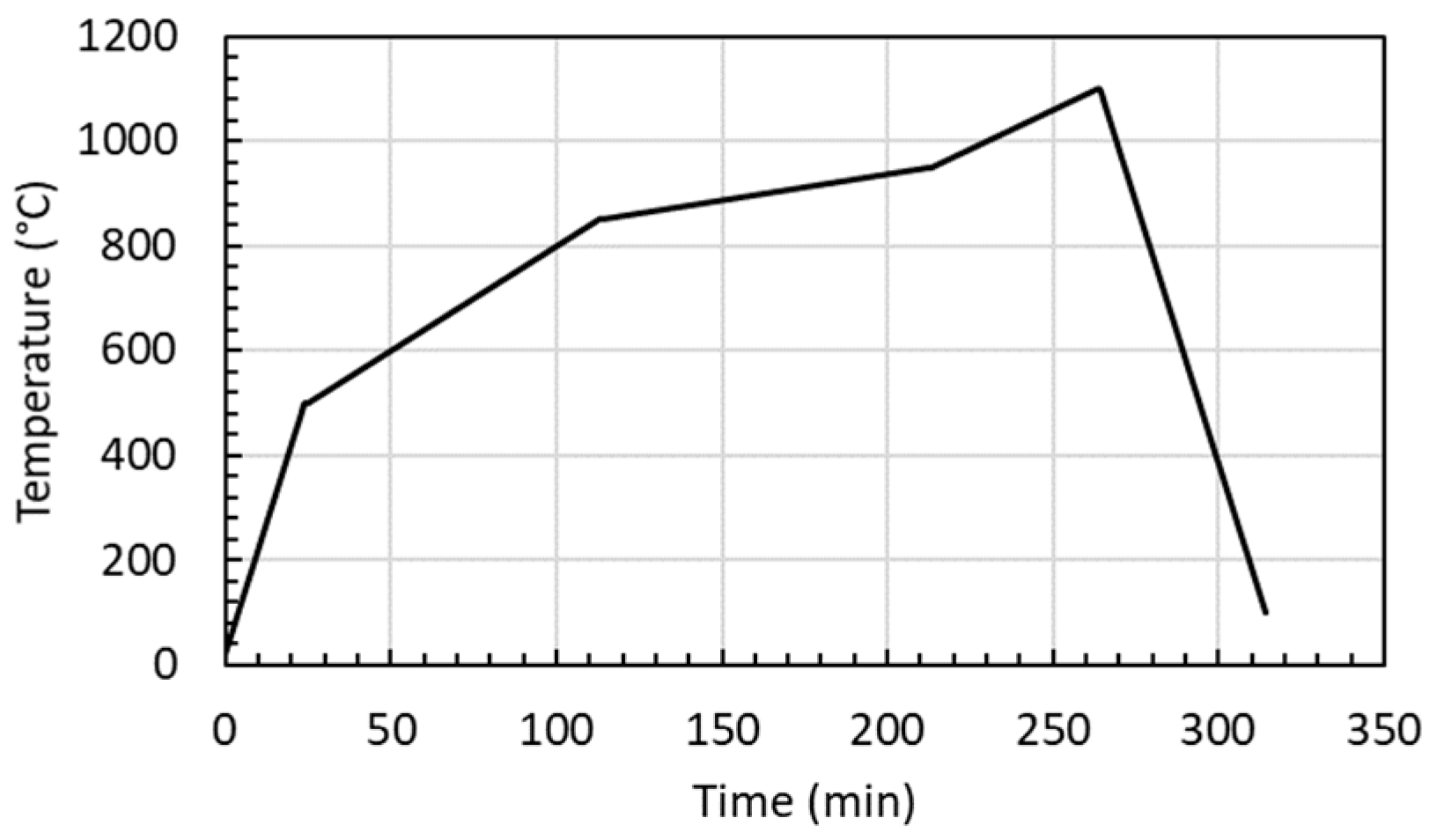
Figure 1.
Applied thermal profile during the TGA experiments.
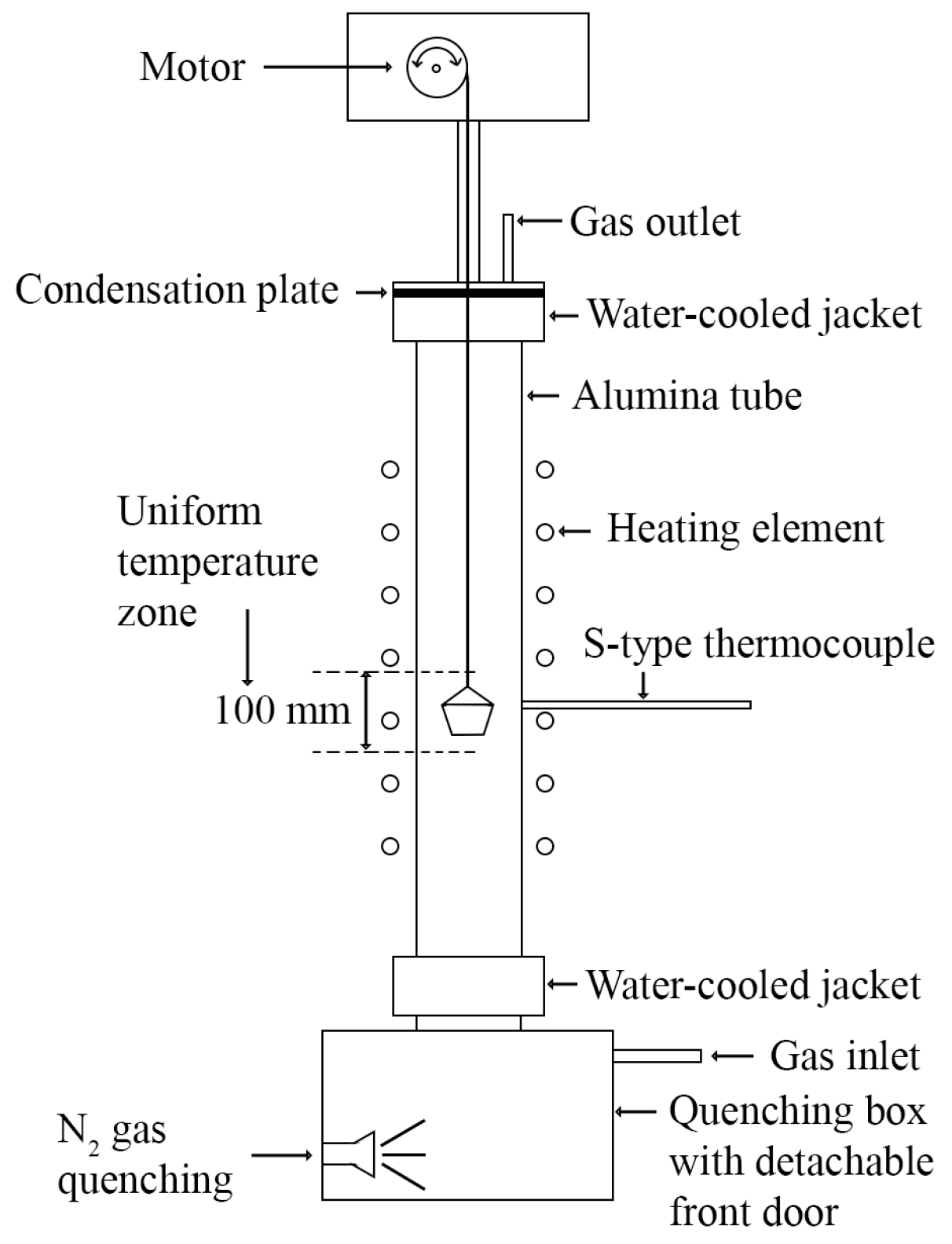
Figure 2.
