Dissimilar Resistance Spot Weld of Ni-Coated Aluminum to Ni-Coated Magnesium Using Cold Spray Coating Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. The Materials
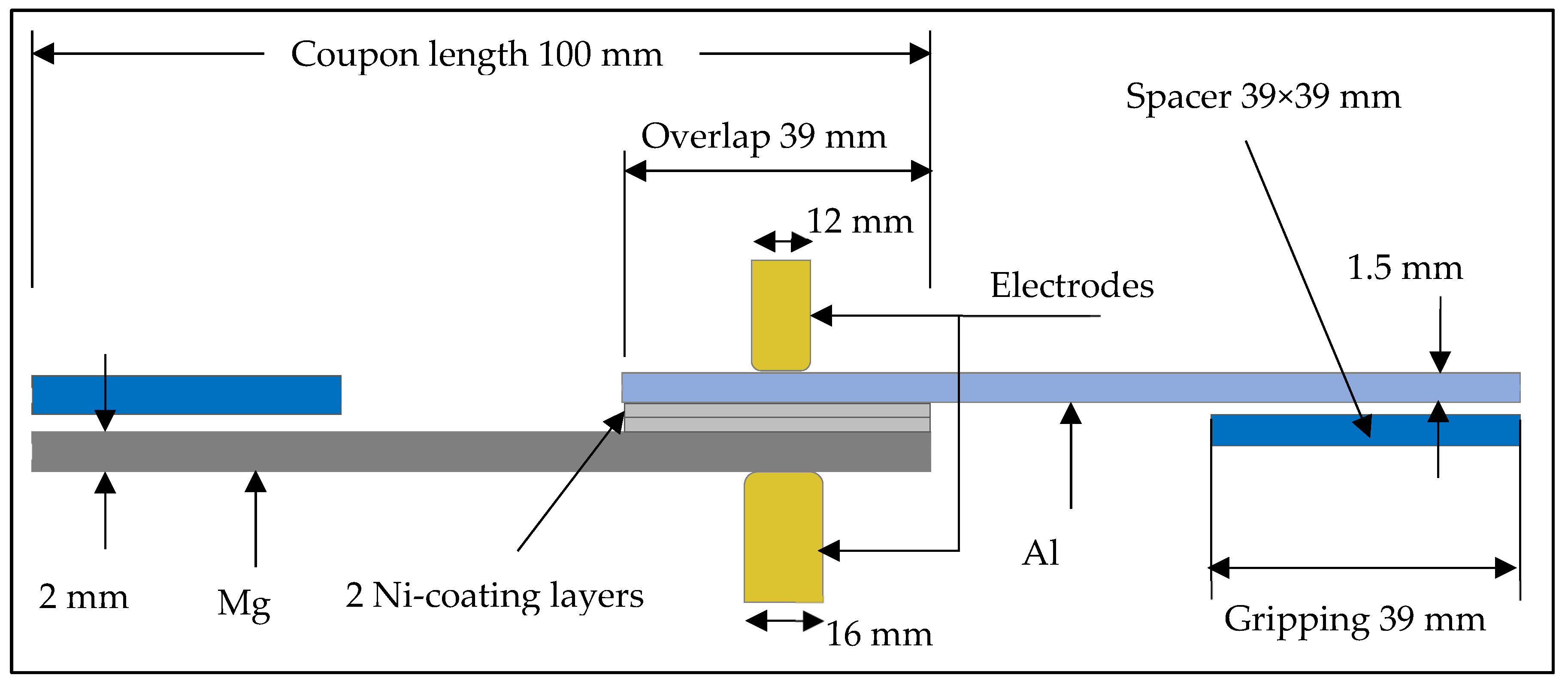
