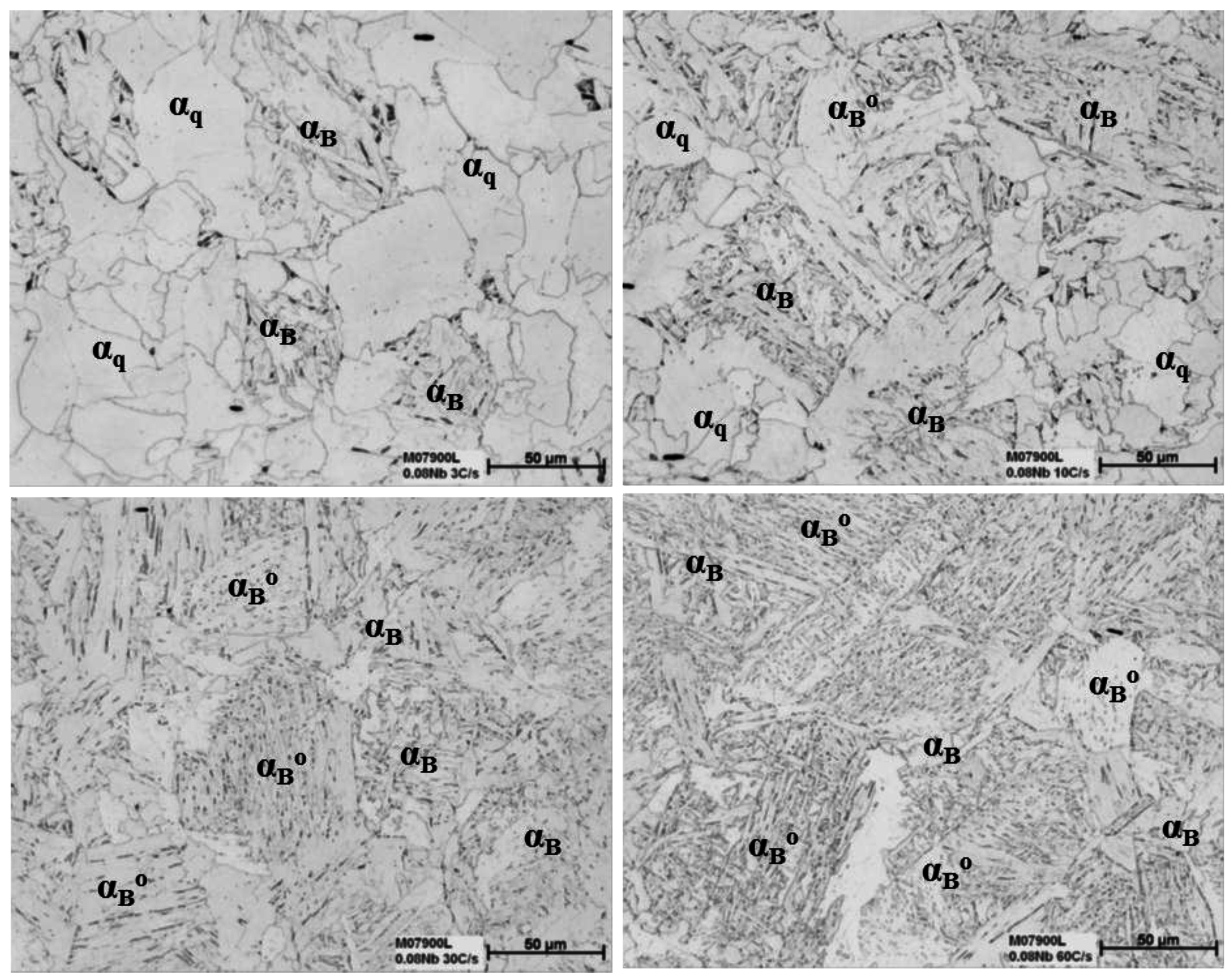
3.4. Effect of Nb on Hardenability

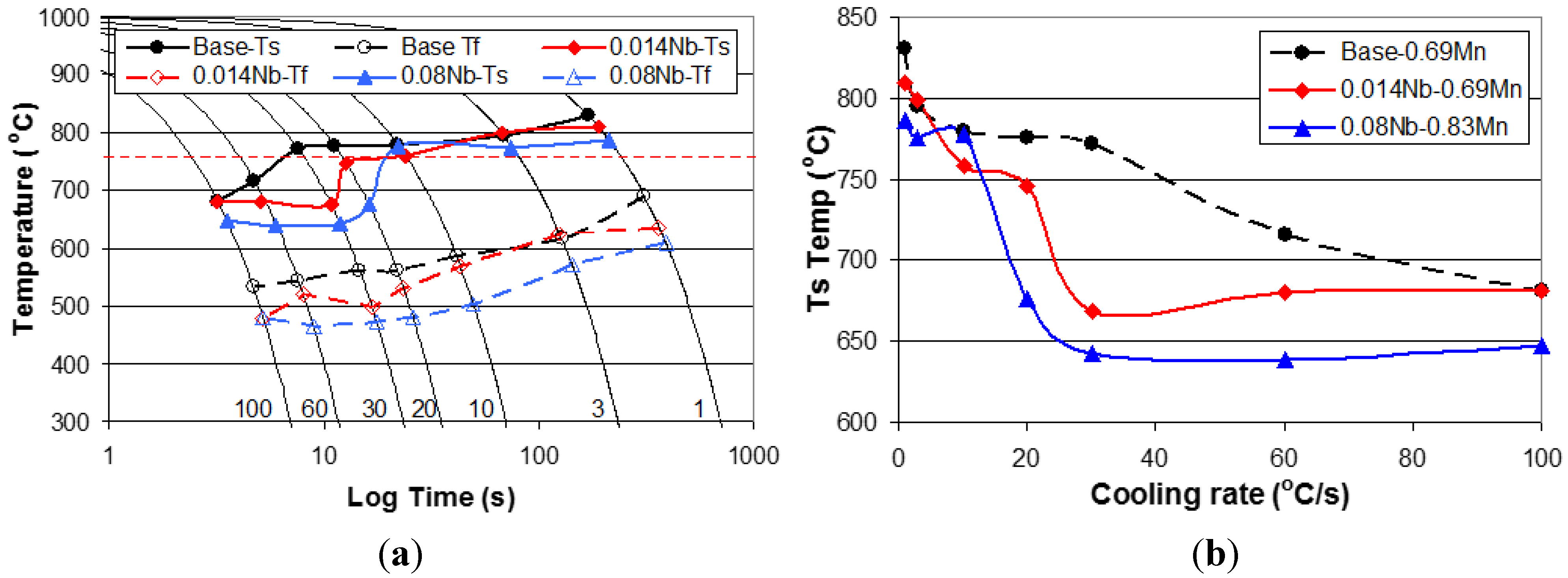
3.5. Effect of Cooling Rate and Increasing Nb Additions on Hardness of Dilatometry Samples
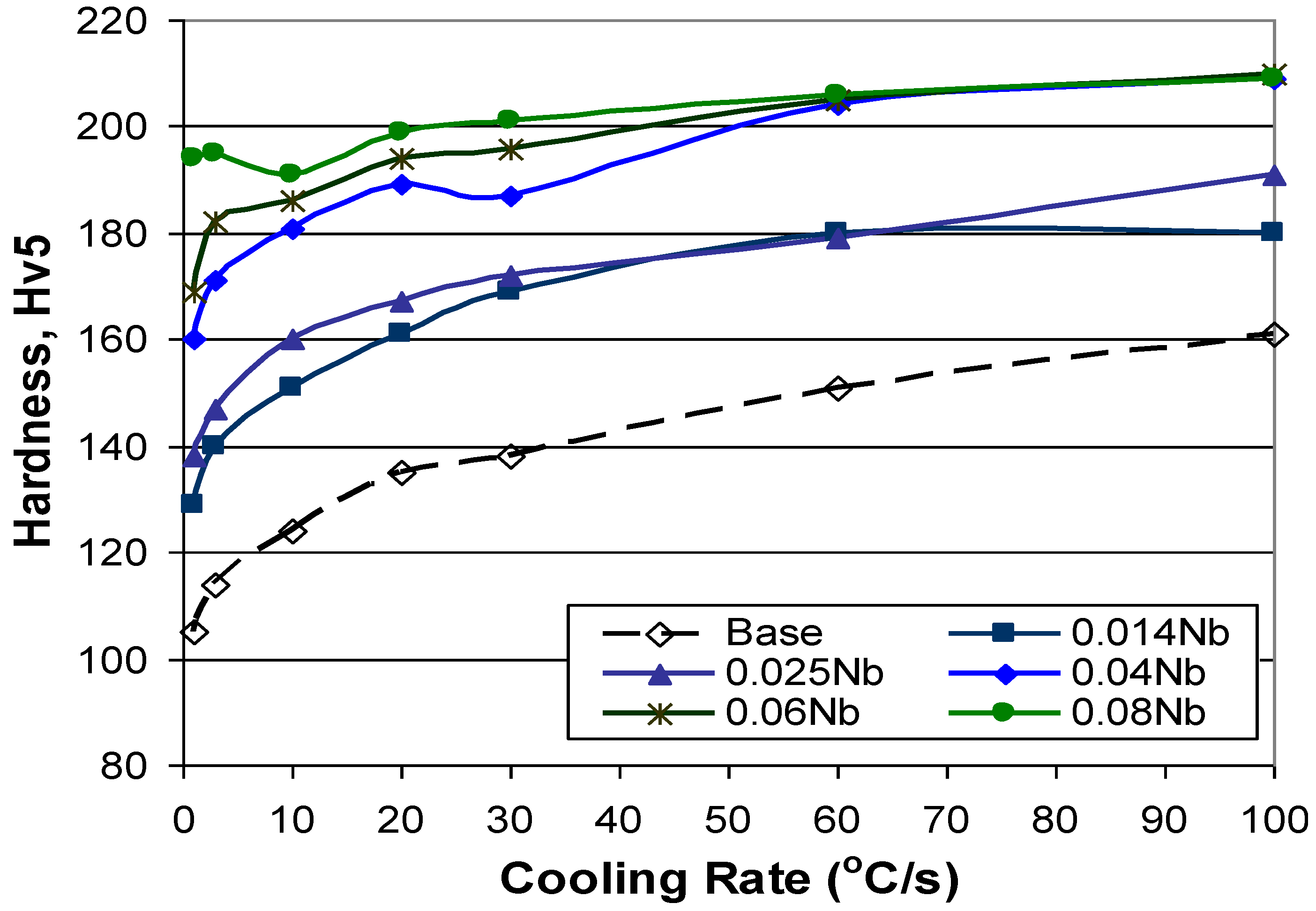
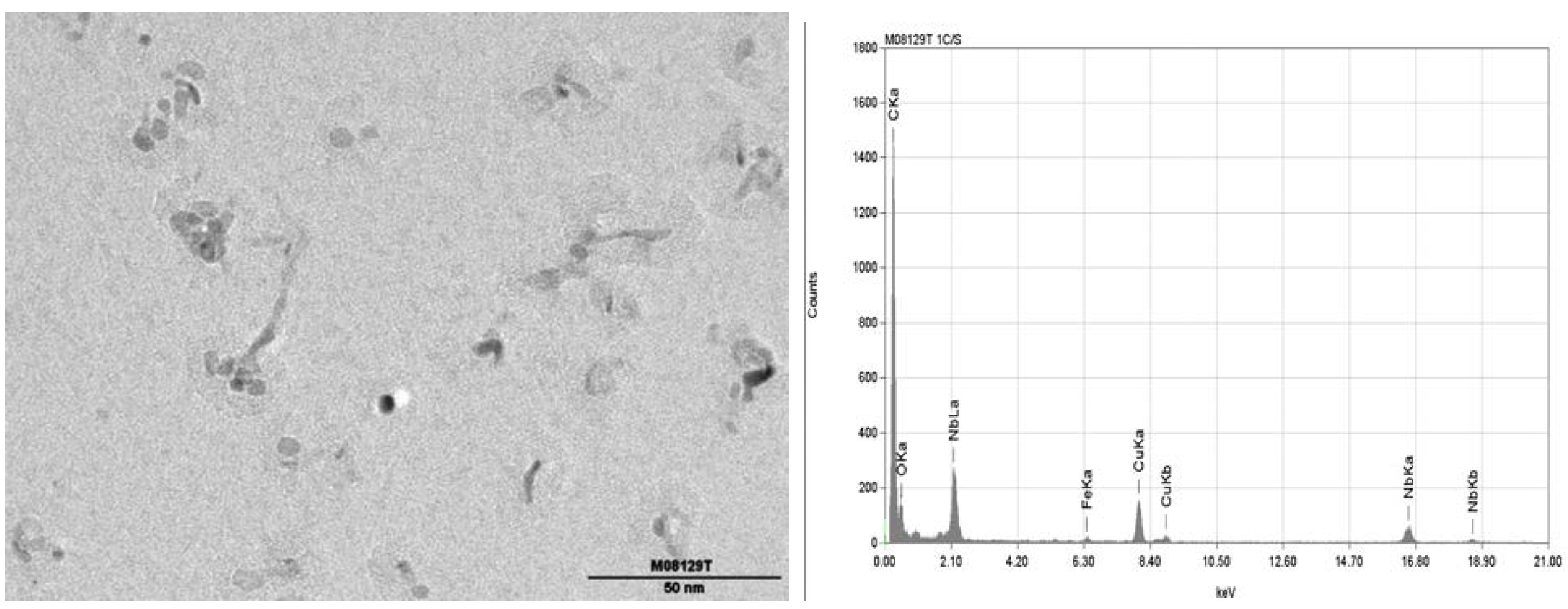
3.6. Comparison of CCT Microstructures with Production Produced Grades
| Grade | Nb (wt%) | Mn (wt%) | YS (Mpa) | TS (Mpa) | TE% | %HR | Coil Temp |
|---|---|---|---|---|---|---|---|
| Base | 0 | 0.69 | 379.2 | 482.6 | 21 | 34% | 646°C |
| 0.015 Nb | 0.014 | 0.69 | 434.4 | 517.1 | 17 | 35% | 495°C |
| 0.024 Nb | 0.024 | 0.73 | 420.6 | 515.7 | 17.6 | 42% | 492°C |