A schematic drawing of the vertical tube furnace.
Figure 3.
The phase composition of the studied in-plant fines.
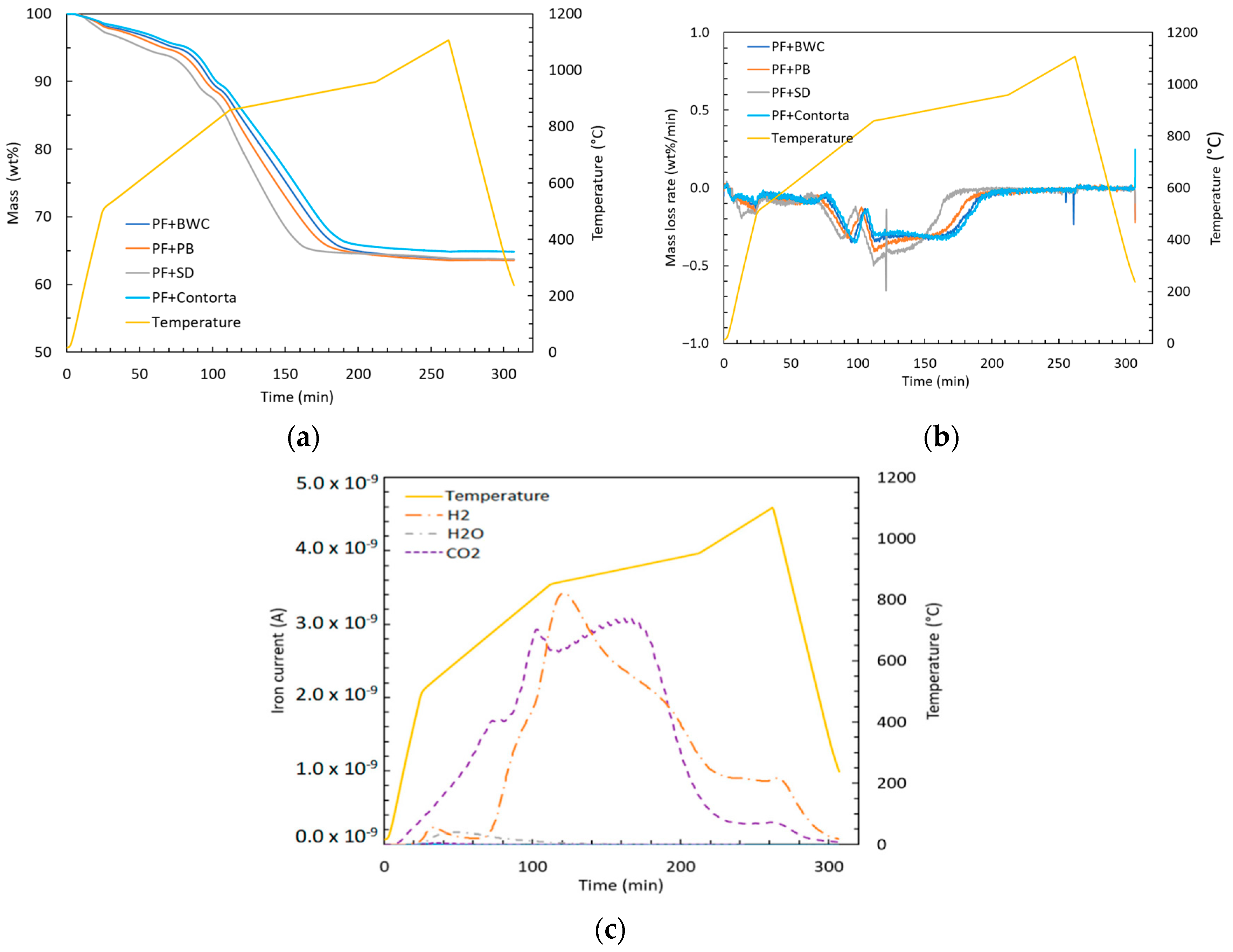
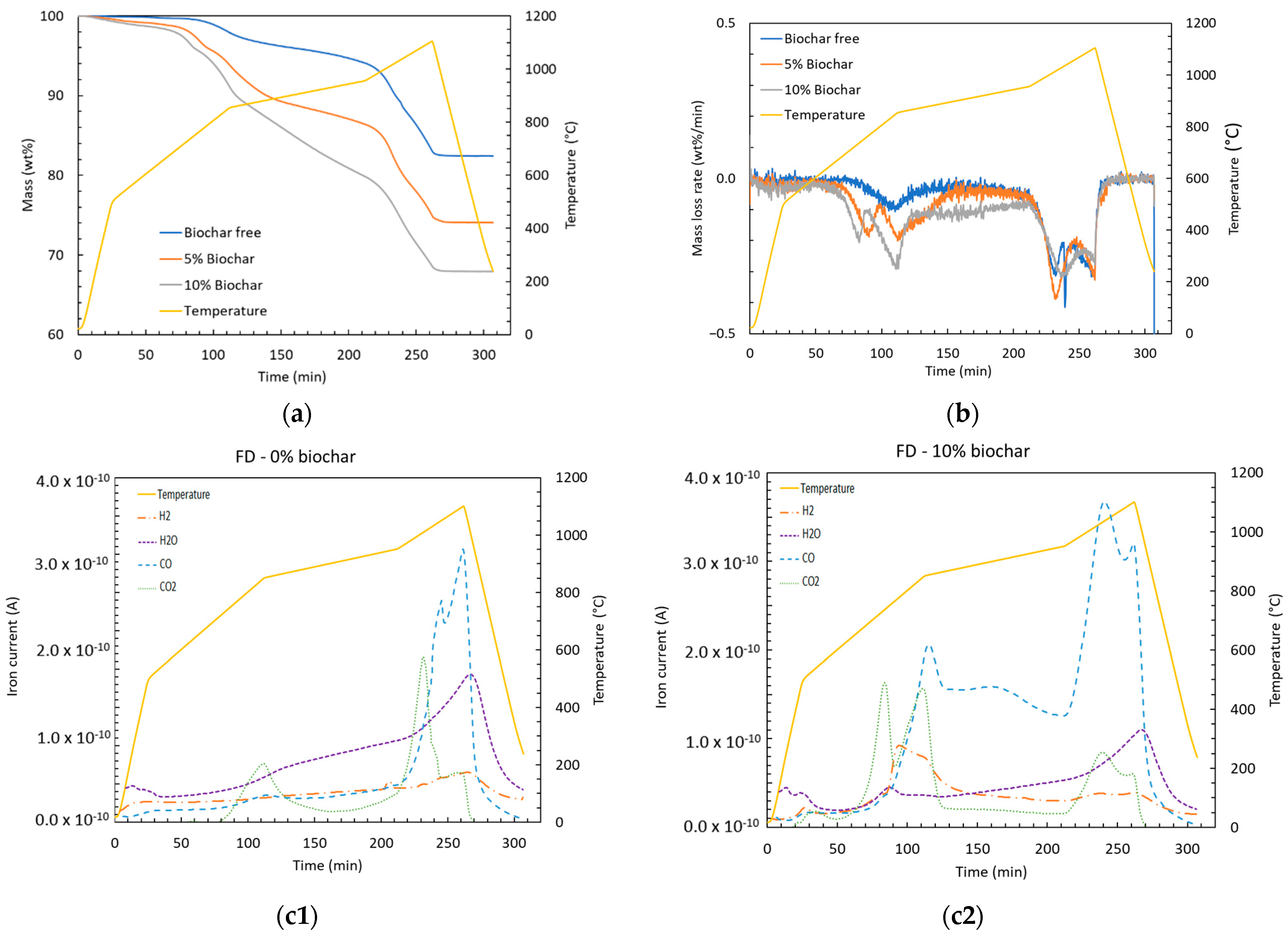
Figure 4.
