Harnessing Hydrothermal Treatments to Control Magnesium Alloy Degradation for Bioresorbable Implants: A Comprehensive Evaluation of Bath Chemistry Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Hydrothermal Treatment Protocol
2.3. Coating Characterization
2.4. Corrosion/Degradation Assessment
2.5. Statistics
3. Results
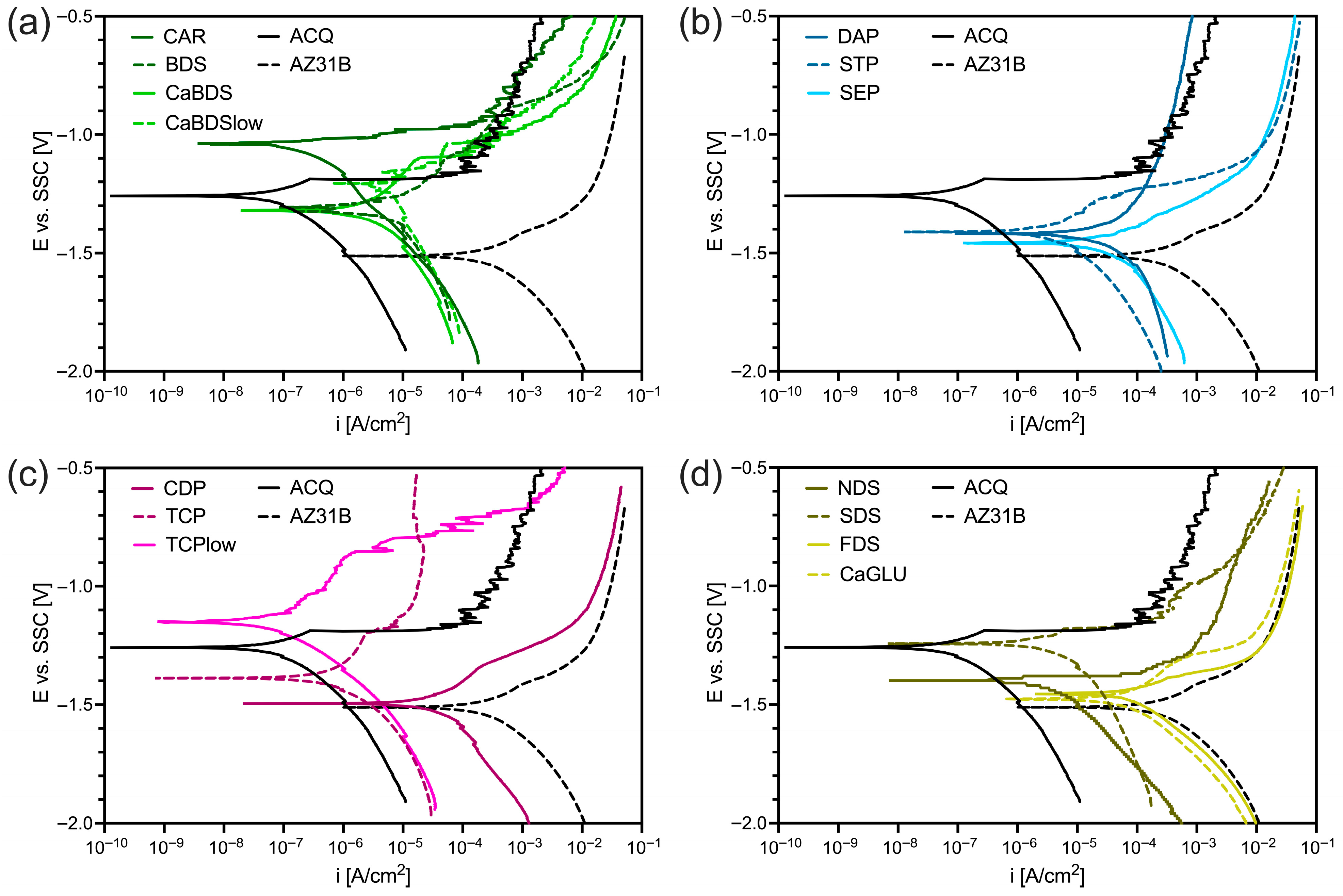
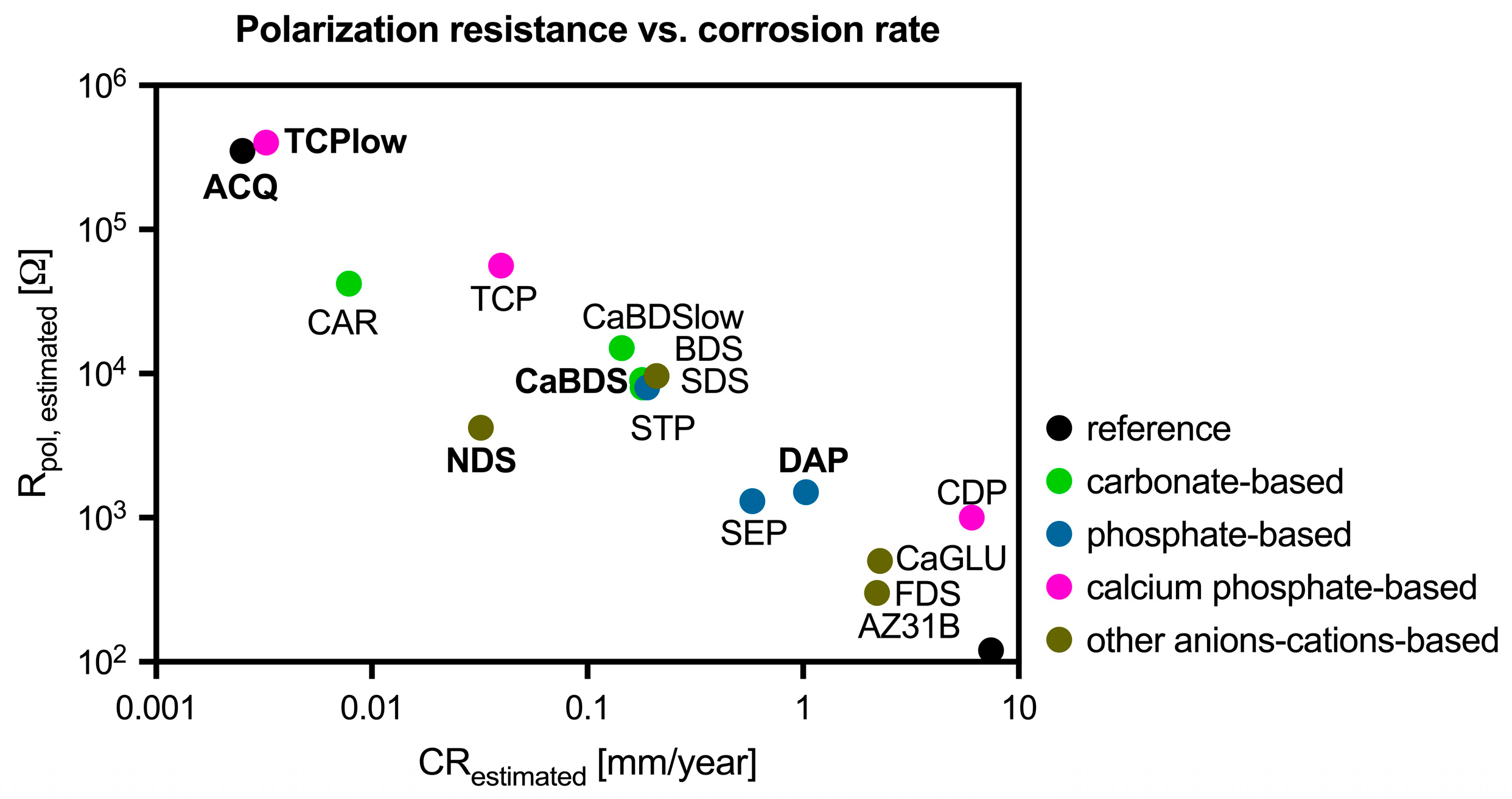