| 0.04 Nb | 0.041 | 0.87 | 472.3 | 562.6 | 14.3 | 36% | 590°C |
| 0.06 Nb | 0.059 | 0.74 | 479.9 | 553.0 | 14.3 | 35% | 570°C |
| 0.08 Nb | 0.084 | 0.83 | 480.9 | 575.7 | 16.4 | 40% | 560°C |

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tanaka, T.; Tabata, N.; Hatomura, T.; Shiga, C. Three Stages of the Controlled-Rolling Process. In Proceedings of Conference on Microalloying, New York, NY, USA, 1–3 October, 1975; pp. 107–119.
- DeArdo, A.J. Niobium in modern steels. Int. Mater. Rev. 2003, 48, 371–402. [Google Scholar] [CrossRef]
- Gladman, T. The Physical Metallurgy of Microalloyed Steels; Institute of Materials: London, UK, 1996; pp. 213–260. [Google Scholar]
- Akben, M.G.; Weiss, I.; Jonas, J.J. Dynamic precipitation and solute hardening in AV microalloyed steel and two Nb steels containing high levels of Mn. Acta Metall. 1981, 29, 111–121. [Google Scholar] [CrossRef]
- Dutta, B.; Palmiere, E.J. Effect of prestrain and deformation temperature on the recrystallization behavior of steels microalloyed with niobium. Metall. Mater. Trans. 2003, 34, 1237–1247. [Google Scholar] [CrossRef]
- Klinkenberg, C. Niobium in Microalloyed Structural and Engineering Steels. Mater. Sci. Forum 2007, 539–543, 4261–4266. [Google Scholar] [CrossRef]
- Tanaka, T. Controlled Rolling of Steel Plate and Strip. Int. Met. Rev. 1981, 4, 185–212. [Google Scholar]
- Abe, T.; Tsukada, K.; Kozasu, I. Role of Interrupted Accelerated Cooling and Microalloying on Weldable HSLA Steels. In HSLA Steels: Metallurgy and Applications; Gray, J.M., Ko, T., Zhang, S., Wu, B., Xie, X., Eds.; ASM International Publication: Beijing, China, 1985; pp. 103–112. [Google Scholar]
- Subramanian, S.V.; Zeng, X.; Collins, L.E.; Bucholtz, M.; Kostic, M. Effect of Niobium on the Transformation Behaviour of Nb-Ti Microalloyed Steels. In Proceedings of the International Symposium on Low-Carbon Steels for the 90’s, Pittsburgh, PA, USA, 18–21 October 1993; Asfahani, R., Tither, G., Eds.; Minerals, Metals & Materials Society (TMS): Warrendale, PA, USA; pp. 313–322.
