Recovery of Cu and Fe from a Sphalerite Concentrate by the MnO2–KI Leaching Oxidation System
Abstract
1. Introduction
2. Materials and Methods
2.1. Structural Characterization
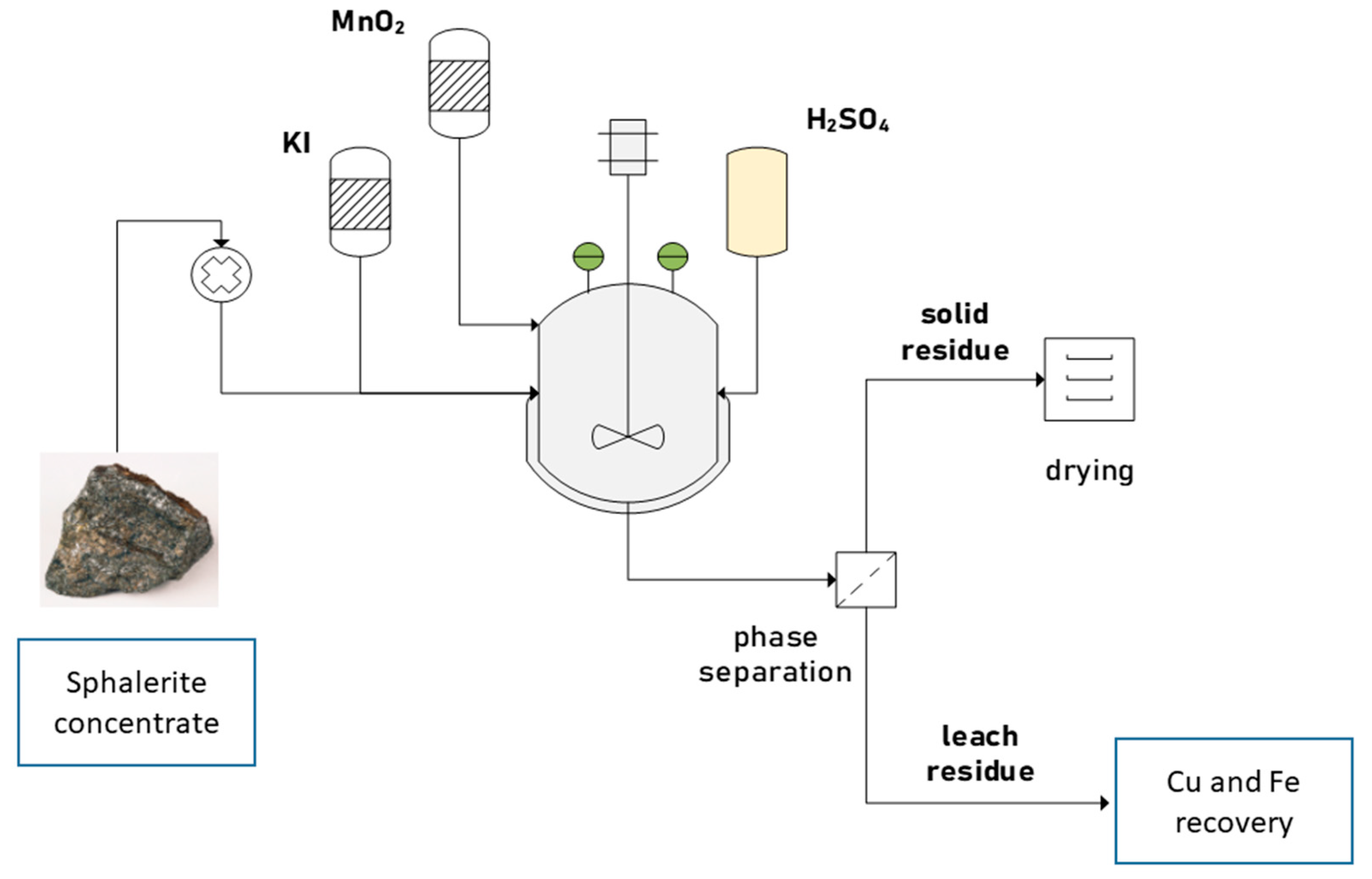
2.2. Leaching Experiments
3. Results and Discussion
3.1. Material Characterization
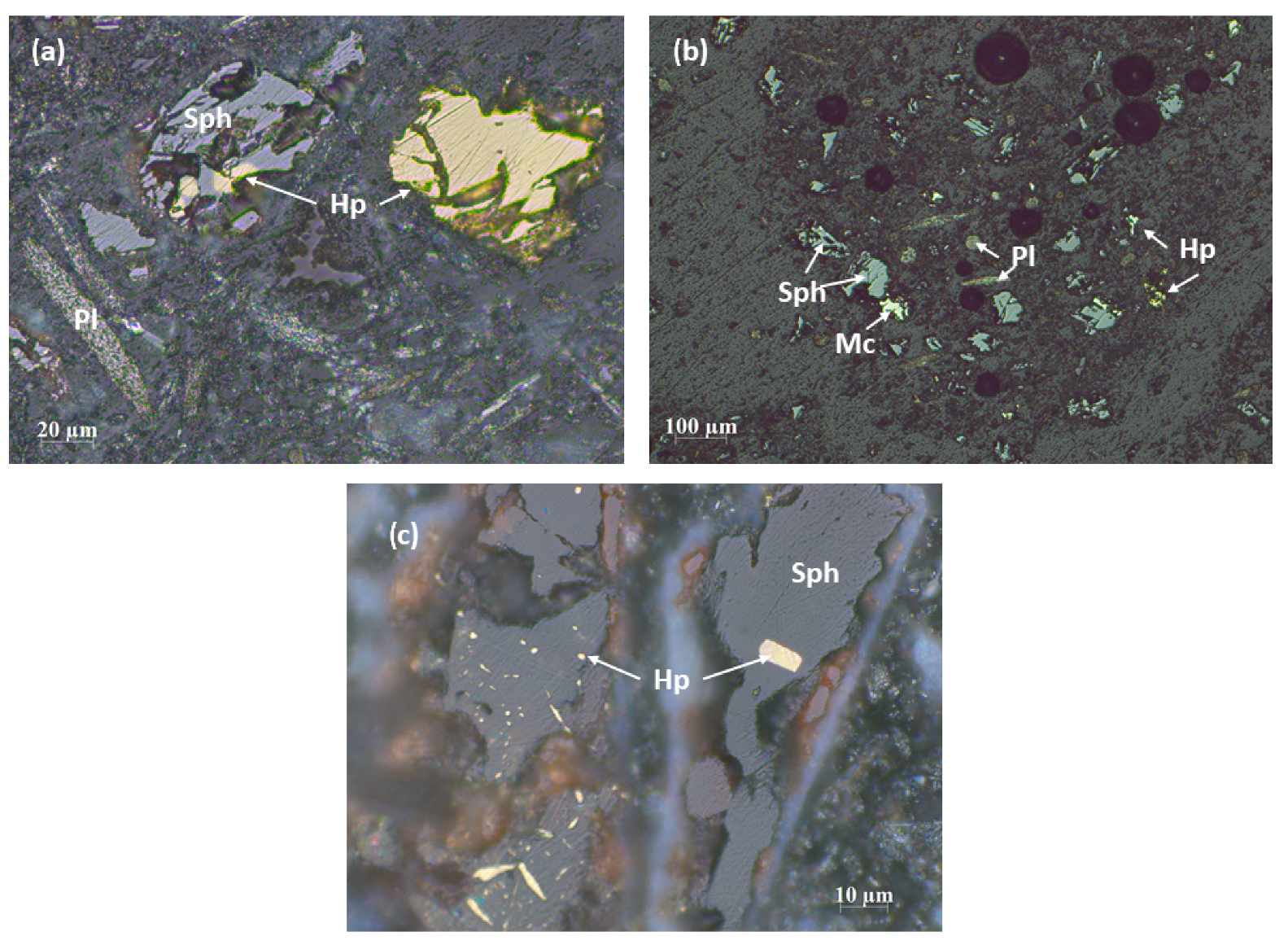
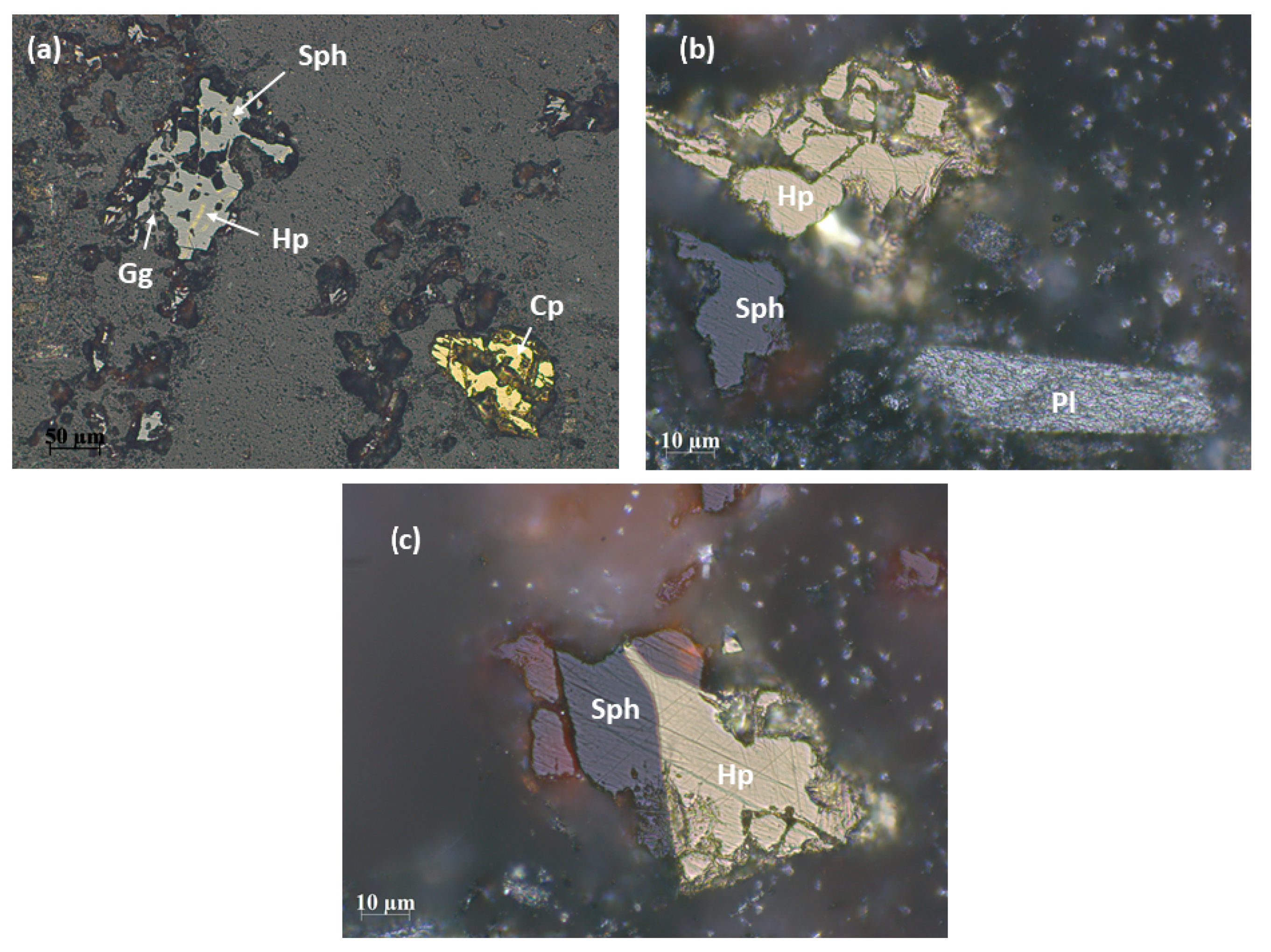
3.1.1. Optical Microscopy
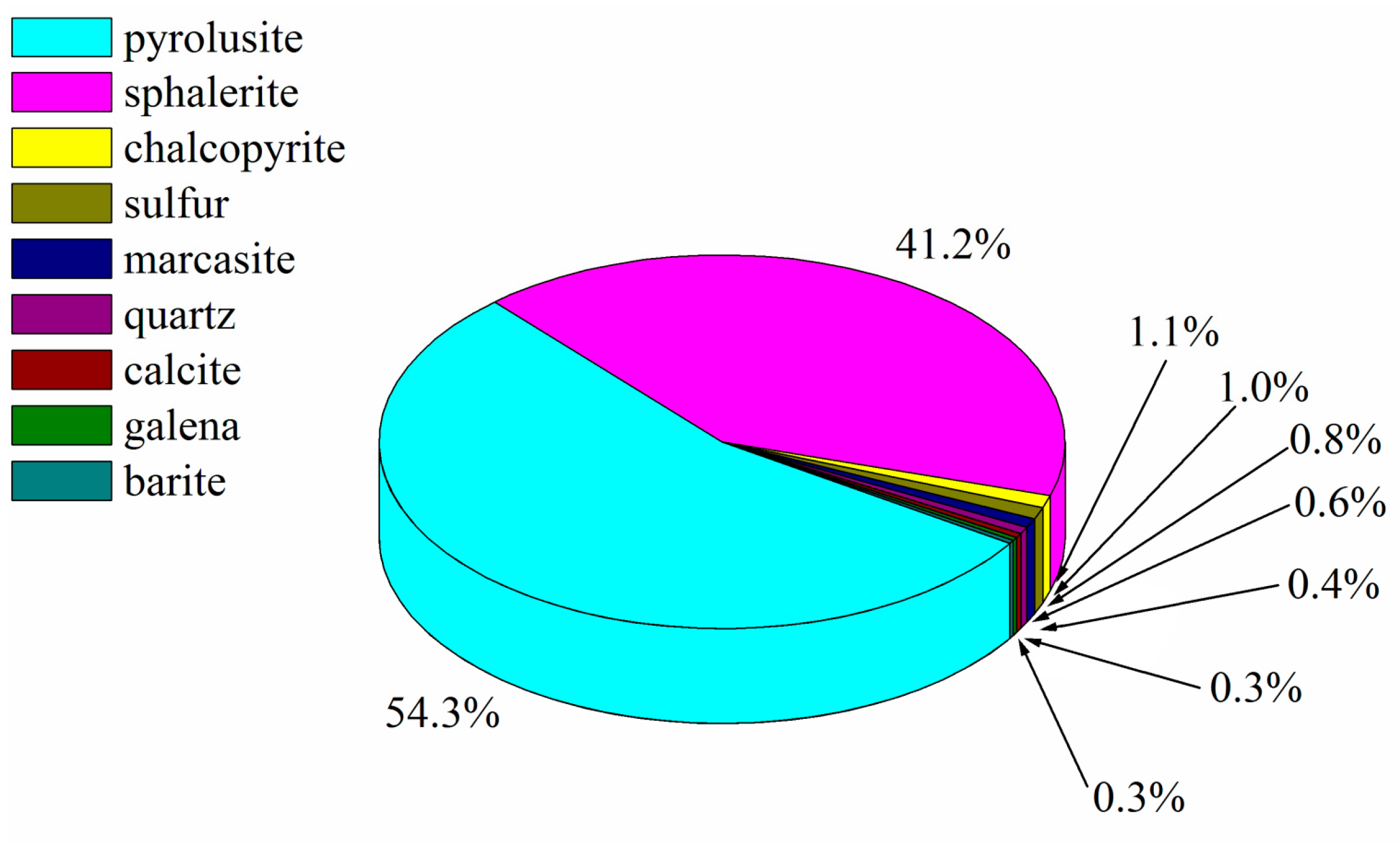
3.1.2. XRD Analysis
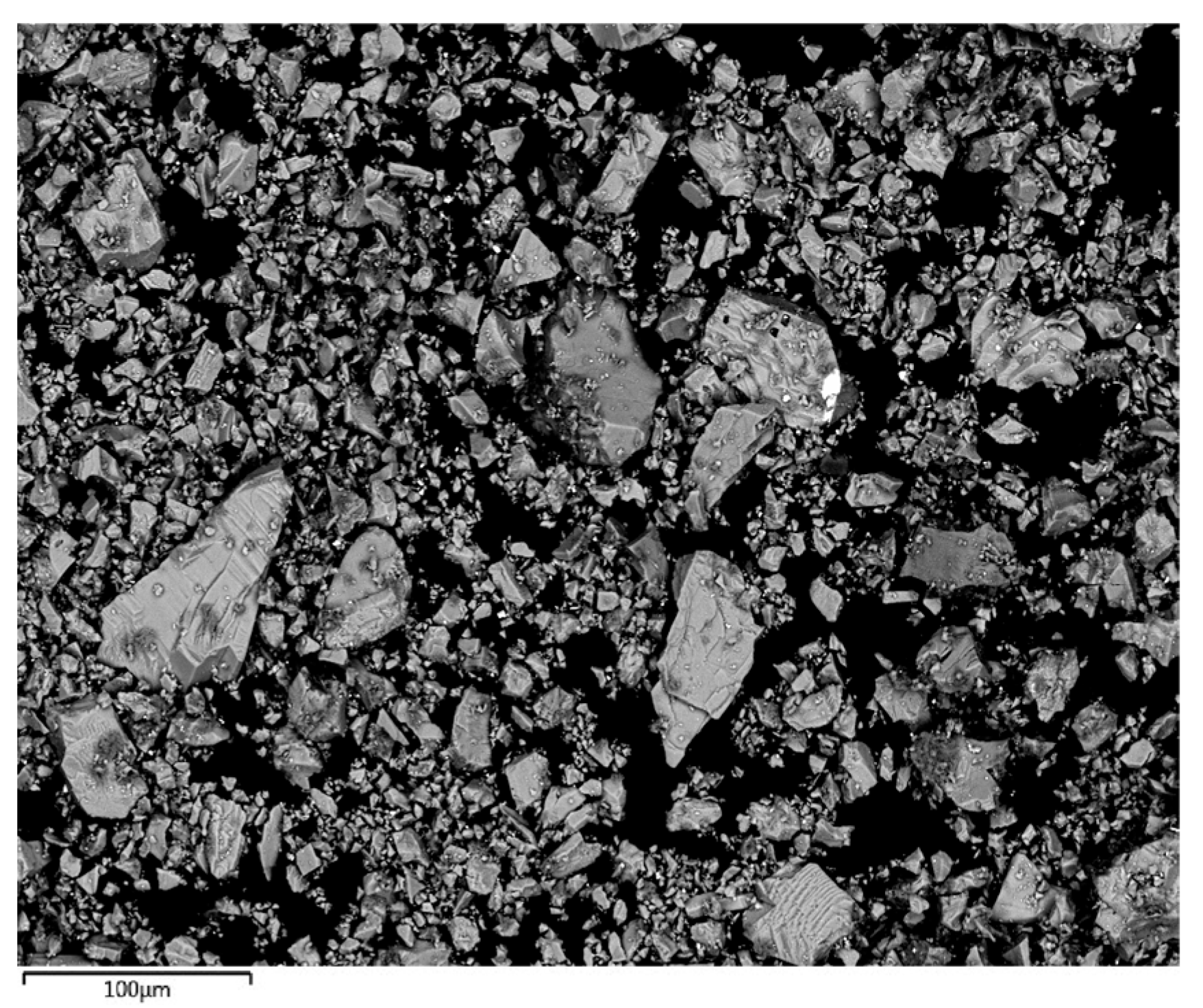
3.1.3. Field Emission SEM
3.2. Leaching Assays
3.2.1. Advanced Oxidative Leaching of Initial Concentrate with MnO2/KI
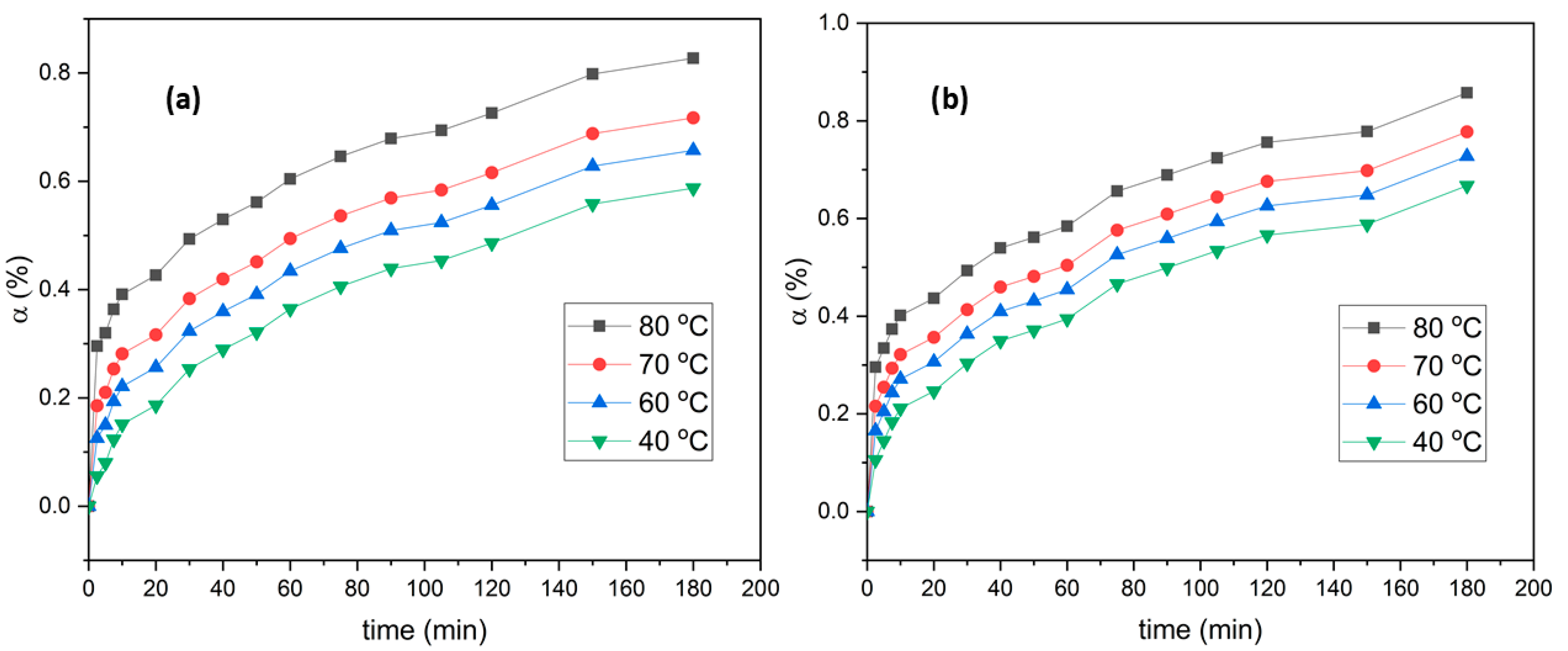
Impact of Temperature on Leaching Degree
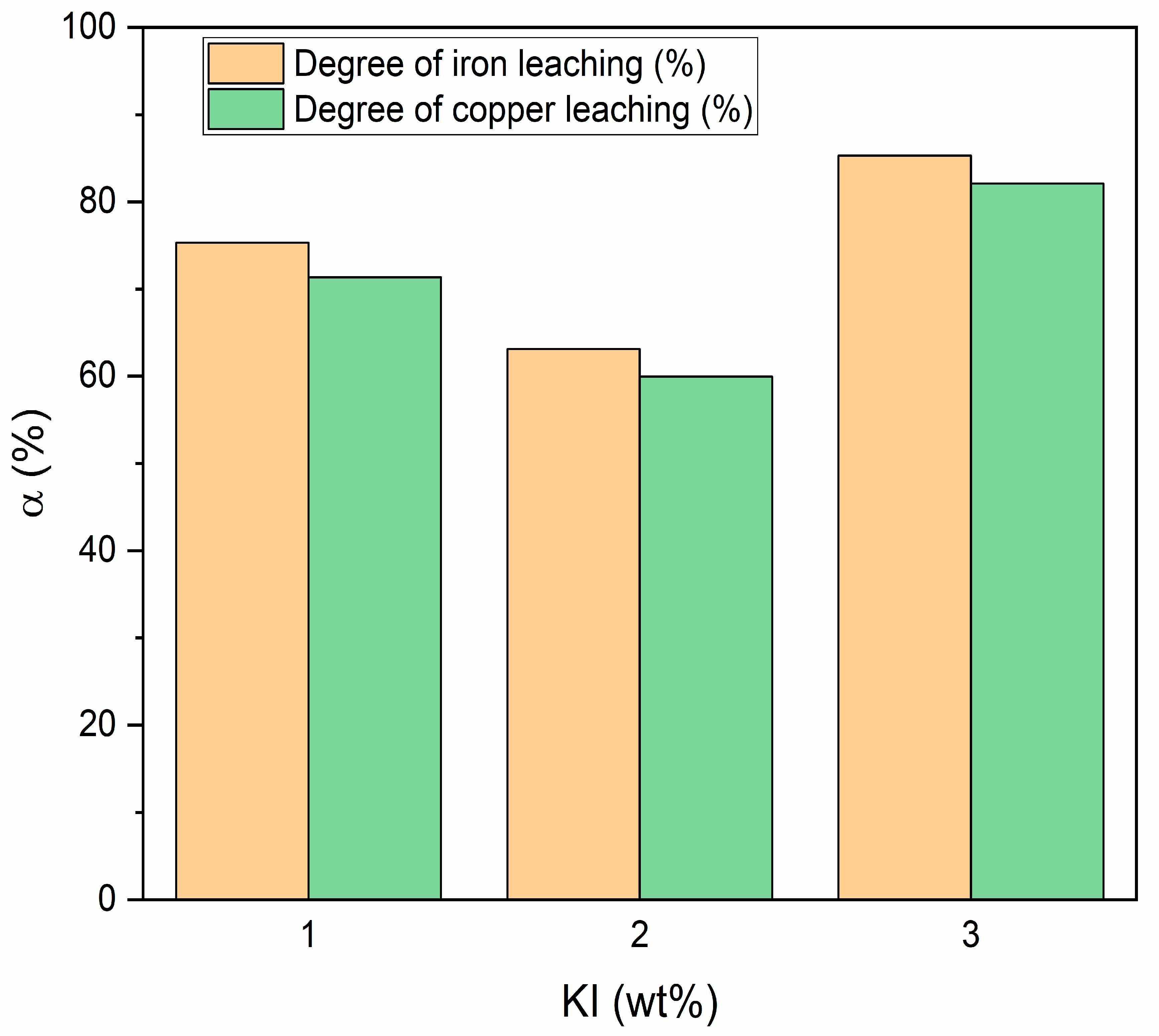
3.2.2. Influence of KI Amount on Leaching Effectiveness
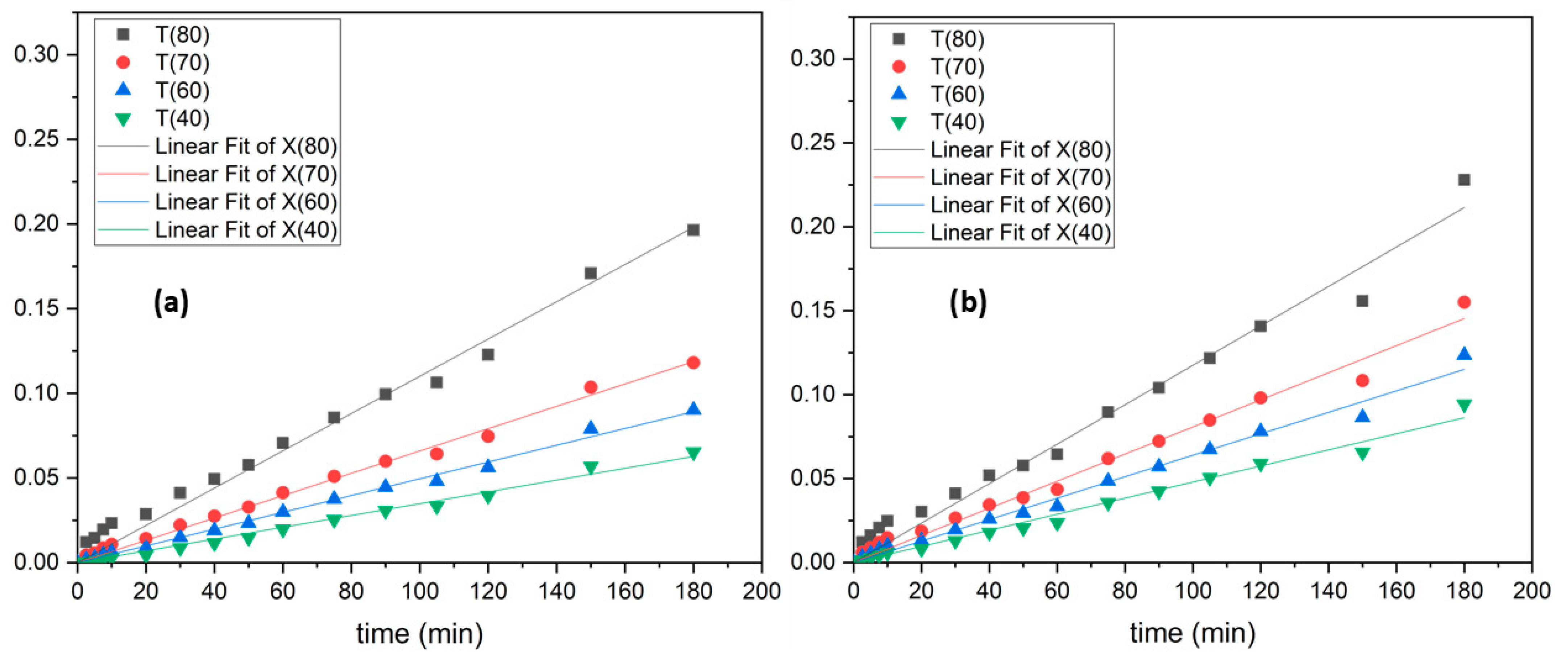
3.3. Leaching Kinetics
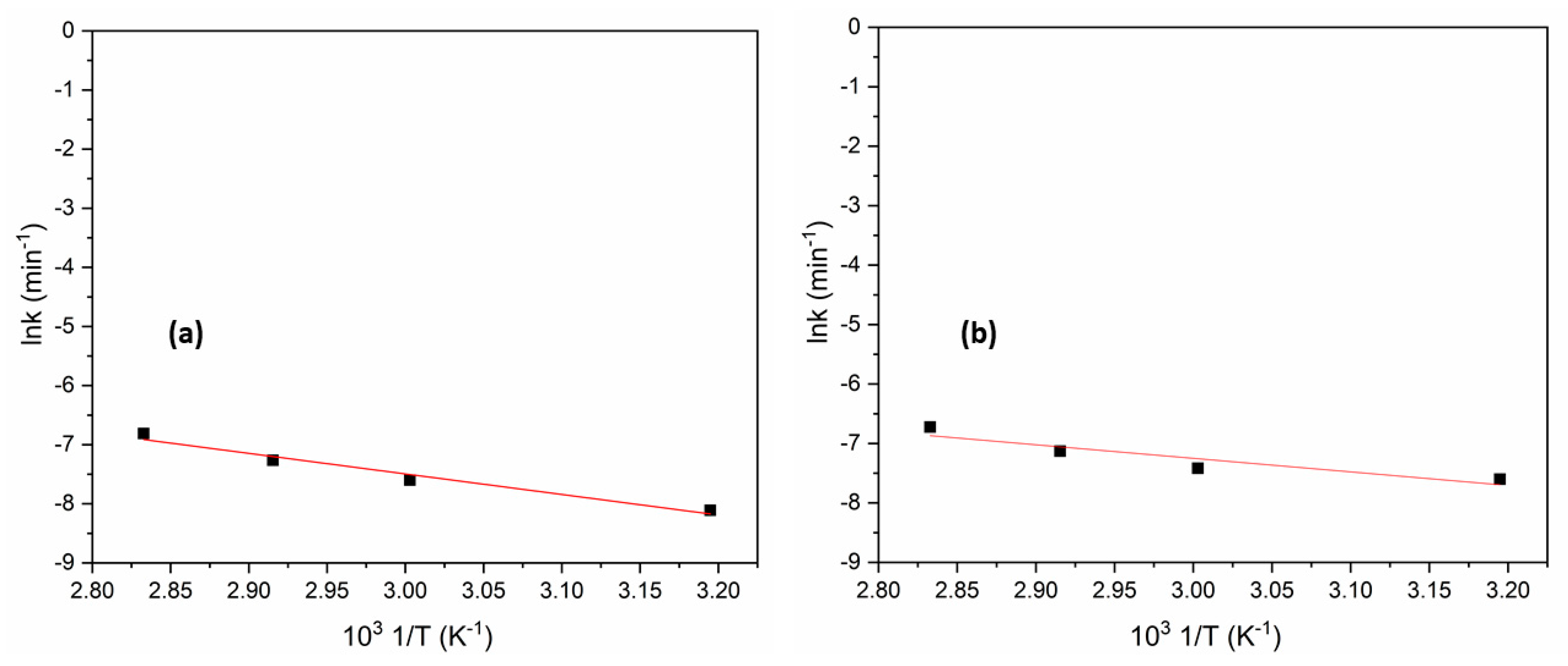
3.4. Determination of Activation Energy for Concentrate Leaching
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jaramillo, K.; Gómez, M.; Colet-Lagrille, M. From Copper Ores to E-Waste Processing: Can Urban Mining Practices Be Actually Integrated into Idle Capacity in Acid-Ferric Hydrometallurgical Facilities? Miner. Eng. 2025, 229, 109355. [Google Scholar] [CrossRef]
- Bogdanović, G.D.; Petrović, S.; Sokić, M.; Antonijević, M.M. Chalcopyrite Leaching in Acid Media: A Review. Metall. Mater. Eng. 2020, 26, 177–198. [Google Scholar] [CrossRef]
- Sokić, M.D.; Bugarčić, M.; Jovanović, A.A. Sphalerite Leaching in Acid Media: A Review. Metall. Mater. Data 2023, 1, 33–43. [Google Scholar] [CrossRef]
