A New Approach in Hydrometallurgy for the Solvent Extraction of Cu(II) from Alkaline Solutions Leached with Tartrate Using Phenyl-2-Pyridyl Ketoxime
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
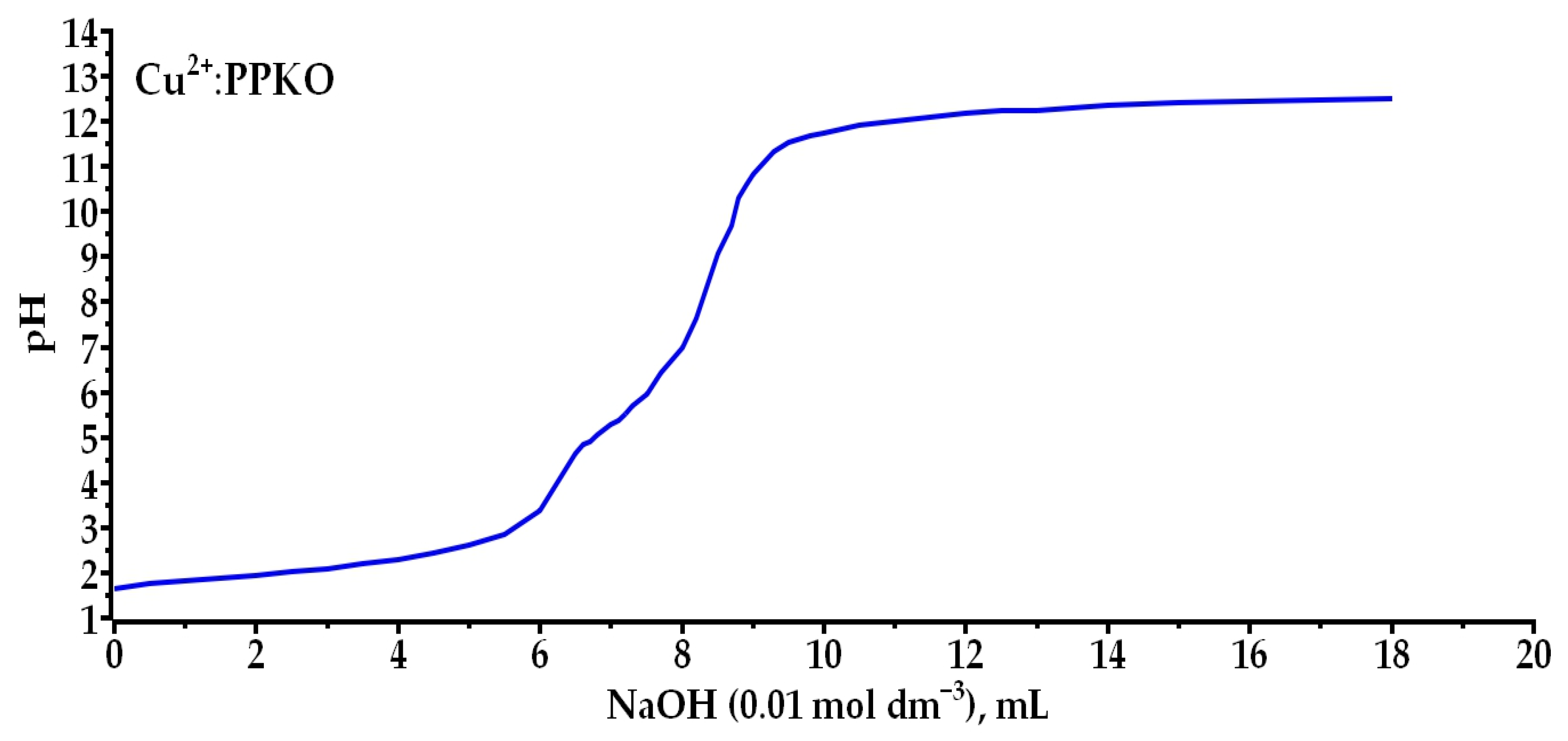
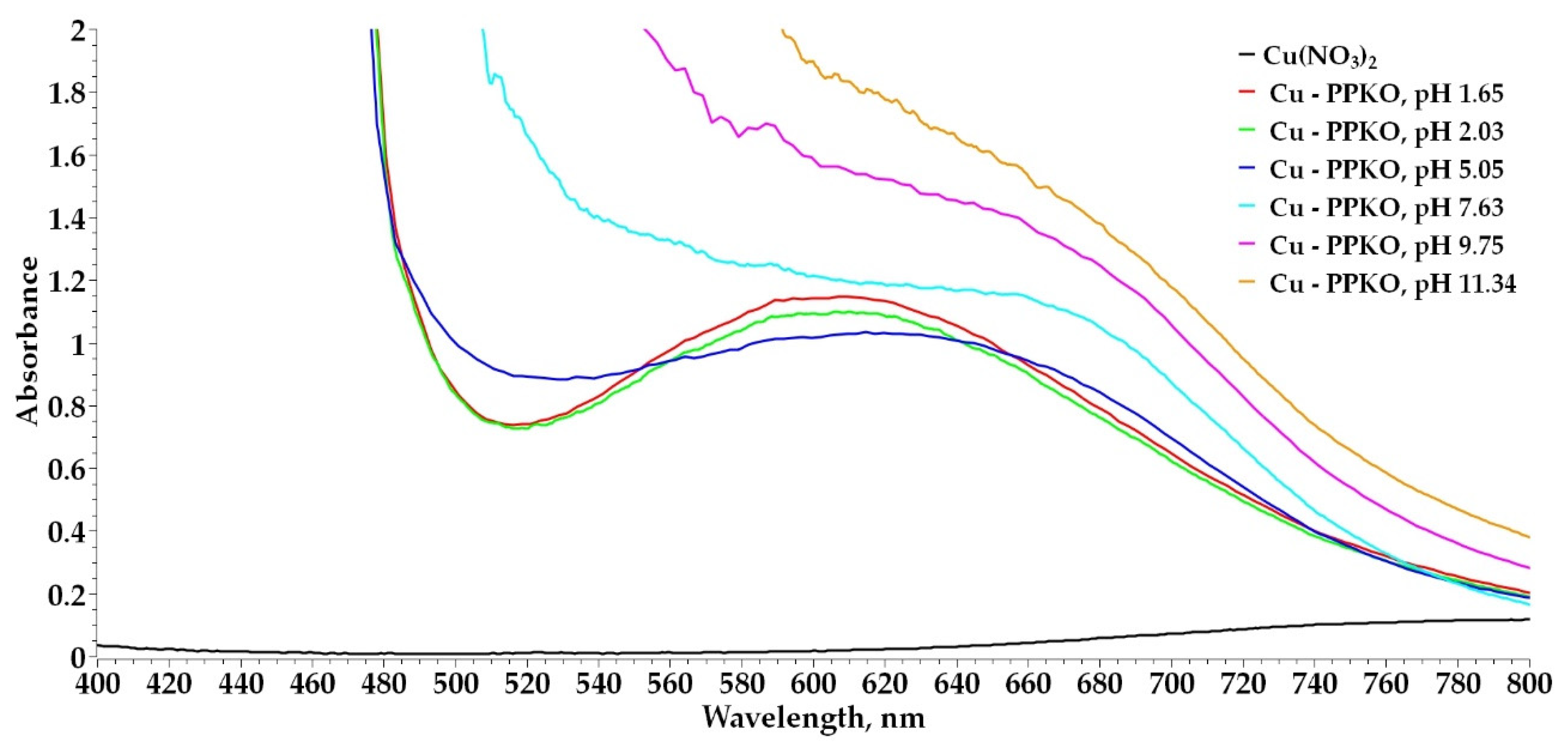
2.2.1. Experimental Determination of the Coordination Number of the Cu(II):PPKO Complex Through Simultaneous Potentiometric and Spectrophotometric Analysis in Ethanolic Solution
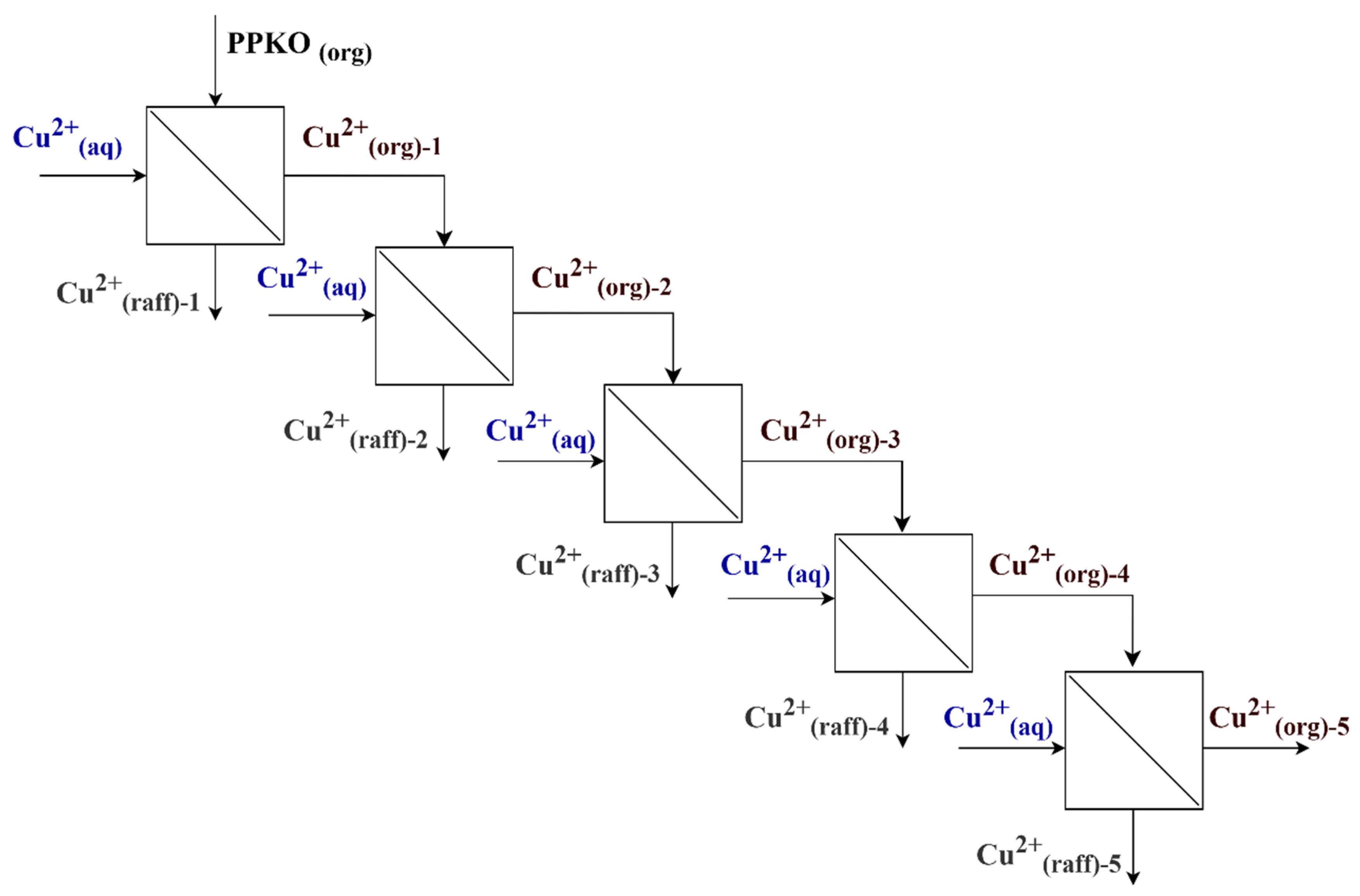
2.2.2. Evaluation of Equilibrium Time and Maximum Loading Capacity in the Extraction of Cu(II) Using Phenyl-2-Pyridyl Ketoxime (PPKO)
- is the mass of Cu(II) in the aqueous phase entering stage n.
- is the mass of copper ions in the raffinate at the outlet of the stage.
- is the mass of copper ions in the organic phase at the outlet of stage “n”.
- V is the volume of the phase.
2.2.3. Determination of the Extraction and Stripping Isotherms
2.2.4. Reconditioning Procedure for Extractant Regeneration and Reuse
2.2.5. Evaluation of the Temperature Effect on the Complexation Equilibrium of Cu(II)
- t1 and t2 are the times required to reach the steady-state potential at temperatures T1 and T2, respectively.
