Abstract
For the first time, an alternative and sustainable approach is reported using phenyl-2-pyridyl ketoxime (PPKO) as a selective extracting agent for the recovery of Cu(II) from alkaline solutions in the presence of tartrate ions. The advantages relative to conventional processes carried out in acidic media are outlined. Through potentiometric and spectrophotometric analyses, the sequential formation of a 1:2 metal cation–ligand Cu(II)-(PPKO)2 complex was identified as the predominant species in alkaline aqueous solutions. The high removal capacity of the extractant for Cu(II), as assessed from liquid–liquid extraction, and its efficient performance are comparable to widely used commercial extractants. Thermodynamic studies of the complexation between the copper(II) ion and PPKO demonstrated that the process exhibits an endothermic character. A progressive decrease in the performance of the extractant was observed after reuse without a regenerative treatment. This deterioration was partially reversed through a controlled reprotonation process using an acetate buffer solution. Overall, the results support the potential of PPKO as an effective and selective alternative ligand for hydrometallurgical applications in alkaline medium.
1. Introduction
The extraction of copper ions from aqueous media has been an area of interest in hydrometallurgy and remains essential due to the high dynamic demand from the electronics industry, which has significantly driven the advancement of science and technology [1]. Currently, 20% of the world’s primary copper production is processed through leaching, solvent extraction, and electrowinning (SX-EW) [2,3]. Traditionally, acidic leaching with sulfuric acid has dominated the processing of oxidized copper ores. However, acid leaching still presents significant challenges, including the high consumption of sulfuric acid, the generation of leach residues, and acid mine drainage. These by-products, resulting from improper disposal practices or the abandonment of mining operations during closure stages, can become persistent sources of heavy metals and pollutants, posing a considerable environmental risk to surrounding ecosystems [4,5,6]. Alkaline alternatives have been reported in scientific studies, such as the recovery of copper from oxidized or mixed ores using leaching solutions based on glycinate [7], tartrate, oxime derivatives [8], and ammonia [9,10], have emerged as options to improve selectivity and to reduce negative environmental impacts on aquatic ecosystems and soils in case of spillage. However, these emerging leaching solutions present both advantages and disadvantages compared to the conventional use of sulfuric acid in leaching processes; therefore, their application must be evaluated within an operational and environmental context.
It has been demonstrated that tartrate solutions in alkaline medium possess the capacity to form complexes with metal ions at high pH values above 10, through the formation of polydentate complexes, favoring the existence of the [Cu(OH)2C4H4O6]2− anion in solution and preventing precipitation as copper hydroxide [11]. This property has been investigated in the environmental remediation of contaminated waters [12], reflecting the potential of tartrate use in leaching processes.
Likewise, solvent extraction (SX) continues to play a fundamental role in the recovery of valuable metals. In industrial practice, mixtures of aldoximes and ketoximes, such as LIX 54-100, LIX 984, LIX 84-I, and Acorga M5640, have demonstrated an improvement in kinetics extraction, phase stability, and reasonable operating costs. This context has also spurred great interest in scientific research, proposing extractants such as 2,6-Bis(4-Methoxybenzoyl)-Diaminopyridine [13]; 1,1′-dialkyl-2,2′-bibenzimidazoles [14] for the recovery of Cu (II); and N-benzoyl-N′,N′-diethyl thiourea, N-benzoyl-N′,N′-dibutyl thiourea, and N-benzoyl-N′-butyl thiourea, which have been applied to water treatment [15] in acidic mediums, and complementarily, the solvent extraction of copper(II) from alkaline aqueous solutions has shown high efficiencies when using oxime-based extractants [16], with the aim of improving selectivity against interfering species, increasing loading capacity, or adapting to specific process conditions. Although the commercial viability of these compounds remains limited, recent studies on novel extractants in hydrometallurgical processes provide an opportunity to deepen our understanding of metal–ligand coordination principles. Moreover, these advances allow for the assessment of extractant performance under controlled conditions or model systems, offering a foundation for future developments that could enhance the efficiency and sustainability of industrial processes.
Extractants such as phenyl-2-pyridyl ketoxime (PPKO), as proposed in the present study, have demonstrated considerable potential as selective ligands in coordination chemistry and trace metal analysis. This capability arises from the presence of donor atoms, specifically the nitrogen of the pyridine ring, as well as the nitrogen and oxygen atoms of the oxime group. These features enable the ligand to adopt bidentate (N, N) or even tridentate (N, N, O) coordination modes. Such configurations are influenced by the protonation state of the ligand and the nature of the metal ion involved. Previous studies have reported the formation of stable complexes with metal ions such as Ni2+, Cd2+, Ln3+, Co2+, and Zn2+, which typically adopt mononuclear structures in which PPKO acts predominantly as a bidentate ligand, coordinating through pyridine nitrogen and oxime nitrogen. Recent advances in the field of hydrometallurgy have focused on optimizing extraction operating conditions and developing extractants with enhanced selectivity [17,18,19], capable of adapting to aqueous solutions derived from leaching processes carried out in acidic, neutral, and alkaline media [20,21,22,23]. Precisely within this context, the aim of this work is to investigate, through liquid–liquid extraction, the recovery of copper (II) from a leach solution produced by the combined action of tartrate in alkaline medium, as well as phenyl-2-pyridyl ketoxime (PPKO) as an extractant in chloroform, as an approach to develop a promising alternative to the conventional use of acidic leaching systems, opening new perspectives on the practice of sustainable hydrometallurgical processes.
Therefore, the experimental approach encompassed the determination of the stoichiometry of the Cu(II):PPKO complex by potentiometric and spectrophotometric analyses, the evaluation of the equilibrium time and maximum loading capacity, the construction of extraction and stripping isotherms, and the implementation of a reconditioning procedure to assess the regeneration and reusability of the extractant over multiple cycles. In addition, the effect of temperature on the complexation–extraction process was examined.
2. Materials and Methods
2.1. Materials
The experimental tests developed in the present research were carried out using analytical-grade chemical reagents without prior treatment. These included sodium potassium tartrate, C4H4KNaO6·4H2O (SOLUTEST—Lab Chem, Zelienople, PA, USA); sodium hydroxide, NaOH (Riedel-de Haën, Hannover, Germany, 98% purity); potassium chloride, KCl (JT Baker, Phillipsburg, NJ, USA, 100.3% purity); glacial acetic acid, C2H4O2 (JT Baker, Phillipsburg, NJ, USA, ≥99.9% purity); sodium acetate, NaCH3COO (JT Baker, Phillipsburg, NJ, USA, ≥99% purity); disodium salt of ethylenediaminetetraacetic acid dihydrate, C10H14O8Na2N2·2H2O (Daryaganj, New Delhi, India (CDH, 99.5% purity)); basic copper carbonate (malachite), CuCO3·Cu(OH)2 (Phillipsburg, NJ, USA (JT Baker)); phenyl-2-pyridyl ketoxime, C12H10N2O (Saint Louis, MO 63103, USA (Sigma-Aldrich)); chloroform, CHCl3 (Phillipsburg, NJ, USA (JT Baker)), and ethanol, C2H6O (Phillipsburg, NJ, USA (JT Baker, 96% purity)).