- Moskalyk, R.R.; Alfantazi, A.M. Review of Copper Pyrometallurgical Practice: Today and Tomorrow. Miner. Eng. 2003, 16, 893–919. [Google Scholar] [CrossRef]
- Gok, O.; Anderson, C.G. Dissolution of Low-Grade Chalcopyrite Concentrate in Acidified Nitrite Electrolyte. Hydrometallurgy 2013, 134–135, 40–46. [Google Scholar] [CrossRef]
- Shiri, H.R.; Mokmeli, M.; Ghadamgahi, S.M.; Babakhani, A. Deep Eutectic Solvents (DESs) for Chalcopyrite Concentrate Extraction: Leaching, Optimization and Kinetics Mechanism. J. Environ. Chem. Eng. 2025, 13, 117779. [Google Scholar] [CrossRef]
- Ali, Z.; Wilkes, N.; Raza, N.; Omar, M. Modified Hydrometallurgical Approach for the Beneficiation of Copper from Its Low-Grade Ore. ACS Omega 2025, 10, 14826–14834. [Google Scholar] [CrossRef]
- Flores, D.J.; Graber, T.A.; Angel-Castillo, A.H.; Hernández, P.C.; Taboada, M.E. Use of Hydrogen Peroxide as Oxidizing Agent in Chalcopyrite Leaching: A Review. Metals 2025, 15, 531. [Google Scholar] [CrossRef]
- Aghazadeh-Ghomi, M.; Adli-Mehr, A.; Mozammel, M. A Kinetic Study on the Atmospheric Leaching of Chalcopyrite Concentrate in Sulfuric Acid-Hydrogen Peroxide-Isopropanol Medium. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2025, 56, 300–306. [Google Scholar] [CrossRef]
- Panda, S.; Akcil, A.; Pradhan, N.; Deveci, H. Current Scenario of Chalcopyrite Bioleaching: A Review on the Recent Advances to Its Heap-Leach Technology. Bioresour. Technol. 2015, 196, 694–706. [Google Scholar] [CrossRef]
- Behnajady, B.; Najafi, M.; Karimi, S. A New Approach to Direct Chemical Leaching of Sungun Chalcopyrite Concentrate via Green Deep Eutectic Solvent Choline Chloride-ρ-Toluenesulfonic Acid and MD Simulation. J. Taiwan Inst. Chem. Eng. 2025, 172, 106118. [Google Scholar] [CrossRef]