3.1. Hydrothermal Coatings Screening
- 1.
- ACQ (reference).
- 2.
- CaBDS, representing carbonate-based coatings. The CAR coating was both macroscopically and in terms of corrosion resistance (corrosion rate, polarization resistance) very similar to the ACQ sample, likely due to the low solubility of carbonate ions. Such an observation was confirmed by preliminary SEM/EDS evaluation (Figure S1) which showed no significant differences between the two.
- 3.
- DAP, representing phosphate-based coatings (chosen despite the overall poor electrochemical results of this family of coatings, in order to investigate their long-term degradation behavior).
- 4.
- TCPlow, representing calcium phosphate-based coatings.
- 5.
- NDS, as a different anion-based coating.
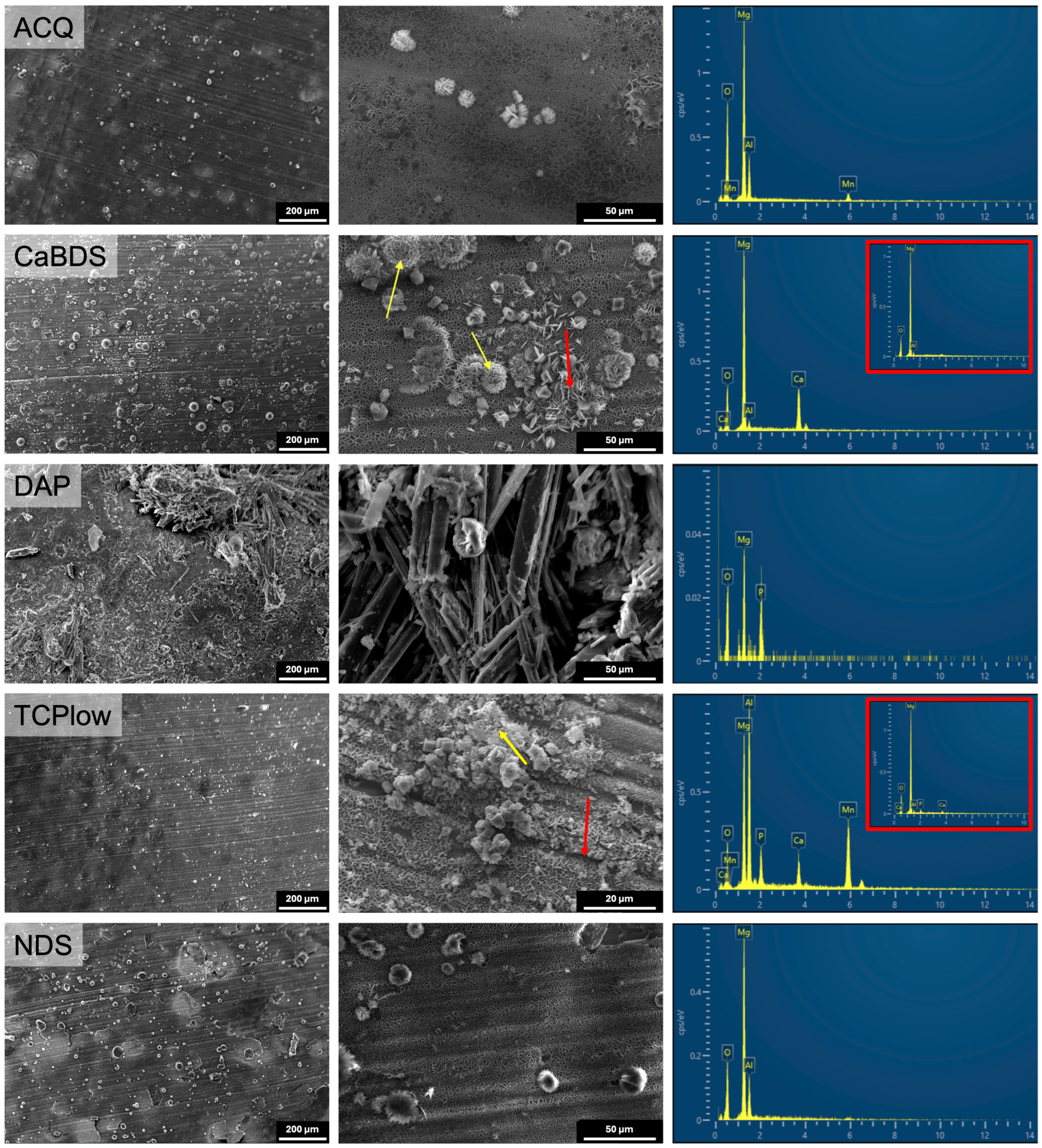
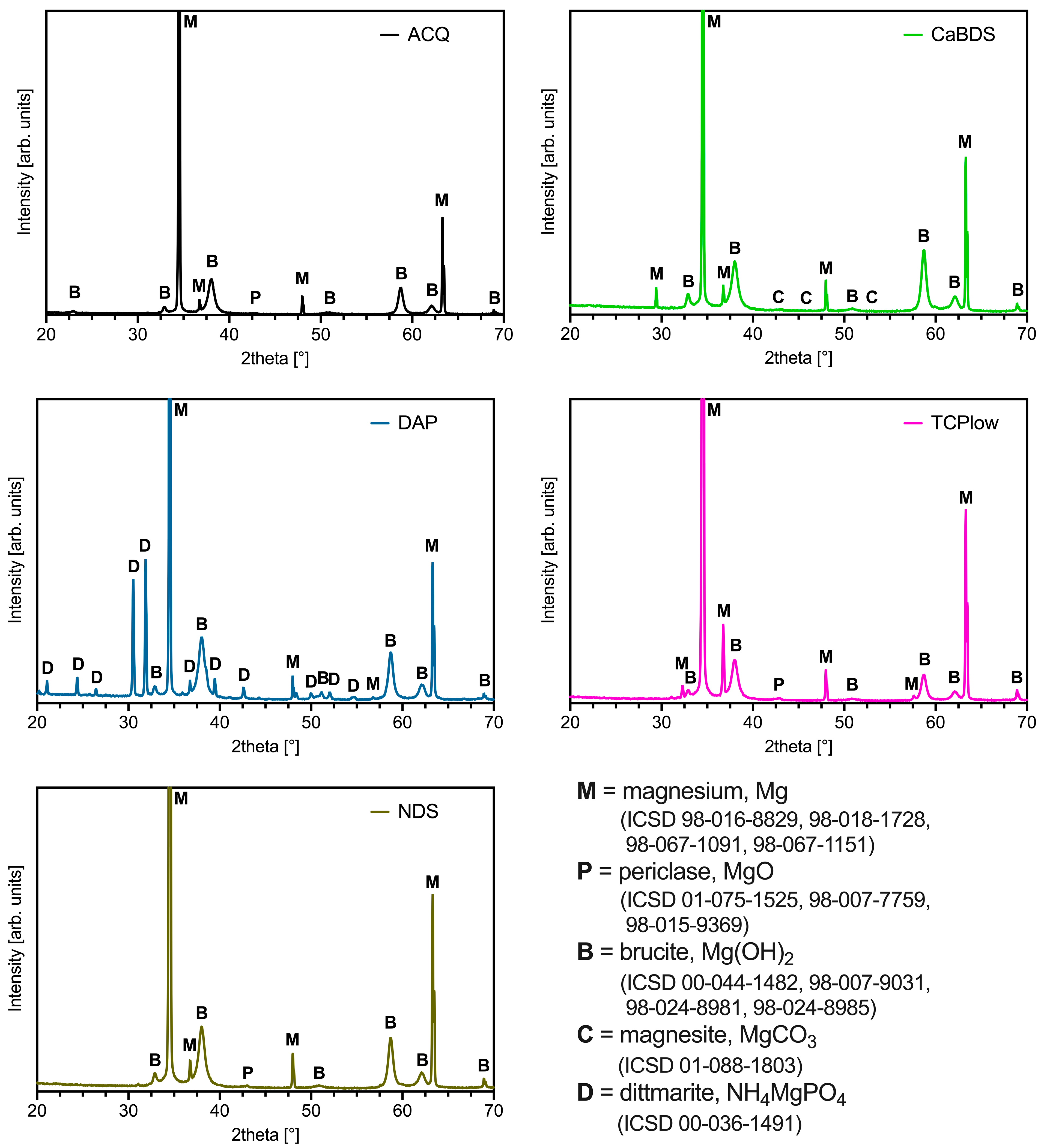