Additionally, potentiometric measurements were conducted using a digital pH meter, JENCO, model 1671, Shanghai, China, with a combined Ag/AgCl glass electrode (HANNA—HI 1610D, Mexico City, Mexico); a Cu(II) ion-selective electrode (HANNA—HI 4108, Woonsocket, RI, USA); and a scanning spectrophotometer (VSP-UV—VERNIER, Beaverton, OR, USA) (equipped with Vernier Spectral Analysis software, available online: https://www.vernier.com/product/spectral-analysis/ (accessed on 26 August 2024)), fitted with a quartz cell with a 1.0 cm path length. For the homogenization of reaction mixtures, a digital mixer (Cole Parmer EW-50006-03, Vernon Hills, IL, USA), a magnetic stirrer (IKA—COMBIMAG-RCH, Burladingen, Germany), and a vortex mixer (GENIE—WINN, Tolbert, Netherlands) were used. For the precise quantification of copper(II) ions present in the pregnant leach solution, raffinate, and enriched (stripping) solution, a centrifuge (HETTICH–ROTOFIX 32A, Tuttlingen, Germany) and an atomic absorption spectrophotometer (PerkinElmer AA PinAAcle 500, Waltham, MA, USA) were employed.
2.2. Methodology
2.2.1. Experimental Determination of the Coordination Number of the Cu(II):PPKO Complex Through Simultaneous Potentiometric and Spectrophotometric Analysis in Ethanolic Solution
In order to establish the stoichiometry or composition of the complex Cu(II) ion and phenyl-2-pyridyl ketoxime (PPKO), potentiometric measurements were conducted at 298.15 K, over a 1.5–12 pH range. The experiments were carried out in a glass vessel with a water jacket, under constant magnetic stirring, using a calibrated digital pH meter. A standard NaOH solution (CNaOH = 0.010 mol·dm−3) was gradually added via a piston burette connected to a fine capillary, which remained submerged in the reaction medium. pH readings were recorded three minutes after each addition, allowing chemical equilibrium to be established, following procedures described in the literature [24]. The experimental system consisted of a solution of PPKO (CL = 0.010 mol·dm−3) dissolved in ethanol and Cu(II) (CM = 0.005 mol·dm−3), with a total reaction volume of 100 cm3. NaOH was added until a pH of 11 was reached, a condition that simulates the alkaline environment of a loaded leach solution, as employed in liquid–liquid extraction tests. This setup enabled the study of the complexation behavior under representative conditions for the selective extraction of Cu(II). During the titration process, parallel spectrophotometric measurements were performed to analyze the formation of the Cu–PPKO complex throughout the pH adjustment. These readings confirmed the presence of a complex of 1:2 stoichiometry Cu:(PPKO)2.
2.2.2. Evaluation of Equilibrium Time and Maximum Loading Capacity in the Extraction of Cu(II) Using Phenyl-2-Pyridyl Ketoxime (PPKO)
The equilibrium time study for the extraction of Cu(II) ions was carried out using a synthetic aqueous solution prepared from copper sulfate, with a Cu(II) concentration of 2.00 g/L, in the presence of tartrate ions and adjusted to pH ≈ 11. The organic phase consisted of a 2.0% (w/v) solution of phenyl-2-pyridyl ketoxime (PPKO) dissolved in chloroform. The mixtures were agitated at 600 rpm and maintained at 298.15 ± 1 K, conditions that were consistently applied across all experiments. In all trials, an organic-to-aqueous phase ratio (O:A) of 1:1 was maintained, with a total system volume of 40 mL. To evaluate the effect of contact time, a batch experimental design was implemented with agitation intervals of 1.0, 2.0, 3.0, 4.0, 5.0, and 6.0 min. At the end of each interval, the phases were separated using a separatory funnel. The residual Cu(II) concentration in the aqueous phase was determined by atomic absorption spectroscopy (AAS). Based on these measurements, the extraction efficiency at each time point was calculated in order to determine the minimum contact time required to reach extraction equilibrium.
Subsequently, the maximum loading capacity of phenyl-2-pyridyl ketoxime (PPKO) was assessed using a liquid–liquid extraction system designed to facilitate the transfer of copper ions from an aqueous phase to an organic phase. The same concentration of extractant (2.0% w/v in chloroform) was used for the organic phase, while the aqueous phase consisted of alkaline copper (II) solutions prepared from basic copper carbonate (2.0 g/L), in the presence of tartrate, to simulate conditions representative of an oxidized copper ore leachate. The phases were agitated for 5 min, a duration considered optimal for the extraction process based on previously established procedures in the literature [25], even in systems different from the one evaluated in this study. An organic-to-aqueous phase ratio of 1:1 was maintained in all experiments.
The loading capacity was evaluated through successive extraction cycles. In each cycle, the phases were separated by decantation, and the loaded organic phase was brought into contact with a fresh Cu(II) solution, maintaining identical conditions for each evaluated cycle. This procedure was repeated for five successive cycles without including a stripping stage, allowing the progressive accumulation of copper in the organic phase to be assessed (Figure 1). The aqueous solutions that were not extracted in each cycle were collected in a single container, and their concentration was quantified at the end of each cycle. The obtained data allowed for the precise determination of the amount of copper accumulated in the loaded organic phase, based on the amount of aqueous phase feed accumulated and introduced into the system. A metallurgical mass balance was conducted for each stage using Equation (1), and copper concentration profiles (g/L) were calculated according to Equation (2).
where
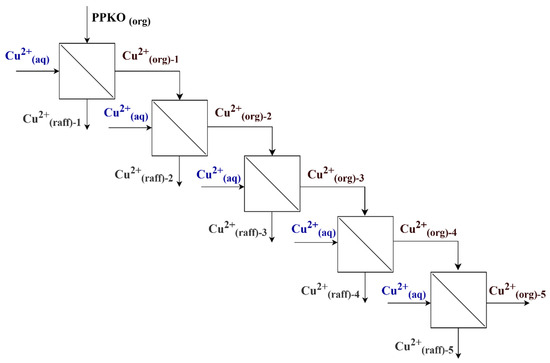
Figure 1.
Solvent extraction flow diagram for the determination of the maximum loading capacity of PPKO.
- is the mass of Cu(II) in the aqueous phase entering stage n.
- is the mass of copper ions in the raffinate at the outlet of the stage.
- is the mass of copper ions in the organic phase at the outlet of stage “n”.
- V is the volume of the phase.
2.2.3. Determination of the Extraction and Stripping Isotherms
For the determination of the extraction isotherms, an aqueous phase obtained from the leaching process was prepared in a 700 cm3 reactor equipped with a vertical stirrer. The reactor was loaded with oxidized ore (50 g; particle size, 45 µm) and 500 cm3 of a leaching solution containing tartrate (0.425 mol dm−3, 80 g/L) in NaOH (0.06 mol dm−3, 2.5 g/L, pH ≈ 11). The reaction mixture was stirred at 600 rpm for 48 h and then centrifuged at 2500 rpm for 15 min. The supernatant was filtered under reduced pressure. The supernatant was quantified by complexometric titration using an EDTA solution (0.01 mol dm−3), a 3% Fast Sulphon Black F indicator, and a pH 10 buffer solution for copper ion quantification. To complement the quantification of copper present in the pregnant leach solution, an atomic absorption spectrophotometry analysis was also conducted.