- Fossaert, C.; Rees, G.; Maurickx, T.; Bhadeshia, H.K.D.H. The effect of niobium on the hardenability of microalloyed austenite. Metall. Mater. Trans. 1995, 26, 21–30. [Google Scholar] [CrossRef]
- Thomas, M.H.; Michal, G.M. The Influence of Niobium and Nb(C,N) Precipitation on the Formation of Proeutectic Ferrite in Low-Alloy Steels. In Proceedings of the Solid to Solid Phase Transformation, Pittsburgh, PA, USA, 10–14 August, 1981; pp. 469–473.
- Manohar, P.A.; Chandra, T.; Killmore, C.R. Continuous Cooling Transformation Behaviour of Microalloyed Steels Containing Ti, Nb, Mn and Mo. ISIJ Int. 1996, 36, 1486–1493. [Google Scholar] [CrossRef]
- DeArdo, A.J.; Gray, J.M.; Meyer, L. Fundamental Metallurgy of Niobium in Steel. In Niobium-Proceedings of the International Symposium, San Francisco, CA, USA, 8–11 November 1981; Stuart, H., Ed.; Metallurgical Society of AIME: Warrendale, PA, USA; pp. 685–759.
- Felfer, P.J.; Killmore, C.R.; Williams, J.G.; Carpenter, K.R.; Ringer, S.P.; Cairney, J.M. A quantitative atom probe study of the Nb excess at prior austenite grain boundaries in a Nb microalloyed strip-cast steel. Acta Mater. 2012, 60, 5049–5055. [Google Scholar] [CrossRef]
- Yan, P.; Bhadeshia, H.K.D.H. Austenite-ferrite transformation in enhanced niobium, low carbon steel. Mater. Sci. Technol. 2015, 31, 1066–1076. [Google Scholar] [CrossRef]
- Hulka, K.; Gray, J.M.; Heisterkamp, F. High Temperature Thermomechanical Processing of Pipe Steel—Technical Basis and Production Experience. In Proceedings of the 3rd International Pipeline Technology Conference, Vol II, Brugge, Belgium, 21–24 May 2000; pp. 291–306.
- Graf, M.; Schroder, J.; Schwinn, V.; Hulka, K. Production of Large Diameter Pipes Grade X 70 with High Toughness Using Acicular Ferrite Microstructures. In Proceedings of the International Conference on Application and Evaluation of High Grade Linepipes in Hostile Environments, Yokohama, Japan, 8–9 November, 2002; pp. 1–13.
- DeArdo, A.J.; Marraccini, R.; Hua, M.J.; Garcia, C.I. Optimization of Nb HSLA Microstructure Using Advanced Thermomechanical Processing in a CSP Plant. Mater. Sci. Forum 2007, 539–543, 28–35. [Google Scholar] [CrossRef]
- Blejde, W.; Mahapatra, R.; Fukase, H. Application of Fundamental Research at Project ‘M’. In Proceedings of the Belton Memorial Symposium Proceedings, Sydney, Australia, 10–11 January 2000; pp. 253–261.
- Dunne, D.P. Ferrite Morphology and residual phases in continuously cooled low carbon steels. Mater. Forum 1999, 23, 63–76. [Google Scholar]
- Bhadeshia, H.K.D.H. Bainite in Steels; Institute of Materials: Cambridge, UK, 1992. [Google Scholar]
- Bramfitt, B.L.; Speer, J.G. A perspective on the morphology of Bainite. Met. Trans. A 1990, 21, 817–829. [Google Scholar] [CrossRef]
- Ohtani, H.; Okaguchi, S.; Fujihiro, Y.; Ohmori, Y. Morphology and properties of low-carbon bainite. Met. Trans. A 1990, 21, 877–887. [Google Scholar] [CrossRef]
- Araki, T.; Kozasu, I.; Takechi, H.; Shibata, K.; Enomoto, M.; Tamehiro, H.; Yamamoto, S.; Katumata, M.; Okaguchi, H.; Amao, K.; et al. Atlas For Bainitic Microstructures. Iron Steel Inst. Jpn. 1992, 1, 1–20. [Google Scholar]
- Killmore, C.R.; Creely, H.; Phillips, A.; Kaul, H.; Campbell, P.; Schueren, M.; Williams, J.G.; Blejde, W. Development of Ultra-Thin Cast Strip Products by the CASTRIP Process. In Proceedings of the AISTech 2007 Proceedings of the Iron and Steel Technology Conference, Indianapolis, IN, USA, 7–10 May 2007; p. 465.