- ΔH is the reaction enthalpy (J·mol−1).
- R is the universal gas constant (8.314 J·mol−1·K−1).
3. Results and Discussion
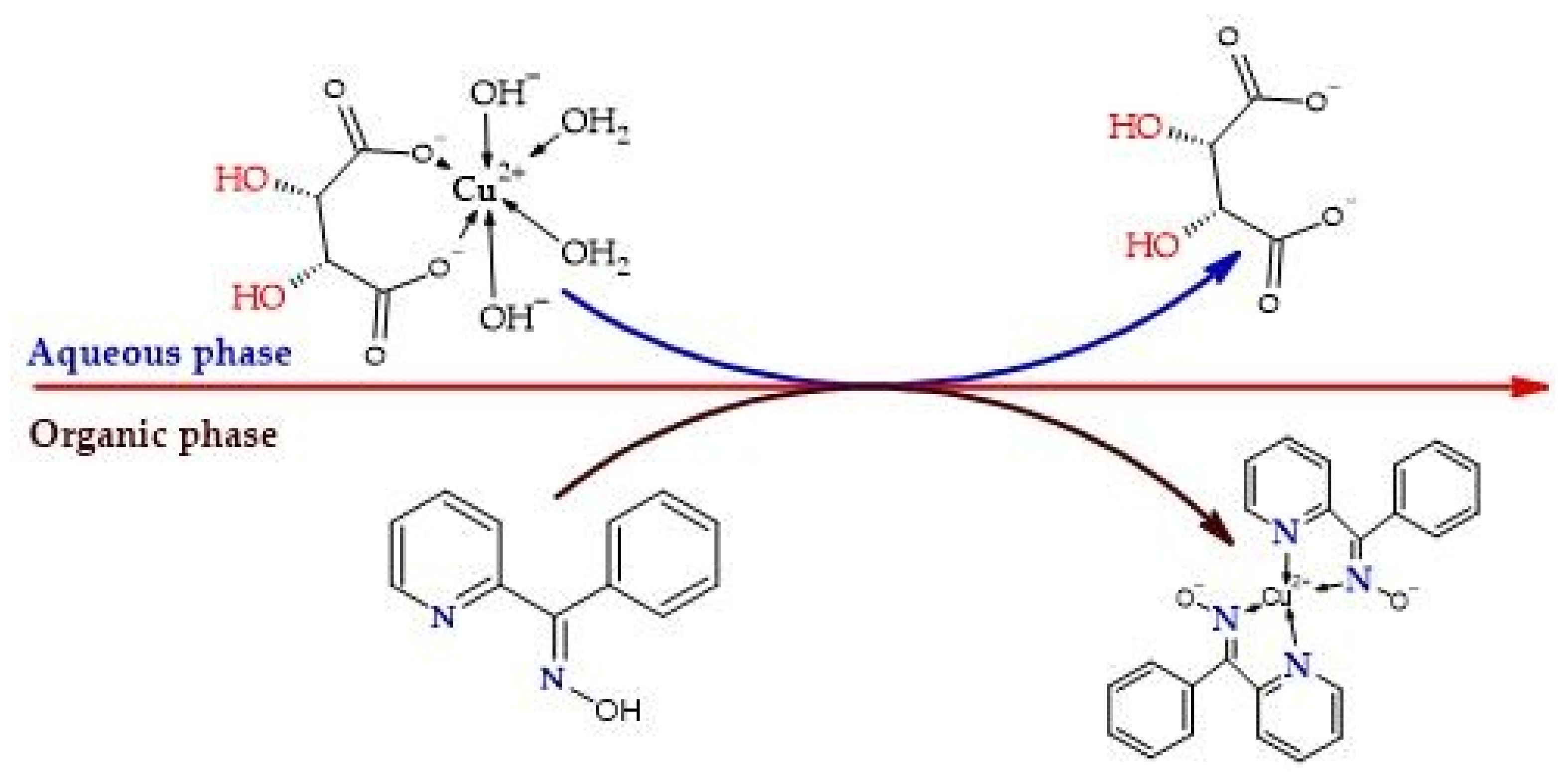
3.1. Formation and Stability of the Cu2+ Complex with Phenyl-2-Pyridyl Ketoxime for Solvent Extraction in the Hydrometallurgical Process
3.2. Determination of the Coordination Number of the Cu(II):PPKO Complex to Be Extracted
3.3. Effect of Contact Time
3.4. Evaluation of Cu(II) Transfer Efficiency and Extractant Performance
3.5. Leaching and Selective Extraction of Copper(II) Ions
3.6. Extraction and Stripping Isotherm