2.2. Methods
3. Results and Discussion
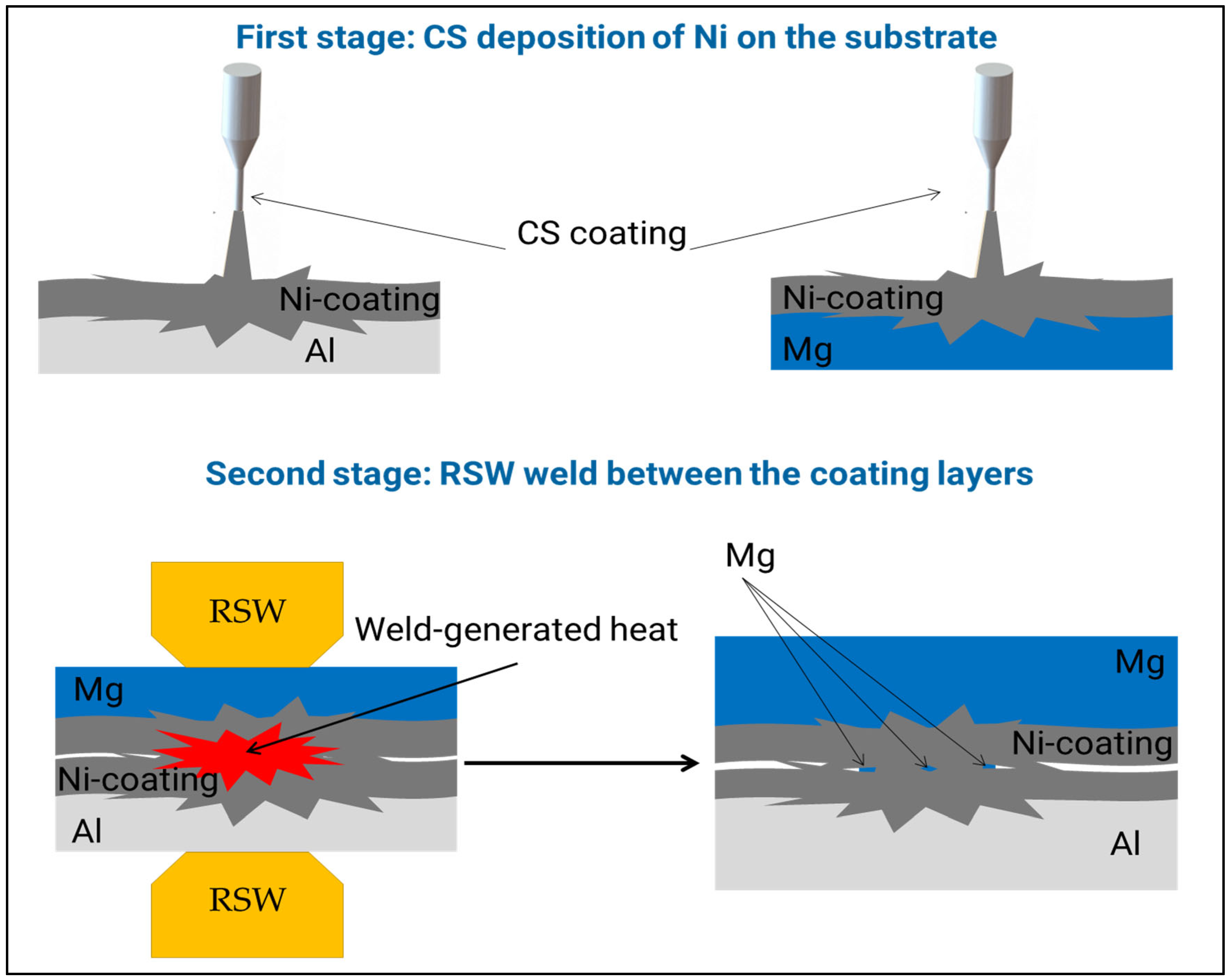
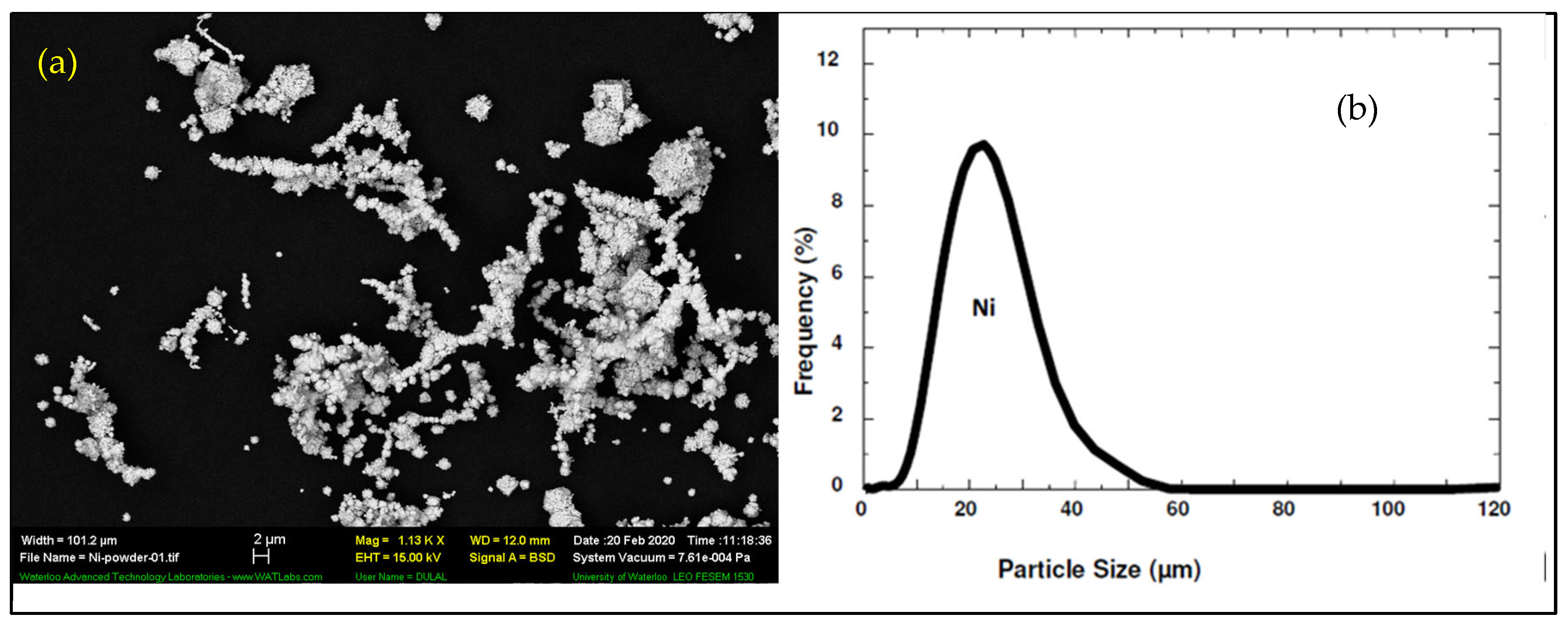
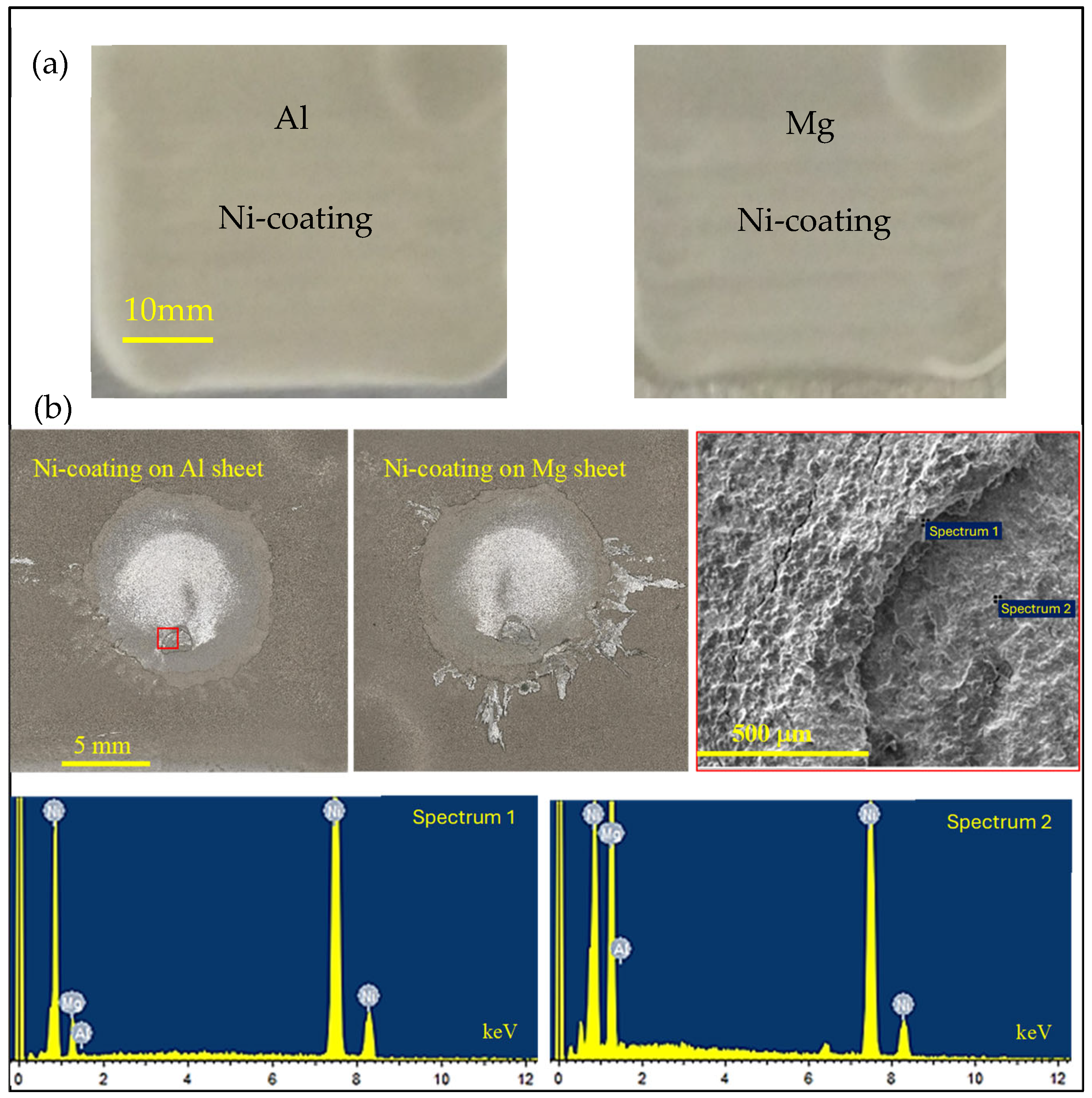
3.1. Cold Spray Coating
3.2. Resistance Spot Weld
