Surface Modification of Inconel 625 in Nitrate Environment
Abstract
1. Introduction
2. Materials and Method
3. Results and Discussion
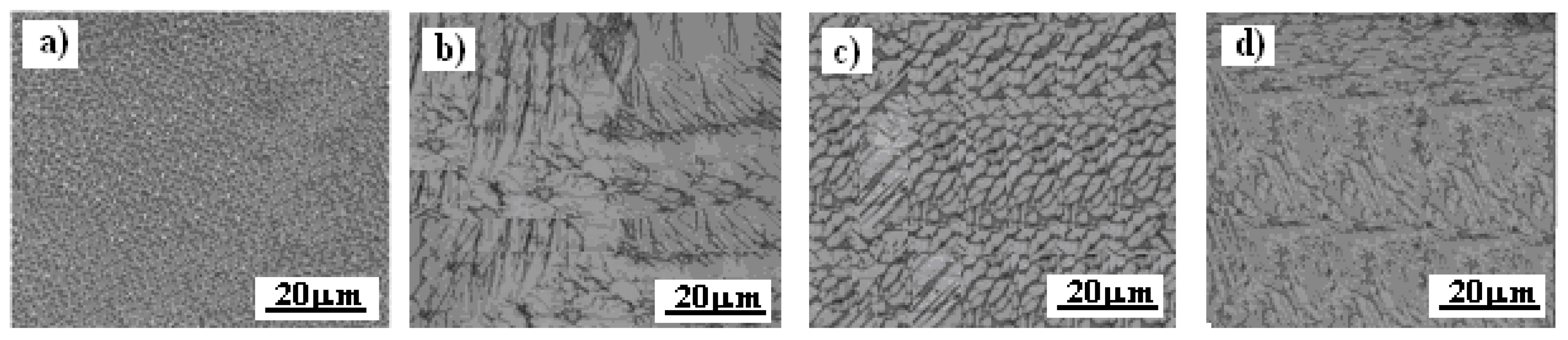
3.1. Microstructure Inconel 625 After Exposure in Nitrate Environment
3.2. Microhardness
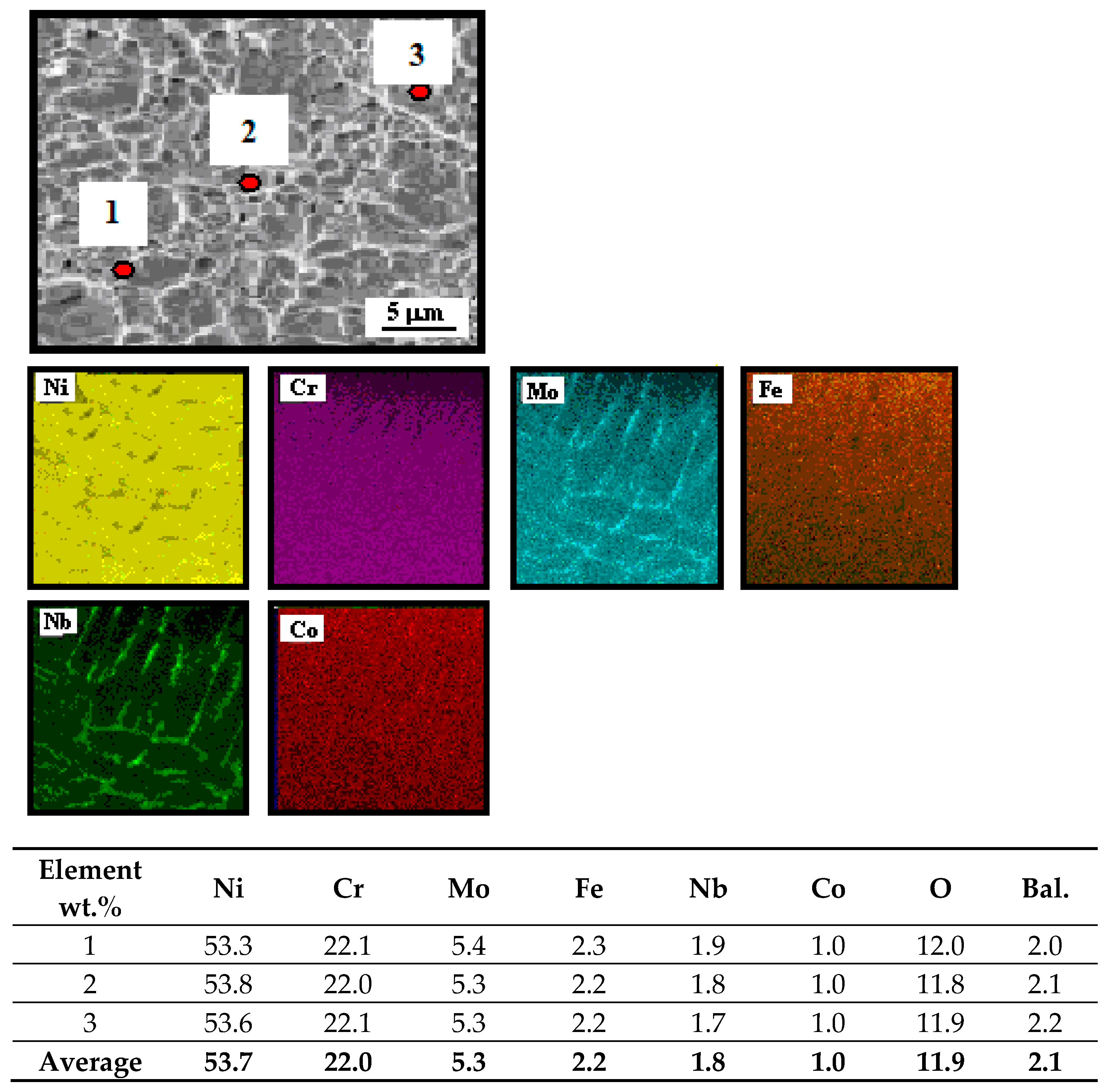