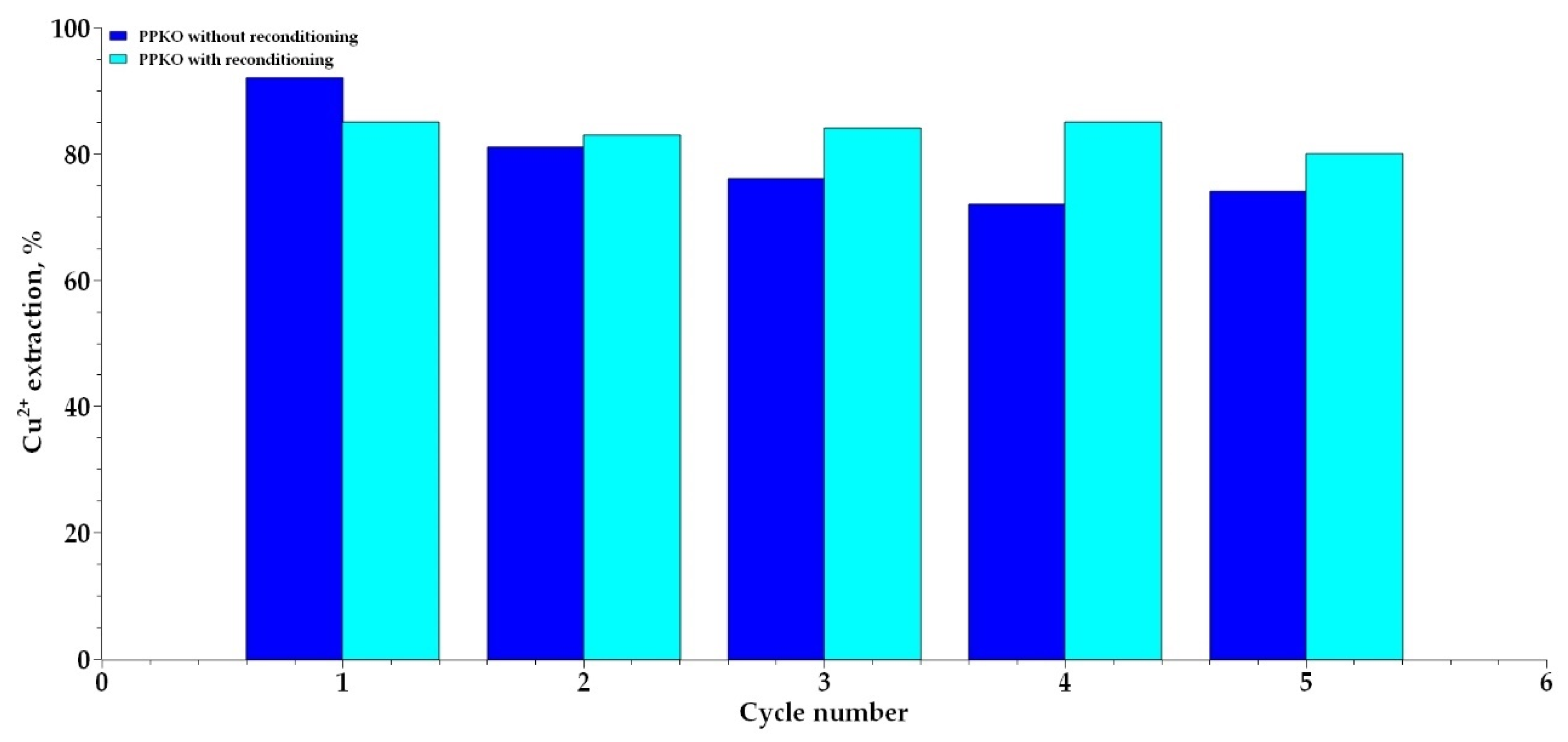
3.7. Effect of Reconditioning on the Reusability of the PPKO Extractant
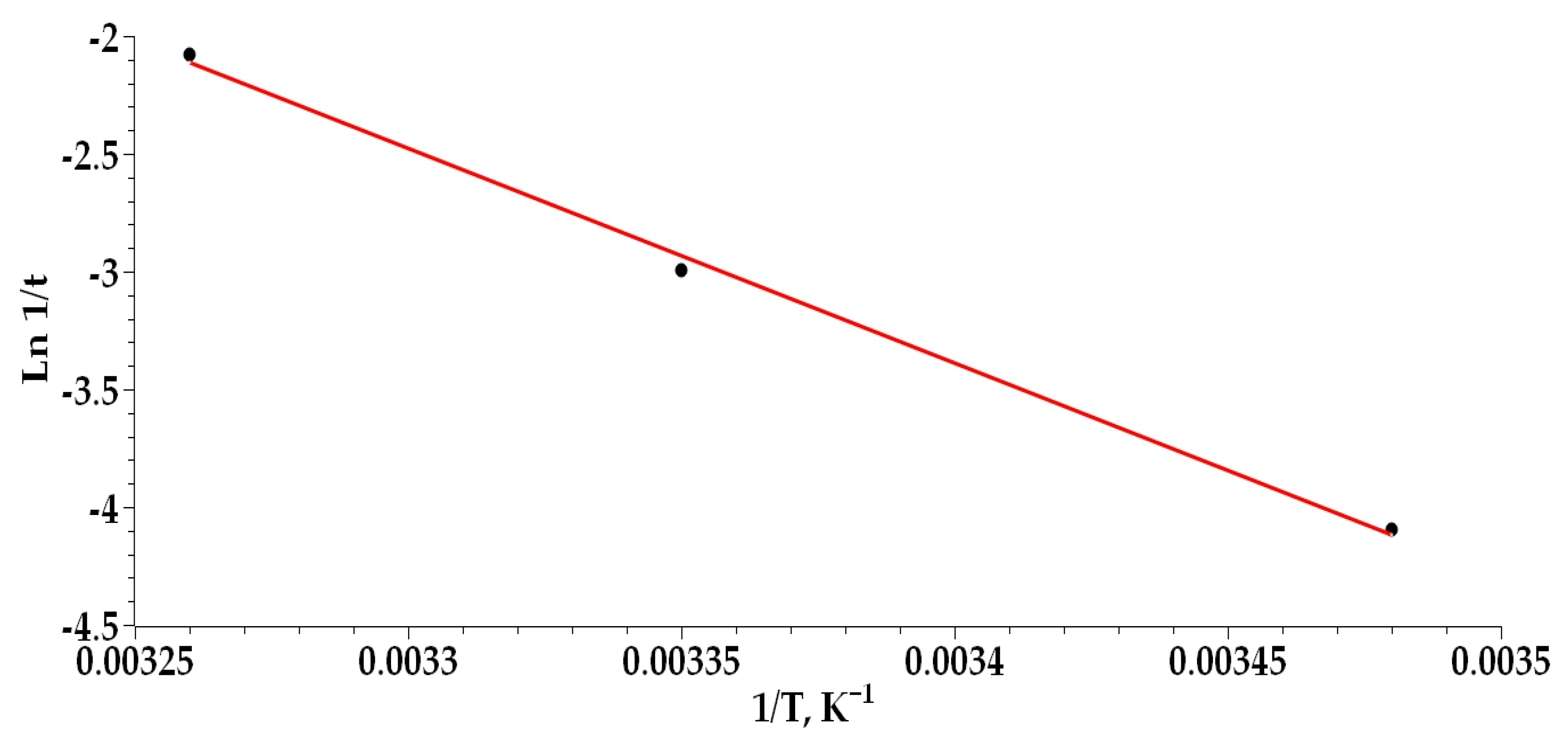
3.8. Thermodynamic Study of the Cu(II)–PPKO System in Ethanolic Medium
4. Conclusions
- i.
- A new methodology was successfully developed based on a solvent, using phenyl-2-pyridyl ketoxime (PPKO) as a selective extractant of Cu2+ from leach solutions containing tartrate in an alkaline medium derived from oxidized ores. PPKO demonstrated high efficiency in the transfer of copper(II) without the need for acidic media or conventional commercial extractants.