3.3. Tensile Test
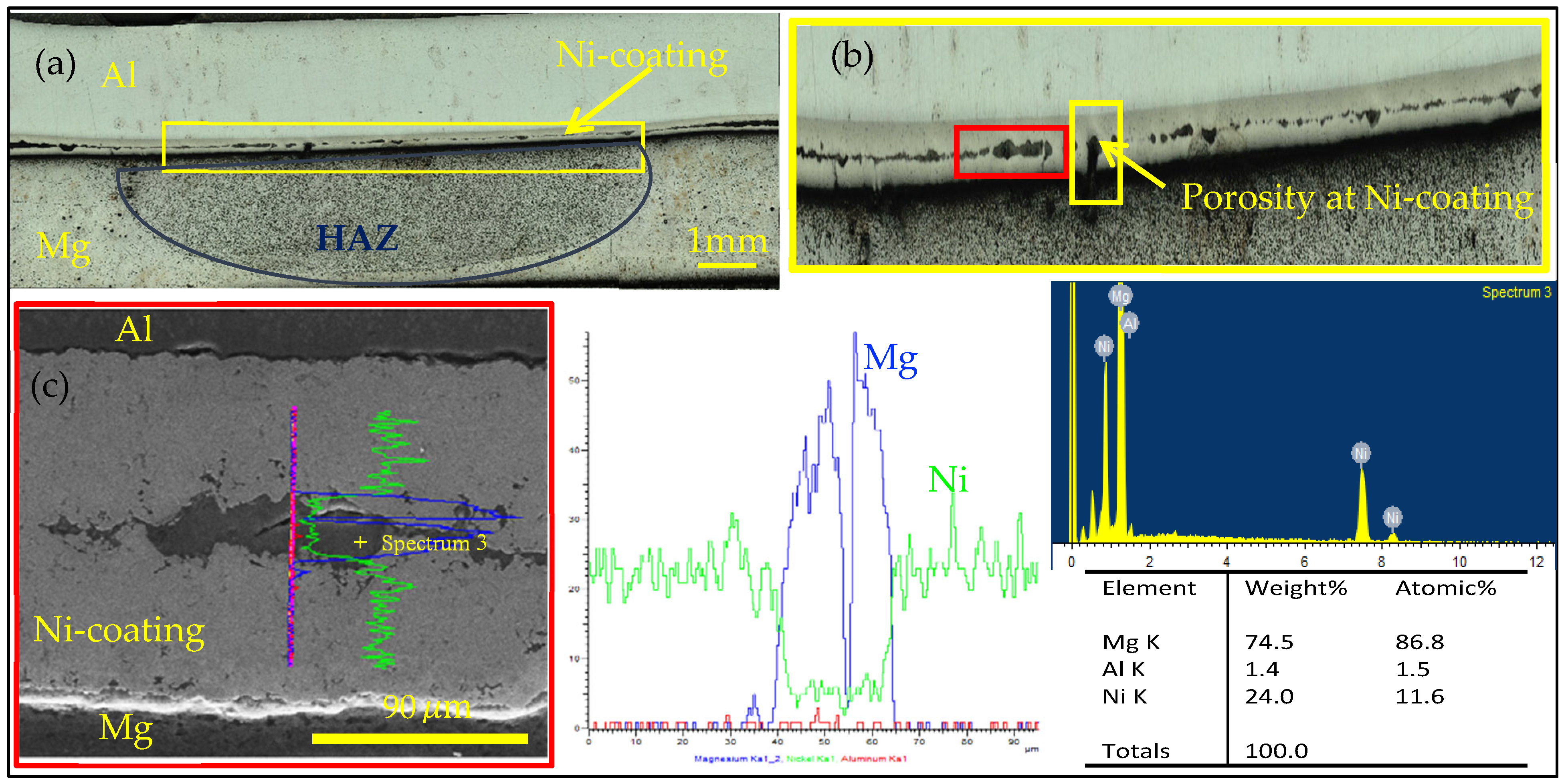
3.4. Microstructure Characterization
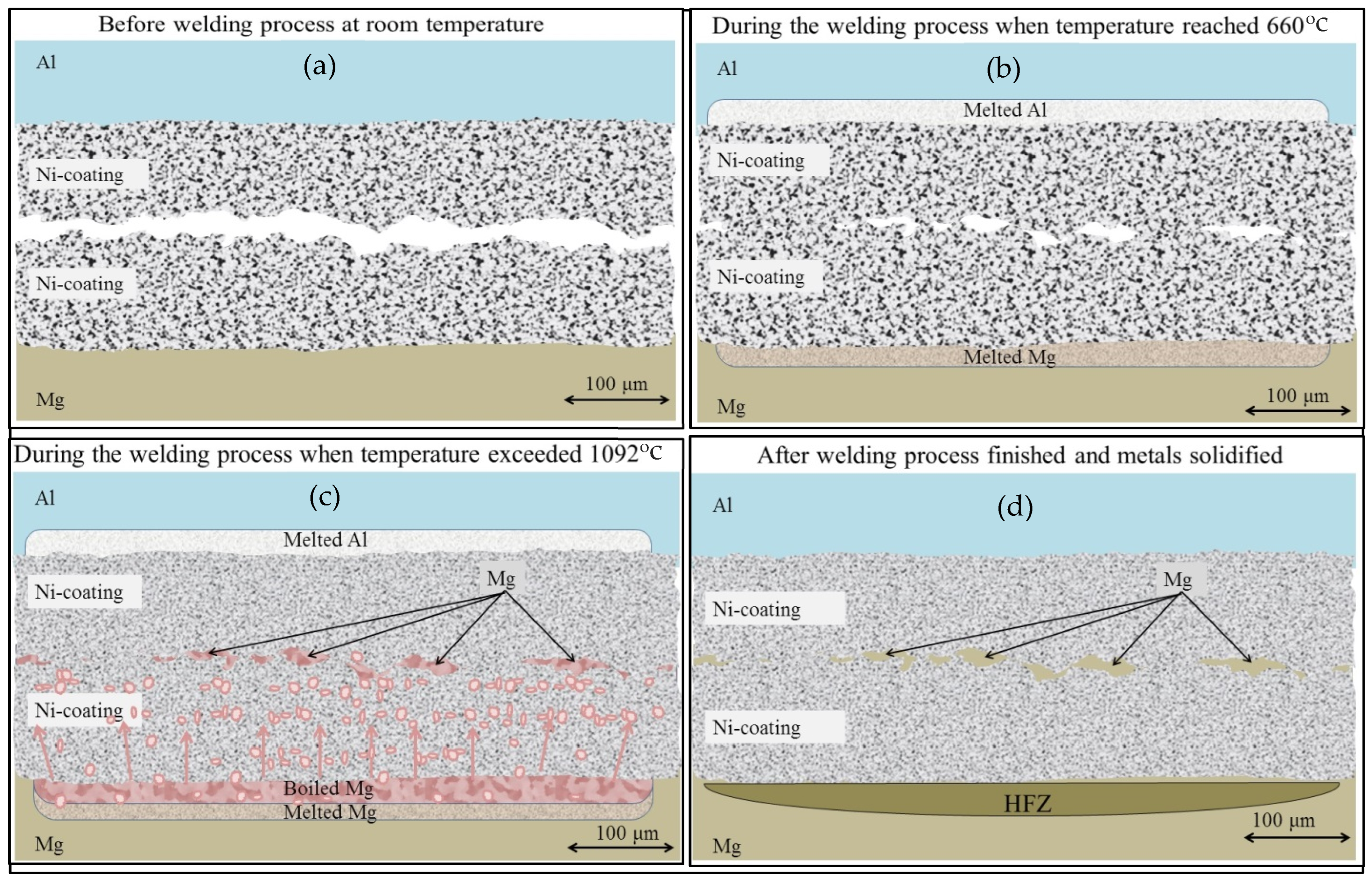
3.5. Joining Mechanism
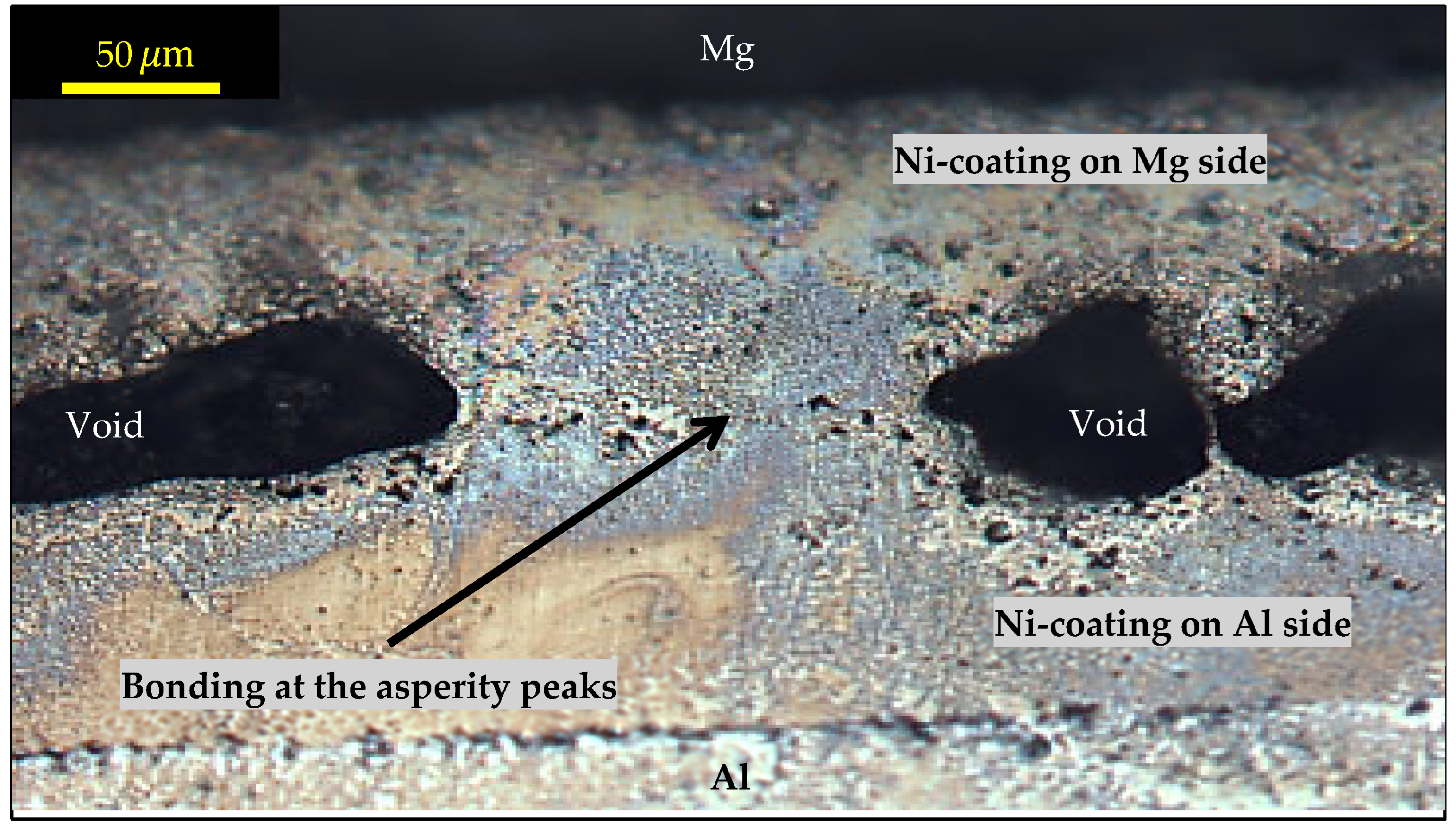
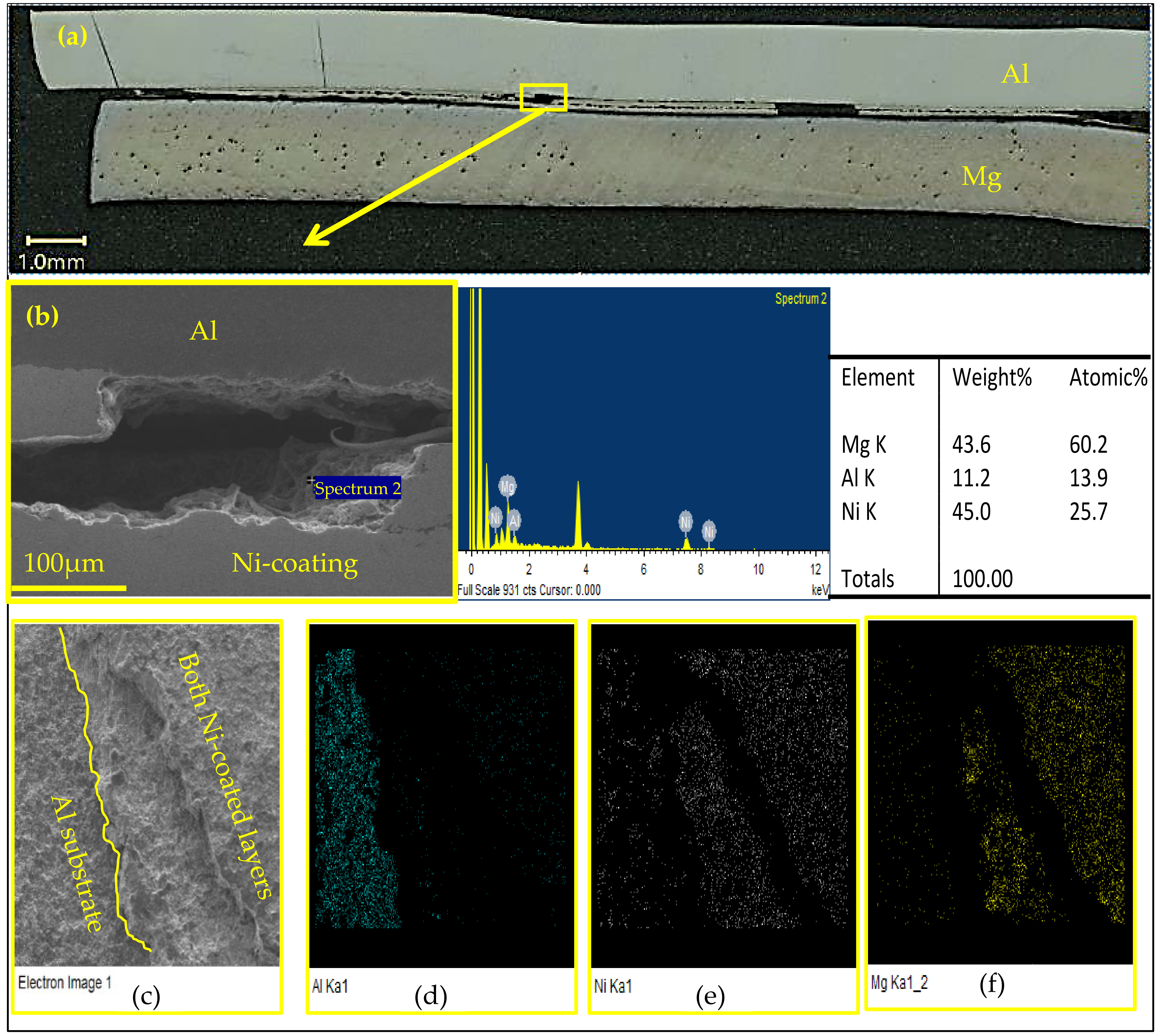
3.6. Fracture Mechanism
4. Conclusions
- Ni-coated layer successfully prevented the direct interaction between the Al and Mg after the welding process, and the formation of undesirable Al/Mg IMCs.