- Adebayo, A.O.; Ipinmoroti, K.O.; Ajayi, O.O. Dissolution Kinetics of Chalcopyrite with Hydrogen Peroxide in Sulphuric Acid Medium. Chem. Biochem. Eng. Q. 2003, 17, 213–218. [Google Scholar]
- Agacayak, T.; Aras, A.; Aydogan, S.; Erdemoglu, M. Leaching of Chalcopyrite Concentrate in Hydrogen Peroxide Solution. Physicochem. Probl. Miner. Process. 2014, 50, 657–666. [Google Scholar]
- Laubertová, M.; Velgosová, O.; Pirošková, J.; Briančin, J. Effects of Microwave Energy and MnO2 from Deep-Sea Polymetallic Nodules as an Oxidizing Agent on the Leaching of Chalcopyrite Concentrate. Minerals 2025, 15, 914. [Google Scholar] [CrossRef]
- Du, Y.; Wang, L.; Zhang, W.; Chen, Y.; Ma, B.; Wang, C. Disparity in Leaching Behavior of Representative Copper Sulfide Minerals with Dilute H2SO4 and the Underlying Microscopic Mechanism. Appl. Surf. Sci. 2025, 706, 163413. [Google Scholar] [CrossRef]
- Cháidez, J.; Parga, J.; Valenzuela, J.; Carrillo, R.; Almaguer, I. Leaching Chalcopyrite Concentrate with Oxygen and Sulfuric Acid Using a Low-Pressure Reactor. Metals 2019, 9, 189. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, J.; Xu, Z.; Wang, J.; Qiu, J.; Xie, Y. Accelerated Metal Separation from Chalcopyrite Assisted with Ozone. Chem. Eng. Process. Process Intensif. 2025, 212, 110263. [Google Scholar] [CrossRef]
- Petrović, S.J.; Bogdanović, G.D.; Antonijević, M.M.; Vukčević, M.; Kovačević, R. The Extraction of Copper from Chalcopyrite Concentrate with Hydrogen Peroxide in Sulfuric Acid Solution. Metals 2023, 13, 1818. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, Á.; Lapidus, G.T. Study of Chalcopyrite Leaching from a Copper Concentrate with Hydrogen Peroxide in Aqueous Ethylene Glycol Media. Hydrometallurgy 2017, 169, 192–200. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, A.; Lázaro, I.; Lapidus, G.T. Improvement Effect of Organic Ligands on Chalcopyrite Leaching in the Aqueous Medium of Sulfuric Acid-hydrogen Peroxide-Ethylene Glycol. Hydrometallurgy 2020, 193, 105293. [Google Scholar] [CrossRef]
- Taboada, M.E.; Jamett, N.E.; Moraga, G.A.; Hernández, P.C.; Graber, T.A. Obtention of Suitable Pregnant Leach Solution (PLS) for Copper Solvent Extraction Plants from Copper Concentrate Using Hydrogen Peroxide and Iodine in a Sulfuric Acid–Chloride Medium. Metals 2024, 14, 817. [Google Scholar] [CrossRef]
- Granata, G.; Miura, A.; Liu, W.; Pagnanelli, F.; Tokoro, C. Iodide-Assisted Leaching of Chalcopyrite in Acidic Ferric Sulfate Media. Hydrometallurgy 2019, 186, 244–251. [Google Scholar] [CrossRef]
- Nurtazina, N.; Uvarov, N.; Azhigulova, R.; Tyapkin, P. Chalcopyrite Leaching by Amino Acid Solutions in the Presence of Hydrogen Peroxide. Physicochem. Probl. Miner. Process. 2022, 58, 157067. [Google Scholar] [CrossRef]
- Laskar, C.; Dakkoune, A.; Julcour, C.; Bourgeois, F.; Biscans, B.; Cassayre, L. Case-Based Analysis of Mechanically-Assisted Leaching for Hydrometallurgical Extraction of Critical Metals from Ores and Wastes: Application in Chalcopyrite, Ferronickel Slag, and Ni-MH Black Mass. Comptes Rendus. Chimie 2024, 27, 37–52. [Google Scholar] [CrossRef]
- Zandevakili, S.; Akhondi, M.R. Microwave-Assisted Leaching for Copper Recovery from the Chalcopyrite Concentrate of Sarcheshmeh Copper Complex. Int. J. Min. Geo-Eng. 2022, 56, 277–284. [Google Scholar]