(a) Remaining mass, (b) mass loss rate, and (c) representative example of evolved gases composition during the reduction test.
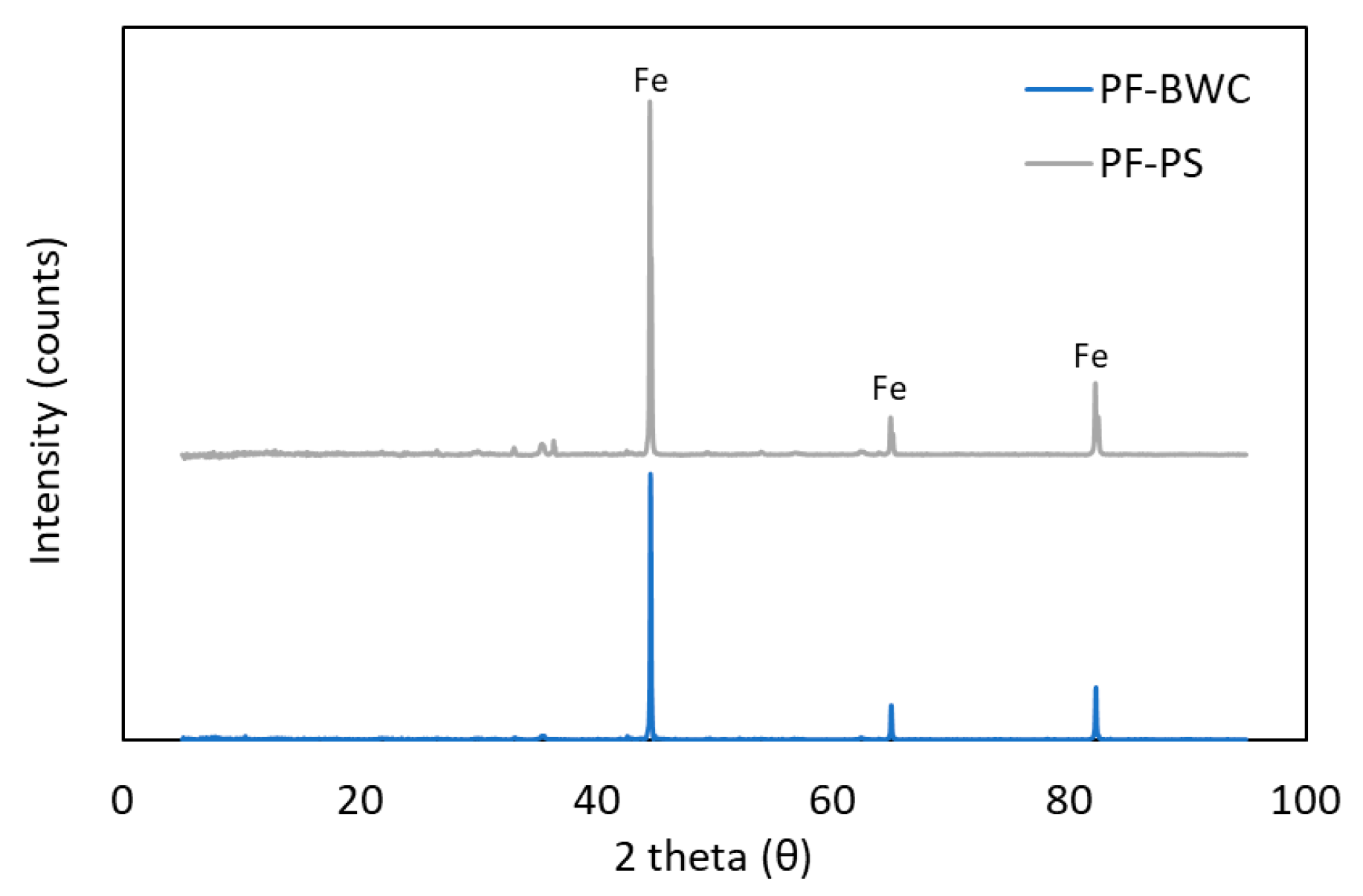
Figure 5.
XRD pattern of pellet fines–biochar sample products.
Figure 6.
(a) Remaining mass percent, (b) mass loss rate, and (c1,c2) off-gas composition of filter dust with/with no added biochar.
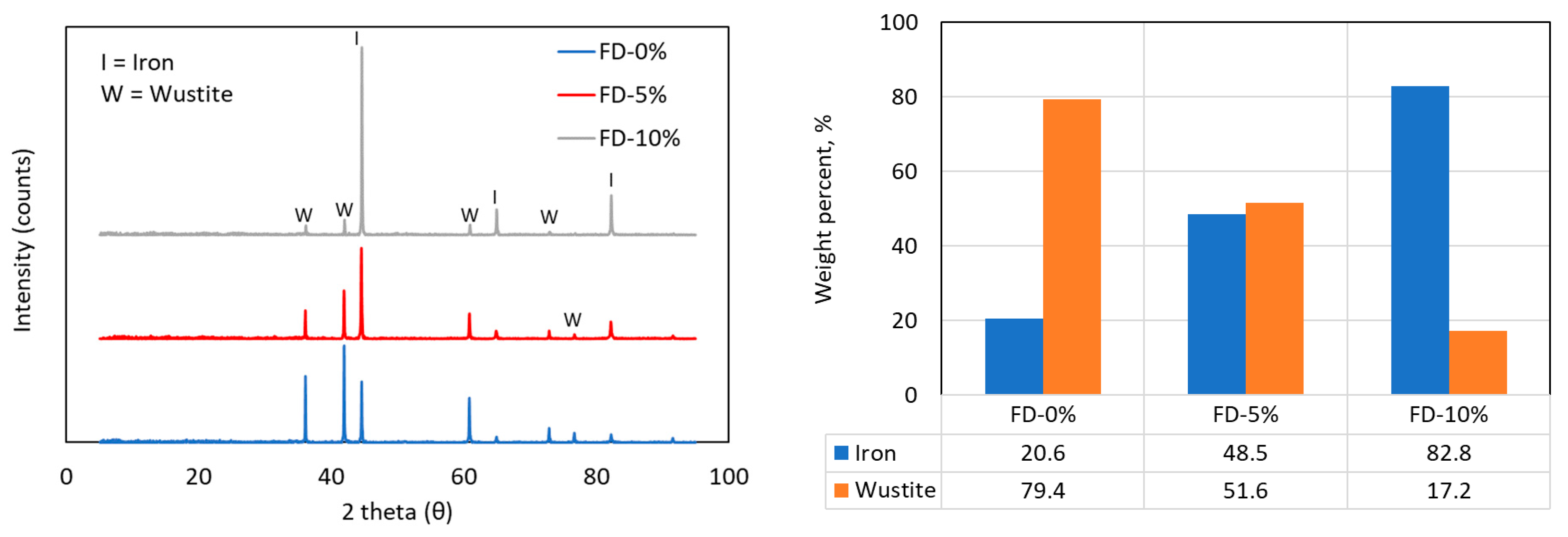
Figure 7.