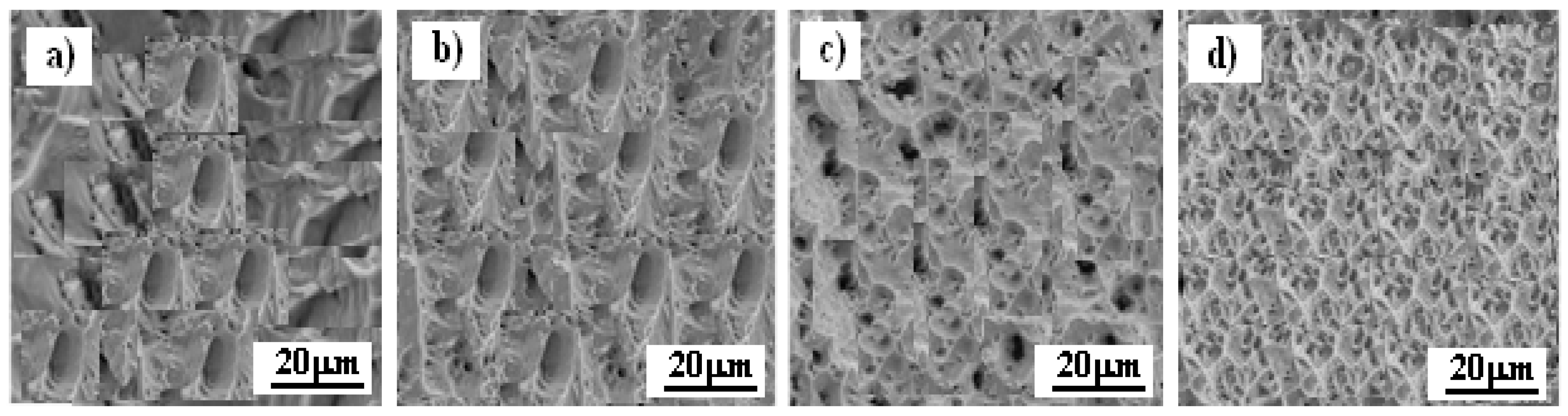
3.3. Microstructure of Inconel 625 After Exposure in Corrosive Environment
3.4. Corrosion Test
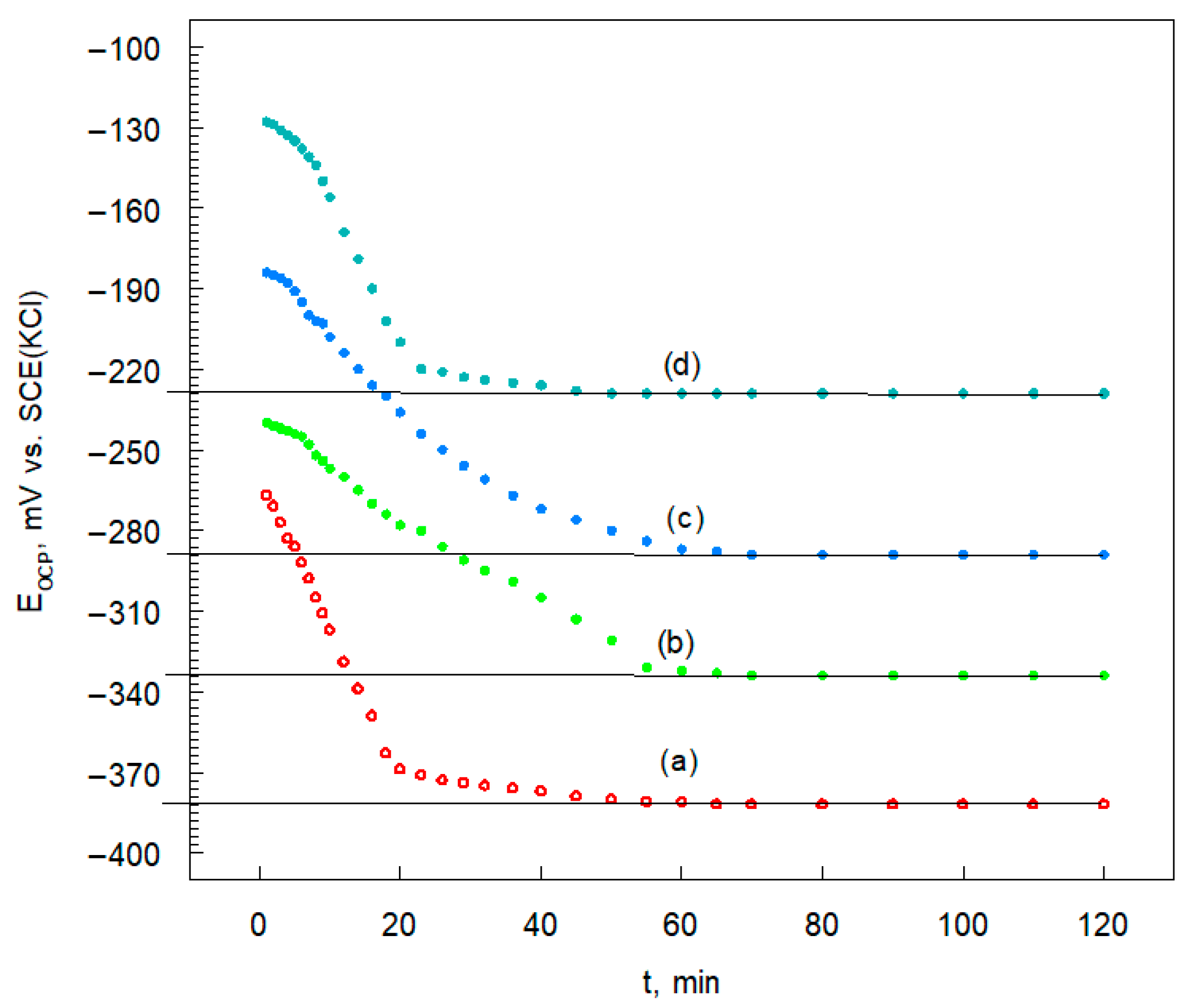
3.4.1. Open Circuit Potential
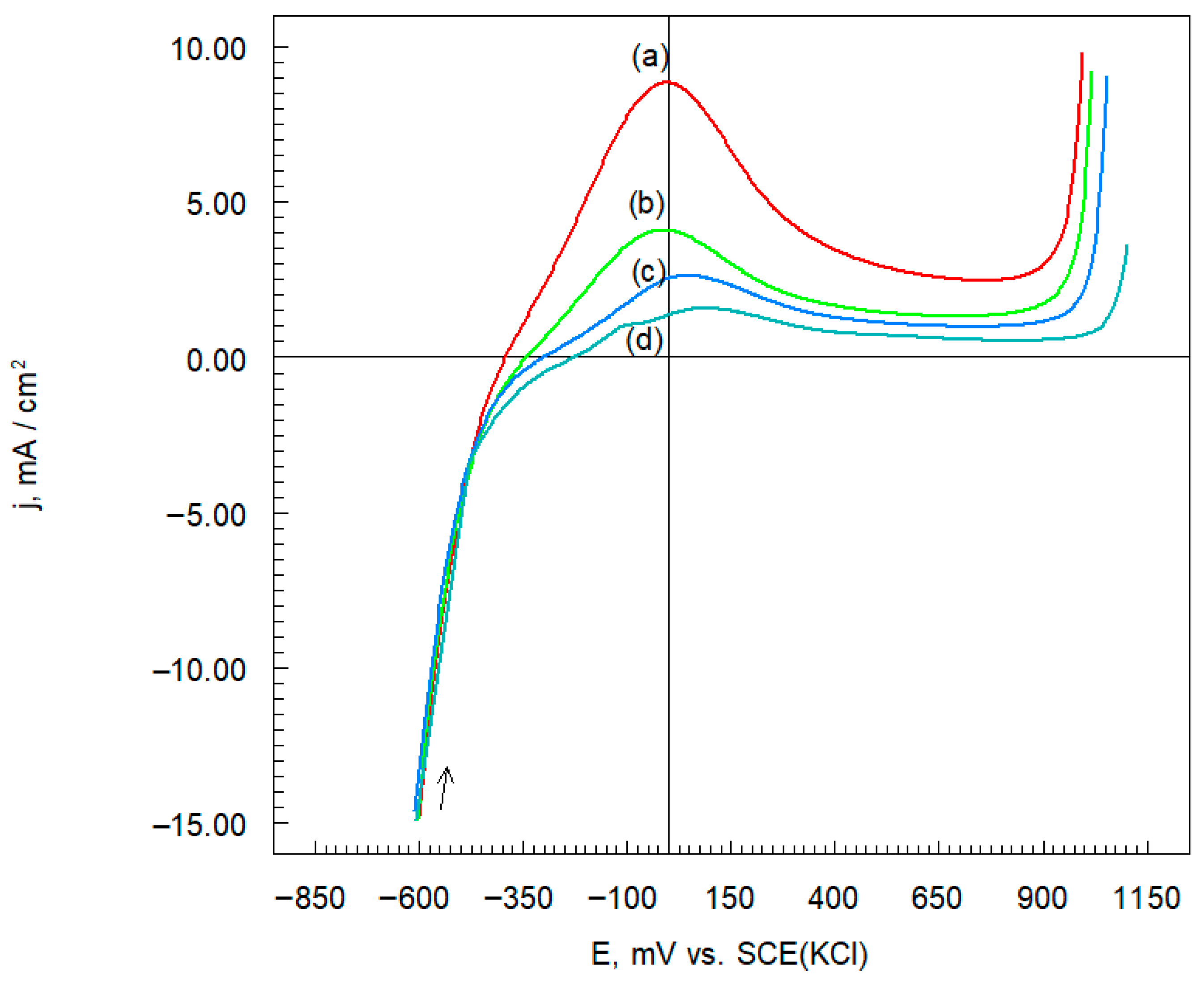
3.4.2. Potentiodynamic Polarization Curves