- The peak load of 4.2 kN was achieved in the tensile lap-shear test of Al/Mg RSW samples with cold-sprayed Ni-coating interlayer, which meets the required load capacity based on AWS standards for similar sheet joints.
- The mechanism of Mg penetrating into the Ni/Ni weld region was established. It was shown that, due to excessive local heat, vaporized Mg found its way through the coating porosity.
- The fracture mechanism of joints during mechanical testing was attributed to the roughness of the coated Ni layer, which caused voids/porosity at the weld surface, acting as the stress concentration area, in addition to the presence of porosity within the interlayer, which contained Mg that migrated from the sheet during welding.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alkhimov, A.P.; Kosarev, V.F.; Papyrin, A.N. A method of “cold” gas-dynamic spraying. Dokl. Akad. Nauk. SSSR 1990, 315, 1062–1065. [Google Scholar]
- Alkimov, A.N.P.A.P.; Kosarev, V.F.; Nesterovich, N.I. Method of Applying Coatings. Russian Patent No. 1,618,778; SU 1618778, 7 January 1991. [Google Scholar]
- Hassani-Gangaraj, M.; Veysset, D.; Nelson, K.A.; Schuh, C.A. In-situ observations of single micro-particle impact bonding. Scr. Mater. 2018, 145, 9–13. [Google Scholar] [CrossRef]
- Meng, F.; Yue, S.; Song, J. Quantitative prediction of critical velocity and deposition efficiency in cold-spray: A finite-element study. Scr. Mater. 2015, 107, 83–87. [Google Scholar] [CrossRef]
- Hassani-Gangaraj, M.; Veysset, D.; Nelson, K.A.; Schuh, C.A. Melting Can Hinder Impact-Induced Adhesion. Phys. Rev. Lett. 2017, 119, 175701. [Google Scholar] [CrossRef] [PubMed]
- Grujicic, M.; Zhao, C.L.; DeRosset, W.S.; Helfritch, D. Adiabatic shear instability based mechanism for particles/substrate bonding in the cold-gas dynamic-spray process. Mater. Des. 2004, 25, 681–688. [Google Scholar] [CrossRef]
- Assadi, H.; Schmidt, T.; Richter, H.; Kliemann, J.-O.; Binder, K.; Gärtner, F.; Klassen, T.; Kreye, H. On parameter selection in cold spraying. J. Therm. Spray Technol. 2011, 20, 1161–1176. [Google Scholar] [CrossRef]
- Yin, S.; Wang, X.; Suo, X.; Liao, H.; Guo, Z.; Li, W.; Coddet, C. Deposition behavior of thermally softened copper particles in cold spraying. Acta Mater. 2013, 61, 5105–5118. [Google Scholar] [CrossRef]
- Yin, S.; Meyer, M.; Li, W.; Liao, H.; Lupoi, R. Gas Flow, Particle Acceleration, and Heat Transfer in Cold Spray: A review. J. Therm. Spray Technol. 2016, 25, 874–896. [Google Scholar] [CrossRef]
- Moridi, A.; Hassani-Gangaraj, S.M.; Guagliano, M.; Dao, M. Cold spray coating: Review of material systems and future perspectives. Surf. Eng. 2014, 30, 369–395. [Google Scholar] [CrossRef]
- Villafuerte, J. Modern Cold Spray: Materials, Process, and Applications, 1st ed.; Springer International Publishing: Windsor, ON, Canada, 2015. [Google Scholar] [CrossRef]