XRD patterns of filter dust samples after reduction, with Rietveld analysis showing the quantities of Fe and FeO for filter dust mixed with 0, 5, or 10 wt.% of biochar.
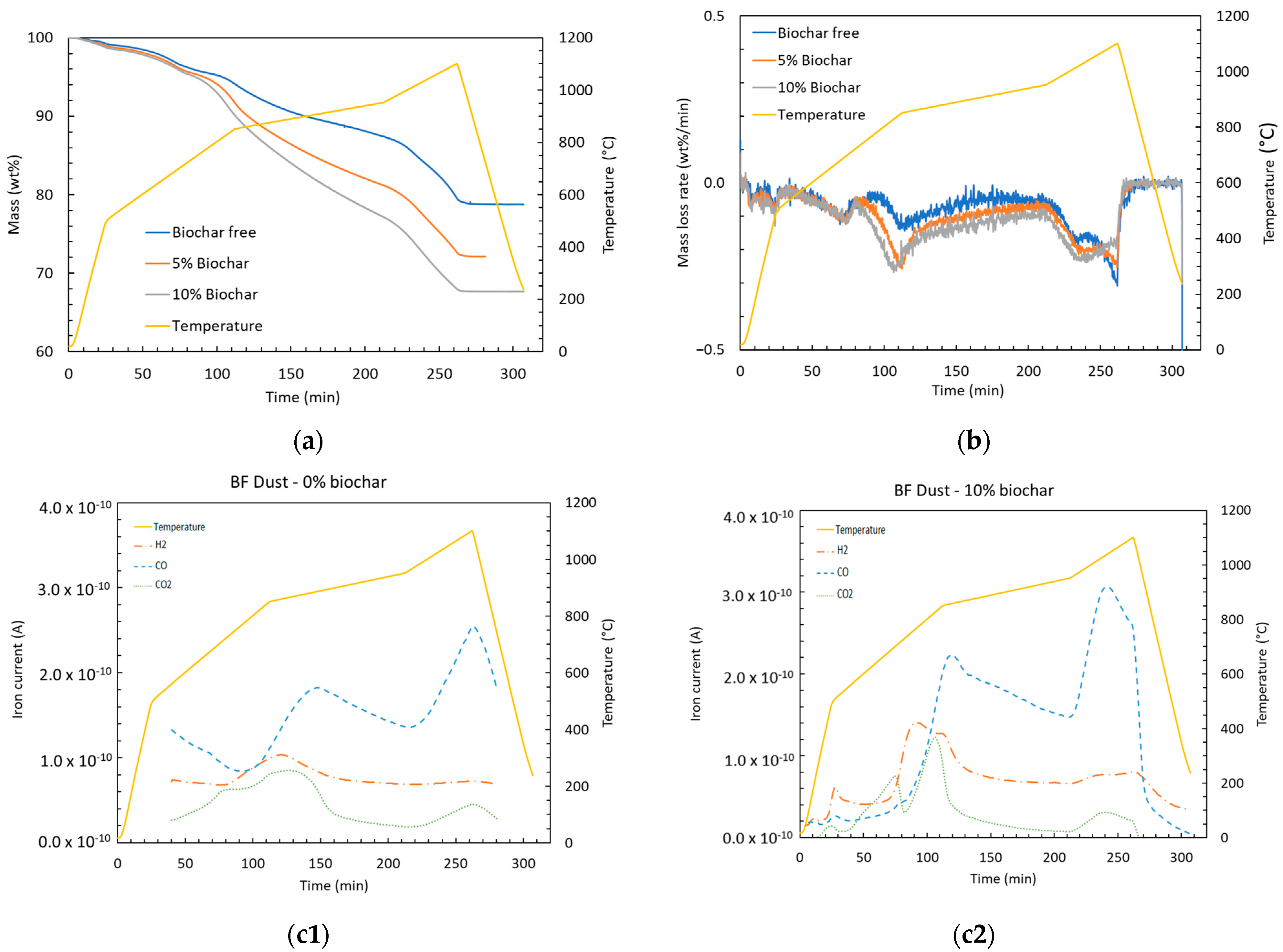
Figure 8.
(a) Remaining mass percent, (b) mass loss rate, and (c1,c2) off-gas composition of BF dust with 0 or 10 wt.% of biochar.
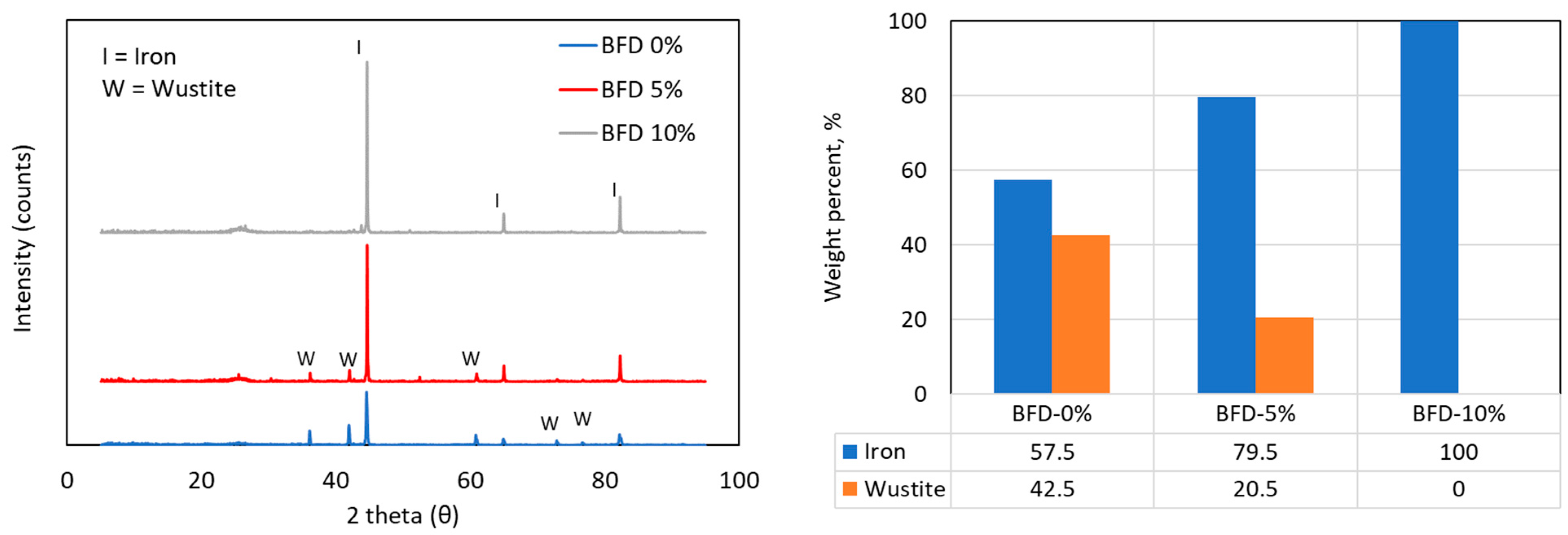
Figure 9.
XRD patterns of BF dust samples after reduction, with Rietveld analysis showing the quantities of Fe and FeO for samples with 0, 5, or 10 wt.% of biochar added.
Figure 10.