- Karimov, K.; Tretiak, M.; Rogozhnikov, D. The Dissolution Behavior of Pyrite and Chalcopyrite in Their Mixture During Low-Temperature Pressure Oxidation: A Kinetic Analysis. Materials 2025, 18, 551. [Google Scholar] [CrossRef]
- Aydoğan, S.; Aras, A.; Canbazoğlu, M. Oxidative Ammonia Leaching of Sphalerite Concentrate. J. Fac. Eng. Arch. Selcuk Univ. 2005, 20, 55–62. [Google Scholar]
- Mubarok, M.Z.; Sukamto, K.; Ichlas, Z.T.; Sugiarto, A.T. Direct Sulfuric Acid Leaching of Zinc Sulfide Concentrate Using Ozone as Oxidant under Atmospheric Pressure. Miner. Metall. Process. 2018, 35, 133–140. [Google Scholar] [CrossRef]
- Wang, J.; Faraji, F.; Ghahreman, A. Evaluation of Ozone as an Efficient and Sustainable Reagent for Chalcopyrite Leaching: Process Optimization and Oxidative Mechanism. J. Ind. Eng. Chem. 2021, 104, 333–344. [Google Scholar] [CrossRef]
- Conić, V.; Stanković, S.; Marković, B.; Božić, D.; Stojanović, J.; Sokić, M. Investigation of the Optimal Technology for Copper Leaching from Old Flotation Tailings of the Copper Mine Bor (Serbia). Metall. Mater. Eng. 2020, 26, 209–222. [Google Scholar] [CrossRef]
- Stanković, S.; Goldmann, S.; Kraemer, D.; Ufer, K.; Schippers, A. Bioleaching of a Lateritic Ore (Piauí, Brazil) in Percolators. Hydrometallurgy 2024, 224, 106262. [Google Scholar] [CrossRef]
- Bakhti, A.; Moghimi, H.; Bozorg, A.; Stankovic, S.; Manafi, Z.; Schippers, A. Comparison of Bioleaching of a Sulfidic Copper Ore (Chalcopyrite) in Column Percolators and in Stirred-Tank Bioreactors Including Microbial Community Analysis. Chemosphere 2024, 349, 140945. [Google Scholar] [CrossRef]
- Cruells, M.; Roca, A. Jarosites: Formation, Structure, Reactivity and Environmental. Metals 2022, 12, 802. [Google Scholar] [CrossRef]
