High-Temperature Corrosion Behavior of 12Cr18Ni10Ti Grade Austenitic Stainless Steel Under Chlorination Conditions
Abstract
1. Introduction
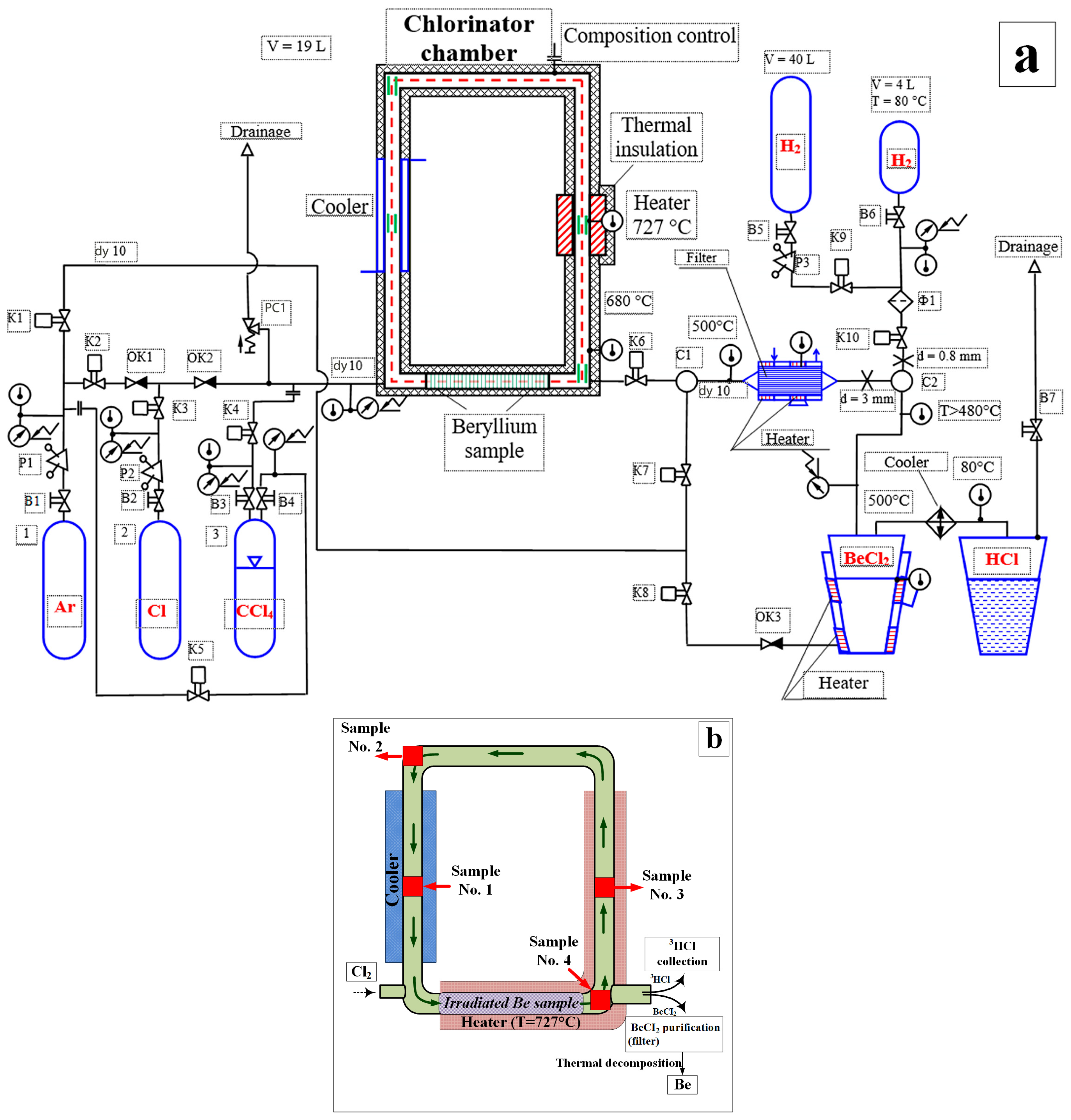
2. Materials and Methods
- Sample No. 1—tube section from the cooler zone;
- Sample No. 2—weld joint located in the cooler zone;
- Sample No. 3—tube section from the heater zone;
- Sample No. 4—weld joint located in the heater zone.
3. Results
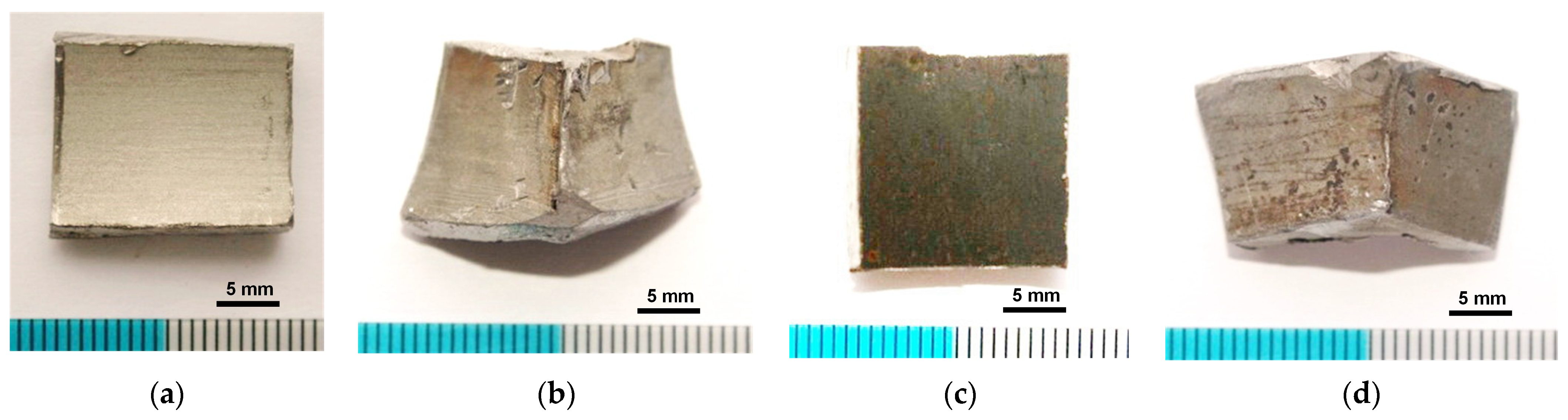
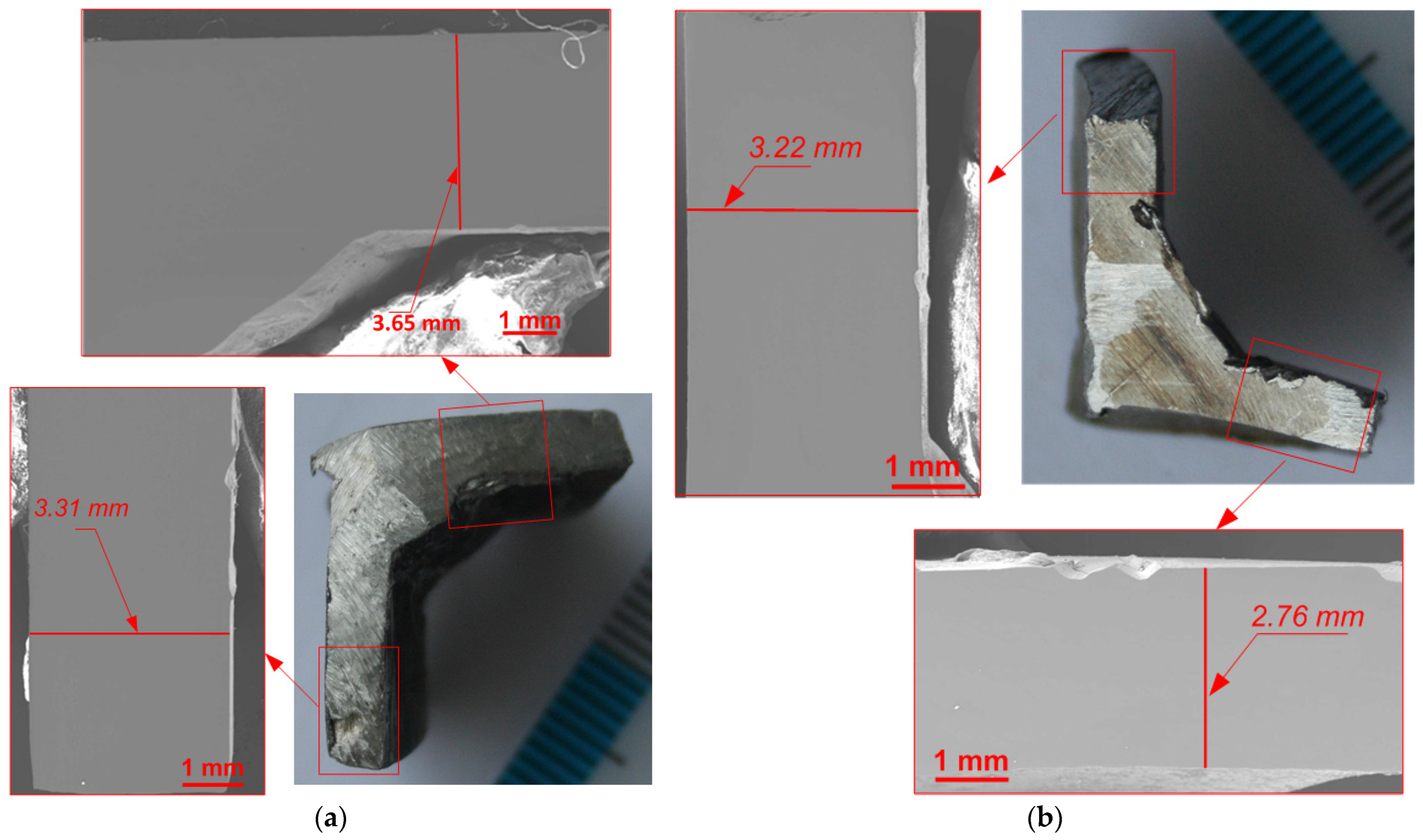
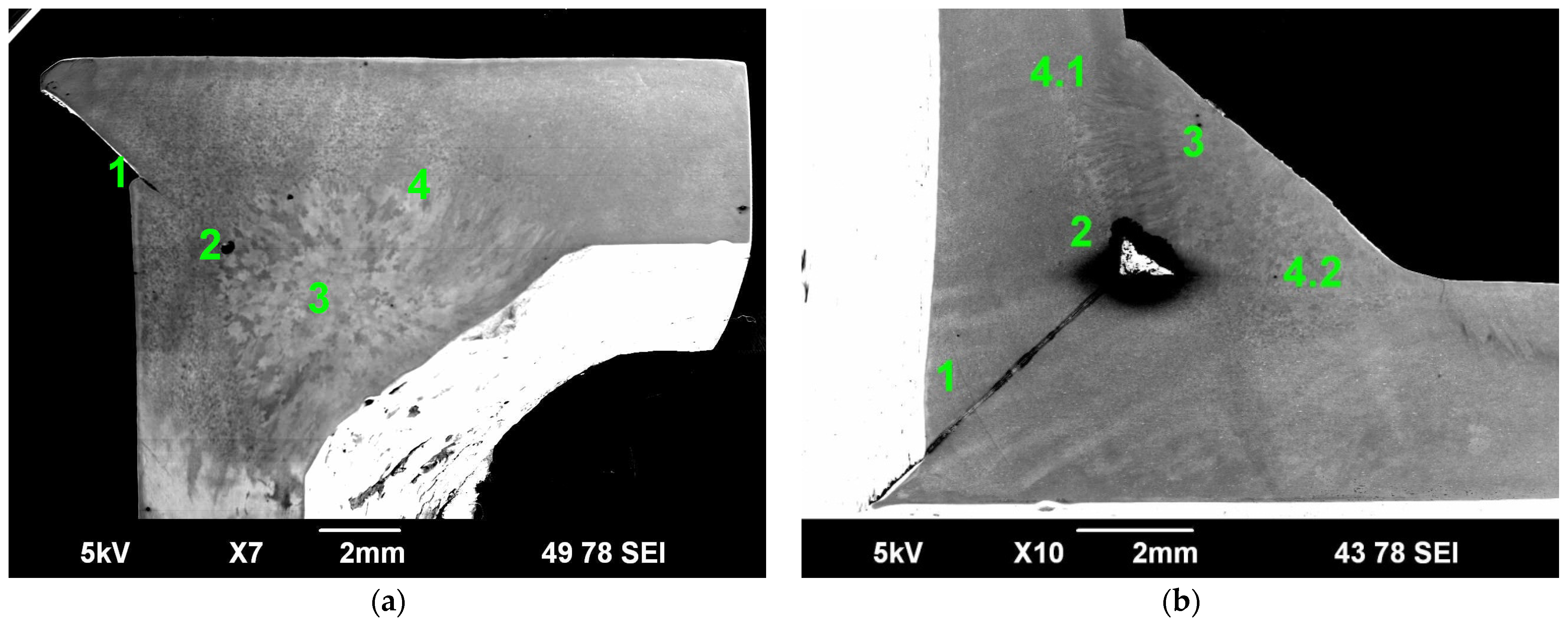
3.1. Macroscopic Analysis
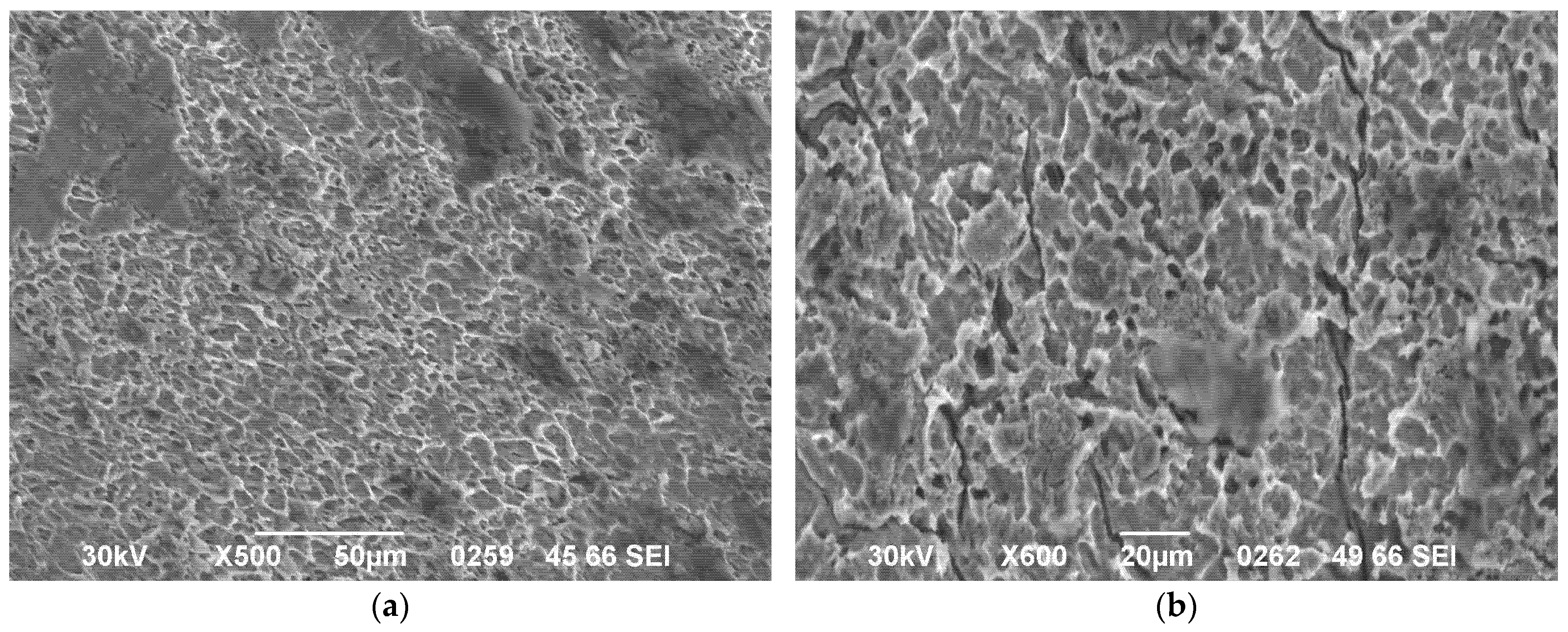
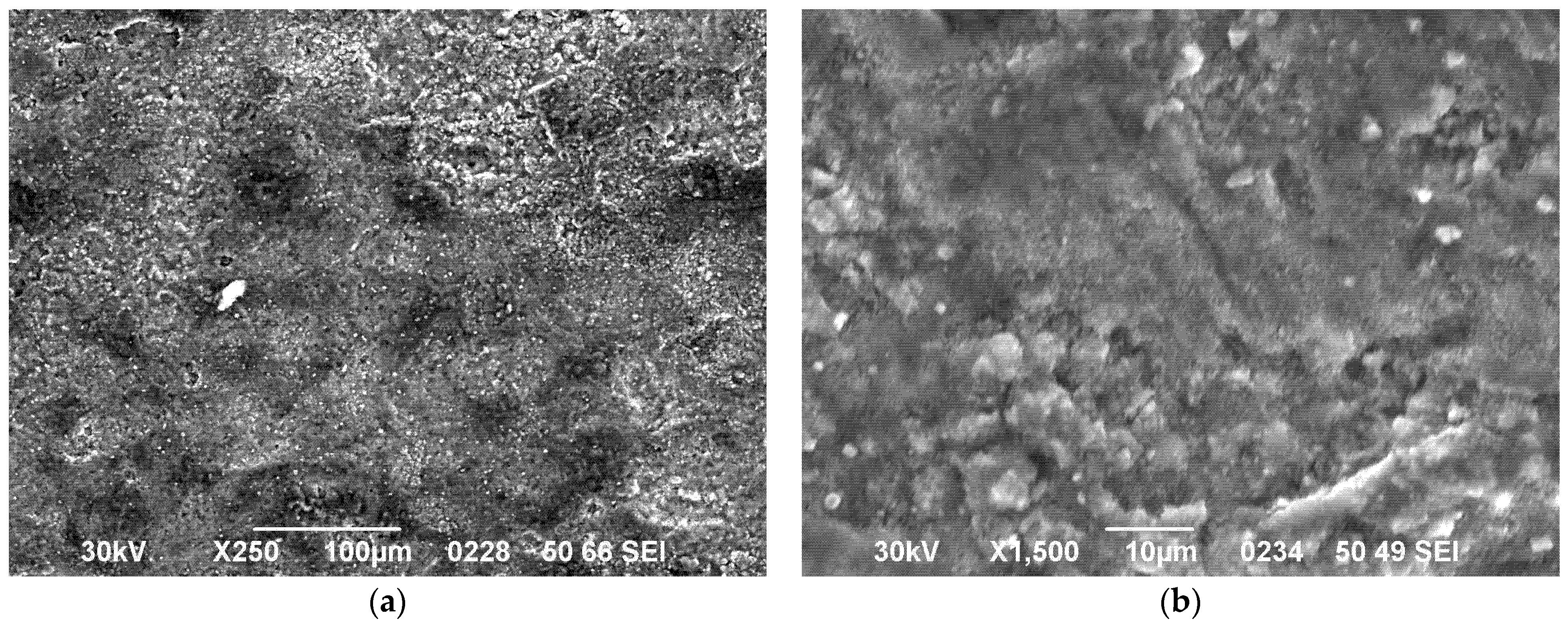
3.2. Microstructural Analysis
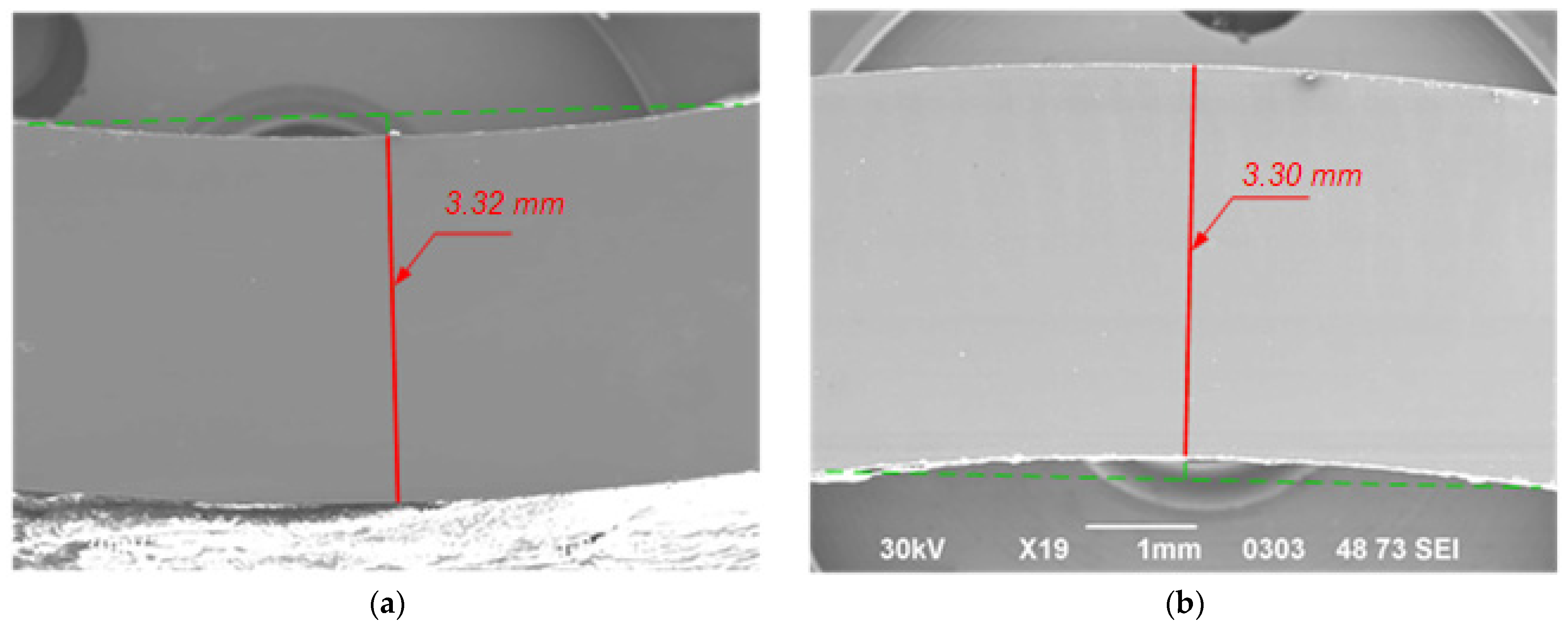
3.3. Wall Thickness Evaluation
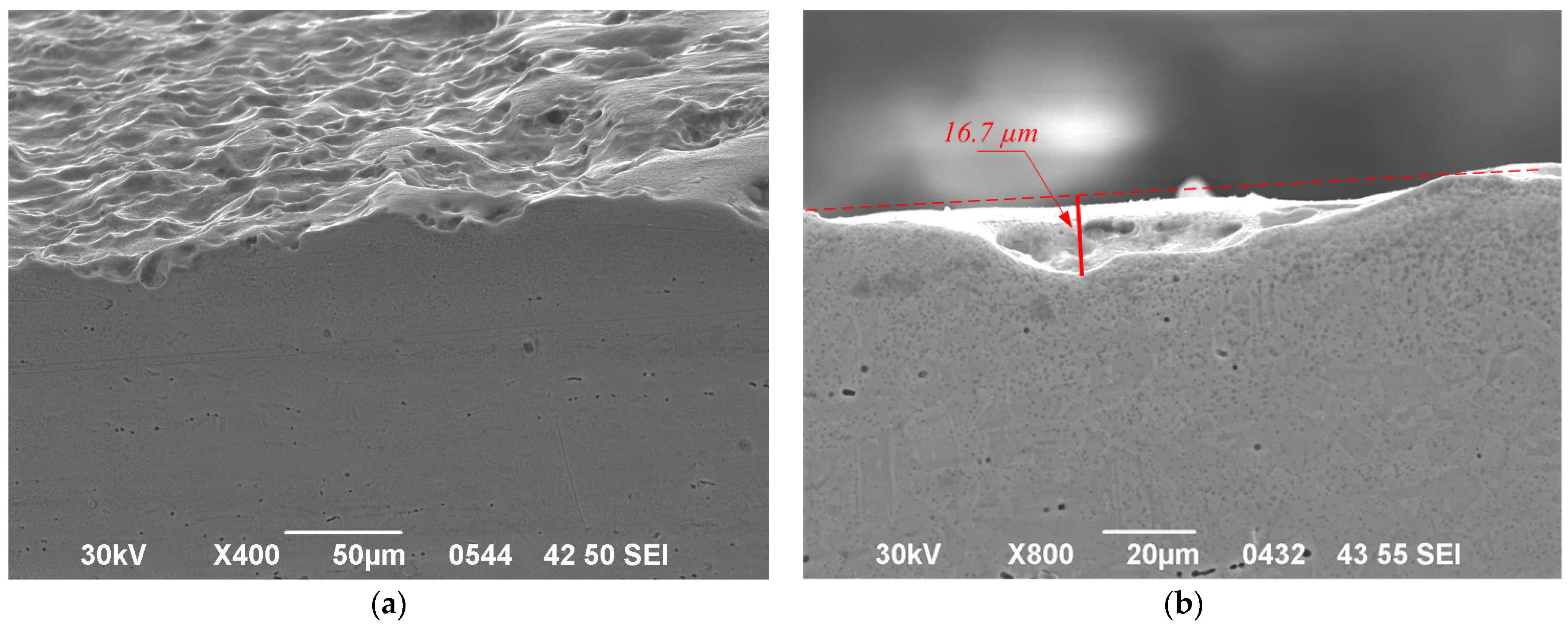
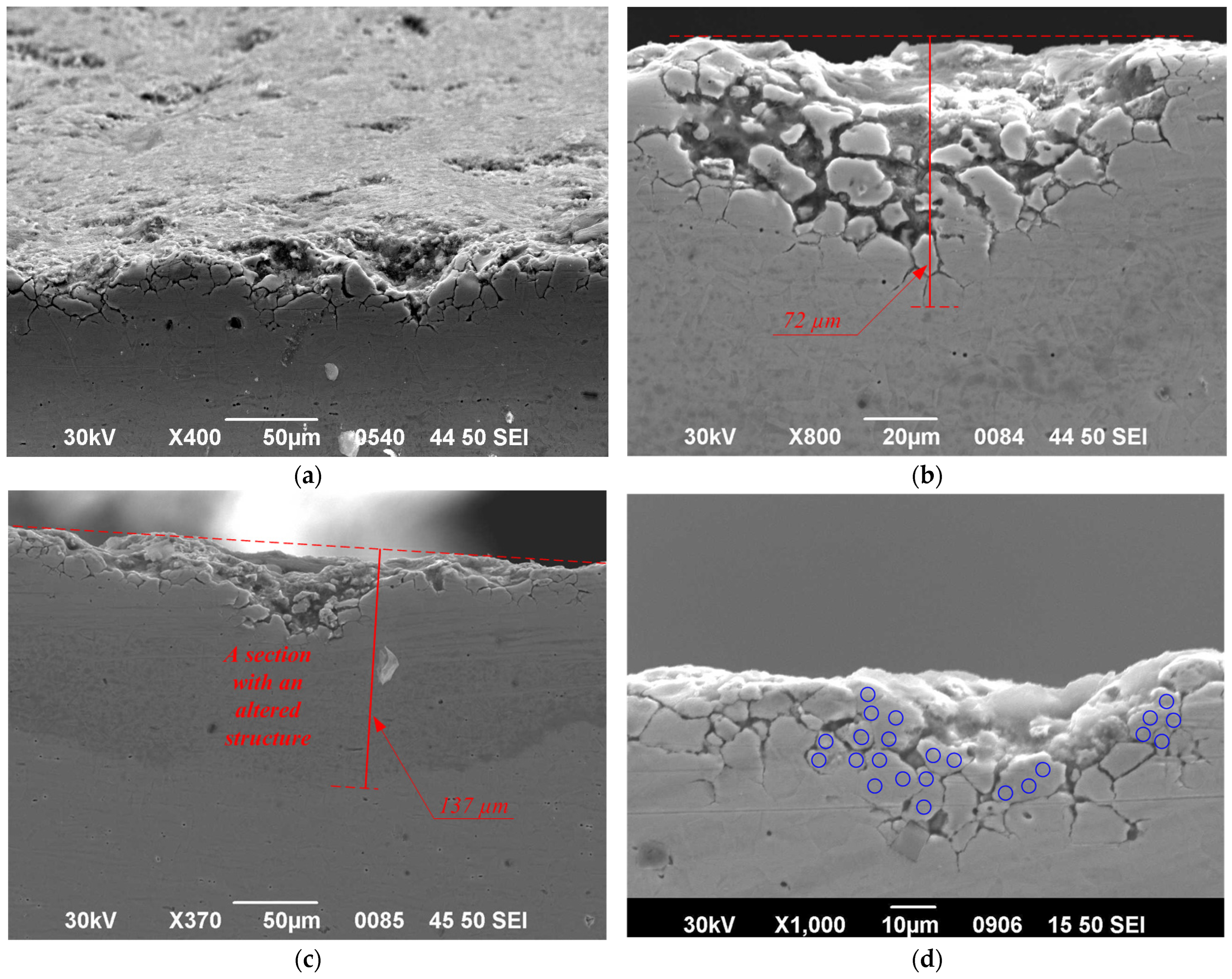
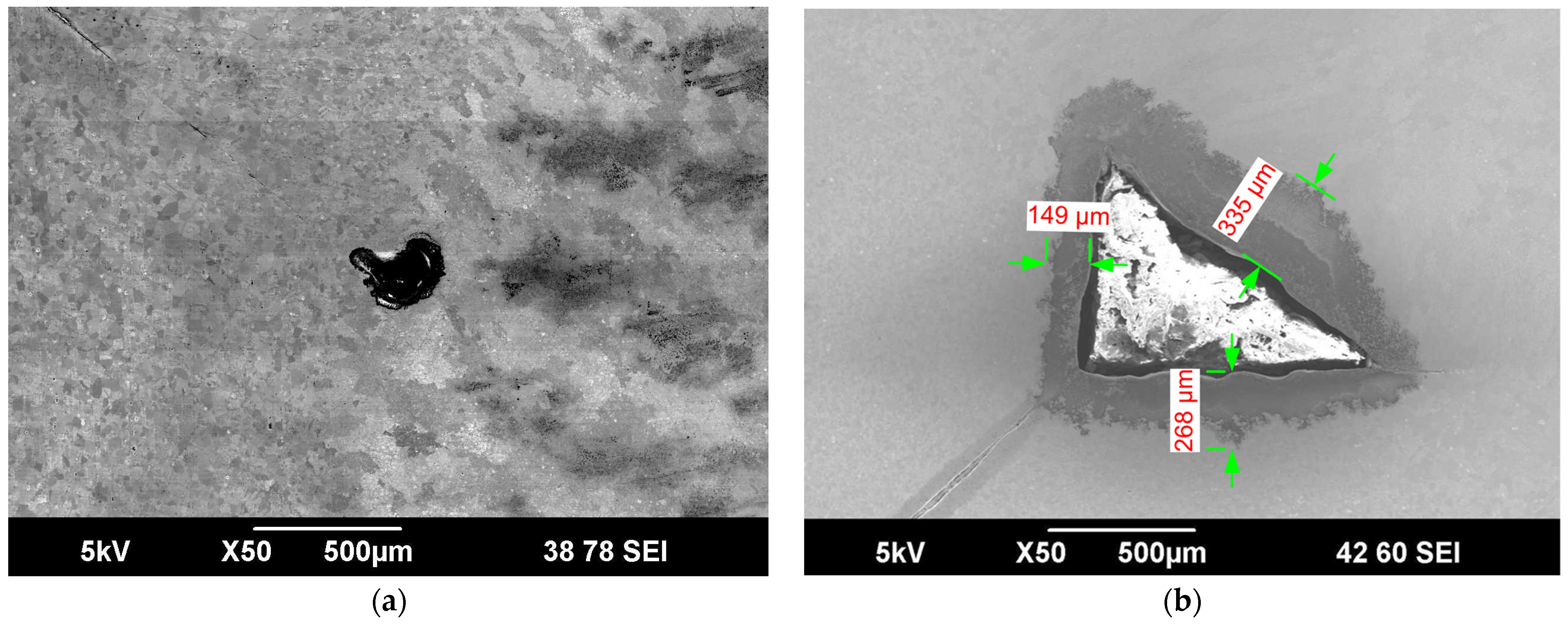
3.4. Localized Damage and Structural Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| JMTR | Japan Materials Testing Reactor |
| SEM | Scanning electron microscopy |
References
- Fernández-Arias, P.; Vergara, D.; Antón-Sancho, Á. Global Review of International Nuclear Waste Management. Energies 2023, 16, 6215. [Google Scholar] [CrossRef]
- Terranova, M.L.; Tavares, O.A.P. Trends and Perspectives on Nuclear Waste Management: Recovering, Recycling, and Reusing. J. Nucl. Eng. 2024, 5, 299–317. [Google Scholar] [CrossRef]
- Lee, S.G.; Cheong, J.H. Neutron Activation of Structural Materials of a Dry Storage System for Spent Nuclear Fuel and Implications for Radioactive Waste Management. Energies 2020, 13, 5325. [Google Scholar] [CrossRef]
- Tang, W.; Chatzidakis, S.; Schrad, C.M.; Miller, R.G.; Howard, R. Study of Mechanical Properties, Microstructure, and Residual Stresses of AISI 304/304L Stainless Steel Submerged Arc Weld for Spent Fuel Dry Storage Systems. Metals 2024, 14, 262. [Google Scholar] [CrossRef]