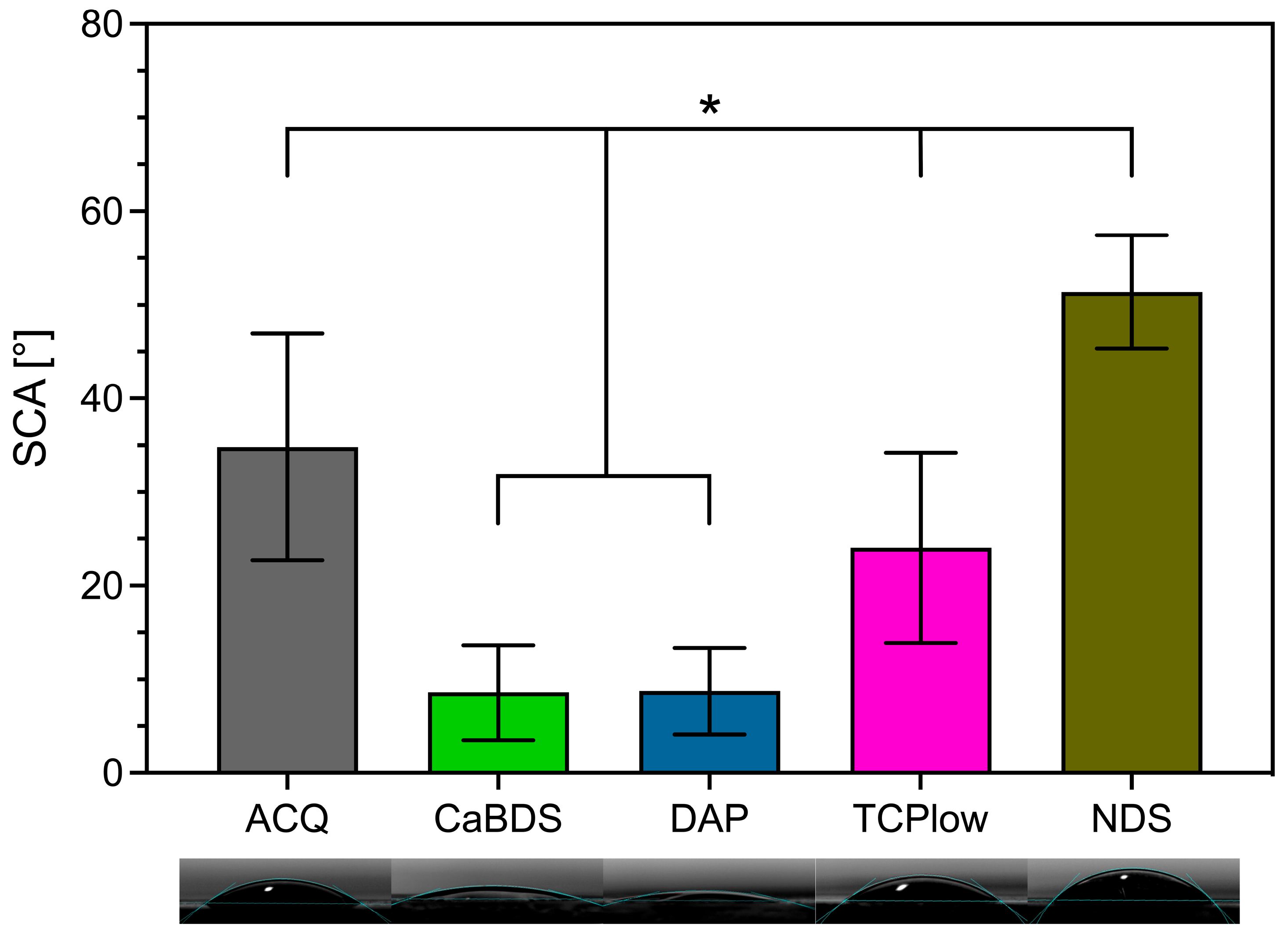
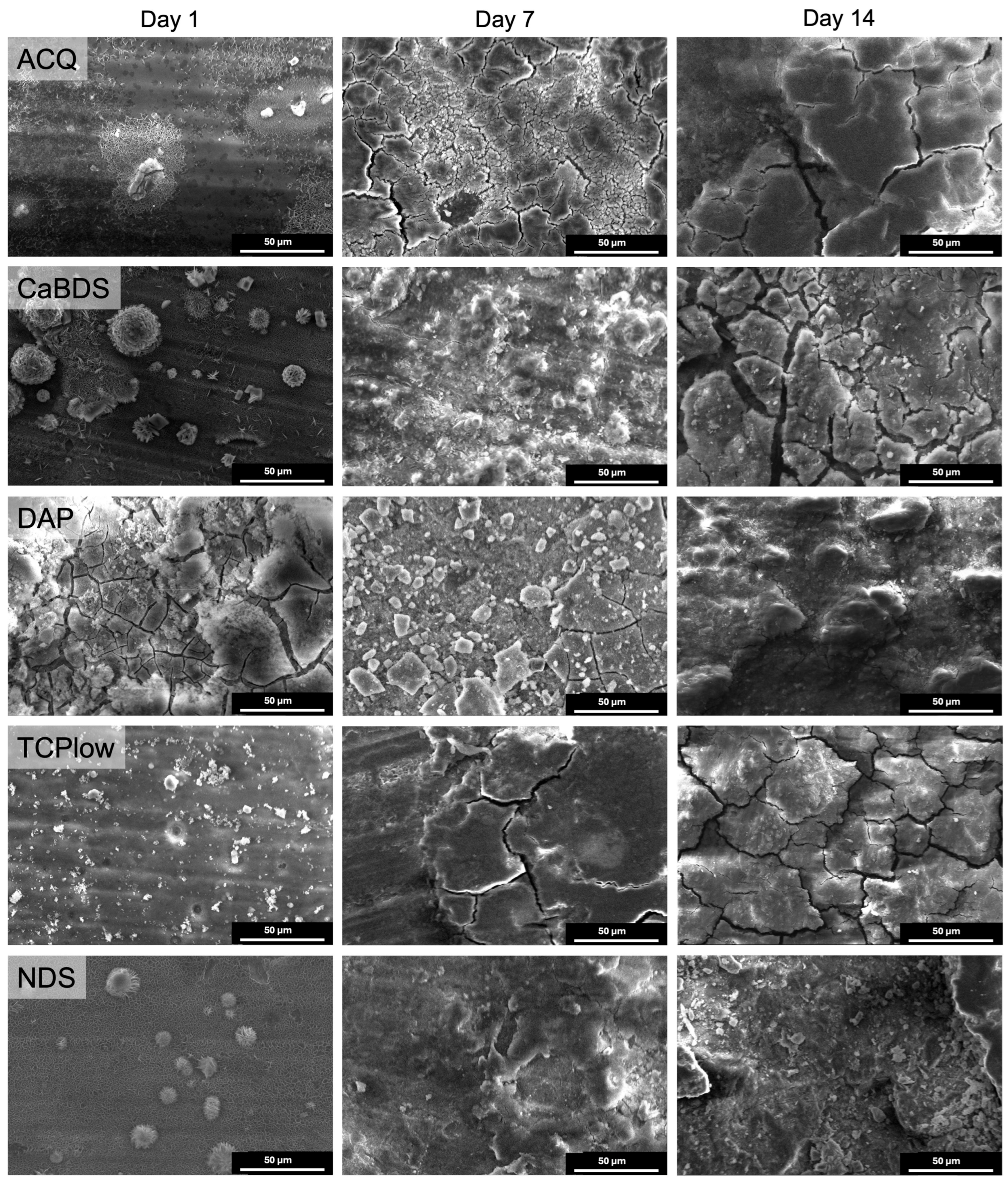
3.2. Morphological and Physical–Chemical Characterization
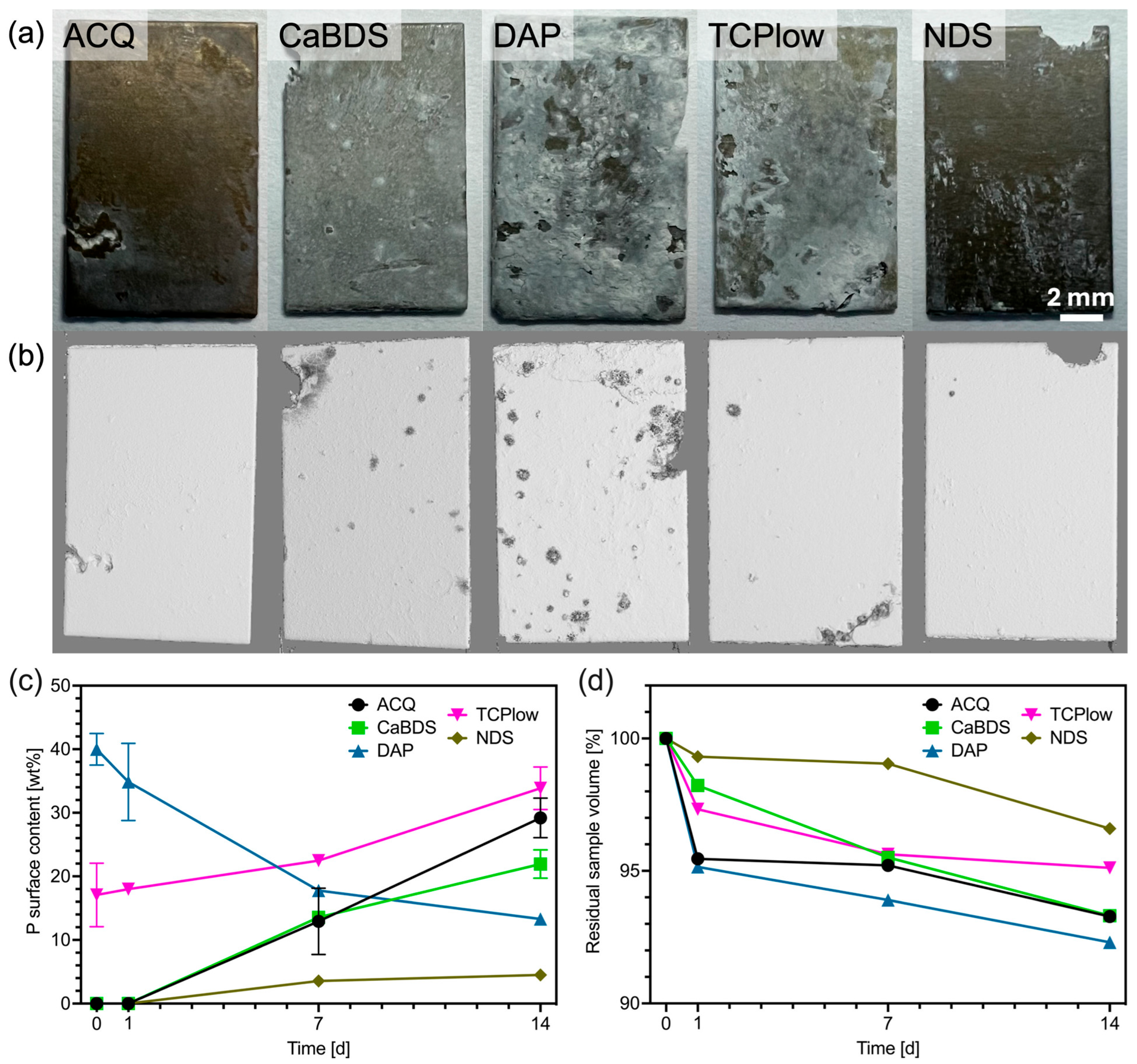
3.3. In Vitro Degradation
4. Discussion
4.1. The Primacy of Coating Quality over Thickness
4.2. The Critical Role of Magnesium Oxide
4.3. Performance Analysis of the Individual Coating Systems
- ACQ (deionized water): The simple treatment in water produced a markedly effective coating. Its good performance (icorr of 0.11 μA/cm2, SCA of about 35°, in vitro corrosion rate of 0.39 mm/year) can be ascribed to the formation of a stable MgO phase and to its overall uniform and almost defectless structure, although its inherently simple chemical structure limited its overall performance compared to other tested sample groups.