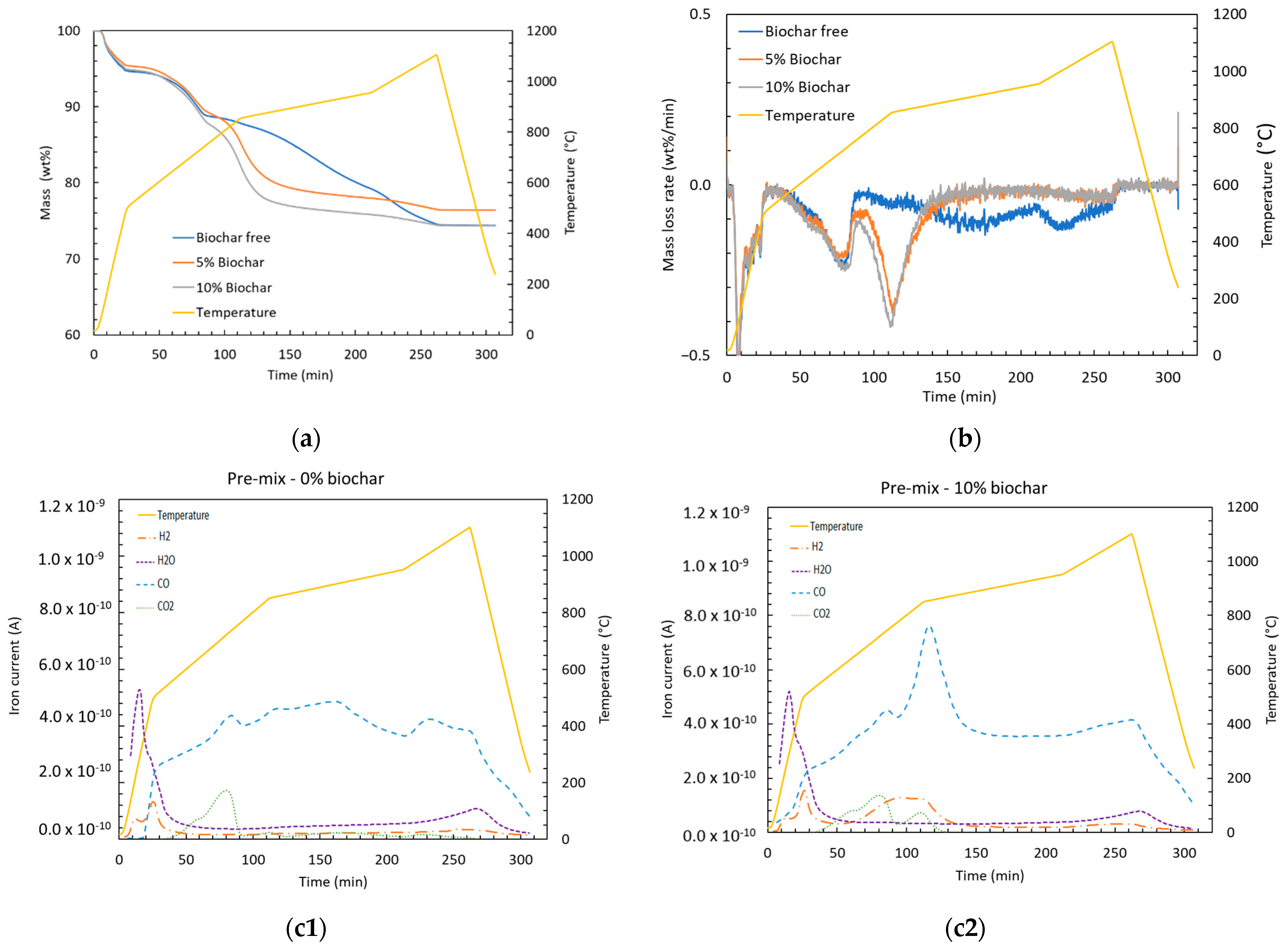
(a) Remaining mass percent, (b) mass loss rate, and (c1,c2) off-gas composition of pre-mix with 0 or 10 wt.% added biochar.
Figure 11.
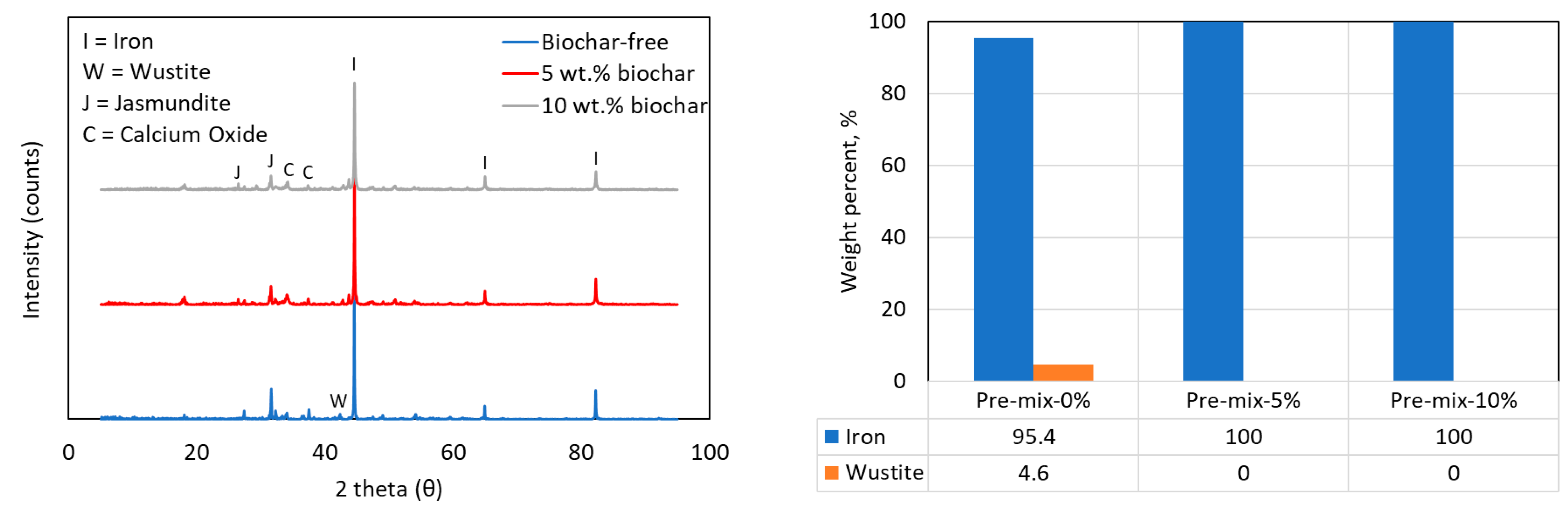
XRD pattern of pre-mix-containing samples after reaction and the corresponding Rietveld analysis indicating quantities of Fe and FeO.
Figure 12.