- Alwaeli, M.; Mannheim, V. Investigation into the Current State of Nuclear Energy and Nuclear Waste Management—A State-of-the-Art Review. Energies 2022, 15, 4275. [Google Scholar] [CrossRef]
- Merk, B.; Detkina, A.; Atkinson, S.; Litskevich, D.; Cartland-Glover, G. Innovative Investigation of Reflector Options for the Control of a Chloride-Based Molten Salt Zero-Power Reactor. Appl. Sci. 2021, 11, 6795. [Google Scholar] [CrossRef]
- Dylst, K.; Seghers, J.; Druyts, F.; Braet, J. Removing Tritium and Other Impurities During Industrial Recycling of Beryllium from a Fusion Reactor. Fusion Sci. Technol. 2008, 54, 215–218. [Google Scholar] [CrossRef]
- Kolbasov, B.N.; Khripunov, V.I.; Biryukov, A.Y. On use of beryllium in fusion reactors: Resources, impurities, and necessity of detritiation after irradiation. Fusion Eng. Des. 2016, 109–111, 480–484. [Google Scholar] [CrossRef]
- Suleimenov, N.A.; Kotov, V.M.; Vurim, A.D.; Baklanova, Y.Y. Operating parameters of a direct flow irradiated beryllium chlorination plant. NNC RK Bull. 2020, 1, 12–18. (In Russian) [Google Scholar] [CrossRef]
- Kotov, V.; Savchyk, V.; Zorin, B.; Tazhibayeva, I.; Kawamura, H.; Tsuchiya, K.; Druyts, F. Research and development of recycling technology of irradiated beryllium. In Proceedings of the 14th International Conference on Fusion Reactor Materials (ICFRM-14), Sapporo, Japan, 10–11 September 2009. [Google Scholar]
- Kotov, V.M.; Supprunov, V.I.; Baklanova, Y.Y.; Vityuk, G.A.; Suraev, A.S. Beryllium chloride plant temperature conditions. NNC RK Bull. 2013, 1, 70–77. (In Russian) [Google Scholar]
- Baklanova, Y.Y.; Vurim, A.D.; Kotov, V.M.; Surayev, A.S.; Prozorova, I.V. Work safety during purification of irradiated beryllium by chlorination. J. Phys. Conf. Ser. 2020, 1443, 012018. [Google Scholar] [CrossRef]