- Lara, B.; Giorjao, R.; Ramirez, A. Resistance spot welding of printed interlayers to join Al–Fe sheets. Sci. Technol. Weld. Join. 2023, 28, 18–26. [Google Scholar] [CrossRef]
- Szwajka, K.; Zielińska-Szwajka, J.; Szewczyk, M.; Mezher, M.T.; Trzepieciński, T. Analysis of the Microstructure and Mechanical Performance of Resistance Spot-Welding of Ti6Al4V to DP600 Steel Using Copper/Gold Cold-Sprayed Interlayers. Materials 2024, 17, 3251. [Google Scholar] [CrossRef]
- Hagen, C.; Klinkenberg, F.-J.; Ossenbrink, R.; Michailov, V. Resistance spot welding of dissimilar material joints with a cold-gas-sprayed inlayer. Int. J. Adv. Manuf. Technol. 2023, 127, 5679–5690. [Google Scholar] [CrossRef]
- Liu, L.; Ren, D.; Liu, F. A review of dissimilar welding techniques for magnesium alloys to aluminum alloys. Materials 2014, 7, 3735–3757. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, Z.; Li, Y.; Liu, Z.M.; Huang, Z.Y. Microstructure characterization and tensile properties of Mg/Al dissimilar joints manufactured by thermo-compensated resistance spot welding with Zn interlayer. Mater. Des. 2015, 75, 166–173. [Google Scholar] [CrossRef]
- Penner, P.; Liu, L.; Gerlich, A.; Zhou, Y. Feasibility study of resistance spot welding of dissimilar Al/Mg combinations with Ni based interlayers. Sci. Technol. Weld. Join. 2013, 18, 541–550. [Google Scholar] [CrossRef]
- Penner, P.; Liu, L.; Gerlich, A.; Zhou, Y. Dissimilar resistance spot welding of aluminum to magnesium with Zn-coated steel interlayers. Weld. J. 2014, 93, 225–231. [Google Scholar]
- Sun, M.; Niknejad, S.T.; Zhang, G.; Lee, M.K.; Wu, L.; Zhou, Y. Microstructure and mechanical properties of resistance spot welded AZ31/AA5754 using a nickel interlayer. Mater. Des. 2015, 87, 905–913. [Google Scholar] [CrossRef]
- Sun, M.; Niknejad, S.T.; Gao, H.; Wu, L.; Zhou, Y. Mechanical properties of dissimilar resistance spot welds of aluminum to magnesium with Sn-coated steel interlayer. Mater. Des. 2016, 91, 331–339. [Google Scholar] [CrossRef]
- Sun, M.; Behravesh, S.B.; Wu, L.; Zhou, Y.; Jahed, H. Fatigue behaviour of dissimilar Al 5052 and Mg AZ31 resistance spot welds with Sn-coated steel interlayer. Fatigue Fract. Eng. Mater. Struct. 2017, 40, 1048–1058. [Google Scholar] [CrossRef]
- Manladan, S.M.; Yusof, F.; Ramesh, S.; Fadzil, M. A review on resistance spot welding of magnesium alloys. Int. J. Adv. Manuf. Technol. 2016, 86, 1805–1825. [Google Scholar] [CrossRef]
- Cooke, K.O.; Khan, T.I. Resistance spot welding aluminium to magnesium using nanoparticle reinforced eutectic forming interlayers. Sci. Technol. Weld. Join. 2018, 23, 271–278. [Google Scholar] [CrossRef]
- Panteli, A.; Chen, Y.-C.; Strong, D.; Zhang, X.; Prangnell, P.B. Optimization of aluminium-to-magnesium ultrasonic spot welding. JOM 2012, 64, 414–420. [Google Scholar] [CrossRef]