- CaBDS (sodium bicarbonate and calcium nitrate): This coating successfully incorporated calcium into its flower-like carbonate structures, offering potential bioactivity. However, its corrosion resistance was significantly inferior compared to the top performers (icorr of 7.82 μA/cm2). The absence of an MgO phase and its highly hydrophilic nature (SCA of about 9°) likely contributed to its faster degradation. The complex, high-surface-area morphology of the flower-like crystals may have also provided a greater number of sites for localized corrosion to occur.
- DAP (diammonium hydrogen phosphate): This coating performed poorly across all metrics (icorr of 44.9 μA/cm2, in vitro corrosion rate of 0.49 mm/year) despite its high thickness (about 35 μm) and crystalline nature. The dittmarite phase formed revealed to be not stable in the PBS solution, as shown by the rapid initial volume loss (with a 5% volume reduction after only one day of immersion) and the decrease in surface phosphorus concentration over time (from 40wt% to 13wt%). Its highly hydrophilic character (SCA of about 9°) and acicular morphology, prone to cracking, likely created a porous structure that offered limited barrier against penetration of the degradation medium.
- TCP (tricalcium phosphate): This coating showed excellent initial electrochemical resistance, nearly matching the ACQ control (icorr of 0.14 μA/cm2 vs. 0.11 μA/cm2). It successfully incorporated Ca and P and formed a protective MgO-containing layer. However, its long-term degradation was slightly more pronounced than that of NDS (with an estimated CR of 0.30 mm/year vs. 0.20 mm/year). The presence of distinct Ca-P agglomerates again led to compositional heterogeneities that acted as initiation sites for localized corrosion over time [38].
- NDS (sodium nitrate): This coating showed the best overall performance in long-term degradation tests (volume loss of about 3.5% and estimated CR of 0.20 mm/year after 14 days of immersion). Its optimal behavior can be attributed to a combination of factors: the formation of a stable MgO-containing layer, a relatively hydrophobic surface (confirmed by the highest SCA among groups, of about 51°), and a uniform morphology. While its initial electrochemical corrosion current was not the lowest (1.42 μA/cm2), its ability to form a stable, slowly degrading passive corrosion layer in PBS proved to be the most important characteristic for long-term protection.
4.4. Degradation Mechanism and Implications
4.5. µ-CT Scanning as a Promising Alternative for Corrosion Assessment
5. Conclusions
- Coating quality, not thickness, dictates performance. This study shows that the protective efficacy of a hydrothermal coating is governed by its homogeneity, density, and chemical stability, rather than by its bulk thickness. Notably, the NDS coating, regardless being one of the thinnest investigated (about 17 µm, compared to thicknesses up to about 50 µm in other sample groups), exhibited the overall lowest long-term corrosion rate (0.20 mm/year) after in vitro testing.
- The presence of MgO is critical for stability. A correlation was found between enhanced corrosion resistance and the presence of a crystalline magnesium oxide (MgO) phase within the coating. The most stable coatings (NDS, ACQ, TCP) contained both MgO and Mg(OH)2 phases, suggesting that the presence of MgO, even if not predominant, provides a more robust barrier to corrosion than Mg(OH)2 alone.
- A trade-off exists between bioactivity and corrosion resistance. While coatings containing calcium and phosphate (CaBDS, TCP) successfully incorporated bioactive elements, they exhibited slightly compromised corrosion performance (with in vitro corrosion rates of 0.43 and 0.30 mm/year, respectively), highlighting the need to carefully balance these competing properties in coating design.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SEM | Scanning Electron Microscopy |
| PBS | Dulbecco’s Phosphate-Buffered Saline Solution |
| SBF | Simulated Body Fluid |
| SCA | Static Contact Angle |
| µ-CT | Micro-Computed Tomography |
References
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti Based Biomaterials, the Ultimate Choice for Orthopaedic Implants—A Review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A. Biomedical Applications of Titanium Alloys: A Comprehensive Review. Materials 2023, 17, 114. [Google Scholar] [CrossRef]
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in Orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef]
- Böstman, O.; Pihlajamäki, H. Clinical Biocompatibility of Biodegradable Orthopaedic Implants for Internal Fixation: A Review. Biomaterials 2000, 21, 2615–2621. [Google Scholar] [CrossRef]