- GOST 5632-1997; High-Alloy Steels and Corrosion-Resistant, Heat-Resisting and High-Temperature Alloyed Grades. Fushun Special Steel: Fushun, China, 1997; p. 20. Available online: https://www.fushunspecialsteel.ru/upload/userfiles/files/%D0%93%D0%9E%D0%A1%D0%A2%205632_1997%20%D0%B2%D1%8B%D1%81%D0%BE%D0%BA%D0%BE%D0%BB%D0%B5%D0%B3%D0%B8%D1%80%D0%BE%D0%B2%D0%B0%D0%BD%D0%BD%D0%B0%D1%8F%20%D1%81%D1%82%D0%B0%D0%BB%D1%8C%20%D0%B8%20%D0%BA%D0%BE%D1%80%D1%80%D0%BE%D0%B7%D0%B8%D0%BE%D0%BD%D0%BD%D0%BE%D1%81%D1%82%D0%BE%D0%B9%D0%BA%D0%B8%D0%B5%20%D0%B6%D0%B0%D1%80%D0%BE%D0%BF%D1%80%D0%BE%D1%87%D0%BD%D1%8B%D0%B5%20%D0%B8%20%D0%B2%D1%8B%D1%81%D0%BE%D0%BA%D0%BE%D1%82%D0%B5%D0%BC%D0%BF%D0%B5%D1%80%D0%B0%D1%82%D1%83%D1%80%D0%BD%D1%8B%D0%B5%20%D0%BB%D0%B5%D0%B3%D0%B8%D1%80%D0%BE%D0%B2%D0%B0%D0%BD%D0%BD%D1%8B%D0%B5%20%D0%BC%D0%B0%D1%80%D0%BA%D0%B8.pdf (accessed on 19 August 2025).
- Lehmusto, J.; Skrifvars, B.J.; Yrjas, P.; Hupa, M. Comparison of potassium chloride and potassium carbonate with respect to their tendency to cause high-temperature corrosion of stainless 304L steel. Fuel Process. Technol. 2013, 105, 98–105. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Y.; Cheng, Y.; Li, J.; Jiang, Y. The Intergranular Corrosion Susceptibility of Metastable Austenitic Cr–Mn–Ni–N–Cu High-Strength Stainless Steel under Various Heat Treatments. Materials 2019, 12, 1385. [Google Scholar] [CrossRef]
- Nowik, K.; Zybała, R.; Sztorch, B.; Oksiuta, Z. Corrosion Behavior of Ferritic 12Cr ODS and Martensitic X46Cr13 Steels in Nitric Acid and Sodium Chloride Solutions. Materials 2024, 17, 3466. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-Y.; Jeong, C.; Kim, Y.-J.; Jang, C. Effect of Stress Magnitude on Pit Growth Rate of 304 Austenitic Stainless Steel in Chloride Environments. Metals 2021, 11, 1415. [Google Scholar] [CrossRef]
- Wang, L.; Zanna, S.; Mercier, D.; Maurice, V.; Marcus, P. Early-stage surface oxidation of the equiatomic CoCrFeMnNi high entropy alloy studied in situ by XPS. Corros. Sci. 2023, 220, 111310. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Qian, J.; Pessu, F. Investigation of the early stage corrosion characteristics of molten (nitrate) salt–oxide–metal interfaces, and the links to interfacial thermo-mechanical stress. Corros. Sci. 2023, 220, 111282. [Google Scholar] [CrossRef]