- ii.
- It was confirmed that phenyl-2-pyridyl ketoxime (PPKO) forms a stable 1:2 stoichiometric complex with Cu(II) under alkaline conditions, identified as [Cu(PPKO)2], with maximum stability at pH 11, as evidenced by UV–Vis spectra and potentiometric curves.
- iii.
- The effective loading capacity of PPKO reached an extraction efficiency of approximately 0.44 gpl per v/o, comparable to commercial systems, validating its competitiveness in terms of performance.
- iv.
- A progressive decrease in the extraction efficiency of PPKO was observed after several operational cycles, attributed to the sustained deprotonation of the oxime functional group during the extraction process. However, reconditioning of the extractant with an acetate buffer solution at pH 5 enabled the restoration of up to 80% of its original extractive capacity through a reprotonation process associated with an ion exchange mechanism. This strategy contributes to preserving the functionality of the extractant and ensures its efficient reuse in successive extraction cycles.
- v.
- The Cu(II)–PPKO complexation is an endothermic process, strongly favored by increasing temperature, that reduces the reaction time and enhances the efficiency of solvent extraction under hydrometallurgical conditions.
- vi.
- Furthermore, the results of this research lay the groundwork for implementing the Cu(II)–PPKO system in a tartrate–alkaline medium, demonstrating it as a promising alternative for the selective recovery of copper in a novel, environmentally friendly hydrometallurgical process.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, L.; Li, S.; He, Z.; Fu, Y.; Qiu, F.; Liu, R.; Yang, G. Electroplated Copper Additives for Advanced Packaging: A Review. ACS Omega 2024, 9, 20637–20647. [Google Scholar] [CrossRef]
- Singer, D.A. Future Copper Resources. Ore. Geol. Rev. 2017, 86, 271–279. [Google Scholar] [CrossRef]
- Castellón, C.I.; Taboada, M.E. Leaching of Copper Concentrates with Iodized Salts in a Saline Acid Medium: Part 2—Effect on Chloride Concentration and an Aerated System. Materials 2023, 16, 5940. [Google Scholar] [CrossRef]
- Izydorczyk, G.; Mikula, K.; Skrzypczak, D.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Potential Environmental Pollution from Copper Metallurgy and Methods of Management. Environ. Res. 2021, 197, 111050. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Du, X.; Wang, X.; Zeng, Y.; Fan, S. Accumulated Copper Tailing Solid Wastes with Specific Compositions Encourage Advances in Microbial Leaching. Minerals 2024, 14, 1051. [Google Scholar] [CrossRef]
- Martínez, J.I.; Teja, A.M.; Reyes, M.; Toro, N.; Cisneros, G.; Flores, U.M.; Labra, M.P.; Urbano, G.; Juarez, J.C. Optimization of Sustainable Copper Leaching Using Glycine and Oxidizing Agents in an Alkaline Medium. Metals 2025, 15, 617. [Google Scholar] [CrossRef]
- Tanda, B.C.; Oraby, E.A.; Eksteen, J.J. Recovery of Copper from Alkaline Glycine Leach Solution Using Solvent Extraction. Sep. Purif. Technol. 2017, 187, 389–396. [Google Scholar] [CrossRef]
- Sueros Velarde, F.J.; Siles, H.O.; Danil De Namor, A.F. The Use of a Novel Oxime Derivative [Phenyl(N-Methylaniline) Ketooxime] as Leach Reagent in the Copper(II) Hydrometallurgy. Miner. Eng. 2005, 18, 213–218. [Google Scholar] [CrossRef]
- Radmehr, V.; Koleini, S.M.J.; Khalesi, M.R.; Tavakoli Mohammadi, M.R. Ammonia Leaching: A New Approach of Copper Industry in Hydrometallurgical Processes. J. Inst. Eng. Ser. D 2013, 94, 95–104. [Google Scholar] [CrossRef]
- Conejeros, V.; Pérez, K.; Jeldres, R.I.; Castillo, J.; Hernández, P.; Toro, N. Novel Treatment for Mixed Copper Ores: Leaching Ammonia—Precipitation—Flotation (L.A.P.F.). Miner. Eng. 2020, 149, 106242. [Google Scholar] [CrossRef]
- Velarde, F.J.S.; Ortiz, J.A.Q.; Apaza, A.A.H.; de Namor, A.F.D. A New Approach for Increasing the Chelating Capacity of the Tartrate Ion in the Extraction of Copper from Ores. Metals 2023, 13, 1672. [Google Scholar] [CrossRef]