- Dakubo, F.; Baygents, J.C.; Farrell, J. Peroxodisulfate Assisted Leaching of Chalcopyrite. Hydrometallurgy 2012, 121–124, 68–73. [Google Scholar] [CrossRef]
- Turan, M.D.; Arslanoğlu, H.; Altundoğan, H.S. Optimization of the Leaching Conditions of Chalcopyrite Concentrate Using Ammonium Persulfate in an Autoclave System. J. Taiwan Inst. Chem. Eng. 2015, 50, 49–55. [Google Scholar] [CrossRef]
- Jorjani, E.; Ghahreman, A. Challenges with Elemental Sulfur Removal during the Leaching of Copper and Zinc Sulfides, and from the Residues; a Review. Hydrometallurgy 2017, 171, 333–343. [Google Scholar] [CrossRef]
- Li, L.; Soleymani, M.; Ghahreman, A. New Insights on the Role of Lattice-Substituted Silver in Catalytic Oxidation of Chalcopyrite. Electrochim. Acta 2021, 369, 137652. [Google Scholar] [CrossRef]
- Fukano, Y.; Miura, A. Chalcopyrite Leaching with Iodine (JX Iodine Process) for Various Ore Types. Hydrometallurgy 2021, 206, 105752. [Google Scholar] [CrossRef]
- Winarko, R.; Dreisinger, D.B.; Miura, A.; Fukano, Y.; Liu, W. Characterization of the Solid Leach Residues from the Iodine-Assisted Chalcopyrite Leaching in Ferric Sulfate Media. Hydrometallurgy 2024, 226, 106302. [Google Scholar] [CrossRef]
- Manabe, M. Method of Leaching Copper Sulfide Ore with the Use of Iodine. U.S. Patent No. 8,163,063, 24 April 2012. [Google Scholar]
- Kuwano, K.; Abe, A.; Manabe, M.; Miura, A. Method of Leaching Copper Sulfide Ore. U.S. Patent No. 20110229385, 4 September 2012. [Google Scholar]
- Bugarčić, M.; Jovanović, A.; Anđić, D.; Jelić, I.; Miletić, M.; Marković, B.; Sokić, M. Kinetics of Sphalerite Leaching by MnO2-KI Oxidation System in Sulfuric Acid. Metals 2025, 15, 50. [Google Scholar] [CrossRef]