- Migai, L.L.; Taritsyna, T.A. Corrosion Resistance of Materials in Chlorine and Its Compounds; Metallurgy: Moscow, Russia, 1976; pp. 1–120. (In Russian) [Google Scholar]
- Tomashov, N.D. Theory of Corrosion and Protection of Metals: The Science of Corrosion; Macmillan: New York, NY, USA, 1966; pp. 1–672. [Google Scholar]
- Gerard, A.Y.; Kautz, E.J.; Schreiber, D.K.; Han, J.; McDonnell, S.; Ogle, K.; Lu, P.; Saal, J.E.; Frankel, G.S.; Scully, J.R. The role of chromium content in aqueous passivation of a non-equiatomic Ni38Fe20CrxMn21-0.5xCo21-0.5x multi-principal element alloy (x = 22, 14, 10, 6 at%) in acidic chloride solution. Acta Mater. 2023, 245, 118607. [Google Scholar] [CrossRef]
- Tiamiyu, A.A.; Eduok, U.; Szpunar, J.A.; Zeng, C.H. Corrosion Behavior of Metastable AISI 321 Austenitic Stainless Steel: Investigating the Effect of Grain Size and Prior Plastic Deformation on Its Degradation Pattern in Saline Media. Sci. Rep. 2019, 9, 12116. [Google Scholar] [CrossRef] [PubMed]
- Narivs’kyi, O.; Atchibayev, R.; Kemelzhanova, A.; Yar-Mukhamedova, G.; Snizhnoi, G.; Subbotin, S.; Beisebayeva, A. Mathematical Modeling of the Corrosion Behavior of Austenitic Steels in Chloride-Containing Media during the Operation of Plate-Like Heat Exchangers. Eurasian Chem. Technol. J. 2022, 24, 295–302. [Google Scholar] [CrossRef]
- Snizhnoi, G.V.; Snizhnoi, V.L. Magnetometric assessment of the influence of chemical elements on the corrosion of austenitic Fe-Cr-Ni alloys. Mater. Sci. 2024, 60, 91–96. [Google Scholar] [CrossRef]
- Narivskyi, O.E.; Belikov, S.B.; Subbotin, S.A.; Pulina, T.V. Influence of chloride-containing media on the pitting resistance of AISI 321 steel. Mater. Sci. 2021, 57, 291–297. [Google Scholar] [CrossRef]