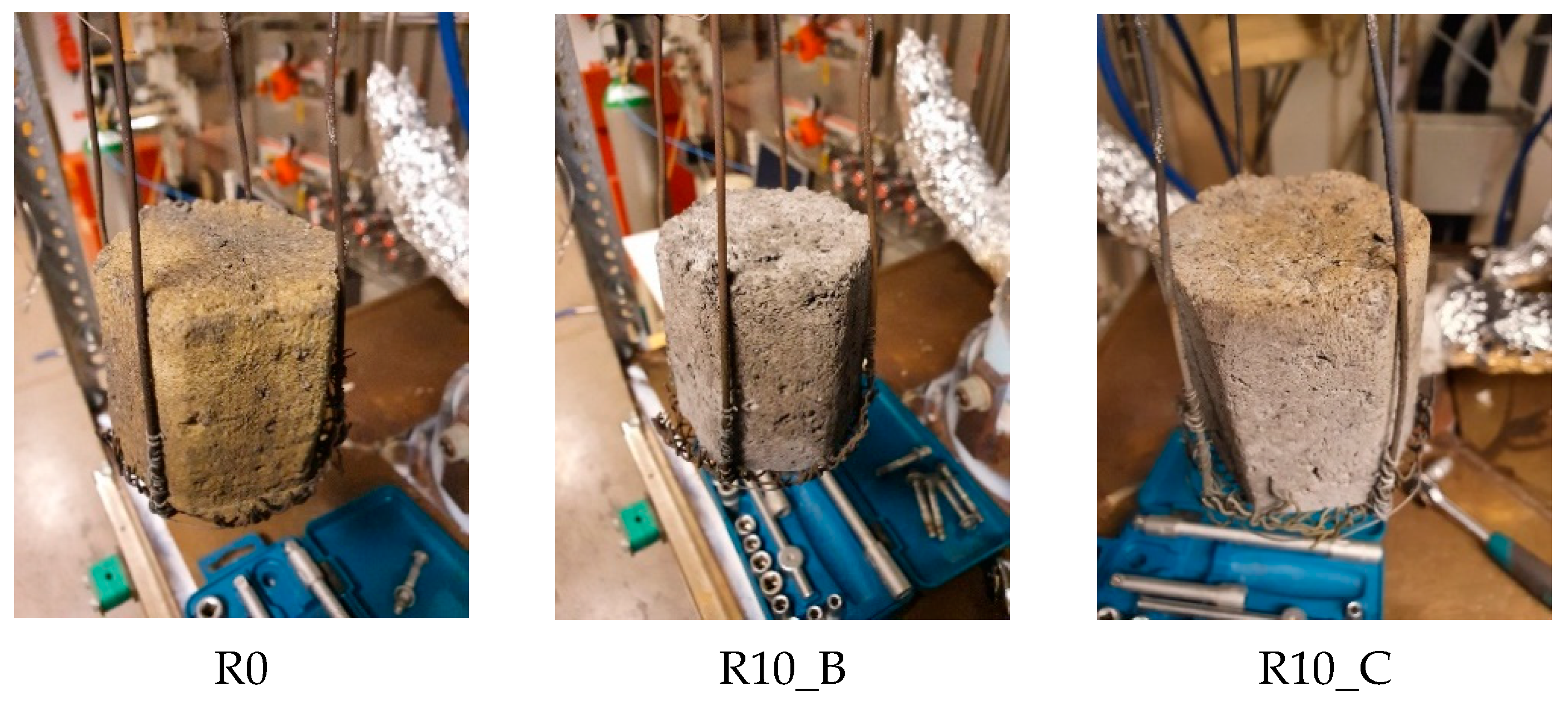
Typical pictures of the studied briquettes after heating in inert atmosphere up to 1100 °C.
Figure 13.
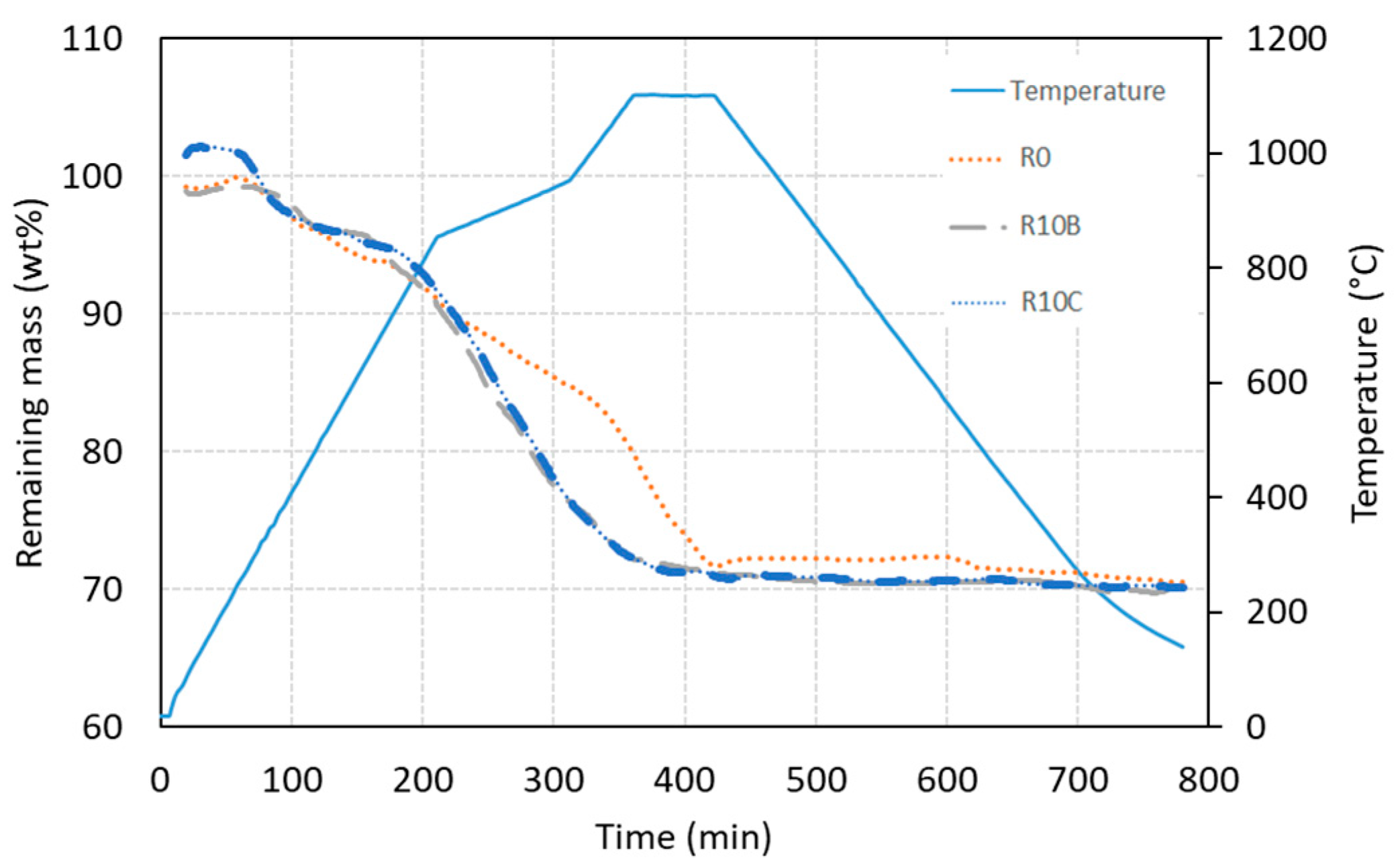
The remaining mass loss of the briquettes as a function of time and temperature.