The extraction distribution isotherm was obtained by mixing the organic phase (2.0% w/v phenyl-2-pyridyl ketoxime dissolved in chloroform) with a diluted aqueous leach solution containing 2.00 g/L of Cu(II), in the presence of tartrate ions, and adjusted to pH ≈ 11. The experiments were conducted at 298.15 ± 1 K using various organic-to-aqueous phase ratios (O:A) ranging from 1:1 to 1:2. Each system was stirred for 5 min, after which copper concentrations in both phases were quantified. The obtained data were used to construct a McCabe–Thiele extraction diagram, reflecting the distribution behavior of Cu(II) between the organic and aqueous phases.
The stripping distribution isotherm was determined using a previously loaded organic phase and a 15% (w/v) aqueous oxalic acid solution as the stripping agent. The procedure was performed under a volumetric aqueous-to-organic ratio (A:O) of 1:1 with a contact time of 5 min. Copper recovery in the aqueous stripping phase was quantified via atomic absorption spectroscopy (AAS).
2.2.4. Reconditioning Procedure for Extractant Regeneration and Reuse
Subsequently, to maintain the efficiency of the extractant phenyl-2-pyridyl ketoxime (PPKO) and enable its reuse over multiple cycles, a chemical reconditioning procedure was implemented at the end of each stripping stage. This consisted of washing the organic phase with a sodium acetate buffer solution adjusted to pH 5 in order to restore the protonated state of the oxime functional group, thereby compensating for the loss of protonation and the positive charge caused during the stripping step with oxalic acid. In order to evaluate the recycling capacity of the extractant, five consecutive extraction–stripping–reconditioning cycles were carried out. In each cycle, the reconditioned organic phase was reused under identical experimental conditions (Figure 2). The copper-loading capacity of the extractant in each cycle was determined indirectly by measuring the concentration of Cu(II) in the aqueous phase before and after contact with the organic phase, using atomic absorption spectroscopy (AAS). This information allowed for the calculation of the amount of copper transferred to the organic phase at each stage.
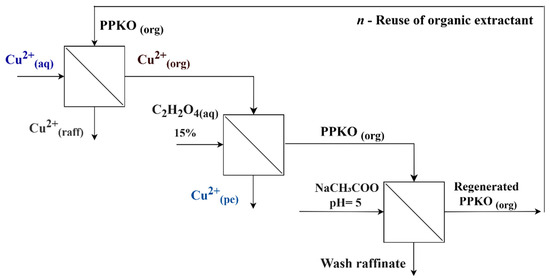
Figure 2.
Flow diagram of the extraction, stripping, and reconditioning cycle of the PPKO extractant.
2.2.5. Evaluation of the Temperature Effect on the Complexation Equilibrium of Cu(II)
To determine the reaction enthalpy (endothermic or exothermic) for the Cu(II) ion complexation process, the effect of temperature on the reaction equilibrium was evaluated. A combined Cu(II) ion-selective electrode coupled to a data acquisition system (model 1671, JENCO Instruments) was employed. The experimental assays consisted of a solution containing 20 mL of 10% w/v potassium nitrate, 20 mL of 96% v/v ethanol, and 1.0 mL of 0.3 mol dm−3 copper (II) sulfate, placed in a double-jacketed glass vessel. Measurements were performed at three temperatures: 288.15 K, 298.15 K, and 308.15 K.
For the thermodynamic analysis, the modified Van ’t Hoff equation (Equation (4)) [26] was applied, potential values were monitored over time, and the reaction time was defined as the moment the system reached a potential of 246 mV, assuming that the reaction with the complexing agent followed first-order kinetics. Measurements were initiated immediately after the addition of 6.0 mL of a 2.0% w/v PPKO solution in ethanol.
Since, in a first-order reaction, the rate constant, k, is inversely proportional to the characteristic reaction time (k ∝ 1/t), the equation can be rewritten as
where
- t1 and t2 are the times required to reach the steady-state potential at temperatures T1 and T2, respectively.
- ΔH is the reaction enthalpy (J·mol−1).
- R is the universal gas constant (8.314 J·mol−1·K−1).
3. Results and Discussion
3.1. Formation and Stability of the Cu2+ Complex with Phenyl-2-Pyridyl Ketoxime for Solvent Extraction in the Hydrometallurgical Process
The potentiometric titration of the Cu2+–phenyl-2-pyridyl ketoxime (PPKO) system in absolute ethanol showed two inflection points corresponding to the sequential formation of complexes [CuHL]2+, [CuL]+, and [CuL2]. These complexes are formed through coordination with the nitrogen atoms of the pyridine ring and the oxime group. In Figure 3, it is evident that upon the addition of NaOH, the first inflection region in the curve occurred at 6.5 mL, with a resulting pK1 ≈ 4.65, marking the formation of the [CuL]+ system (Equation (6)). This involves the formation of a monovalent positively charged complex due to the loss of the first hydrogen from the oxime group and the possible formation of a hydrogen bridge with the proton and available oxygen atoms in the system. The second inflection occurred upon the addition of 9.5 mL of NaOH, reaching pK2 ≈ 11.5, indicating the formation of the second system [CuL2] (Equation (7)), where the ligand loses the second hydrogen from the oxime group, resulting in a neutral complex whose charge is balanced by the Cu2+ ion. These results are similar to those reported by [26], and the observed outcome is significant because neutral complexes favor solubility in hydrocarbons commonly used in hydrometallurgical recovery processes [27,28].
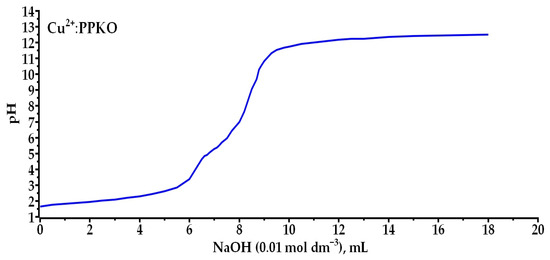
Figure 3.
Potentiometric titration curves of the Cu:PPKO system in 96% ethanol: CM = 5.00 × 10−3 mol dm−3, CL = 0.01 mol dm−3, CNaOH = 0.01 mol dm−3, T = 298.15 K, V = 0.1 dm3.
3.2. Determination of the Coordination Number of the Cu(II):PPKO Complex to Be Extracted
The combined application of potentiometric and spectrophotometric techniques [29,30,31] enabled the characterization of the sequential formation of complexed species between the Cu(II) ion and the ligand phenyl-2-pyridyl ketoxime (PPKO), revealing that pH greatly influences the type of complexes formed [32,33]. At pH 1.65, the formation of a monoligand complex, [CuHL]2+, was observed, evidenced by a well-defined UV–Vis spectrum, consistent with a coordinated species still protonated at the oxime group. The subsequent addition of NaOH up to pH 2.03 showed a decrease in absorbance without a shift in the maximum wavelength, which can be attributed to a dilution effect in the system rather than significant structural changes. With a progressive increase in pH up to 5.05, close to the first experimentally determined pKa value (4.65), a bathochromic shift in the UV–Vis spectrum was identified, indicating the deprotonation of the oxime group and the formation of the [CuL]+ species. This spectral change supports the existence of an acid–base equilibrium that modulates the coordination to the metal ion. Finally, at pH 9.75, close to the second pKa (11.5), a significant change in the spectral profile was recorded, attributable to the formation of a bidentate complex [CuL2], characterized by its high stability in an alkaline medium. These findings confirm the existence of a dynamic pH-dependent equilibrium among Cu–PPKO species, in which [CuHL]2+, [CuL]+, and [CuL2] predominate in acidic, neutral, and alkaline ranges, respectively. The 1:2 metal-to-ligand coordination ratio under basic conditions was confirmed by the UV–Vis spectrum recorded at pH 11, which shows a characteristic profile of the [Cu(PPKO)2] species (Figure 4).