- Nejati, S.; Tavangar, R.; Pour-Ali, S.; Hejazi, S. Improved Resistance to Chloride-Induced Corrosion through the Combined Influence of Surface Nanocrystallization and Thermal Oxidation Treatments on AISI 321 Stainless Steel. Mater. Chem. Phys. 2023, 309, 128404. [Google Scholar] [CrossRef]
- Kawamura, H.; Sakamoto, N.; Tatenuma, K. Reprocessing Technology Development for Irradiated Beryllium. In Proceedings of the International Conference on Nuclear Engineering (ICONE), Kyoto, Japan, 23–27 April 1995; pp. 261–268. [Google Scholar]
- Inaba, Y.; Ishihara, M.; Niimi, M.; Kawamura, H. Present status of refurbishment and irradiation technologies in JMTR. J. Nucl. Mater. 2011, 417, 1348–1351. [Google Scholar] [CrossRef]
- Mukhamedov, N.; Toleubekov, K.; Vityuk, G.; Bekmuldin, M.; Dolzhikov, S. Decommissioning of the BN-350 Fast Neutron Reactor: History Review and Current Status. Energies 2025, 18, 3486. [Google Scholar] [CrossRef]
- Mukhamedov, N.; Baklanov, V.; Moldagulov, M.; Toleubekov, K.; Surayev, A.; Yagudin, A.; Kanatnikov, S. The BN-350 Reactor Decommissioning: Quantitative Analysis and Prospects for Solid Radioactive Waste Management. Energies 2025, 18, 4651. [Google Scholar] [CrossRef]
- Koyanbayev, Y.T.; Skakov, M.K.; Batyrbekov, E.G.; Deryavko, I.I.; Sapatayev, Y.Y.; Kozhahmetov, Y.A. The Forecasting of Corrosion Damage of Structural Materials during Dry Long-Term Storage of RD BN-350 SNF with CC-19 SFA. Sci. Technol. Nucl. Ins. 2019, 2019, 1293060. [Google Scholar] [CrossRef]
- Koyanbayev, Y.T.; Skakov, M.K.; Ganovichev, D.A.; Martynenko, Y.A.; Sitnikov, A.A. Simulation of the Thermal Conditions of Cask with Fuel Assemblies of BN-350 Reactor for Dry Storage. Sci. Technol. Nucl. Ins. 2019, 2019, 3045897. [Google Scholar] [CrossRef]
- Koyanbayev, Y. Applying the Hollomon-Jaffe parameter to predict changes in mechanical properties of irradiated austenitic chromium-nickel steels during isothermal exposure. AIMS Mater. Sci. 2024, 11, 216–230. [Google Scholar] [CrossRef]