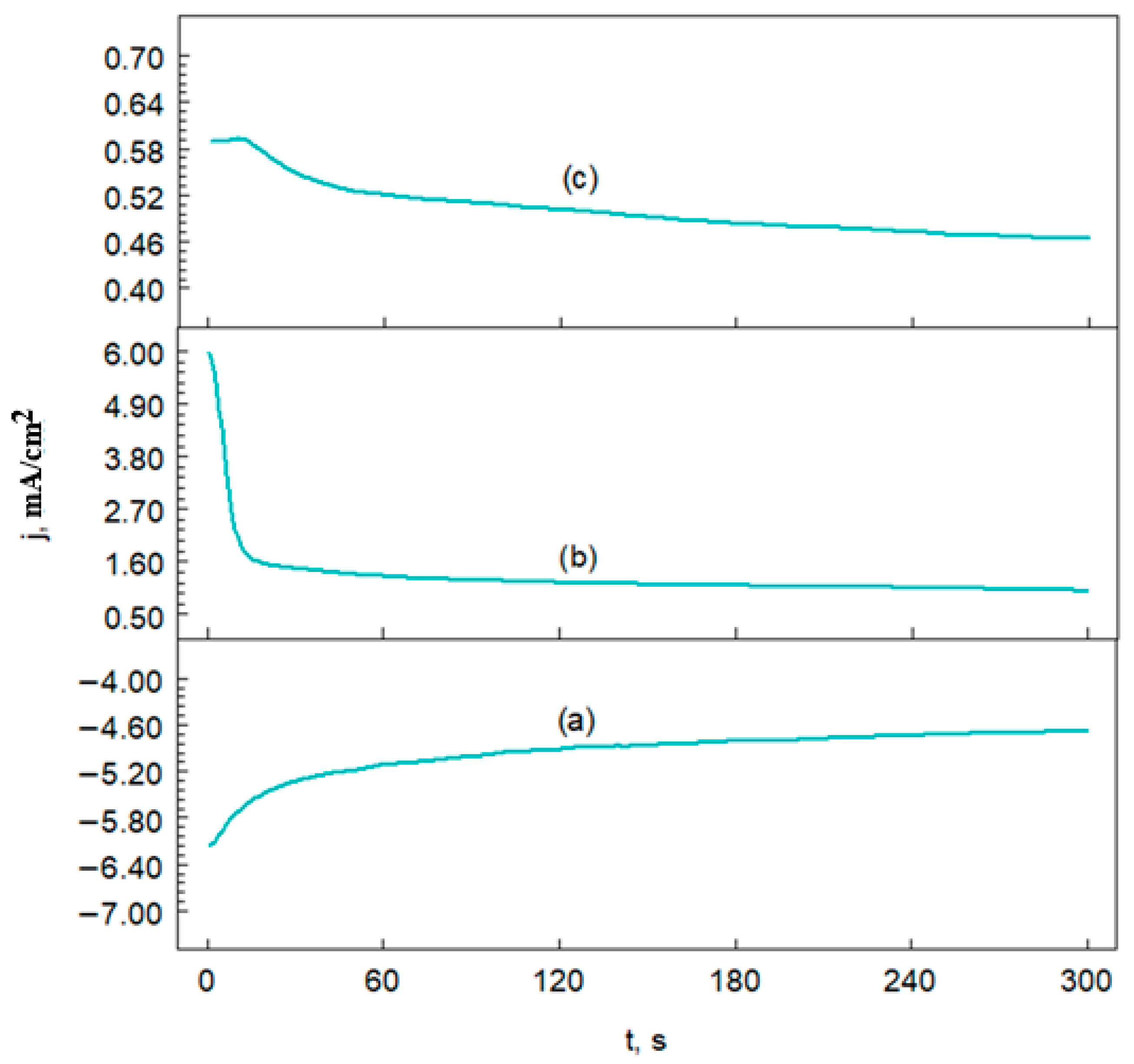
3.4.3. Chronoamperometric Curves
3.5. Corrosion Electrochemical Parameters
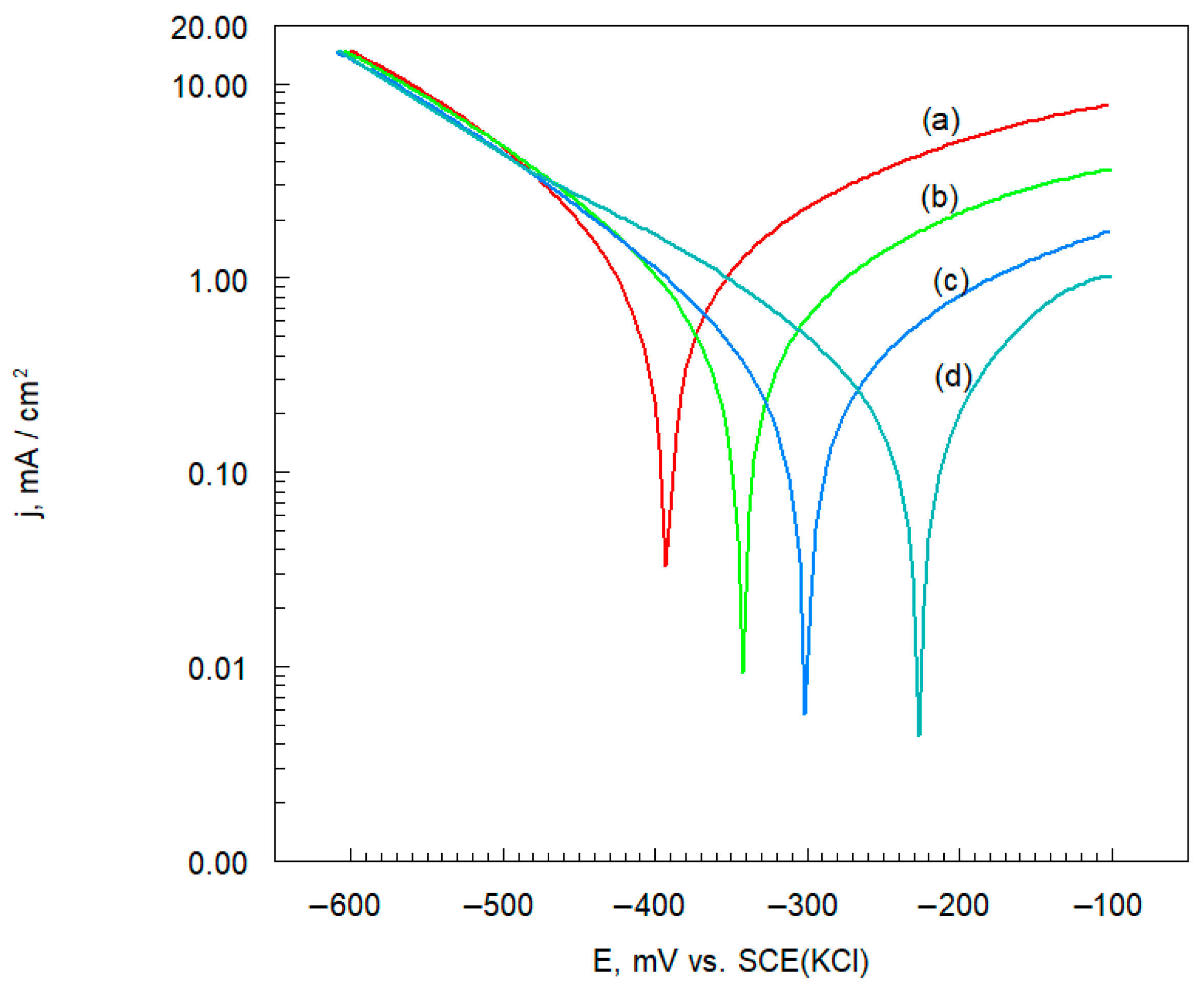
3.5.1. Polarization Resistance
3.5.2. Corrosion Rate
3.5.3. Surface Coverage Degree
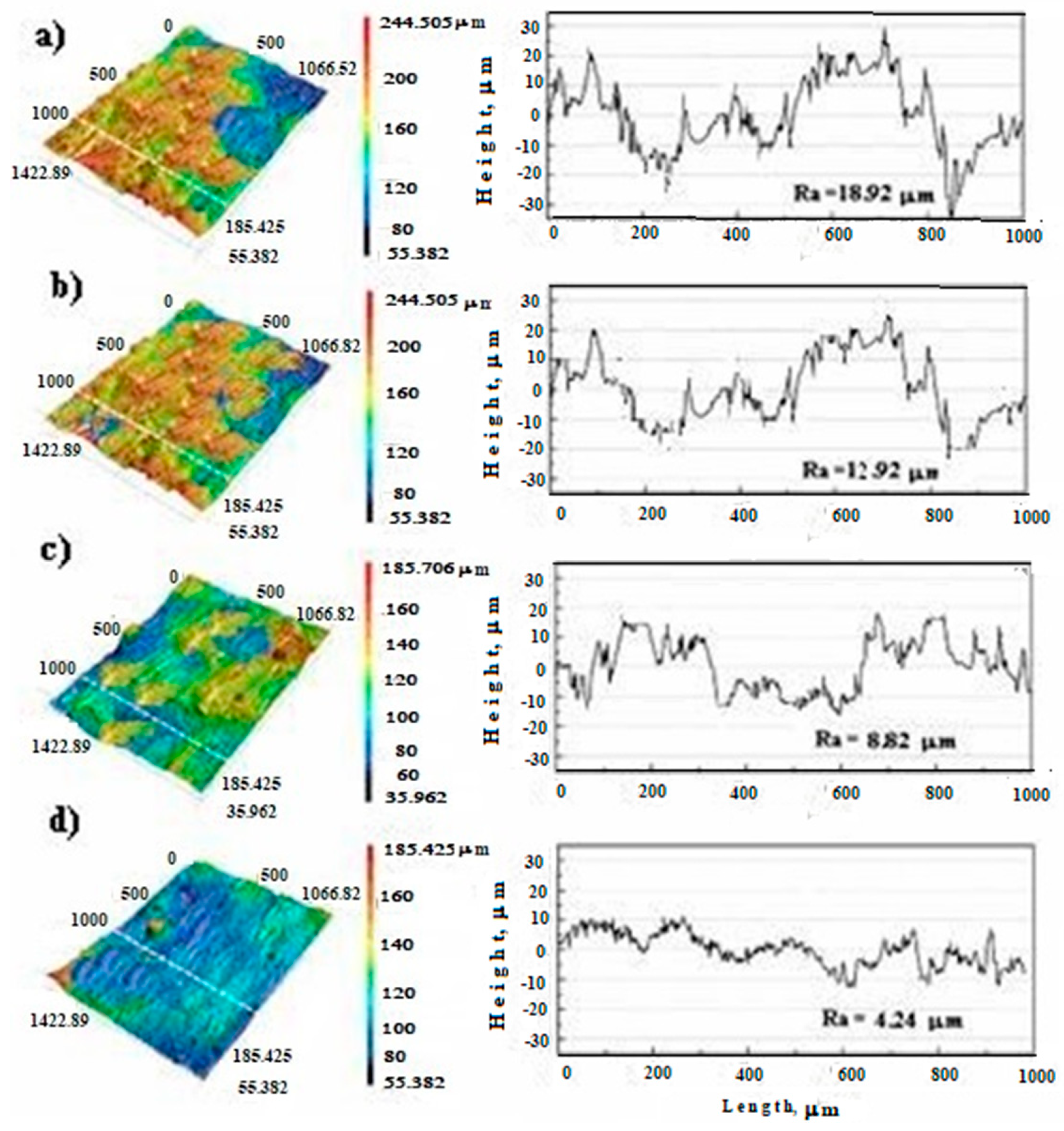
3.5.4. Surface Roughness
4. Conclusions
- The surfaces of Inconel 625 were chemically modified by oxidation in an alkaline sodium nitrate(V) solution. The surfaces were covered with a thin, smooth, and well-adhered layer of (MemOn)ads as passive coatings.
- As the NO3− concentration increases, the microhardness (HV10) of Inconel 625 surfaces increases slightly.
- The adsorption of the (MemOn)ads effectively separates the Inconel 625 surface from contact with the aggressive corrosive environment.
- The oxide coatings on the Inconel 625 surface were destroyed in the acidic chloride solution. The least amount of destruction to the coating was observed for the Inconel 625-1.00 sample after exposure in a solution containing 1.00 M NO3−.
- The highest polarization resistance (Rp) and the lowest corrosion rate (CW) values were recorded for the Inconel 625 sample after exposure in the solution with the highest concentration of nitrate in an alkaline environment.