- Sharp, J.H.; Brindley, G.W.; Achar, B.N.N. Numerical Data for Some Commonly Used Solid State Reaction Equations. J. Am. Ceram. Soc. 1966, 49, 379–382. [Google Scholar] [CrossRef]
- Ojeda, C.; Jaques, A.; Aracena, A. Optimal Sampling Times for Leaching Experiments. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2022, 53, 1082–1088. [Google Scholar] [CrossRef]
- Fu, W.; Meng, L.; Qu, J. Sintering Mechanism and Leaching Kinetics of Low-Grade Mixed Lithium Ore and Limestone. Metals 2024, 14, 1075. [Google Scholar] [CrossRef]
- Liu, X.; Du, Z.; Sun, C.; Zhang, N. A Review on the Electrochemical Analysis of Sulfide Minerals—Pyrite, Chalcopyrite, and Galena. Green Smart Min. Eng. 2025, 2, 18–31. [Google Scholar] [CrossRef]
- Liu, X.J.; Liao, Y.; Ma, H.; Liu, Q. Electrochemical Characterizations and Galvanic Effect of Chalcopyrite Leaching and Passivation-A Review. Miner. Eng. 2024, 210, 108673. [Google Scholar] [CrossRef]
- Ramette, R.W.; Sandford, R.W. Thermodynamics of Iodine Solubility and Triiodide Ion Formation in Water and in Deuterium Oxide. J. Am. Chem. Soc. 1955, 77, 1068. [Google Scholar] [CrossRef]
- Winarko, R.; Dreisinger, D.B.; Miura, A.; Fukano, Y.; Liu, W. Iodine-Assisted Chalcopyrite Leaching in Ferric Sulfate Media: Kinetic Study under Fully Controlled Redox Potential and PH. Hydrometallurgy 2022, 208, 105797. [Google Scholar] [CrossRef]
- Kauffman, G.B.; Fang, L.Y.; Viswanathan, N.; Townsend, G. Purification of Copper (I) Iodide. Inorg. Synth. 1984, 22, 101–103. [Google Scholar]
- Breed, A.W.; Harrison, S.T.L.; Hansford, G.S. A Preliminary Investigation of the Ferric Leaching of a Pyrite/Arsenopyrite Flotation Concentrate. Miner. Eng. 1997, 10, 1023–1030. [Google Scholar] [CrossRef]