- Han, M.; He, J.; Sun, W.; Li, S.; Yu, H.; Yue, T.; Wei, X.; Zhang, C. Coordination Configurations of Cupric Tartrate in Electronic Industry Wastewater. Trans. Nonferrous Met. Soc. China 2022, 32, 3753–3766. [Google Scholar] [CrossRef]
- Bozejewicz, D.; Witt, K.; Kaczorowska, M.A.; Osmiałowski, B. The Copper(II) Ions Solvent Extraction with a New Compound: 2,6-Bis(4-Methoxybenzoyl)-Diaminopyridine. Processes 2019, 7, 954. [Google Scholar] [CrossRef]
- Madrzak-Litwa, I.; Borowiak-Resterna, A. Solvent Extraction of Copper(II) from Chloride Solutions Using 1,1′-Dialkyl-2,2′-Bibenzimidazoles as Extractants. Physicochem. Probl. Miner. Process. 2019, 55, 1165–1178. [Google Scholar] [CrossRef]
- Huang, X.; Jin, K.; Zhang, R.; Gong, Y.; Zeng, J.; Zhang, R.; Liu, Y.; Xue, J. Selective Solvent Extraction of Cu(II) from Aqueous Solutions Using an Acyl-Based Thiourea: Extraction Study and DFT Analysis of Reaction Mechanism. Hydrometallurgy 2024, 223, 106226. [Google Scholar] [CrossRef]
- Mohammed, T.; Bezuidenhout, G.A.; Oraby, E.A.; Eksteen, J.J. Sequential Separation of Cobalt, Copper, and Nickel from Alkaline Glycinate Solutions Using Solvent Extraction. J. Sustain. Metall. 2024, 10, 2455–2468. [Google Scholar] [CrossRef]
- Mazarakioti, E.C.; Soto Beobide, A.; Angelidou, V.; Efthymiou, C.G.; Terzis, A.; Psycharis, V.; Voyiatzis, G.A.; Perlepes, S.P. Modeling the Solvent Extraction of Cadmium(II) from Aqueous Chloride Solutions by 2-pyridyl Ketoximes: A Coordination Chemistry Approach. Molecules 2019, 24, 2219. [Google Scholar] [CrossRef]
- Shokrollahi, A.; Ghaedi, M.; Rajabi, H.R.; Niband, M.S. Potentiometric Study of Binary Complexes of Methyl-2-Pyridyl Ketone Oxime, Phenyl-2-Pyridyl Ketone Oxime and Diacetyl Monooxime with Some Transition and Heavy Metal Ions in Aqueous Solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 655–662. [Google Scholar] [CrossRef]
- Mądrzak-Litwa, I.; Borowiak-Resterna, A. Solvent Extraction of Zinc from Chloride Solutions Using Dialkyl Derivatives of 2,2′-Bibenzimidazole as Extractants. Hydrometallurgy 2018, 182, 8–20. [Google Scholar] [CrossRef]
- Ferreira, A.E.; Agarwal, S.; MacHado, R.M.; Gameiro, M.L.F.; Santos, S.M.C.; Reis, M.T.A.; Ismael, M.R.C.; Correia, M.J.N.; Carvalho, J.M.R. Extraction of Copper from Acidic Leach Solution with Acorga M5640 Using a Pulsed Sieve Plate Column. Hydrometallurgy 2010, 104, 66–75. [Google Scholar] [CrossRef]
- Sengupta, B.; Sengupta, R.; Bhakhar, M.S. Extraction-Stripping Patterns during Co-Extraction of Copper and Nickel from Ammoniacal Solutions into Emulsion Liquid Membranes Using LIX 84I®. J. Membr. Sci. Res. 2017, 3, 71–77. [Google Scholar] [CrossRef]
- Ruiz, M.C.; González, I.; Rodriguez, V.; Padilla, R. Solvent Extraction of Copper from Sulfate-Chloride Solutions Using LIX 84-IC and LIX 860-IC. Miner. Process. Extr. Metall. Rev. 2021, 42, 1–8. [Google Scholar] [CrossRef]
- Chepushtanova, T.; Yessirkegenov, M.; Bochevskaya, Y.; Sharipova, A.; Baigenzhenov, O.; Merkibayev, Y.; Altmyshbayeva, A. The Testing Results of ACORGA, LIX Extractants and CR60 Crud Mitigation Reagent Influence During SX-EW Copper Extraction. Sustainability 2024, 16, 7815. [Google Scholar] [CrossRef]
- U. S. Geological Survey. National Field Manual for the Collection of Water-Quality Data Book 9, Handbooks for Water-Resources Investigations. In Measurement of PH; Chapter 6.4; U.S. Geological Survey: Reston, VA, USA, 2021. [Google Scholar]
- Kasongo, K.B.; Mwanat, M.H.M.; Mbuya, B.; Makhatha, M.E.; Mulaba-Bafubiandi, A.F.; Zeka, M.L. Minimization of Organic-in-Aqueous (O-in-A) Entrainments and Improvement of Solvent Extraction Efficiency by Optimizing Key Parameters Using Response Surface Methodology (RSM). J. Sustain. Metall. 2025, 11, 1542–1553. [Google Scholar] [CrossRef]
- Smith, J.M. Chemical Engineering Kinetics, 3rd ed.; McGraw-Hill: New York, NY, USA, 1981. [Google Scholar]
- Meek, T.L.; Cheney, G.E. The Copper(II) Syn-Phenyl-2-Pyridyl Ketoxime System Part I. Can. J. Chem. 2011, 43, 64–74. [Google Scholar] [CrossRef]