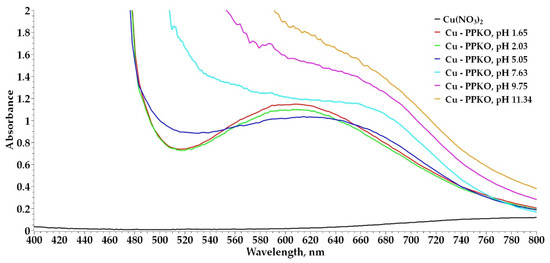
Figure 4.
Identification by UV–visible spectrophotometry of the species formed in the Cu:PPKO system.
From a hydrometallurgical perspective, the results obtained allow this study to assess the significant implications involved in the design and optimization of liquid–liquid extraction processes. The predominance of the [Cu(PPKO)2] complex under alkaline conditions, particularly in media enriched with tartrate ions and at pH ≈ 11, indicates that this species constitutes the most stable and effective form for transferring Cu(II) ions from the aqueous phase to the organic phase. This observation is supported by previous studies using commercial extractants such as Mextral 84H and Mextral 54-100 [7], which have demonstrated high extraction efficiencies of Cu(II) in alkaline glycinate solutions. Taking into account the methodology employed, the results obtained contribute to the rational design of more selective and efficient extractive systems for the recovery of copper(II) from leach solutions in alkaline medium, positioning PPKO as a competitive and versatile ligand for selective hydrometallurgical applications for metals.
3.3. Effect of Contact Time
In the present study, it was observed that the extraction of Cu(II) ions using phenyl-2-pyridyl ketoxime (PPKO) reached equilibrium, with no significant changes detected between 3 and 5 min of agitation. Extending the contact time beyond this range did not result in any additional increase in extraction efficiency or adverse effects, indicating that the system stabilizes within a short period. This behavior is consistent with the findings reported by [34], who investigated oxime-derived extractants dissolved in heptane and toluene and concluded that the extraction equilibrium is achieved within the first minute of agitation, with no improvements observed at prolonged contact times. Similarly, ref. [35] documented that commercial extractant systems typically reach equilibrium between 1 and 5 min, depending on the type of organic diluent and operational temperature.
In our system, the rapid stabilization can be attributed to diffusion-controlled mass transfer, where the interfacial transport of Cu(II) to the organic phase occurs rapidly due to the high affinity of the oxime group for the metal ion. This phenomenon highlights the operational advantage of using fast-kinetic extractants, particularly in hydrometallurgical processes where contact time efficiency is critical. In this context, the molecular structure of the extractant and its selectivity toward the metal ion play a decisive role in determining the time required to reach equilibrium [36].
The selection of a standard agitation time of 5 min (Figure 5) for all subsequent experiments ensured equilibrium conditions without the need to apply complex kinetic models. This decision is supported by previous studies indicating that, in systems employing extractants with high affinity for Cu(II), such as oxime-based compounds, detailed kinetic modeling may be unnecessary and may not provide additional value in terms of process optimization [37].
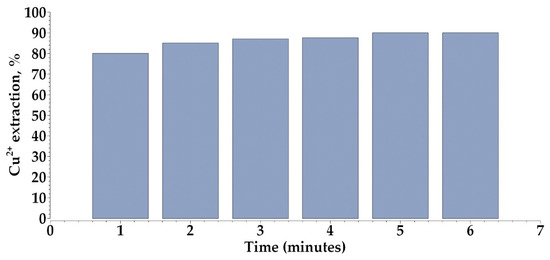
Figure 5.
Influence of shaking time on Cu(II) extraction with PPKO.
3.4. Evaluation of Cu(II) Transfer Efficiency and Extractant Performance
The experimental findings enabled the determination of the maximum loading capacity of the extractant phenyl-2-pyridyl ketoxime (PPKO) for Cu(II) ions in organic–aqueous biphasic systems. Notable variations in copper accumulation within the organic phase were identified as a function of the organic-to-aqueous phase ratio (O/A), thereby underscoring the significant impact of the phase ratio on the loading performance of the extraction system.
At an organic-to-aqueous phase ratio (O/A) of 1:1, the copper concentration in the organic phase reached a maximum of 4.25 g/L during the third contact cycle and remained stable in subsequent cycles. This behavior suggests that the extractant approached its saturation point under these conditions. In contrast, when using an O/A ratio of 1:2, a more pronounced increase in loading capacity was observed, also reaching 7.00 g/L in the third cycle, with no significant changes in the following cycles. This difference can be attributed to the greater excess of aqueous phase available, which enhances the mass transfer of Cu(II) ions into the organic phase, thereby promoting more efficient saturation of the extractant. Figure 6 shows the evolution of copper loading over successive contact cycles, while Table 1 summarizes the hydrometallurgical balance, specifying the accumulated concentrations per cycle in the aqueous and loaded organic phases. Furthermore, an effective loading capacity of 0.44 gpl of Cu(II) per volume of organic phase (gpl per v/o) was calculated, normalized with respect to the volumetric phase ratio. This parameter provides a valuable metric for comparing extractant performance under equivalent experimental conditions. Additionally, Table 2 presents comparative values derived indirectly from experimental data reported in the literature, processed using an analytical approach analogous to that employed in the present study. The results confirm that PPKO exhibits a high affinity for Cu(II) ions, achieving higher loading capacities than those reported for other oxime-based extractants under comparable conditions.
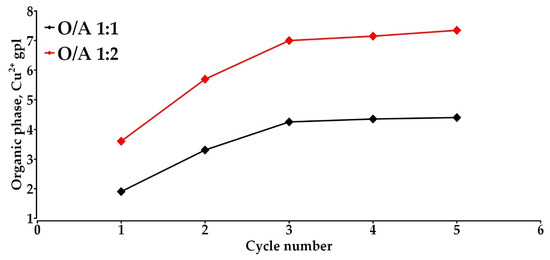
Figure 6.
Extraction loading capacity: [Cu2+]o = 2.0 gpl, [PPKO] = 2.0% w/v, pH = 11, T = 298.15 K. Contact time = 5 min.

Table 1.
Distribution of Cu2+ between organic and aqueous phases during successive extraction cycles at different O:A ratios.

Table 2.
Comparative evaluation of the maximum effective loading capacity (g/L per v/o) between commercial extractants and the Cu2+:PPKO system for copper(II) recovery.