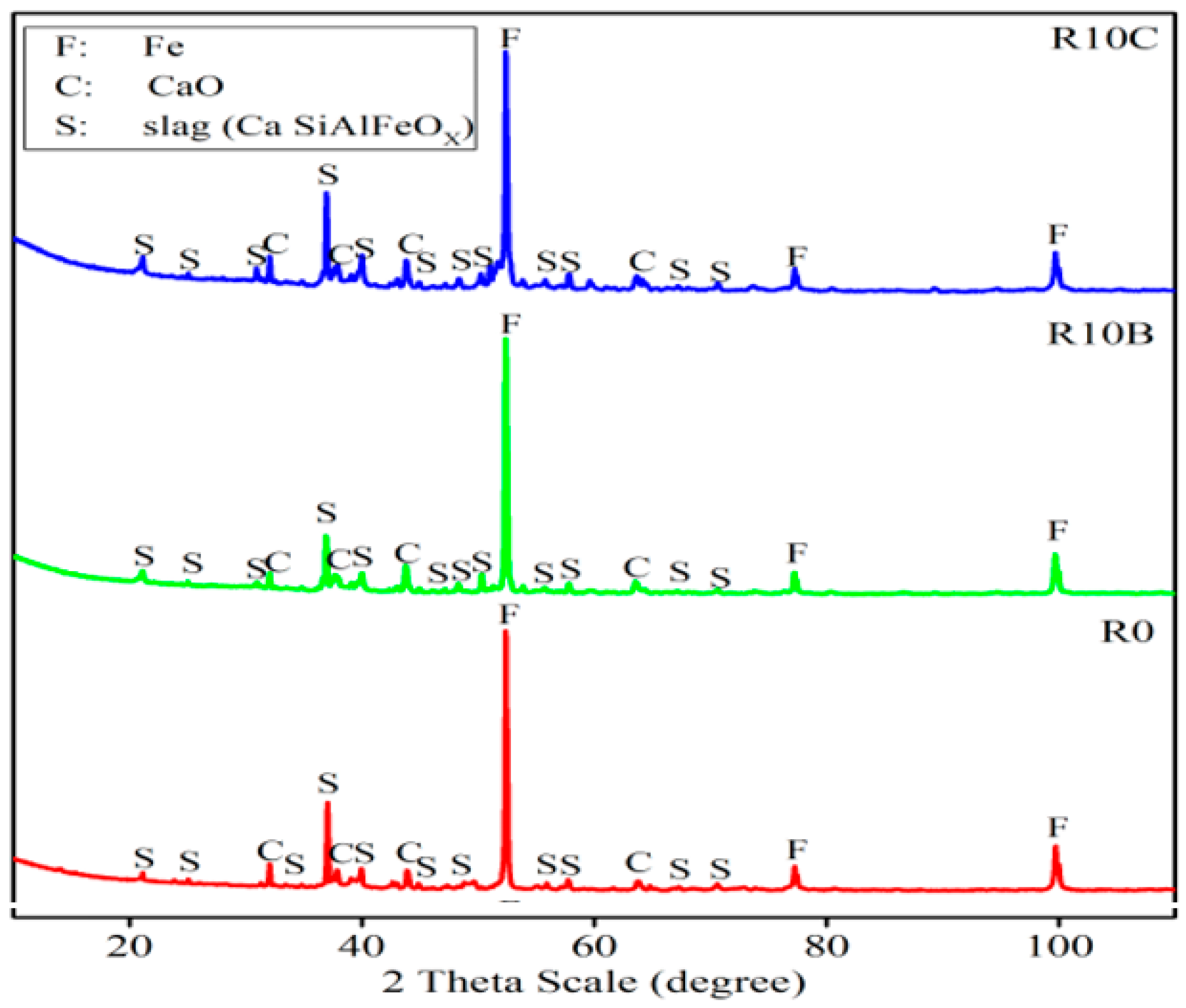
Figure 14.
XRD patterns of the reduced briquettes.
Figure 15.
Pictures of the arrested briquettes (R0) at predefined temperatures.
Figure 16.
Pictures of the arrested briquettes (R10B) at predefined temperatures.
Figure 17.
Allocation of the mass loss values of arrested experiments on the mass loss curve of the full-length experiment, (a) R0 and (b) R10B.
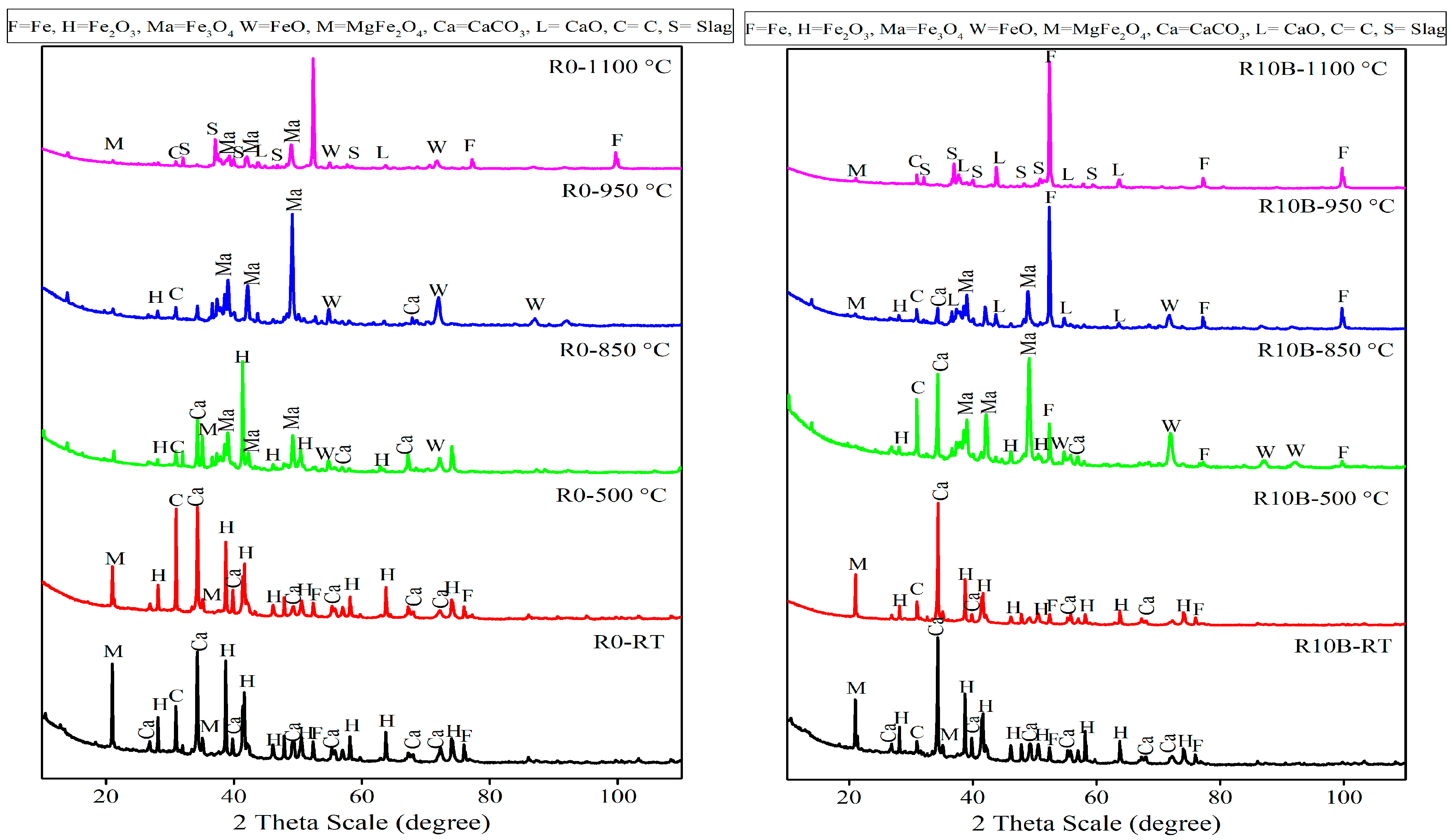
Figure 18.
XRD patterns of the arrested briquettes produced according to R0 and R10B recipes.