- Wilson, A.M.; Bailey, P.J.; Tasker, P.A.; Turkington, J.R.; Grant, R.A.; Love, J.B. Solvent Extraction: The Coordination Chemistry Behind Extractive Metallurgy. Chem. Soc. Rev. 2013, 43, 123–134. [Google Scholar] [CrossRef]
- Li, Z.; Dewulf, B.; Binnemans, K. Nonaqueous Solvent Extraction for Enhanced Metal Separations: Concept, Systems, and Mechanisms. Ind. Eng. Chem. Res. 2021, 60, 17285–17302. [Google Scholar] [CrossRef]
- Simonsen, S.H.; Burnett, H.M. Spectrophotometric Determination of Copper with Salicylaldoxime Application to Analysis of Aluminum Alloys. Anal. Chem. 1955, 27, 1336–1339. [Google Scholar] [CrossRef]
- Sliva, T.Y.; Duda, A.M.; Glowiak, T.; Fritsky, I.O.; Amirkhanov, V.M.; Mokhir, A.A.; Kozlowski, H. Co-Ordination Ability of Amino Acid Oximes. Potentiometric, Spectroscopicand Structural Studies of Complexes of 2-Cyano-2-(Hydroxyimino)Acetamide. J. Chem. Soc. Dalton Trans. 1997, 273–276. [Google Scholar] [CrossRef]
- Silva, T.Y.; Dobosz, A.; Jerzykiewicz, L.; Karaczyn, A.; Moreeuw, A.M.; Świątek-Kozłowska, J.; Głowiak, T.; Kozłowski, H. Copper(II) and Nickel(II) Complexes with Oxime Analogues of Amino Acids. Potentiometric, Spectroscopic and X-Ray Studies of Complexes with 2-Cyano-2-(Hydroxyimino)Acetic Acid and Its Ethane-1,2-Diamine Derivative. J. Chem. Soc. Dalton Trans. 1998, 1863–1868. [Google Scholar] [CrossRef]
- Chylewska, A.; Ogryzek, M.; Chmurzyński, L.; Makowski, M. Spectrophotometric, Potentiometric, and Conductometric Studies of Binary Complex Formation Between Copper(II) and Three Forms of Vitamin B 6 in Aqueous Solutions. J. Coord. Chem. 2015, 68, 3761–3775. [Google Scholar] [CrossRef]
- Szczęsny, R.; Muzioł, T.M.; Gregory, D.H.; Szłyk, E. Structural and Thermal Characterization of Copper(II) Complexes with Phenyl-2-Pyridylketoxime and Deposition of Thin Films by Spin Coating. Chem. Pap. 2015, 69, 569–579. [Google Scholar] [CrossRef]
- Aksamitowski, P.; Wieszczycka, K.; Wojciechowska, I. Selective Copper Extraction from Sulfate Media with N,N-Dihexyl-N′-Hydroxypyridine-Carboximidamides as Extractants. Sep. Purif. Technol. 2018, 201, 186–192. [Google Scholar] [CrossRef]
- Mondal, S.; Majumder, S.K. Studies on the Copper Extraction in a Channel-Based Packed Extraction Device. Miner. Eng. 2018, 126, 194–206. [Google Scholar] [CrossRef]
- Deep, A.; Kumar, P.; Carvalho, J.M.R. Recovery of Copper from Zinc Leaching Liquor Using ACORGA M5640. Sep. Purif. Technol. 2010, 76, 21–25. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.; Petersen, J.; Azizi, A. Solvent Extraction Studies of Copper from a Heap Leach Liquor Using Mextral 5640H. Minerals 2022, 12, 1322. [Google Scholar] [CrossRef]
- Wang, L.P.; Lin, J.Y.; Chen, Y.J.; Tseng, B.C.; Hsu, C.H.; Kou, M.; Zhou, H.; Sreearunothai, P. Separation and Recovery of Copper and Nickel in the Leachate of a Waste IC Lead Frame through Synergistic Solvent Extraction Using a Binary Extractant Containing LIX984N and Cyanex302 Followed by Selective Stripping. Sustainability 2023, 16, 77. [Google Scholar] [CrossRef]
- Kumar, V.; Pandey, B.D.; Bagchi, D. Application of LIX 84 for Separation of Copper, Nickel and Cobalt in Ammoniacal Leaching of Ocean Nodules. Mater. Trans. JIM 1991, 32, 157–163. [Google Scholar] [CrossRef]
- Kul, M.; Çetinkaya, Ü. Recovery of Copper by LIX 984N-C from Electroplating Rinse Bath Solution. Hydrometallurgy 2009, 98, 86–91. [Google Scholar] [CrossRef]
- Ochromowicz, K.; Chmielewski, T. Solvent Extraction of Copper(II) from Concentrated Leach Liquors. Physicochem. Probl. Miner. Process. 2013, 49, 357–367. [Google Scholar] [CrossRef]
- Agarwal, S.; Reis, M.T.A.; Ismael, M.R.C.; Correia, M.J.N.; Carvalho, J.M.R. Modeling of the Extraction Equilibrium of Copper from Sulfate Solutions with Acorga M5640. Solvent Extr. Ion Exch. 2012, 30, 536–551. [Google Scholar] [CrossRef]
- El Hady, S.M. Solvent Extraction of Yttrium from Rare Earths Concentrate Using Cyanex 272 and PC88A. Bull. Fac. Sci. Zagazig Univ. 2024, 2023, 190–200. [Google Scholar] [CrossRef]