- Dorn, C.K.; Tsuchiya, K.; Kawamura, H.; Hashiguchi, D.H.; Sayer, A.B.; Haws, W.J. Material Selection for Extended Life of the Beryllium Reflectors in the JMTR. In Proceedings of the International Symposium on Materials Testing Reactors, Oarai, Japan, 16–17 July 2008; JAEA Oarai R&D Center: Oarai, Japan, 2008; pp. 59–65. [Google Scholar]
- Baklanova, Y. Studying the Decontamination Process of an Irradiated Beryllium Reflector in a Chlorine Environment. PLoS ONE 2025, 20, e0322723. [Google Scholar] [CrossRef]
- GOST 14771-76; Arc Welding in Protective Gas. Welded Joints. Basic Types, Design Elements, and Dimensions. Standards Publishing House: Strathfield South, Australia, 1977. Available online: https://auremo.biz/gosts/gost-14771-76.html (accessed on 19 August 2025).
- GOST R 50.05.09-2021; Conformity Assessment in the Form of Control. Unified Methods. Capillary Control. Standartinform: Moscow, Russia; p. 32. Available online: https://files.stroyinf.ru/Data2/1/4293732/4293732617.htm (accessed on 19 August 2025).
- GOST 18442-80; Non-Destructive Testing. Dye Penetrant Methods. General Requirements. USSR State Committee for Standards: Moscow, Russia, 1980. Available online: https://acnkru.ru/wp-content/uploads/2018/07/GOST-18442-80.pdf (accessed on 19 August 2025).
- Silva, R.; Kugelmeier, C.L.; Vacchi, G.S.; Martins Junior, C.B.; Dainezi, I.; Afonso, C.R.M.; Mendes Filho, A.A.; Rovere, C.A.D. A comprehensive study of the pitting corrosion mechanism of lean duplex stainless steel grade 2404 aged at 475 °C. Corros. Sci. 2021, 191, 109738. [Google Scholar] [CrossRef]
- Messinese, E.; Casanova, L.; Paterlini, L.; Capelli, F.; Bolzoni, F.; Ormellese, M.; Brenna, A. A Comprehensive Investigation on the Effects of Surface Finishing on the Resistance of Stainless Steel to Localized Corrosion. Metals 2022, 12, 1751. [Google Scholar] [CrossRef]
- GOST 8734-75; Seamless Steel Tubes Cold Deformed. Range. State Committee of Standards of the USSR: Moscow, Russia, 1975. Available online: https://files.stroyinf.ru/Data1/8/8429/index.htm (accessed on 18 September 2025).