- Panteli, A.; Robson, J.D.; Chen, Y.C.; Prangnell, P.B. The effectiveness of surface coatings on preventing interfacial reaction during ultrasonic welding of aluminum to magnesium. Metall. Mater. Trans. A 2013, 44, 5773–5781. [Google Scholar] [CrossRef]
- Hou, W.; Oheil, M.; Shen, Z.; Shen, Y.; Jahed, H.; Gerlich, A. Enhanced strength and ductility in dissimilar friction stir butt welded Al/Cu joints by addition of a cold-spray Ni interlayer. J. Manuf. Process. 2020, 60, 573–577. [Google Scholar] [CrossRef]
- Hou, W.; Shen, Z.; Huda, N.; Oheil, M.; Shen, Y.; Jahed, H.; Gerlich, A.P. Enhancing metallurgical and mechanical properties of friction stir butt welded joints of Al–Cu via cold sprayed Ni interlayer. Mater. Sci. Eng. A 2021, 809, 140992. [Google Scholar] [CrossRef]
- Dong, S.-K.; Lin, S.; Zhu, H.; Wang, C.-J.; Cao, Z.-L. Effect of Ni interlayer on microstructure and mechanical properties of Al/Mg dissimilar friction stir welding joints. Sci. Technol. Weld. Join. 2022, 27, 103–113. [Google Scholar] [CrossRef]
- CenterLine (Windsor) Limited, Supersonic Spray Technologies Div. SST-TDS-N5001-PR-2.0-0120, Technical Data Sheet: SST-N5001 Powder, Release Date: January 2020. Available online: https://www.supersonicspray.com/uploads/documents/SST-TDS-N5001-PR-2_0-0120.pdf (accessed on 15 August 2025).
- Özbilen, S.; Vasquez, J.F.B.; Abbott, W.M.; Yin, S.; Morris, M.; Lupoi, R. Mechanical milling/alloying, characterization and phase formation prediction of Al0.1–0.5(Mn)CoCrCuFeNi-HEA powder feedstocks for cold spray deposition processing. J. Alloys Compd. 2023, 961, 170854. [Google Scholar] [CrossRef]
- Kang, J.; McDermid, J.R.; Bruhis, M. Determination of the constitutive behaviour of AA6022-T4 aluminium alloy spot welds at large strains. Mater. Sci. Eng. A 2013, 567, 95–100. [Google Scholar] [CrossRef]
- Giraud, E.; Rossi, F.; Germain, G.; Outeiro, J.C. Constitutive modelling of AZ31B-O magnesium alloy for cryogenic machining. Procedia CIRP 2013, 8, 522–527. [Google Scholar] [CrossRef]
- Tang, Q.; Veysset, D.; Assadi, H.; Ichikawa, Y.; Hassani, M. Strength gradient in impact-induced metallic bonding. Nat. Commun. 2024, 15, 9630. [Google Scholar] [CrossRef]
- Assadi, H.; Gärtner, F.; Stoltenhoff, T.; Kreye, H. Bonding mechanism in cold gas spraying. Acta Mater. 2003, 51, 4379–4394. [Google Scholar] [CrossRef]
- Ito, K. Mechanistic Analysis of Cold Sprayed Particle Deposition Behavior by Single Particle Impact Testing System and Improvement of Coating Properties. Ph.D. Thesis, Tohoku University, Sendai, Japan, 12 January 2016. [Google Scholar]

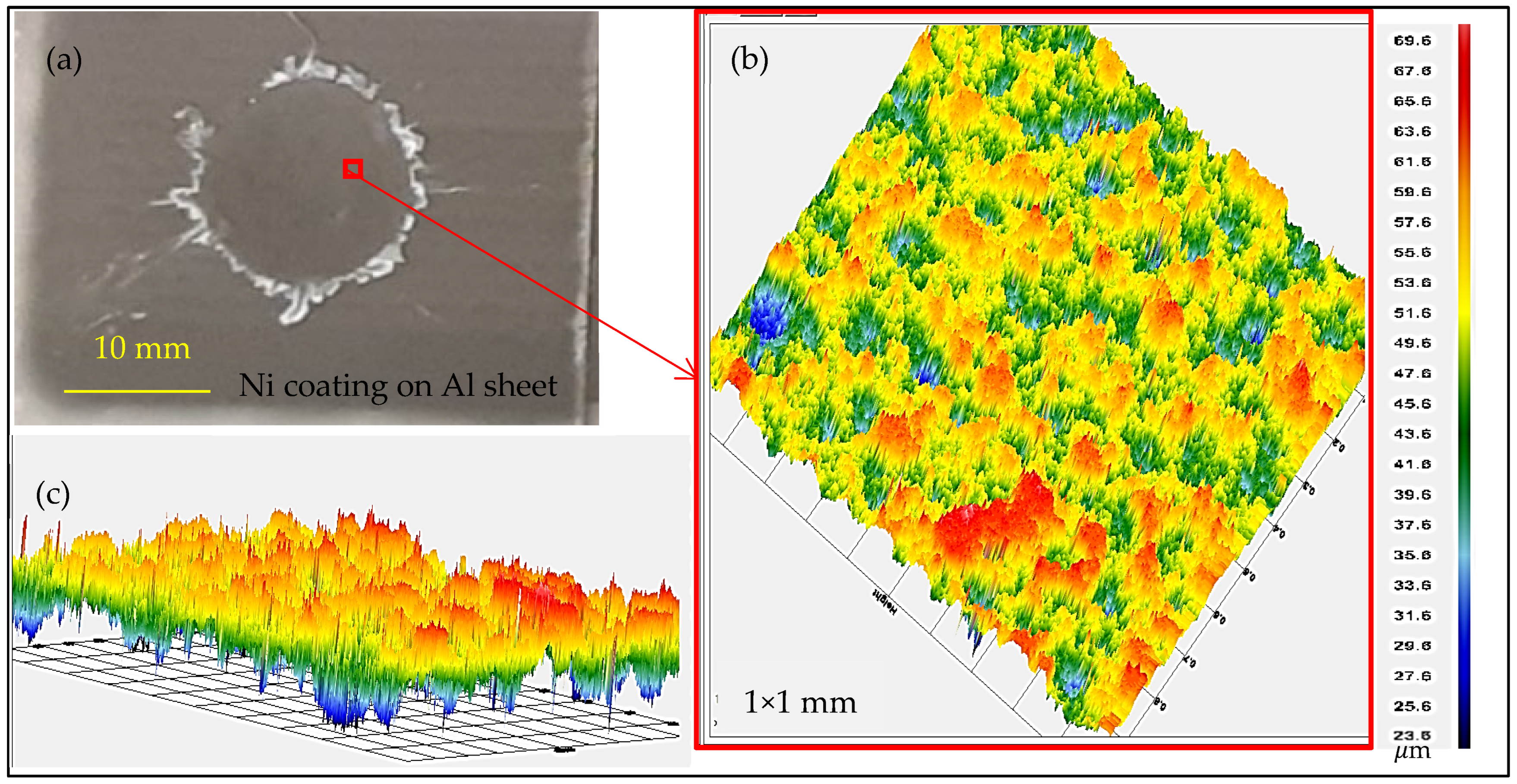
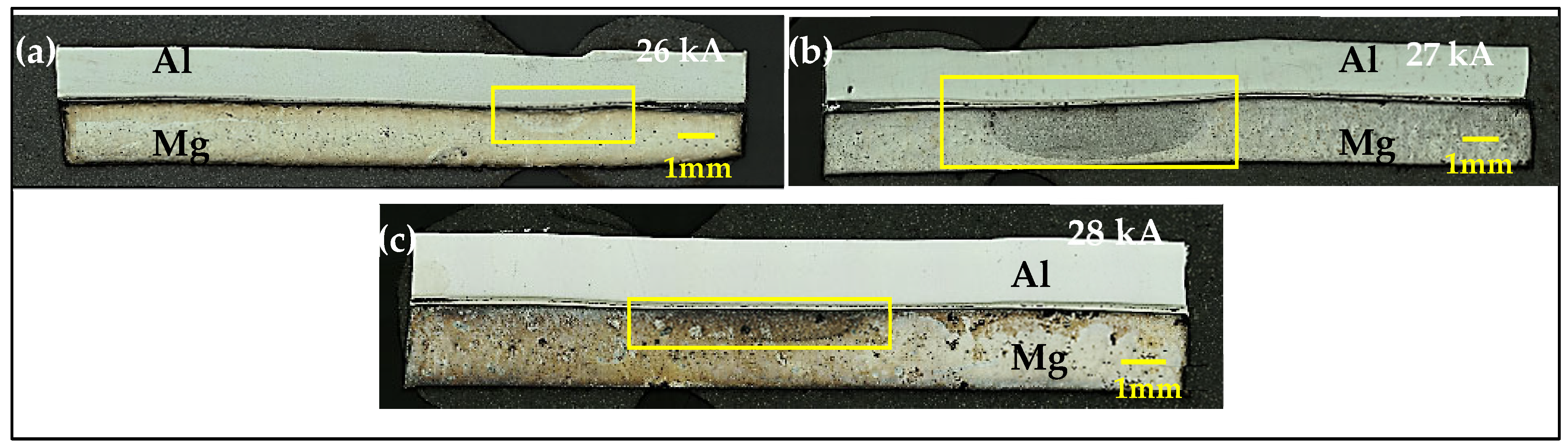
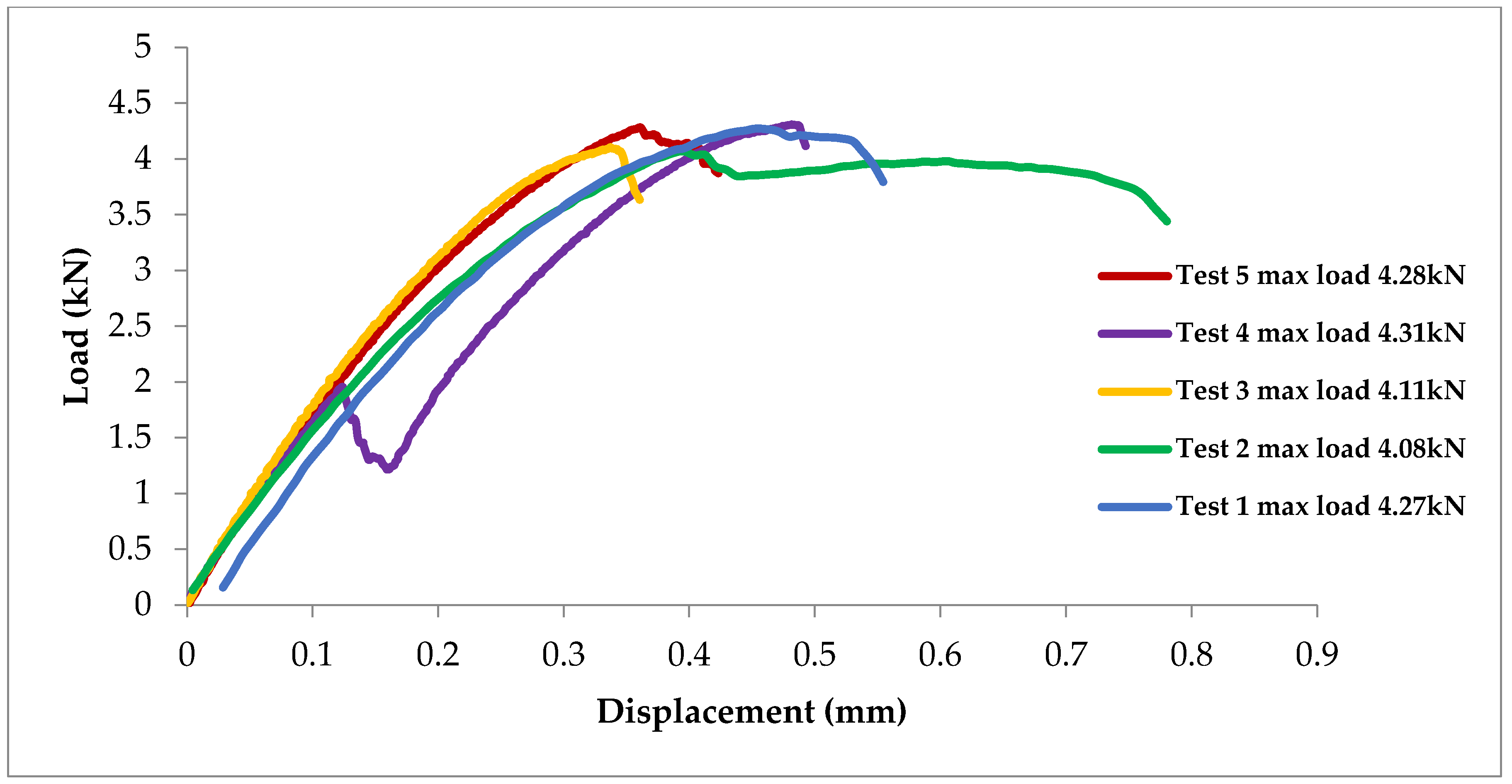

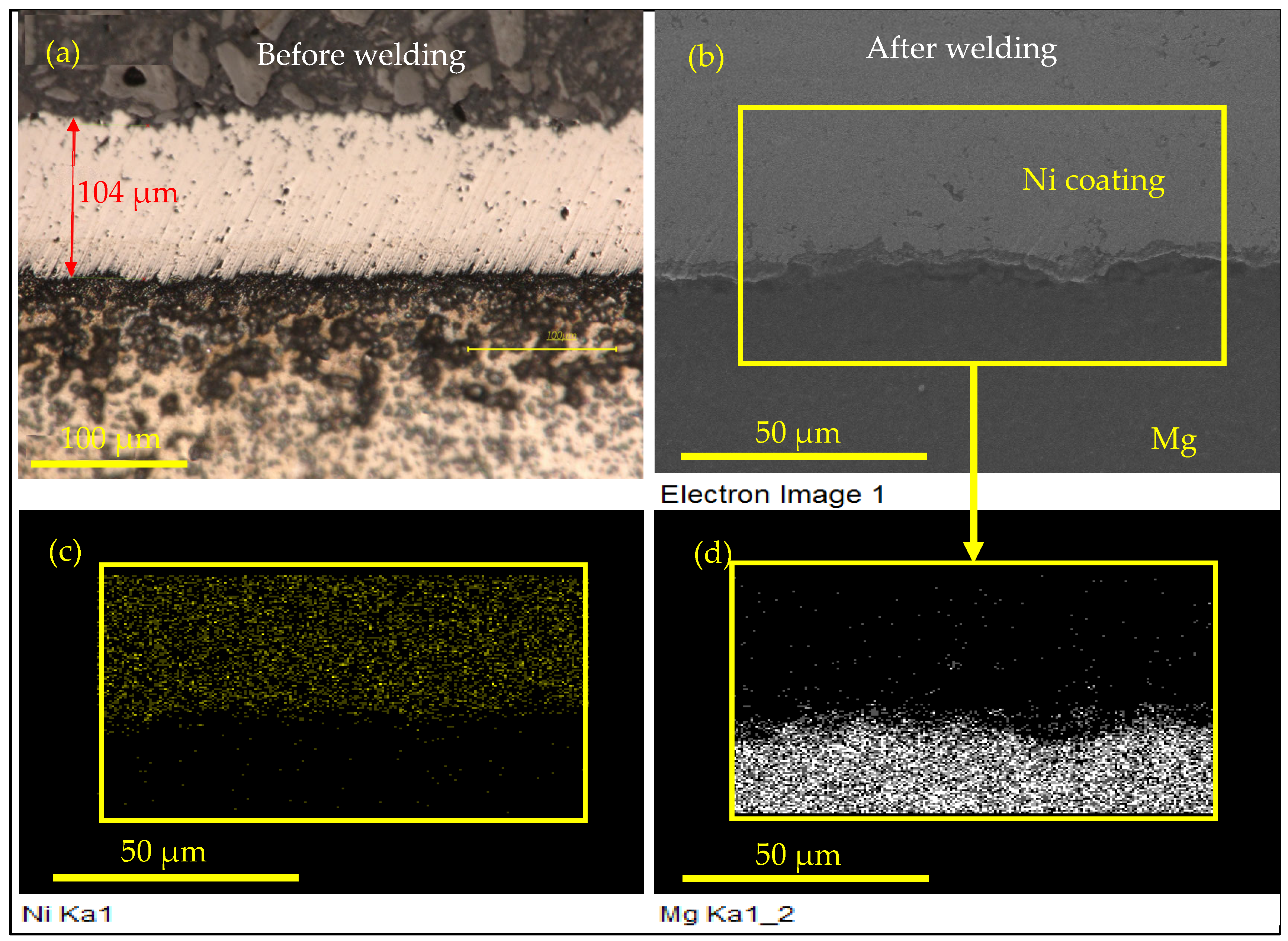
| Weld Process | Sheet Materials | Interlayer Material | Interlayer Type | Strength (kN) | Ref. |
|---|---|---|---|---|---|