- Busam, M.L.; Esther, R.J.; Obremskey, W.T. Hardware Removal: Indications and Expectations. J. Am. Acad. Orthop. Surg. 2006, 14, 113–120. [Google Scholar] [CrossRef]
- Vos, D.; Hanson, B.; Verhofstad, M. Implant Removal of Osteosynthesis: The Dutch Practice. Results of a Survey. J. Trauma. Manag. Outcomes 2012, 6, 6. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable Metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- de Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Wu, S.; Yeung, K.W.K.; Zheng, Y.; Chu, P.K. Design of Magnesium Alloys with Controllable Degradation for Biomedical Implants: From Bulk to Surface. Acta Biomater. 2016, 45, 2–30. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and Its Alloys as Orthopedic Biomaterials: A Review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, T.; Jiang, J.; Xue, W.; Wang, W.; Ni, J.; Zhang, X.; Liu, X. Advances in Magnesium-Based Implants for Biomedical Applications: A Comprehensive Review and Future Perspectives. J. Magnes. Alloys 2025, 13, 2978–3003. [Google Scholar] [CrossRef]
- Nasr Azadani, M.; Zahedi, A.; Bowoto, O.K.; Oladapo, B.I. A Review of Current Challenges and Prospects of Magnesium and Its Alloy for Bone Implant Applications. Prog. Biomater. 2022, 11, 1–26. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and Advances in Magnesium Alloy Corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Gonzalez, J.; Hou, R.Q.; Nidadavolu, E.P.S.; Willumeit-Römer, R.; Feyerabend, F. Magnesium Degradation under Physiological Conditions—Best Practice. Bioact. Mater. 2018, 3, 174–185. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.-A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In Vitro and in Vivo Corrosion Measurements of Magnesium Alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable Magnesium Alloys for Orthopaedic Applications: A Review on Corrosion, Biocompatibility and Surface Modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef]
- Xia, S.; Wang, L.; Wang, Q.; Long, J.; Zhang, Z.; Chai, Y.; Xia, D.; Jiang, B. High-Speed-Extruded BA53 Mg Alloy with Ca-Doping: Multifunctional Gains in Strength, Corrosion Resistance and Thermal Stability. J. Alloys Compd. 2025, 1039, 182999. [Google Scholar] [CrossRef]
- Wang, L.; Xia, S.; Wang, Q.; Qin, X.; Jiang, B. Simultaneously Enhancing the Mechanical Properties and Corrosion Resistance in a Selective Laser Melting AZ91 Magnesium Alloy by Hot Rolling and Subsequent Annealing. J. Mater. Res. Technol. 2025, 36, 8503–8516. [Google Scholar] [CrossRef]
- Johari, N.A.; Alias, J.; Zanurin, A.; Mohamed, N.S.; Alang, N.A.; Zain, M.Z.M. Anti-Corrosive Coatings of Magnesium: A Review. Mater. Today Proc. 2022, 48, 1842–1848. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, K. Corrosion and Protection of Magnesium Alloys: Recent Advances and Future Perspectives. Coatings 2023, 13, 1533. [Google Scholar] [CrossRef]
- Yin, Z.-Z.; Qi, W.-C.; Zeng, R.-C.; Chen, X.-B.; Gu, C.-D.; Guan, S.-K.; Zheng, Y.-F. Advances in Coatings on Biodegradable Magnesium Alloys. J. Magnes. Alloys 2020, 8, 42–65. [Google Scholar] [CrossRef]
- Miklaszewski, A.; Kowalski, K.; Jurczyk, M. Hydrothermal Surface Treatment of Biodegradable Mg-Materials. Metals 2018, 8, 894. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, G.; Zhang, Y.-H.; Zhao, Q. Growth and Characterization of Mg(OH)2 Film on Magnesium Alloy AZ31. Appl. Surf. Sci. 2011, 257, 6129–6137. [Google Scholar] [CrossRef]
- Yoshimura, M.; Byrappa, K. Hydrothermal Processing of Materials: Past, Present and Future. J. Mater. Sci. 2008, 43, 2085–2103. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Q.; Zhang, Y.-H.; Wu, G. Hydrothermal Synthesis of Protective Coating on Magnesium Alloy Using De-Ionized Water. Surf. Coat. Technol. 2012, 206, 2961–2966. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical Coatings on Magnesium Alloys—A Review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, Y.-K.; Ryu, M.-H.; Bae, T.-S.; Lee, M.-H. Corrosion Resistance and Bioactivity Enhancement of MAO Coated Mg Alloy Depending on the Time of Hydrothermal Treatment in Ca-EDTA Solution. Sci. Rep. 2017, 7, 9061. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Iqbal, F.; Ahmad, A.; Ikram, F.; Nawaz, A.; Chaudhry, A.A.; Siddiqi, S.A.; Rehman, I. Hydrothermal Deposition of High Strength Calcium Phosphate Coatings on Magnesium Alloy for Biomedical Applications. Surf. Coat. Technol. 2019, 357, 716–727. [Google Scholar] [CrossRef]
- Jiang, P.; Hou, R.; Zhu, S.; Guan, S. A Robust Calcium Carbonate (CaCO3) Coating on Biomedical MgZnCa Alloy for Promising Corrosion Protection. Corros. Sci. 2022, 198, 110124. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting in Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Liu, L.; Gebresellasie, K.; Collins, B.; Zhang, H.; Xu, Z.; Sankar, J.; Lee, Y.-C.; Yun, Y. Degradation Rates of Pure Zinc, Magnesium, and Magnesium Alloys Measured by Volume Loss, Mass Loss, and Hydrogen Evolution. Appl. Sci. 2018, 8, 1459. [Google Scholar] [CrossRef]