The reported information confirms the high affinity of phenyl-2-pyridyl ketoxime for Cu2+ ions due to the presence of the oxime group and the pyridine ring, and that the organic-to-aqueous ratio has a significant impact on the extraction process. Loading capacity data have also been reported for commercial oximes such as LIX 984 N and LIX 64 N at equilibrium pH values between 1.7 and 2.1 [40,41,42]. Additionally, during the evaluation cycles, limitations in extraction were observed starting from the third cycle, where a third component appeared at the organic–aqueous interface. This phenomenon indicates non-ideal behavior in the system due to the formation of dimers or supramolecular aggregates, which are activated by the presence of hydrogen bonding, competing with active sites for coordination with the metal ion and reducing extraction efficiency [43,44]. Although the literature suggests adding octanol or isotridecanol to improve phase separation efficiency—as studied with Acorga M5640 in kerosene and demonstrated experimentally [45]—the objective of our work was not to develop and validate a phase equilibrium model but rather to demonstrate the feasibility of phenyl-2-pyridyl ketoxime for the recovery of Cu2+ ions from a loaded leach solution in the presence of tartrate ions under alkaline conditions (Figure 7).
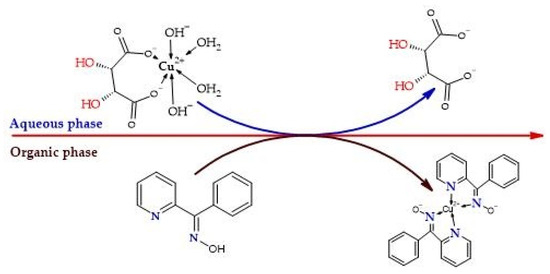
Figure 7.
Transfer of Cu2+ ions from an alkaline aqueous solution in the presence of tartrate ions to an organic phase of phenyl-2-pyridyl ketoxime (PPKO) as extractant in chloroform.
3.5. Leaching and Selective Extraction of Copper(II) Ions
The leachate was generated from an oxidized ore subjected to a leaching process in the presence of tartrate ions in an alkaline medium. The resulting concentration of the loaded leachate was 6.285 g/L. From this loaded leach solution, experimental tests were carried out after diluting it with distilled water to reach a concentration of 2 g/L of copper ions. Preliminary extraction results using phenyl-2-pyridyl ketoxime in chloroform show that copper is strongly extracted at a pH close to 11 in a hydrometallurgical process, which is consistent with expectations for the extractant based on its structure due to the presence of the oxime group (–C=NOH), which is highly effective for the formation of the Cu:PPKO complex. This efficiency is also supported by the presence of tartrate ions in the solution, which contributes to the stability of copper in solution, allowing phenyl-2-pyridyl ketoxime to selectively extract copper(II) ions. The formation of the Cu:PPKO complex from the leach solution is shown in Figure 8. Copper extraction using hydroxyoximes has been widely studied and reported [46], although ketoximes have received attention in coordination and structural chemistry approaches [47,48,49], their application in hydrometallurgical processes under alkaline conditions has not been addressed and constitutes the main focus of the present work.
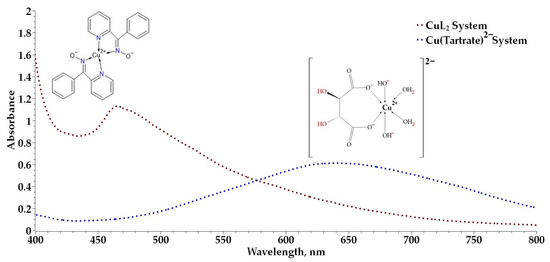
Figure 8.
Comparative UV–Vis profile of the Cu(PPKO)2 and Cu(tartrate)2− systems.
3.6. Extraction and Stripping Isotherm
The number of stages required for copper extraction was determined, and a McCabe–Thiele diagram was constructed for organic-to-aqueous phase ratios (O:A) ranging from 1:1 to 1:2 while keeping the total phase volume constant. Figure 9 indicates the presence of 0.005 and 0.1 g/L of copper(II) ions in the raffinate after the second and first extraction stages for O:A ratios of 1:2 and 1:1, respectively, suggesting the potential to increase copper concentration in the organic phase. The analysis of copper ion concentrations in the organic phase confirmed extractions of 4 and 2 g/L of copper(II) ions in the tests conducted at O:A ratios of 1:2 and 1:1, respectively. During the stripping process, a stripping efficiency of 99.5% was observed at an aqueous-to-organic phase ratio (A:O) of 1:1. Figure 10 shows that stripping occurs in two stages for organic phases loaded with 4.0 g/L of copper ions, and the organic phase was subsequently recovered and reused.
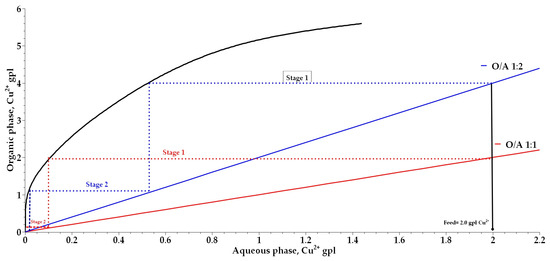
Figure 9.
McCabe–Thiele diagram for copper extraction using 2% (w/v) phenyl-2-pyridyl ketoxime.
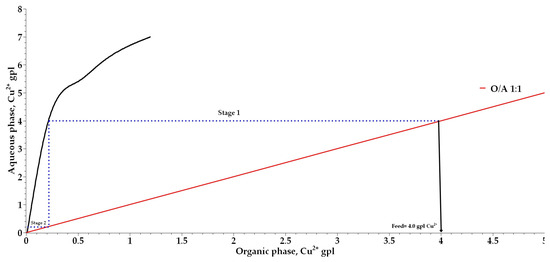
Figure 10.
McCabe–Thiele diagram for the stripping of loaded copper from 2% (w/v) phenyl-2-pyridyl ketoxime using 15% (w/v) oxalic acid (C2H2O4).
3.7. Effect of Reconditioning on the Reusability of the PPKO Extractant
The results obtained reveal a 20% decrease in the copper-loading capacity of the extractant phenyl-2-pyridyl ketoxime (PPKO) by the fifth reuse cycle compared to the initial maximum value. This loss of efficiency suggests a progressive reduction in the number of active sites available on the extractant for the formation of the Cu2+:PPKO complex. This behavior is consistent with previous reports on oxime-type extractants, in which gradual chemical degradation of the oxime functional group has been documented after multiple extraction and stripping cycles, thereby compromising its coordination capacity with Cu2+ ions [50,51,52].
However, treatment of the extractant with an acetate buffer solution at pH 5 enabled the recovery of approximately 75% to 80% of its original extraction capacity. This restoration can be attributed to the effective reprotonation of the oxime group in PPKO, which undergoes deprotonation during the liquid–liquid extraction process (Figure 11). Although oxalic acid, employed as a stripping agent, facilitates the removal of Cu2+ ions from the metal–ligand complex, it does not guarantee complete ionic exchange to restore the protonated form of the extractant. In contrast, the acetate buffer—by maintaining a pH close to the first experimentally determined pKa of PPKO (~4.65), as established through potentiometric titrations—provides more favorable conditions for reprotonation of the oxime functional group (–C=NOH). This strategy contributes to a more complete regeneration of the extractant in its active form and enhances its performance across successive extraction cycles.
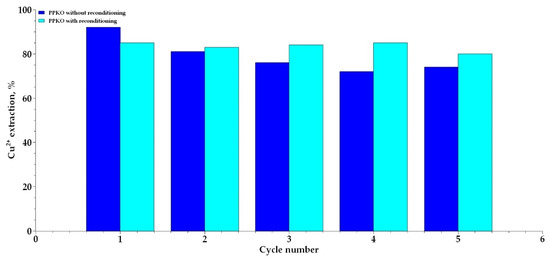
Figure 11.