Table 1.
Constituents of in-plant fines mixtures with 0, 5, or 10 wt.% of biochar added.
| Components | Pre-Mix. Mixtures | Filter Dust Mixtures | Blast Furnace Dust Mixture |
|---|
| Pre-mix, g | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Filter dust, g | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| BF dust, g | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| Pellet fines, g | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 2 |
| Coke fines, g | 0.04 | 0.04 | 0.04 | 0.11 | 0.11 | 0.11 | 0 | 0 | 0 |
| Biochar, wt.% | 0 | 5% | 10% | 0 | 5% | 10% | 0 | 5% | 10% |
| Biochar, g | 0 | 0.05 | 0.10 | 0 | 0.06 | 0.11 | 0 | 0.15 | 0.3 |
| C/O molar ratio | 0.50 | 0.81 | 1.11 | 0.50 | 0.74 | 0.98 | 0.50 | 0.73 | 0.96 |
Table 2.
The corresponding recipes of the selected briquettes for lab-scale reduction.
| Recipe No. | Cement, wt.% | Biochar, wt.% | Pre-Mix, wt.% | BF Dust, wt.% | Filter Dust, wt.% |
|---|
| R0 | 12.5 | 0.0 | 73.5 | 9.6 | 4.4 |
| R10B | 12.5 | 10.0 * | 65.1 | 8.5 | 3.9 |
| R10C | 12.5 | 10.0 ** | 65.1 | 8.5 | 3.9 |
Table 3.
Proximate and ultimate analyses as well as the high heating value of carbon-bearing materials. All in wt.%, and the HHV is in MJ/kg.
| Raw Materials | Temperature | C | H | N | Ash | (VM) | Fix-C | HHV |
|---|
| Pine sawdust (PS) | 600 °C | 83.40 | 3.30 | 0.70 | 1.10 | 23.80 | 75.00 | 32.7 |
| Birch wood chips (BWC) | 600 °C | 93.00 | 1.70 | 0.80 | 2.50 | 9.70 | 87.80 | 33.6 |
| Pine bark wood chips (PB) | 600 °C | 89.60 | 1.60 | 0.70 | 4.10 | 11.40 | 84.50 | 32.5 |
| Pine contorta wood chips (CWC) | 600 °C | 88.70 | 1.70 | 0.80 | 2.50 | 8.70 | 88.80 | 29.4 |
| Coke | | | | | 11.80 | 0.70 | 79.20 | |
Table 4.
Chemical composition of the carbon-bearing materials’ ash, in wt.%.
| Elements | PS | BWC | PB | CWC | Coke |
|---|
| Al2O3 | 0.21 | 0.34 | 4.05 | 0.62 | 30.94 |
| CaO | 3.67 | 6.06 | 16.10 | 4.34 | 1.32 |
| Fe2O3 | 2.25 | 8.21 | 4.23 | 10.21 | 6.21 |
| K2O | 2.16 | 3.86 | 5.81 | 3.12 | 2.01 |
| MgO | 0.95 | 1.89 | 2.16 | 1.66 | 0.84 |
| MnO | 0.45 | 0.35 | 0.83 | 0.72 | 0.40 |
| Na2O | 0.05 | 0.10 | 0.47 | 0.10 | 0.75 |
| P2O5 | 0.43 | 2.02 | 2.45 | 0.80 | 0.46 |
| S | 0.05 | 0.15 | 0.19 | 0.11 | 5.84 |
| SiO2 | 0.33 | 1.60 | 3.55 | 1.68 | 67.64 |
| Zn | 0.05 | 0.16 | 0.10 | 0.03 | --- |
| Basicity | 11.12 | 3.79 | 4.54 | 2.58 | 0.02 |
Table 5.
Chemical composition of in-plant fines, in wt.%.
| | Pre-Mix (PM) | Filter Dust (FD) | BF Dust (BFD) | Pellet Fines (PF) |
|---|
| Fe total | 46.34 | 63.30 | 39.60 | 68.5 |
| CaO | 19.88 | 0.92 | 4.84 | 0.50 |
| SiO2 | 6.10 | 2.26 | 3.67 | 2.80 |
| MnO | 1.64 | 0.11 | 0.82 | 0.10 |
| P2O5 | 0.17 | 0.01 | 0.05 | 0.033 |
| Al2O3 | 2.14 | 0.46 | 1.32 | 0.60 |
| MgO | 2.39 | 1.43 | 1.56 | 1.50 |
| Na2O | 0.08 | 0.03 | 0.04 | 0.07 |
| K2O | 0.08 | 0.04 | 0.12 | 0.08 |
| V2O5 | 1.32 | 0.24 | 0.38 | 0.24 |
| TiO2 | 0.71 | 0.25 | 0.28 | 0.31 |
| Cr2O3 | 0.13 | 0.03 | 0.05 | 0.03 |
| LoI | 1.00 | −1.80 | −30.10 | |
| Carbon | 4.61 | 1.12 | 28.40 | 0.50 |
| Sulfur | 0.43 | 0.05 | 0.26 | 0.01 |
Table 6.
Calculated alkali index and observed, maximum, reaction rates at 850–950 °C.
| Biochar Origin | Alkali Index | Reaction Rate (%/min) |
|---|
| Birch wood chips | 20 | 0.34 |
| Pine bark wood chips | 18 | 0.38 |
| Pine Saw dust | 19 | 0.47 |
| Pine contorta wood chips | 10 | 0.31 |
Table 7.
List of in-plant fines samples, their constituent materials, the corresponding C/O molar ratio, and bulk density.
| | Biochar | Pre-Mix | Pellet Fines | Coke Fines | C/O | Bulk Density |
|---|
| Filter dust | | | | | | |
| No Biochar | 0.00 g | 1.00 g | --- | 0.11 g | 0.5 | 2.77 g/cm3 |
| 5% Biochar | 0.05 g | 1.00 g | --- | 0.11 g | 0.7 | 2.24 g/cm3 |
| 10% Biochar | 0.11 g | 1.00 g | --- | 0.11 g | 1.0 | 2.14 g/cm3 |
| BF dust | | | | | | |
| No Biochar | 0.00 g | 0.33 g | 0.67 | --- | 0.5 | 2.42 g/cm3 |
| 5% Biochar | 0.05 g | 0.33 g | 0.67 | --- | 0.7 | 2.30 g/cm3 |
| 10% Biochar | 0.10 g | 0.33 g | 0.67 | --- | 1.0 | 2.03 g/cm3 |
| Pre-mix | | | | | | |
| No Biochar | 0.00 g | 1.00 g | --- | 0.036 g | 0.5 | 2.22 g/cm3 |
| 5% Biochar | 0.05 g | 1.00 g | --- | 0.036 g | 0.8 | 1.90 g/cm3 |
| 10% Biochar | 0.10 g | 1.00 g | --- | 0.036 g | 1.1 | 1.74 g/cm3 |
Table 8.
Actual mass loss from arrested experiments and calculated values based on the individual contribution of constituent by-products.
| | 500 °C | 850 °C | 950 °C | 1100 °C |
|---|
| R0, actual | 3.6 wt.% | 13.9 wt.% | 14.4 wt.% | 23 wt.% |
| R0, calculated | 5.7 wt.% | 11.9 wt.% | 20 wt.% | 24.3 wt.% |
| R10B, actual | 4.1 wt.% | 14 wt.% | 21.3 wt.% | 28.2 wt.% |
| R10B, calculated | 5.9 wt.% | 16.5 wt.% | 23.2 wt.% | 26.8 wt.% |