- Killmore, C.R.; Kaul, H.R.; Burg, J.R.; Carpenter, K.R.; Williams, J.G.; Edelman, D.G.; Campbell, P.C.; Blejde, W.N. Development of Plain Carbon and Niobium Microalloyed Ultra-Thin Cast Strip Products Produced by the CASTRIP Process. In Proceedings of the 3rd International Conference on ThermoMechanical Processing of Steels, Padova, Italy, 10–12 September 2008; p. 16.
- Killmore, C.; Carpenter, K.; Kaul, H.; Williams, J.; Edelman, D.; Campbell, P.; Blejde, W. Development of a Family of High Strength Low Carbon Microalloyed Ultra-Thin Cast Strip Products Produced by the CASTRIP® Process. In Proceedings of the Materials Science & Technology Conference, Houston, TX, USA, 17–21 October 2010; pp. 1711–1726.
- Zajac, S.; Schwinn, V.; Tacke, K.H. Characterisation and Quantification of Complex Bainitic Microstructures in High and Ultra-High Strength Linepipe Steels. Mater. Sci. Forum 2005, 500–501, 387–394. [Google Scholar] [CrossRef]
- Schwinn, V.; Fluess, P.; Ormston, D. Low Carbon Bainitic TMCP Plate tor Structural and Line-Pipe Application. In Recent Advances of Niobium Containing Materials in Europe; Hulka, K., Klinkenburg, C., Mohrbacher, H., Eds.; Verlag Stahleisen GmbH: Dusseldorf, Germany, 2005; pp. 45–57. [Google Scholar]
- Zhao, M.; Yang, K.; Xiao, F.; Shan, Y. Continuous cooling transformation of undeformed and deformed low carbon pipeline steels. Mater. Sci. Eng. 2003, 355, 126–136. [Google Scholar] [CrossRef]
- Araki, T.; Enomoto, M.; Shibata, K. Bainitic and Similar Microstructures of Modern Low Carbon HSLA Steels. In Proceedings of the International Conference on Processing, Microstructure and Properties of Microalloyed and Other Modern High Strength Low Alloy Steels, Pittsburgh, PA, USA, 3–6 June 1991; pp. 249–255.
- Jia, T.; Militzer, M. The effect of solute Nb on the austenite-to-ferrite transformation. Metall. Mater. Trans. 2015, 46, 614–621. [Google Scholar] [CrossRef]
- Garcia, C.I.; Hua, M.; DeArdo, A.J. Steel Processing. In Proceedings of the Product and Applications Symposium, Pittsburgh, PA, USA, 25–29 October 2009; pp. 1591–1602.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpenter, K.R.; Killmore, C.R. The Effect of Nb on the Continuous Cooling Transformation Curves of Ultra-Thin Strip CASTRIP© Steels. Metals 2015, 5, 1857-1877. https://doi.org/10.3390/met5041857
Carpenter KR, Killmore CR. The Effect of Nb on the Continuous Cooling Transformation Curves of Ultra-Thin Strip CASTRIP© Steels. Metals. 2015; 5(4):1857-1877. https://doi.org/10.3390/met5041857
Chicago/Turabian StyleCarpenter, Kristin R., and Chris R. Killmore. 2015. "The Effect of Nb on the Continuous Cooling Transformation Curves of Ultra-Thin Strip CASTRIP© Steels" Metals 5, no. 4: 1857-1877. https://doi.org/10.3390/met5041857
APA StyleCarpenter, K. R., & Killmore, C. R. (2015). The Effect of Nb on the Continuous Cooling Transformation Curves of Ultra-Thin Strip CASTRIP© Steels. Metals, 5(4), 1857-1877. https://doi.org/10.3390/met5041857