| Conventional RSW | AZ31B Mg/Al 5754 | Pure nickel | Insert foil | no bonding | [17] |
| Conventional RSW | AZ31B Mg/Al 5754 | Pure nickel | Insert foil | 5.1 | [19] |
| Conventional RSW | AZ31B Mg/Al 5754 | Gold-coated nickel foil | Insert foil | 4.69 | [17] |
| Conventional RSW | AZ31B Mg/Al 5754 | Zinc-coated HSLA steel | Insert foil | 3.86 | [18] |
| Conventional RSW | AZ31B Mg/Al 5754 | (1) Ni (2) Ni-TiO3 (3) Ni-Al2O3 | Electrodeposit | (1) 0.45 (2) 0.48 (3) 0.52 | [23] |
| Conventional RSW | AZ31B Mg/Al 5052-H12 | Zinc interlayer | Insert foil | 0.727 | [16] |
| Thermo-compensated RSW | AZ31B Mg/Al 5052-H12 | Zinc interlayer | Insert foil | 2.199 | [16] |
| Conventional RSW | AZ31B Mg/Al 5052-H12 | No interlayer | No interlayer | 0.833 | [16] |
| Al 6022-T4 (wt. %) | ||||||||
| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Al |
| 0.8–1.5 | 0.05–0.2 | 0.01–0.11 | 0.02–0.1 | 0.45–0.7 | ≤0.1 | ≤0.25 | ≤0.15 | Balance |
| Mg AZ31B-O (wt. %) | ||||||||
| Al | Zn | Mn | Si | Cu | Ca | Ni | Fe | Mg |
| 2.5–3.5 | 0.6–1.4 | ≥0.2 | ≤0.1 | ≤0.005 | ≤0.004 | ≤0.005 | ≤0.005 | balance |
| Coating Materials | Ni on Mg | Ni on Al |
|---|---|---|
| Flow Gas | N2 | N2 |
| Gas Temperature (°C) | 500 | 500 |
| Gas Pressure (MPa) | 1.5 | 1.5 |
| Powder Feed Rate (gr/min) | 8 | 8 |
| Nozzle Speed (mm/s) | 25 | 25 |
| Step Over (mm) | 2.7 | 3 |
| Stand-off Distance (mm) | 12 | 12 |
| Nozzle Length (mm) | 120 | 120 |
| Nozzle Orifice Diameter (mm) | 2 | 2 |
| Nozzle Exit Diameter (mm) | 6.3 | 6.3 |
| Type of Nozzle | de Laval UltiLifeTM | |
| Welding Current (kA) | Two Pulse Welding Time (Cycles) | Delay Time | ||
|---|---|---|---|---|
| 15 | 18 | 20 | (Cycles) | |
| 26 | Test 1 | Test 7 | Test 13 | 5 |
| 27 | Test 2 | Test 8 | Test 14 | 5 |
| 28 | Test 3 | Test 9 | Test 15 | 5 |
| 26 | Test 4 | Test 10 | Test 16 | 10 |
| 27 | Test 5 | Test 11 | Test 17 | 10 |
| 28 | Test 6 | Test 12 | Test 18 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oheil, M.; Saha, D.; Jahed, H.; Gerlich, A. Dissimilar Resistance Spot Weld of Ni-Coated Aluminum to Ni-Coated Magnesium Using Cold Spray Coating Technology. Metals 2025, 15, 940. https://doi.org/10.3390/met15090940
Oheil M, Saha D, Jahed H, Gerlich A. Dissimilar Resistance Spot Weld of Ni-Coated Aluminum to Ni-Coated Magnesium Using Cold Spray Coating Technology. Metals. 2025; 15(9):940. https://doi.org/10.3390/met15090940
Chicago/Turabian StyleOheil, Mazin, Dulal Saha, Hamid Jahed, and Adrian Gerlich. 2025. "Dissimilar Resistance Spot Weld of Ni-Coated Aluminum to Ni-Coated Magnesium Using Cold Spray Coating Technology" Metals 15, no. 9: 940. https://doi.org/10.3390/met15090940
APA StyleOheil, M., Saha, D., Jahed, H., & Gerlich, A. (2025). Dissimilar Resistance Spot Weld of Ni-Coated Aluminum to Ni-Coated Magnesium Using Cold Spray Coating Technology. Metals, 15(9), 940. https://doi.org/10.3390/met15090940