- Moura, V.; Kina, A.Y.; Tavares, S.S.M.; Pardal, J.M.; da Silva, M.R. Influence of Stabilization Heat Treatments on Microstructure, Hardness and Intergranular Corrosion Resistance of the AISI 321 Stainless Steel. J. Mater. Sci. 2008, 43, 536–540. [Google Scholar] [CrossRef]
- Xu, L.; Shao, C.; Tian, L.; Zhang, J.; Han, Y.; Zhao, L.; Jing, H. Intergranular Corrosion Behavior of Inconel 625 Deposited by CMT/GTAW. Corros. Sci. 2022, 201, 110295. [Google Scholar] [CrossRef]
- Alharbi, S.O.; Ahmad, S.; Gul, T.; Khan, M.A.; Aslam, M. The Corrosion Behavior of Low Carbon Steel (AISI 1010) Influenced by Grain Size through Microstructural Mechanical. Sci. Rep. 2024, 14, 5098. [Google Scholar] [CrossRef]
- Ibrahim, M.; Nasir, M.; Rahman, M.; Aung, N.N. Effects of Welding Defects on Corrosion Behavior of Stainless Steel in Chloride Solution. Mater. Sci. Eng. A 2009, 508, 64–69. [Google Scholar] [CrossRef]
- Ramkumar, K.D.; Pavan, B.; Chandrasekar, V. Development of Improved Microstructural Traits and Mechanical Integrity of Stabilized Stainless Steel Joints of AISI 321. J. Manuf. Process. 2018, 32, 582–594. [Google Scholar] [CrossRef]
- Leban, M.B.; Tisu, R. The Effect of TiN Inclusions and Deformation-Induced Martensite on the Corrosion Properties of AISI 321 Stainless Steel. Eng. Fail. Anal. 2013, 33, 430–438. [Google Scholar] [CrossRef]
- Dehgahi, S.; Shahriari, A.; Odeshi, A.; Mohammadi, M. Influence of Ti Content on High Strain Rate Mechanical and Corrosion Behavior of Additively Manufactured Maraging Steels. J. Mater. Eng. Perform. 2023, 32, 1169–1184. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Viejo, F.; Carboneras, M.; Arrabal, R. Influence of Ti, C and N Concentration on the Intergranular Corrosion Behaviour of AISI 316Ti and 321 Stainless Steels. Acta Mater. 2007, 55, 2239–2251. [Google Scholar] [CrossRef]
- Šípová, M.; Marušáková, D.; Aparicio, C.; Procházka, J.; Halodová, P. A study on the corrosion behaviour of stainless steel 08Cr18Ni10Ti in supercritical water. Corros. Sci. 2023, 211, 110853. [Google Scholar] [CrossRef]