Effect of chemical reconditioning of PPKO on Cu2+ extraction efficiency over multiple cycles.
3.8. Thermodynamic Study of the Cu(II)–PPKO System in Ethanolic Medium
The thermodynamic evaluation of the Cu(II)–PPKO complexation system was conducted using an approach analogous to stoichiometric studies of the interaction between Cu(II) ions and PPKO. The results allowed for an accurate characterization of the thermodynamic behavior of the reaction, providing key insights into its role within a liquid–liquid extraction process, where PPKO is dissolved in chloroform in the organic phase under hydrometallurgical conditions.
The temperature-dependent complexation of Cu(II) ions with PPKO was analyzed through a plot of Ln(1/t) versus 1/T (Figure 12), which exhibited a linear fit, indicating a strong correlation between Cu(II)–PPKO complex formation and the system temperature. The reaction times required to reach a potential of 246 mV were found to be 60, 20, and 8 s at 287 K, 298.5 K, and 306 K, respectively, enabling the determination of whether the process is exothermic or endothermic. The linearization yielded a negative slope, directly associated with the −ΔH/R term of the modified Van ’t Hoff equation, from which a reaction enthalpy of +75.73 kJ·mol−1 was calculated. The positive value of ΔH indicates that the Cu(II)–PPKO complexation process is endothermic, implying that an increase in temperature promotes complex formation.
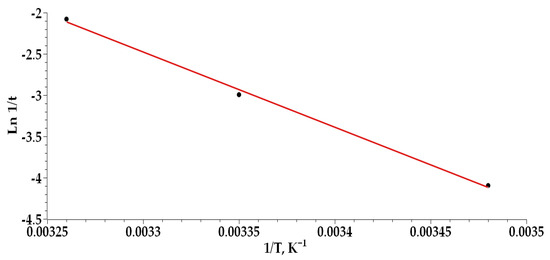
Figure 12.
Plot of ln(1/t) versus 1/T for the determination of the standard reaction enthalpy.
4. Conclusions
Based on the preceding discussions, the following conclusions are established:
- i.
- A new methodology was successfully developed based on a solvent, using phenyl-2-pyridyl ketoxime (PPKO) as a selective extractant of Cu2+ from leach solutions containing tartrate in an alkaline medium derived from oxidized ores. PPKO demonstrated high efficiency in the transfer of copper(II) without the need for acidic media or conventional commercial extractants.
- ii.
- It was confirmed that phenyl-2-pyridyl ketoxime (PPKO) forms a stable 1:2 stoichiometric complex with Cu(II) under alkaline conditions, identified as [Cu(PPKO)2], with maximum stability at pH 11, as evidenced by UV–Vis spectra and potentiometric curves.
- iii.
- The effective loading capacity of PPKO reached an extraction efficiency of approximately 0.44 gpl per v/o, comparable to commercial systems, validating its competitiveness in terms of performance.
- iv.
- A progressive decrease in the extraction efficiency of PPKO was observed after several operational cycles, attributed to the sustained deprotonation of the oxime functional group during the extraction process. However, reconditioning of the extractant with an acetate buffer solution at pH 5 enabled the restoration of up to 80% of its original extractive capacity through a reprotonation process associated with an ion exchange mechanism. This strategy contributes to preserving the functionality of the extractant and ensures its efficient reuse in successive extraction cycles.
- v.
- The Cu(II)–PPKO complexation is an endothermic process, strongly favored by increasing temperature, that reduces the reaction time and enhances the efficiency of solvent extraction under hydrometallurgical conditions.
- vi.
- Furthermore, the results of this research lay the groundwork for implementing the Cu(II)–PPKO system in a tartrate–alkaline medium, demonstrating it as a promising alternative for the selective recovery of copper in a novel, environmentally friendly hydrometallurgical process.
Author Contributions
Conceptualization, F.J.S.V. and J.A.Q.O.; methodology, F.J.S.V., J.A.Q.O., and A.F.D.d.N.; software, J.A.Q.O.; validation, F.J.S.V. and J.A.Q.O.; formal analysis, F.J.S.V. and A.F.D.d.N.; investigation, F.J.S.V.; resources, F.J.S.V. and J.A.Q.O.; data curation, J.A.Q.O.; writing—original draft preparation, F.J.S.V., J.A.Q.O., and A.F.D.d.N.; writing—review and editing, F.J.S.V., J.A.Q.O., and A.F.D.d.N.; visualization, F.J.S.V., J.A.Q.O., and A.F.D.d.N.; supervision, F.J.S.V.; project administration, F.J.S.V.; funding acquisition, F.J.S.V. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support provided by the Universidad Nacional de San Agustin de Arequipa—Perú to conduct this research [Funding number: IBAIB-04-2019-UNSA].
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors wish to express their sincere gratitude to Alejandro Néstor Salas Begazo for his invaluable collaboration and contribution to this research. His dedication was essential to the development of this study during the time he worked with us. Although he is no longer with us, his legacy endures through the lasting impact he made in his field and on all those who had the privilege of working alongside him.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Guo, L.; Li, S.; He, Z.; Fu, Y.; Qiu, F.; Liu, R.; Yang, G. Electroplated Copper Additives for Advanced Packaging: A Review. ACS Omega 2024, 9, 20637–20647. [Google Scholar] [CrossRef]
- Singer, D.A. Future Copper Resources. Ore. Geol. Rev. 2017, 86, 271–279. [Google Scholar] [CrossRef]
- Castellón, C.I.; Taboada, M.E. Leaching of Copper Concentrates with Iodized Salts in a Saline Acid Medium: Part 2—Effect on Chloride Concentration and an Aerated System. Materials 2023, 16, 5940. [Google Scholar] [CrossRef]
- Izydorczyk, G.; Mikula, K.; Skrzypczak, D.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Potential Environmental Pollution from Copper Metallurgy and Methods of Management. Environ. Res. 2021, 197, 111050. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Du, X.; Wang, X.; Zeng, Y.; Fan, S. Accumulated Copper Tailing Solid Wastes with Specific Compositions Encourage Advances in Microbial Leaching. Minerals 2024, 14, 1051. [Google Scholar] [CrossRef]