- Agarwal, S.; Ferreira, A.E.; Santos, S.M.C.; Reis, M.T.A.; Ismael, M.R.C.; Correia, M.J.N.; Carvalho, J.M.R. Separation and Recovery of Copper from Zinc Leach Liquor by Solvent Extraction Using Acorga M5640. Int. J. Miner. Process. 2010, 97, 85–91. [Google Scholar] [CrossRef]
- Panigrahi, S.; Parhi, P.K.; Sarangi, K.; Nathsarma, K.C. A Study on Extraction of Copper Using LIX 84-I and LIX 622N. Sep. Purif. Technol. 2009, 70, 58–62. [Google Scholar] [CrossRef]
- Tarushi, A.; Raptopoulou, C.P.; Psycharis, V.; Kontos, C.K.; Kessissoglou, D.P.; Scorilas, A.; Tangoulis, V.; Psomas, G. Copper(II) Inverse-[9-Metallacrown-3] Compounds Accommodating Nitrato or Diclofenac Ligands: Structure, Magnetism, and Biological Activity. Eur. J. Inorg. Chem. 2016, 2016, 219–231. [Google Scholar] [CrossRef]
- Hołyńska, M. Structural Variety and Magnetic Properties of Oxime-Bridged Copper(II) Complexes. J. Mol. Struct. 2015, 1098, 175–180. [Google Scholar] [CrossRef]
- Chakraborty, A.; Escuer, A.; Ribas, J.; Maji, T.K. A Discrete CuII6 Cluster and a 3D MnII–CuII Framework Based on Assembly of Mn2Cu4 Clusters: Synthesis, Structure and Magnetic Properties. Dalton Trans. 2016, 45, 15523–15531. [Google Scholar] [CrossRef]
- Nobahar, A.; Melka, A.B.; Pusta, A.; Lourenço, J.P.; Carlier, J.D.; Costa, M.C. A New Application of Solvent Extraction to Separate Copper from Extreme Acid Mine Drainage Producing Solutions for Electrochemical and Biological Recovery Processes. Mine Water Environ. 2022, 41, 387–401. [Google Scholar] [CrossRef]
- Whewell, R.J.; Foakes, H.J.; Hughes, M.A. Degradation in Hydroxyoxime Solvent Extraction Systems. Hydrometallurgy 1981, 7, 7–26. [Google Scholar] [CrossRef]
- Xie, Q.; Chen, Q.; Liu, Y.; Yu, Y.; Sun, Q.; Wang, L. Degradation of Hydroxyoxime CP-150 in the Presence of Nitrate and Permanganate Ions Causing Detrimental Effect on Copper Extraction. Hydrometallurgy 2023, 221, 106149. [Google Scholar] [CrossRef]




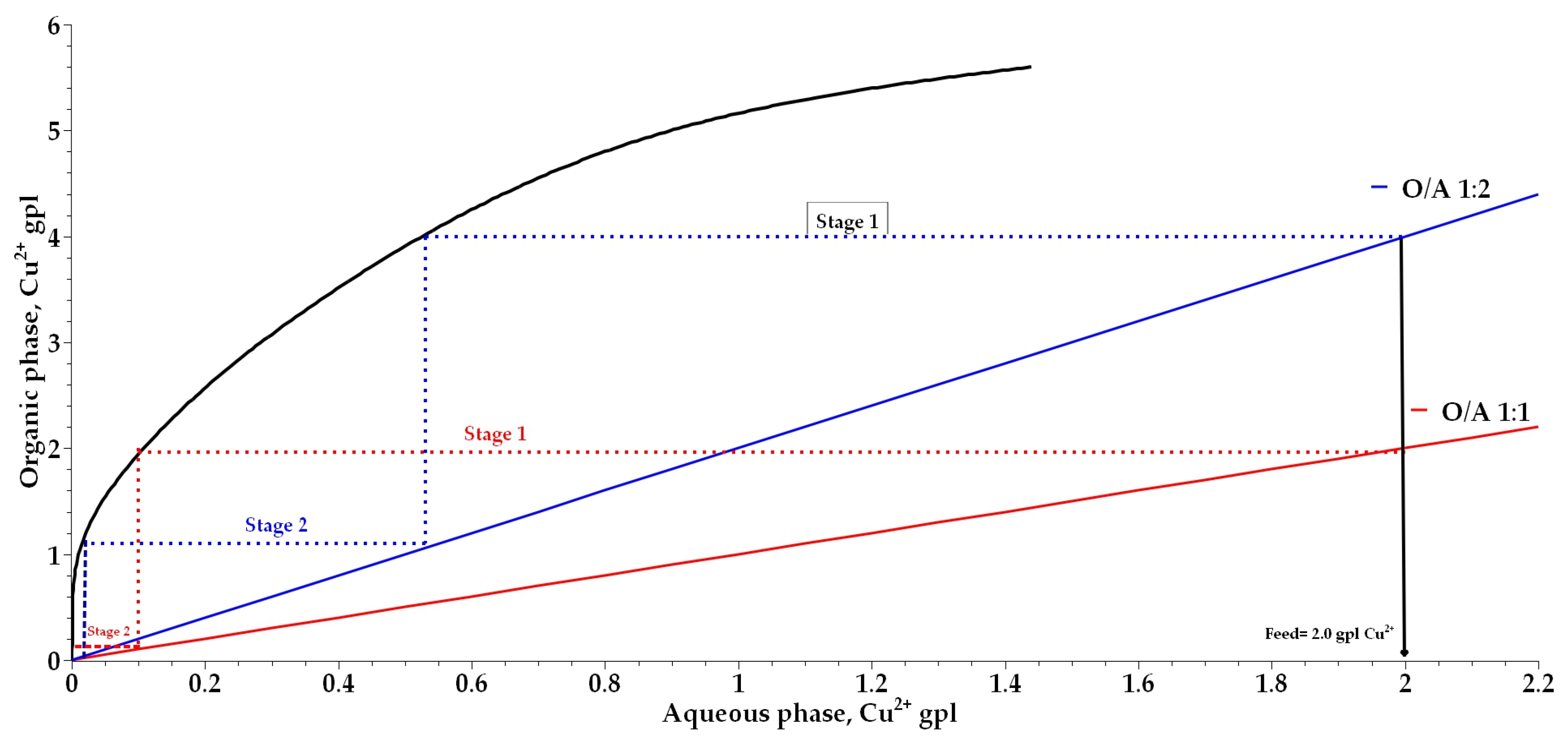

| Nº Cycles | O/A 1:1 | O/A 1:2 | ||||
|---|---|---|---|---|---|---|
| [Cu2+]aq, gpl | [Cu2+]raff, gpl | [Cu2+]org, gpl | [Cu2+]aq, gpl | [Cu2+]raff, gpl | [Cu2+]org, gpl | |
| 1 | 2.00 | 0.10 | 1.90 | 2.00 | 0.20 | 3.60 |
| 2 | 4.00 | 0.70 | 3.30 | 4.00 | 1.15 | 5.70 |
| 3 | 6.00 | 1.75 | 4.25 | 6.00 | 2.50 | 7.00 |
| 4 | 8.00 | 3.65 | 4.35 | 8.00 | 4.43 | 7.15 |
| 5 | 10.00 | 5.60 | 4.40 | 10.00 | 6.33 | 7.35 |
| Extractant | % in Diluent | Aqueous Medium | pH | Uptake (g/L) | gpl Per v/o | Reference |
|---|---|---|---|---|---|---|
| Acorga M5640 | 30% v/v | Alkaline glycinate | ~11 | 17–18 | ~0.57–0.61 | [7] |
| LIX 984N | 10% v/v | Sulfate | ~2.5 | 5–7 | ~0.40–0.45 | [38] |
| LIX 860N-I | 20% v/v | Sulfate–chloride | ~2.2 | 5.5–5.9 | ~0.35–0.38 | [39] |
| PPKO | 2% w/v | Alkaline tartrate | ~11 | 4–7 | ~0.40–0.50 | Our experiment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sueros Velarde, F.J.; Quispe Ortiz, J.A.; Danil de Namor, A.F. A New Approach in Hydrometallurgy for the Solvent Extraction of Cu(II) from Alkaline Solutions Leached with Tartrate Using Phenyl-2-Pyridyl Ketoxime. Metals 2025, 15, 977. https://doi.org/10.3390/met15090977
Sueros Velarde FJ, Quispe Ortiz JA, Danil de Namor AF. A New Approach in Hydrometallurgy for the Solvent Extraction of Cu(II) from Alkaline Solutions Leached with Tartrate Using Phenyl-2-Pyridyl Ketoxime. Metals. 2025; 15(9):977. https://doi.org/10.3390/met15090977
Chicago/Turabian StyleSueros Velarde, Félix José, Jhon Alfredo Quispe Ortiz, and Angela F. Danil de Namor. 2025. "A New Approach in Hydrometallurgy for the Solvent Extraction of Cu(II) from Alkaline Solutions Leached with Tartrate Using Phenyl-2-Pyridyl Ketoxime" Metals 15, no. 9: 977. https://doi.org/10.3390/met15090977
APA StyleSueros Velarde, F. J., Quispe Ortiz, J. A., & Danil de Namor, A. F. (2025). A New Approach in Hydrometallurgy for the Solvent Extraction of Cu(II) from Alkaline Solutions Leached with Tartrate Using Phenyl-2-Pyridyl Ketoxime. Metals, 15(9), 977. https://doi.org/10.3390/met15090977