- The (MemOn)ads layer on the Inconel 625 surface significantly impedes the exchange of mass and electric charge between the electrode and the electrolyte solution.
- For the highest NO3− concentration, a fairly high (approximately 80%) degree of electrode surface coverage (SC) and the lowest value of linear surface roughness (Ra) were achieved.
- To increase the anti-corrosion properties of Inconel 625, it is recommended to modify the superalloy surface by chemical means in an oxidizing environment.
Funding
Data Availability Statement
Conflicts of Interest
References
- Verdi, D.; Garrido, M.A.; Munez, C.J.; Poza, P. Cr3C2 incorporation into an Inconel 625 laser cladded: Effects on matrix microstructure, mechanical properties and local scratch resistance. Mater. Des. 2015, 67, 20–27. [Google Scholar] [CrossRef]
- Cooper, D.; Thornby, J.; Blundell, N.; Henrys, R.; Williams, M.A.; Gibbons, G. Design and manufacture of high performance hollow engine valves by additive layer manufacturing. Mater. Des. 2015, 69, 44–55. [Google Scholar] [CrossRef]
- Petrova, R.S.; Suwattananont, N.; Samardzic, V. The effect of boronizing on metallic alloys for automotive applications. J. Mater. Eng. Perform. 2008, 17, 340–345. [Google Scholar] [CrossRef]
- Makuch, N.; Kulka, M. Microstructural characterization and some mechanical properties of gas borided Inconel 600-alloy. Appl. Surf. Sci. 2014, 314, 1007–1018. [Google Scholar] [CrossRef]
- Dinda, G.; Dasgupta, A.; Majumder, J. Laser aided direct metal deposition of Inconel 625 superalloy: Microstructural evolution and thermal stability. J. Mater. Sci. Eng. A 2009, 509, 98–104. [Google Scholar] [CrossRef]
- Tabernero, I.; Lamikiz, A.; Martínez, S.; Ukar, E.; Figueras, J. Evaluation of the mechanical properties of Inconel 718 components built by laser cladding. J. Int. J. Mach. Tools Manuf. 2011, 51, 465–470. [Google Scholar] [CrossRef]
- Abioye, T.E.; McCartney, D.G.; Clare, A.T. Laser cladding of Inconel 625 wire for corrosion protection. J. Mater. Process. Technol. 2015, 217, 232–240. [Google Scholar] [CrossRef]
- Liu, H.; Tan, C.K.I.; Wei, Y.; Lim, S.H.; Lee, C.J.J. Laser-cladding and interface evolutions of inconel 625 alloy on low alloy steel substrate upon heat and chemical treatments. Surf. Coat. Technol. 2020, 404, 126607. [Google Scholar] [CrossRef]
- Deng, D.; Wang, C.; Liu, Q. Effect of standard heat treatment on microstructure and properties of borided Inconel 718. Trans. Nonferrous Met. Soc. 2015, 25, 25437–25443. [Google Scholar] [CrossRef]
- Kurt, B.; Kucuk, Y.; Gok, M.S. Microabrasion wear behavior of VC and CrC coatings deposited by thermoreactive diffusion technique. Tribol. Trans. 2014, 57, 345–352. [Google Scholar] [CrossRef]
- Ren, Y.; Zhou, G.S.; Li, D.G. A protective state observed for the passive film formed on Alloy 625 in a hydrochloric acid solution. Appl. Surf. Sci. 2018, 431, 197–201. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.-R.; Zhang, L.; Feng, Z.; Lu, M.-X. Comparison study on the semi-conductive and dissolution behaviour of 316 L and Alloy 625 in hydrochloric acid solution. Acta Metall. Sin. 2020, 33, 403–414. [Google Scholar] [CrossRef]
- Dai, H.; Shi, S.; Yang, L.; Hu, J.; Liu, C.; Guo, C.; Chen, X. Effects of elemental composition and microstructure inhomogeneity on the corrosion behavior of nickel-based alloys in hydrofluoric acid solution. Corros. Sci. 2020, 176, 108917. [Google Scholar] [CrossRef]
- Gola, K.; Ledwig, P.; Dubiel, B. Effect of microstructure of additively manufactured Inconel 625 on long-term corrosion behaviour in sulfuric acid media. JOM 2023, 75, 1242–1250. [Google Scholar] [CrossRef]