| Element | Be | BeO | Fe | C | Al | Si | Mg | N | Cl | Ni | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | >98.3 | <1.3 | <0.16 | <0.15 | <0.10 | <0.08 | <0.05 | <0.05 | <0.04 | <0.03 | <0.08 |
| Material | Fe | C | Cr | Ni | Ti | Si | Mn | P | S |
|---|---|---|---|---|---|---|---|---|---|
| 12Cr18Ni10Ti | Base | 0.12 | 17.00 | 10.66 | 0.50 | 0.80 | 1.67 | 0.03 | 0.02 |
| Sample | No. 1 | No. 2 | No. 3 | No. 4 | ||
|---|---|---|---|---|---|---|
| Side | Top | Side | Bottom | |||
| Wall thickness, mm | 3.32 ± 0.005 | 3.31 ± 0.005 | 3.65 ± 0.005 | 3.30 ± 0.005 | 3.22 ± 0.005 | 2.76 ± 0.005 |
| Sample | No. 1 | No. 2 | No. 3 | No. 4 | ||
|---|---|---|---|---|---|---|
| Side | Top | Side | Bottom | |||
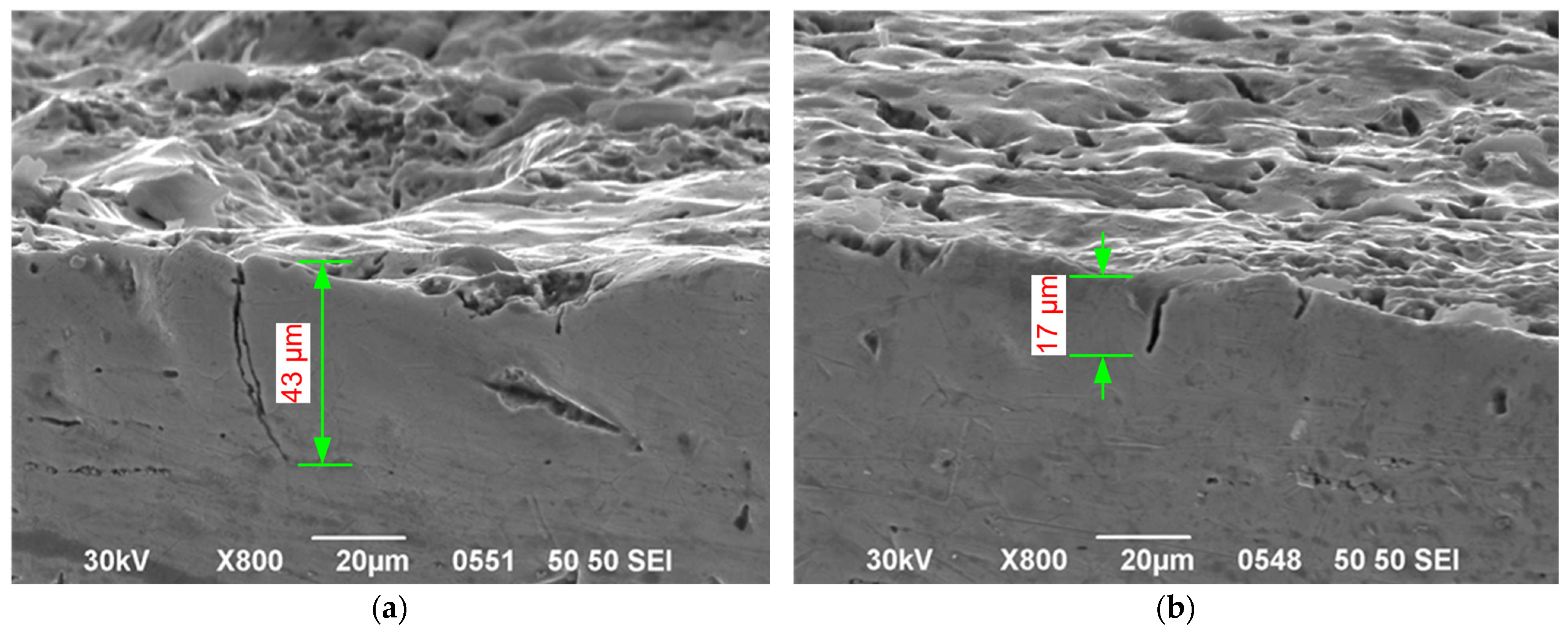
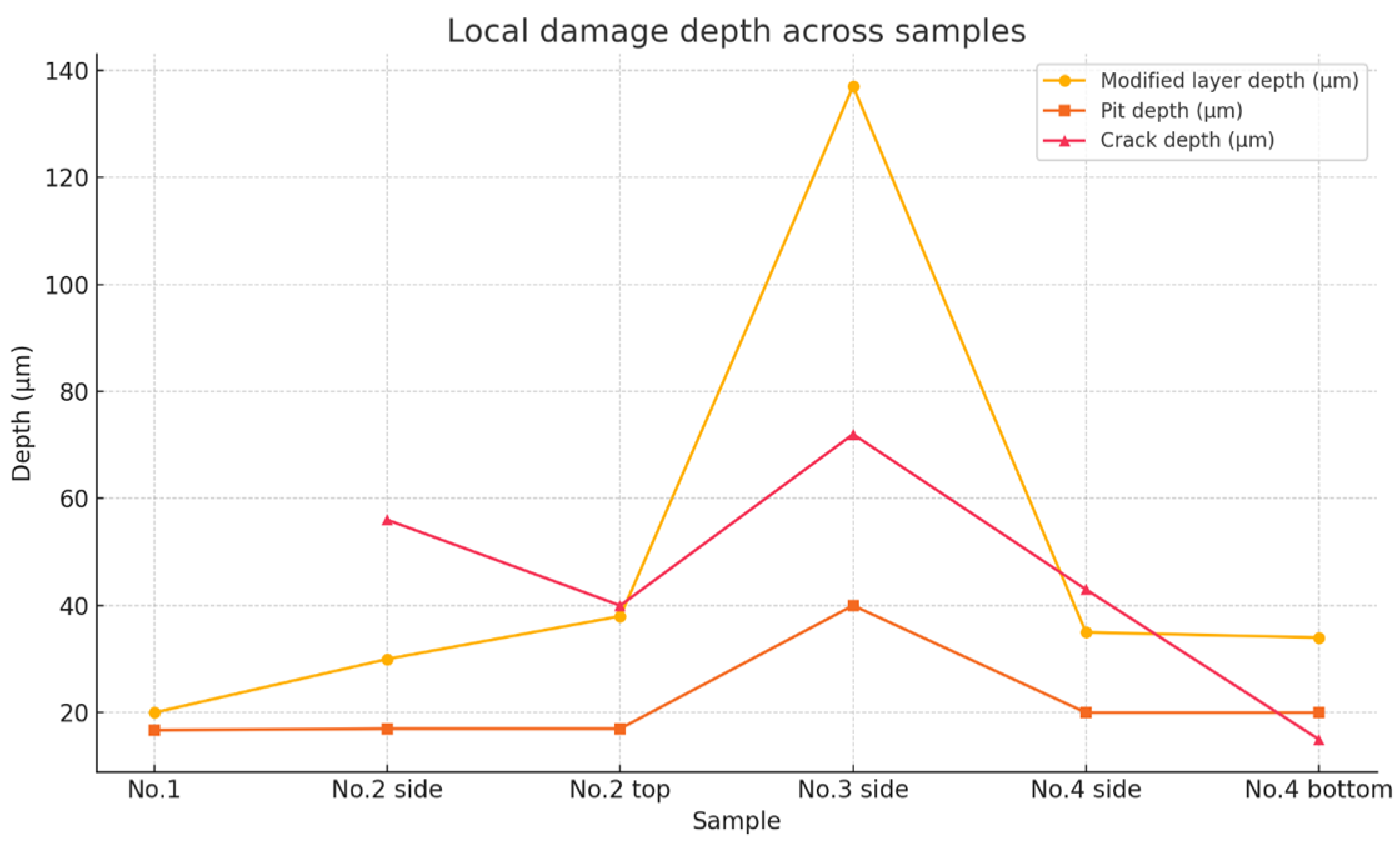
| Maximum depth of modified layer (µm) | 20 | 30 | 38 | 137 | 35 | 34 |
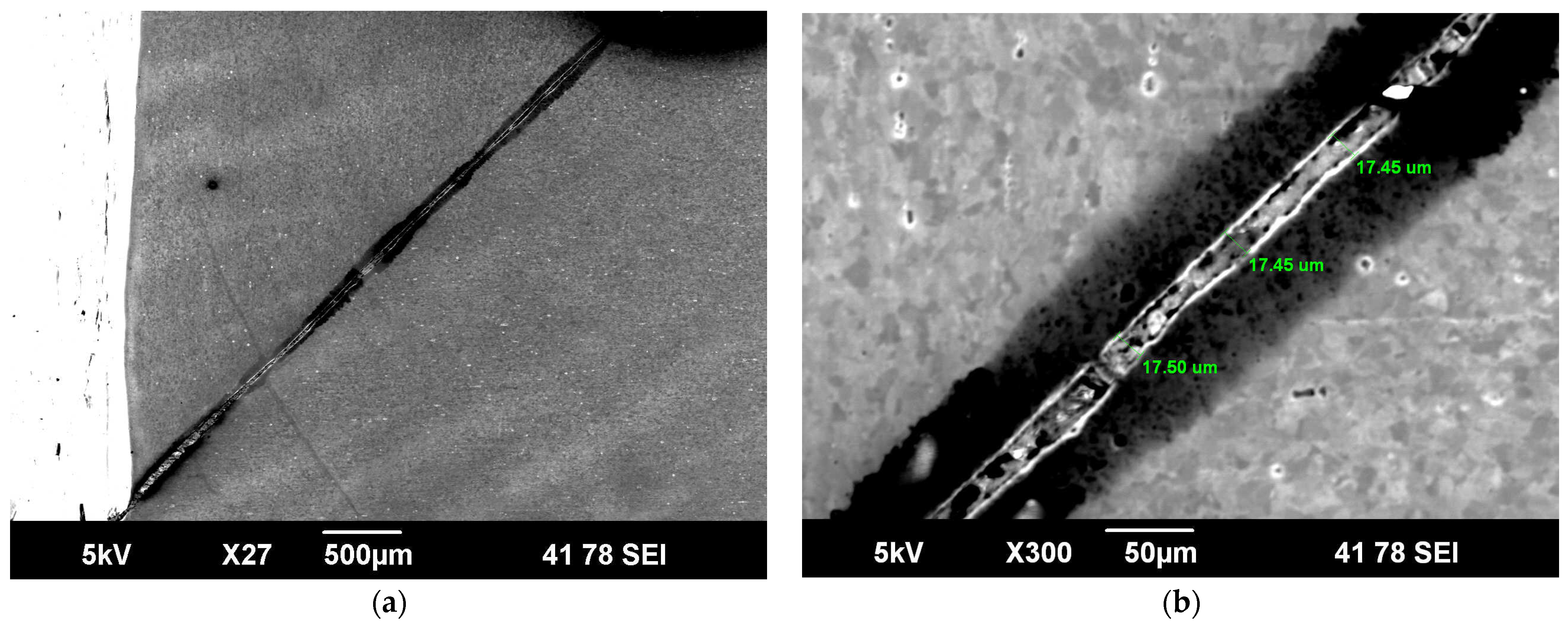
| Maximum pit depth (µm) | 16.7 | 17 | 17 | 40 | 20 | 20 |
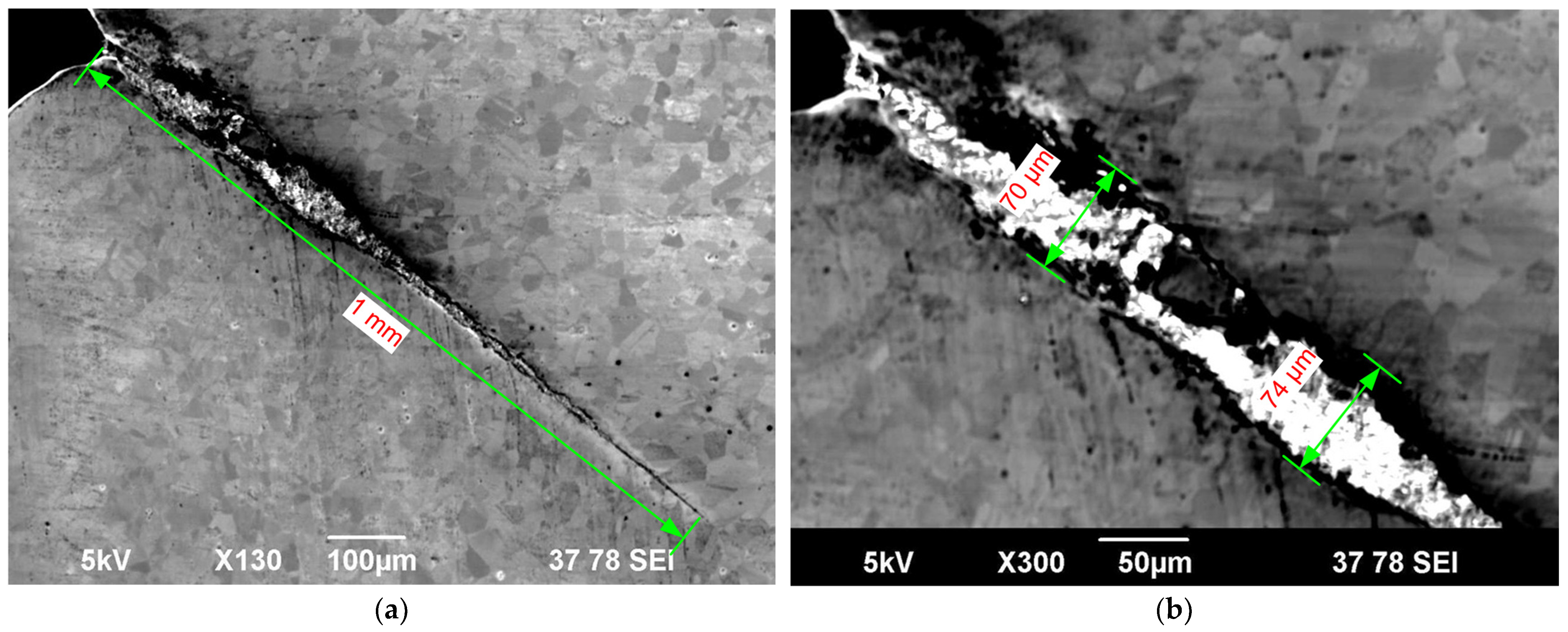
| Maximum crack depth (µm) | No cracking was observed | 56 | 40 | 72 | 43 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baklanova, Y.; Sapatayev, Y.; Samarkhanov, K. High-Temperature Corrosion Behavior of 12Cr18Ni10Ti Grade Austenitic Stainless Steel Under Chlorination Conditions. Metals 2025, 15, 1052. https://doi.org/10.3390/met15091052
Baklanova Y, Sapatayev Y, Samarkhanov K. High-Temperature Corrosion Behavior of 12Cr18Ni10Ti Grade Austenitic Stainless Steel Under Chlorination Conditions. Metals. 2025; 15(9):1052. https://doi.org/10.3390/met15091052
Chicago/Turabian StyleBaklanova, Yuliya, Yerzhan Sapatayev, and Kuanysh Samarkhanov. 2025. "High-Temperature Corrosion Behavior of 12Cr18Ni10Ti Grade Austenitic Stainless Steel Under Chlorination Conditions" Metals 15, no. 9: 1052. https://doi.org/10.3390/met15091052
APA StyleBaklanova, Y., Sapatayev, Y., & Samarkhanov, K. (2025). High-Temperature Corrosion Behavior of 12Cr18Ni10Ti Grade Austenitic Stainless Steel Under Chlorination Conditions. Metals, 15(9), 1052. https://doi.org/10.3390/met15091052