- Martínez, J.I.; Teja, A.M.; Reyes, M.; Toro, N.; Cisneros, G.; Flores, U.M.; Labra, M.P.; Urbano, G.; Juarez, J.C. Optimization of Sustainable Copper Leaching Using Glycine and Oxidizing Agents in an Alkaline Medium. Metals 2025, 15, 617. [Google Scholar] [CrossRef]
- Tanda, B.C.; Oraby, E.A.; Eksteen, J.J. Recovery of Copper from Alkaline Glycine Leach Solution Using Solvent Extraction. Sep. Purif. Technol. 2017, 187, 389–396. [Google Scholar] [CrossRef]
- Sueros Velarde, F.J.; Siles, H.O.; Danil De Namor, A.F. The Use of a Novel Oxime Derivative [Phenyl(N-Methylaniline) Ketooxime] as Leach Reagent in the Copper(II) Hydrometallurgy. Miner. Eng. 2005, 18, 213–218. [Google Scholar] [CrossRef]
- Radmehr, V.; Koleini, S.M.J.; Khalesi, M.R.; Tavakoli Mohammadi, M.R. Ammonia Leaching: A New Approach of Copper Industry in Hydrometallurgical Processes. J. Inst. Eng. Ser. D 2013, 94, 95–104. [Google Scholar] [CrossRef]
- Conejeros, V.; Pérez, K.; Jeldres, R.I.; Castillo, J.; Hernández, P.; Toro, N. Novel Treatment for Mixed Copper Ores: Leaching Ammonia—Precipitation—Flotation (L.A.P.F.). Miner. Eng. 2020, 149, 106242. [Google Scholar] [CrossRef]
- Velarde, F.J.S.; Ortiz, J.A.Q.; Apaza, A.A.H.; de Namor, A.F.D. A New Approach for Increasing the Chelating Capacity of the Tartrate Ion in the Extraction of Copper from Ores. Metals 2023, 13, 1672. [Google Scholar] [CrossRef]
- Han, M.; He, J.; Sun, W.; Li, S.; Yu, H.; Yue, T.; Wei, X.; Zhang, C. Coordination Configurations of Cupric Tartrate in Electronic Industry Wastewater. Trans. Nonferrous Met. Soc. China 2022, 32, 3753–3766. [Google Scholar] [CrossRef]
- Bozejewicz, D.; Witt, K.; Kaczorowska, M.A.; Osmiałowski, B. The Copper(II) Ions Solvent Extraction with a New Compound: 2,6-Bis(4-Methoxybenzoyl)-Diaminopyridine. Processes 2019, 7, 954. [Google Scholar] [CrossRef]
- Madrzak-Litwa, I.; Borowiak-Resterna, A. Solvent Extraction of Copper(II) from Chloride Solutions Using 1,1′-Dialkyl-2,2′-Bibenzimidazoles as Extractants. Physicochem. Probl. Miner. Process. 2019, 55, 1165–1178. [Google Scholar] [CrossRef]
- Huang, X.; Jin, K.; Zhang, R.; Gong, Y.; Zeng, J.; Zhang, R.; Liu, Y.; Xue, J. Selective Solvent Extraction of Cu(II) from Aqueous Solutions Using an Acyl-Based Thiourea: Extraction Study and DFT Analysis of Reaction Mechanism. Hydrometallurgy 2024, 223, 106226. [Google Scholar] [CrossRef]
- Mohammed, T.; Bezuidenhout, G.A.; Oraby, E.A.; Eksteen, J.J. Sequential Separation of Cobalt, Copper, and Nickel from Alkaline Glycinate Solutions Using Solvent Extraction. J. Sustain. Metall. 2024, 10, 2455–2468. [Google Scholar] [CrossRef]
- Mazarakioti, E.C.; Soto Beobide, A.; Angelidou, V.; Efthymiou, C.G.; Terzis, A.; Psycharis, V.; Voyiatzis, G.A.; Perlepes, S.P. Modeling the Solvent Extraction of Cadmium(II) from Aqueous Chloride Solutions by 2-pyridyl Ketoximes: A Coordination Chemistry Approach. Molecules 2019, 24, 2219. [Google Scholar] [CrossRef]
- Shokrollahi, A.; Ghaedi, M.; Rajabi, H.R.; Niband, M.S. Potentiometric Study of Binary Complexes of Methyl-2-Pyridyl Ketone Oxime, Phenyl-2-Pyridyl Ketone Oxime and Diacetyl Monooxime with Some Transition and Heavy Metal Ions in Aqueous Solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 655–662. [Google Scholar] [CrossRef]
- Mądrzak-Litwa, I.; Borowiak-Resterna, A. Solvent Extraction of Zinc from Chloride Solutions Using Dialkyl Derivatives of 2,2′-Bibenzimidazole as Extractants. Hydrometallurgy 2018, 182, 8–20. [Google Scholar] [CrossRef]
- Ferreira, A.E.; Agarwal, S.; MacHado, R.M.; Gameiro, M.L.F.; Santos, S.M.C.; Reis, M.T.A.; Ismael, M.R.C.; Correia, M.J.N.; Carvalho, J.M.R. Extraction of Copper from Acidic Leach Solution with Acorga M5640 Using a Pulsed Sieve Plate Column. Hydrometallurgy 2010, 104, 66–75. [Google Scholar] [CrossRef]
- Sengupta, B.; Sengupta, R.; Bhakhar, M.S. Extraction-Stripping Patterns during Co-Extraction of Copper and Nickel from Ammoniacal Solutions into Emulsion Liquid Membranes Using LIX 84I®. J. Membr. Sci. Res. 2017, 3, 71–77. [Google Scholar] [CrossRef]
- Ruiz, M.C.; González, I.; Rodriguez, V.; Padilla, R. Solvent Extraction of Copper from Sulfate-Chloride Solutions Using LIX 84-IC and LIX 860-IC. Miner. Process. Extr. Metall. Rev. 2021, 42, 1–8. [Google Scholar] [CrossRef]
- Chepushtanova, T.; Yessirkegenov, M.; Bochevskaya, Y.; Sharipova, A.; Baigenzhenov, O.; Merkibayev, Y.; Altmyshbayeva, A. The Testing Results of ACORGA, LIX Extractants and CR60 Crud Mitigation Reagent Influence During SX-EW Copper Extraction. Sustainability 2024, 16, 7815. [Google Scholar] [CrossRef]
- U. S. Geological Survey. National Field Manual for the Collection of Water-Quality Data Book 9, Handbooks for Water-Resources Investigations. In Measurement of PH; Chapter 6.4; U.S. Geological Survey: Reston, VA, USA, 2021. [Google Scholar]
- Kasongo, K.B.; Mwanat, M.H.M.; Mbuya, B.; Makhatha, M.E.; Mulaba-Bafubiandi, A.F.; Zeka, M.L. Minimization of Organic-in-Aqueous (O-in-A) Entrainments and Improvement of Solvent Extraction Efficiency by Optimizing Key Parameters Using Response Surface Methodology (RSM). J. Sustain. Metall. 2025, 11, 1542–1553. [Google Scholar] [CrossRef]
- Smith, J.M. Chemical Engineering Kinetics, 3rd ed.; McGraw-Hill: New York, NY, USA, 1981. [Google Scholar]
- Meek, T.L.; Cheney, G.E. The Copper(II) Syn-Phenyl-2-Pyridyl Ketoxime System Part I. Can. J. Chem. 2011, 43, 64–74. [Google Scholar] [CrossRef]
- Wilson, A.M.; Bailey, P.J.; Tasker, P.A.; Turkington, J.R.; Grant, R.A.; Love, J.B. Solvent Extraction: The Coordination Chemistry Behind Extractive Metallurgy. Chem. Soc. Rev. 2013, 43, 123–134. [Google Scholar] [CrossRef]