- Reffass, M.; Sabot, R.; Jeannin, M.; Berziou, C.; Refait, R. Effects of phosphate species on localized corrosion of steel in NaHCO3 + NaCl electrolytes. Electrochim. Acta 2009, 54, 4389–4396. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Chen, S.; Liu, T.; Wang, L.; Wu, K. Corrosion behaviour and passive film properties of nickel-based alloy in phosphoric acid. Corros. Commun. 2023, 9, 77–88. [Google Scholar] [CrossRef]
- Thomas, A.; El-Wahabi, M.; Cabrera, J.M.; Prado, J.M. High temperature deformation of Inconel 718. J. Mater. Process. Technol. 2006, 177, 469–472. [Google Scholar] [CrossRef]
- Morshed-Behbahani, K.; Nasiri, A. On the corrosion and passivity of Inconel 625 in HNO3 solution. Corros. Commun. 2025, 17, 28–34. [Google Scholar] [CrossRef]
- Scendo, M.; Żórawski, W. Corrosion properties of cold-sprayed Cr3C2-25(Ni20Cr) coatings after heat treatment. Materials 2024, 17, 6289. [Google Scholar] [CrossRef] [PubMed]
- Scendo, M. Influence of sulphide concentration on the properties of Cr3C2-25(Ni20Cr) cermet coatings on Al7075 substrate. Metals 2025, 15, 273. [Google Scholar] [CrossRef]
- Zheng, Z.B.; Zheng, Y.G.; Sun, W.H.; Wang, J.Q. Erosion–corrosion of HVOF-sprayed Fe-based amorphous metallic coating under impingement by a sand-containing NaCl solution. Corros. Sci. 2013, 76, 337–347. [Google Scholar] [CrossRef]
- Bergant, Z.; Trdan, U.; Grum, J. Effect of high-temperature furnace treatment on the microstructure and corrosion behavior of NiCrBSi flame-sprayed coatings. Corros. Sci. 2014, 88, 372–386. [Google Scholar] [CrossRef]
- Lemos, G.V.B.; Farina, A.B.; Piaggio, H.; Bergmann, L.; Ferreira, J.Z.; Fernandez dos Santos, J.; Voort, G.V.; Reguly, A. Mitigating the susceptibility to intergranular corrosion of alloy 625 by friction-stir welding. Sci. Rep. 2022, 12, 3482. [Google Scholar] [CrossRef]
| Element | Ni | Cr | Mo | Fe | Nb | Co | Si | Mn | Al | Ti | C |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | Bal. | 22.2 | 8.7 | 4.3 | 3.7 | 1.1 | 0.6 | 0.5 | 0.4 | 0.3 | 0.03 |
| Sample Name | Microhardness HV10 |
|---|---|
| Inconel 625 | 341 ± 2 |
| Inconel 625-0.25 | 351 ± 2 |
| Inconel 625-0.50 | 372 ± 1 |
| Inconel 625-1.00 | 390 ± 3 |
| Sample Name | EOCP mV vs. SCE(KCl) |
|---|---|
| Inconel 625 | −385 |
| Inconel 625-0.25 | −334 |
| Inconel 625-0.50 | −298 |
| Inconel 625-1.00 | −229 |
| Sample Name | Ecorr mV vs. SCE | jcorr mA cm−2 | −bc | ba |
|---|---|---|---|---|
| mV dec−1 | ||||
| Inconel 625 | −388 | 1.36 | 220 | 240 |
| Inconel 625-0.25 | −342 | 1.00 | 270 | 375 |
| Inconel 625-0.50 | −298 | 0.51 | 260 | 287 |
| Inconel 625-1.00 | −226 | 0.27 | 270 | 198 |
| Sample Name | Rp Ω cm2 |
|---|---|
| Inconel 625 | 36,647 |
| Inconel 625-0.25 | 68,160 |
| Inconel 625-0.50 | 116,150 |
| Inconel 625-1.00 | 183,710 |
| Sample Name | CW mg/Year |
|---|---|
| Inconel 625 | 93.7 |
| Inconel 625-0.25 | 68.9 |
| Inconel 625-0.50 | 35.1 |
| Inconel 625-1.00 | 18.6 |
| Sample Name | SC % |
|---|---|
| Inconel 625-0.25 | 26 |
| Inconel 625-0.50 | 63 |
| Inconel 625-1.00 | 80 |
| Sample Name | Ra µm |
|---|---|
| Inconel 625 | 18.92 ± 6.23 |
| Inconel 625-0.25 | 12.92 ± 4.99 |
| Inconel 625-0.50 | 8.82 ± 1.88 |
| Inconel 625-1.00 | 4.24 ± 2.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Scendo, M. Surface Modification of Inconel 625 in Nitrate Environment. Metals 2026, 16, 112. https://doi.org/10.3390/met16010112
Scendo M. Surface Modification of Inconel 625 in Nitrate Environment. Metals. 2026; 16(1):112. https://doi.org/10.3390/met16010112
Chicago/Turabian StyleScendo, Mieczysław. 2026. "Surface Modification of Inconel 625 in Nitrate Environment" Metals 16, no. 1: 112. https://doi.org/10.3390/met16010112
APA StyleScendo, M. (2026). Surface Modification of Inconel 625 in Nitrate Environment. Metals, 16(1), 112. https://doi.org/10.3390/met16010112