- Song, G.-L.; Atrens, A. Recently Deepened Insights Regarding Mg Corrosion and Advanced Engineering Applications of Mg Alloys. J. Magnes. Alloys 2023, 11, 3948–3991. [Google Scholar] [CrossRef]
- Wei, L.; Gao, Z. Recent Research Advances on Corrosion Mechanism and Protection, and Novel Coating Materials of Magnesium Alloys: A Review. RSC Adv. 2023, 13, 8427–8463. [Google Scholar] [CrossRef]
- Wu, G.; Ibrahim, J.M.; Chu, P.K. Surface Design of Biodegradable Magnesium Alloys—A Review. Surf. Coat. Technol. 2013, 233, 2–12. [Google Scholar] [CrossRef]
- Ghali, E. Activity and Passivity of Magnesium (Mg) and Its Alloys. In Corrosion of Magnesium Alloys; Elsevier: Amsterdam, The Netherlands, 2011; pp. 66–114. [Google Scholar]
- Nahdi, K.; Rouquerol, F.; Trabelsi Ayadi, M. Mg(OH)2 Dehydroxylation: A Kinetic Study by Controlled Rate Thermal Analysis (CRTA). Solid State Sci. 2009, 11, 1028–1034. [Google Scholar] [CrossRef]
- Shkatulov, A.; Krieger, T.; Zaikovskii, V.; Chesalov, Y.; Aristov, Y. Doping Magnesium Hydroxide with Sodium Nitrate: A New Approach to Tune the Dehydration Reactivity of Heat-Storage Materials. ACS Appl. Mater. Interfaces 2014, 6, 19966–19977. [Google Scholar] [CrossRef]
- Lin, Y.; Cai, S.; Jiang, S.; Xie, D.; Ling, R.; Sun, J.; Wei, J.; Shen, K.; Xu, G. Enhanced Corrosion Resistance and Bonding Strength of Mg Substituted β-Tricalcium Phosphate/Mg(OH)2 Composite Coating on Magnesium Alloys via One-Step Hydrothermal Method. J. Mech. Behav. Biomed. Mater. 2019, 90, 547–555. [Google Scholar] [CrossRef]

| Group | Name | Composition | Concentration [M] |
|---|---|---|---|
| Water | ACQ | deionized H2O | - |
| Carbonate-based | CAR | CaCO3 | 0.05 |
| BDS | NaHCO3 | 0.25 | |
| NBDS | NaHCO3 + NaNO3 | 0.25 + 0.05 | |
| CaBDS | NaHCO3 + Ca(NO3)2 4H2O | 0.25 + 0.25 | |
| Ca2BDS | NaHCO3 + Ca(NO3)2 4H2O | 0.25 + 0.50 | |
| CaBDSlow | NaHCO3 + Ca(NO3)2 4H2O | 0.05 + 0.05 | |
| Phosphate-based | DAP | NH4H2PO4 | 0.25 |
| STP | Na5P3O10 | 0.05 | |
| SEP | [Na(PO3)]6 | 0.05 | |
| Calcium phosphate-based | CaDAP | NH4H2PO4 + Ca(NO3)2 4H2O | 0.25 + 0.50 |
| CDP | Ca(H2PO4)2 | 0.05 | |
| TCP | Ca3(PO4)2 | 0.25 | |
| TCPlow | Ca3(PO4)2 | 0.05 | |
| Other anions and cations | NDS | NaNO3 | 0.05 |
| SDS | Na2SiO3 | 0.05 | |
| FDS | NaF | 0.05 | |
| CeN | Ce(NO3)2 6H2O | 0.05 | |
| CuN | Cu(NO3)2 3H2O | 0.05 | |
| CaGLU | (C6H11O7)2Ca H2O | 0.05 |
| Group | Name | Coating Thickness [µm] |
|---|---|---|
| Water | ACQ | 15.27 ± 0.89 |
| Carbonate-based | CAR | 16.84 ± 0.28 |
| BDS | 37.80 ± 1.43 | |
| NBDS | n.a. | |
| CaBDS | 29.71 ± 5.67 | |
| Ca2BDS | n.a. | |
| CaBDSlow | 15.09 ± 0.35 | |
| Phosphate-based | DAP | 34.73 ± 0.07 |
| STP | 16.64 ± 1.85 | |
| SEP | 10.61 ± 1.10 | |
| Calcium phosphate-based | CaDAP | n.a. |
| CDP | 47.28 ± 1.85 | |
| TCP | 30.56 ± 0.23 | |
| TCPlow | 12.76 ± 4.53 | |
| Other anions and cations | NDS | 16.59 ± 0.89 |
| SDS | 10.17 ± 0.16 | |
| FDS | <1 | |
| CeN | n.a. | |
| CuN | n.a. | |
| CaGLU | 6.87 ± 1.05 |
| Name | CR @ 1d [mm/year] | CR @ 7d [mm/year] | CR @ 14d [mm/year] |
|---|---|---|---|
| ACQ | 3.73 ± 0.004 | 0.56 ± 0.004 | 0.39 ± 0.004 |
| CaBDS | 1.62 ± 0.009 | 0.58 ± 0.008 | 0.43 ± 0.006 |
| DAP | 4.33 ± 0.008 | 0.78 ± 0.007 | 0.49 ± 0.006 |
| TCPlow | 2.29 ± 0.005 | 0.54 ± 0.006 | 0.30 ± 0.004 |
| NDS | 0.57 ± 0.002 | 0.11 ± 0.002 | 0.20 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavarini, M.; Milanesi, N.; Moscatelli, M.; Chiesa, R. Harnessing Hydrothermal Treatments to Control Magnesium Alloy Degradation for Bioresorbable Implants: A Comprehensive Evaluation of Bath Chemistry Effect. Metals 2025, 15, 1056. https://doi.org/10.3390/met15091056
Pavarini M, Milanesi N, Moscatelli M, Chiesa R. Harnessing Hydrothermal Treatments to Control Magnesium Alloy Degradation for Bioresorbable Implants: A Comprehensive Evaluation of Bath Chemistry Effect. Metals. 2025; 15(9):1056. https://doi.org/10.3390/met15091056
Chicago/Turabian StylePavarini, Matteo, Nadia Milanesi, Monica Moscatelli, and Roberto Chiesa. 2025. "Harnessing Hydrothermal Treatments to Control Magnesium Alloy Degradation for Bioresorbable Implants: A Comprehensive Evaluation of Bath Chemistry Effect" Metals 15, no. 9: 1056. https://doi.org/10.3390/met15091056
APA StylePavarini, M., Milanesi, N., Moscatelli, M., & Chiesa, R. (2025). Harnessing Hydrothermal Treatments to Control Magnesium Alloy Degradation for Bioresorbable Implants: A Comprehensive Evaluation of Bath Chemistry Effect. Metals, 15(9), 1056. https://doi.org/10.3390/met15091056