- Li, Z.; Dewulf, B.; Binnemans, K. Nonaqueous Solvent Extraction for Enhanced Metal Separations: Concept, Systems, and Mechanisms. Ind. Eng. Chem. Res. 2021, 60, 17285–17302. [Google Scholar] [CrossRef]
- Simonsen, S.H.; Burnett, H.M. Spectrophotometric Determination of Copper with Salicylaldoxime Application to Analysis of Aluminum Alloys. Anal. Chem. 1955, 27, 1336–1339. [Google Scholar] [CrossRef]
- Sliva, T.Y.; Duda, A.M.; Glowiak, T.; Fritsky, I.O.; Amirkhanov, V.M.; Mokhir, A.A.; Kozlowski, H. Co-Ordination Ability of Amino Acid Oximes. Potentiometric, Spectroscopicand Structural Studies of Complexes of 2-Cyano-2-(Hydroxyimino)Acetamide. J. Chem. Soc. Dalton Trans. 1997, 273–276. [Google Scholar] [CrossRef]
- Silva, T.Y.; Dobosz, A.; Jerzykiewicz, L.; Karaczyn, A.; Moreeuw, A.M.; Świątek-Kozłowska, J.; Głowiak, T.; Kozłowski, H. Copper(II) and Nickel(II) Complexes with Oxime Analogues of Amino Acids. Potentiometric, Spectroscopic and X-Ray Studies of Complexes with 2-Cyano-2-(Hydroxyimino)Acetic Acid and Its Ethane-1,2-Diamine Derivative. J. Chem. Soc. Dalton Trans. 1998, 1863–1868. [Google Scholar] [CrossRef]
- Chylewska, A.; Ogryzek, M.; Chmurzyński, L.; Makowski, M. Spectrophotometric, Potentiometric, and Conductometric Studies of Binary Complex Formation Between Copper(II) and Three Forms of Vitamin B 6 in Aqueous Solutions. J. Coord. Chem. 2015, 68, 3761–3775. [Google Scholar] [CrossRef]
- Szczęsny, R.; Muzioł, T.M.; Gregory, D.H.; Szłyk, E. Structural and Thermal Characterization of Copper(II) Complexes with Phenyl-2-Pyridylketoxime and Deposition of Thin Films by Spin Coating. Chem. Pap. 2015, 69, 569–579. [Google Scholar] [CrossRef]
- Aksamitowski, P.; Wieszczycka, K.; Wojciechowska, I. Selective Copper Extraction from Sulfate Media with N,N-Dihexyl-N′-Hydroxypyridine-Carboximidamides as Extractants. Sep. Purif. Technol. 2018, 201, 186–192. [Google Scholar] [CrossRef]
- Mondal, S.; Majumder, S.K. Studies on the Copper Extraction in a Channel-Based Packed Extraction Device. Miner. Eng. 2018, 126, 194–206. [Google Scholar] [CrossRef]
- Deep, A.; Kumar, P.; Carvalho, J.M.R. Recovery of Copper from Zinc Leaching Liquor Using ACORGA M5640. Sep. Purif. Technol. 2010, 76, 21–25. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.; Petersen, J.; Azizi, A. Solvent Extraction Studies of Copper from a Heap Leach Liquor Using Mextral 5640H. Minerals 2022, 12, 1322. [Google Scholar] [CrossRef]
- Wang, L.P.; Lin, J.Y.; Chen, Y.J.; Tseng, B.C.; Hsu, C.H.; Kou, M.; Zhou, H.; Sreearunothai, P. Separation and Recovery of Copper and Nickel in the Leachate of a Waste IC Lead Frame through Synergistic Solvent Extraction Using a Binary Extractant Containing LIX984N and Cyanex302 Followed by Selective Stripping. Sustainability 2023, 16, 77. [Google Scholar] [CrossRef]
- Kumar, V.; Pandey, B.D.; Bagchi, D. Application of LIX 84 for Separation of Copper, Nickel and Cobalt in Ammoniacal Leaching of Ocean Nodules. Mater. Trans. JIM 1991, 32, 157–163. [Google Scholar] [CrossRef][Green Version]
- Kul, M.; Çetinkaya, Ü. Recovery of Copper by LIX 984N-C from Electroplating Rinse Bath Solution. Hydrometallurgy 2009, 98, 86–91. [Google Scholar] [CrossRef]
- Ochromowicz, K.; Chmielewski, T. Solvent Extraction of Copper(II) from Concentrated Leach Liquors. Physicochem. Probl. Miner. Process. 2013, 49, 357–367. [Google Scholar] [CrossRef]
- Agarwal, S.; Reis, M.T.A.; Ismael, M.R.C.; Correia, M.J.N.; Carvalho, J.M.R. Modeling of the Extraction Equilibrium of Copper from Sulfate Solutions with Acorga M5640. Solvent Extr. Ion Exch. 2012, 30, 536–551. [Google Scholar] [CrossRef]
- El Hady, S.M. Solvent Extraction of Yttrium from Rare Earths Concentrate Using Cyanex 272 and PC88A. Bull. Fac. Sci. Zagazig Univ. 2024, 2023, 190–200. [Google Scholar] [CrossRef]
- Agarwal, S.; Ferreira, A.E.; Santos, S.M.C.; Reis, M.T.A.; Ismael, M.R.C.; Correia, M.J.N.; Carvalho, J.M.R. Separation and Recovery of Copper from Zinc Leach Liquor by Solvent Extraction Using Acorga M5640. Int. J. Miner. Process. 2010, 97, 85–91. [Google Scholar] [CrossRef]
- Panigrahi, S.; Parhi, P.K.; Sarangi, K.; Nathsarma, K.C. A Study on Extraction of Copper Using LIX 84-I and LIX 622N. Sep. Purif. Technol. 2009, 70, 58–62. [Google Scholar] [CrossRef]
- Tarushi, A.; Raptopoulou, C.P.; Psycharis, V.; Kontos, C.K.; Kessissoglou, D.P.; Scorilas, A.; Tangoulis, V.; Psomas, G. Copper(II) Inverse-[9-Metallacrown-3] Compounds Accommodating Nitrato or Diclofenac Ligands: Structure, Magnetism, and Biological Activity. Eur. J. Inorg. Chem. 2016, 2016, 219–231. [Google Scholar] [CrossRef]
- Hołyńska, M. Structural Variety and Magnetic Properties of Oxime-Bridged Copper(II) Complexes. J. Mol. Struct. 2015, 1098, 175–180. [Google Scholar] [CrossRef]
- Chakraborty, A.; Escuer, A.; Ribas, J.; Maji, T.K. A Discrete CuII6 Cluster and a 3D MnII–CuII Framework Based on Assembly of Mn2Cu4 Clusters: Synthesis, Structure and Magnetic Properties. Dalton Trans. 2016, 45, 15523–15531. [Google Scholar] [CrossRef]
- Nobahar, A.; Melka, A.B.; Pusta, A.; Lourenço, J.P.; Carlier, J.D.; Costa, M.C. A New Application of Solvent Extraction to Separate Copper from Extreme Acid Mine Drainage Producing Solutions for Electrochemical and Biological Recovery Processes. Mine Water Environ. 2022, 41, 387–401. [Google Scholar] [CrossRef]
- Whewell, R.J.; Foakes, H.J.; Hughes, M.A. Degradation in Hydroxyoxime Solvent Extraction Systems. Hydrometallurgy 1981, 7, 7–26. [Google Scholar] [CrossRef]
- Xie, Q.; Chen, Q.; Liu, Y.; Yu, Y.; Sun, Q.; Wang, L. Degradation of Hydroxyoxime CP-150 in the Presence of Nitrate and Permanganate Ions Causing Detrimental Effect on Copper Extraction. Hydrometallurgy 2023, 221, 106149. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).