- Li, L.; Ghahreman, A. The Synergistic Effect of Cu2+-Fe2+-Fe3+ Acidic System on the Oxidation Kinetics of Ag-Doped Pyrite. J. Phys. Chem. C 2018, 122, 26897–26909. [Google Scholar] [CrossRef]
- Picazo-Rodríguez, N.G.; Soria-Aguilar, M.D.J.; Martínez-Luévanos, A.; Almaguer-Guzmán, I.; Chaidez-Félix, J.; Carrillo-Pedroza, F.R. Direct Acid Leaching of Sphalerite: An Approach Comparative and Kinetics Analysis. Minerals 2020, 10, 359. [Google Scholar] [CrossRef]
- Weisener, C.G.; Smart, R.S.C.; Gerson, A.R. A Comparison of the Kinetics and Mechanism of Acid Leaching of Sphalerite Containing Low and High Concentrations of Iron. Int. J. Miner. Process 2004, 74, 239–249. [Google Scholar] [CrossRef]
- Weisener, C.G.; Smart, R.S.C.; Gerson, A.R. Kinetics and Mechanisms of the Leaching of Low Fe Sphalerite. Geochim. Cosmochim. Acta 2003, 67, 823–830. [Google Scholar] [CrossRef]
- Debernardi, G.; Carlesi, C. Chemical-Electrochemical Approaches to the Study Passivation of Chalcopyrite. Miner. Process. Extr. Metall. Rev. 2013, 34, 10–41. [Google Scholar] [CrossRef]
- Hackl, R.P.; Dreisinger, D.B.; Peters, E.; King, J.A. Passivation of Chalcopyrite during Oxidative Leaching in Sulfate Media. Hydrometallurgy 1995, 39, 25–48. [Google Scholar] [CrossRef]
- Kaplun, K.; Li, J.; Kawashima, N.; Gerson, A.R. Cu and Fe Chalcopyrite Leach Activation Energies and the Effect of Added Fe3+. Geochim. Cosmochim. Acta 2011, 75, 5865–5878. [Google Scholar] [CrossRef]
- Asta, M.P.C.; Acero, P. Dissolution Kinetics of Marcasite at Acidic PH. Eur. J. Mineral. 2010, 22, 49–61. [Google Scholar] [CrossRef]

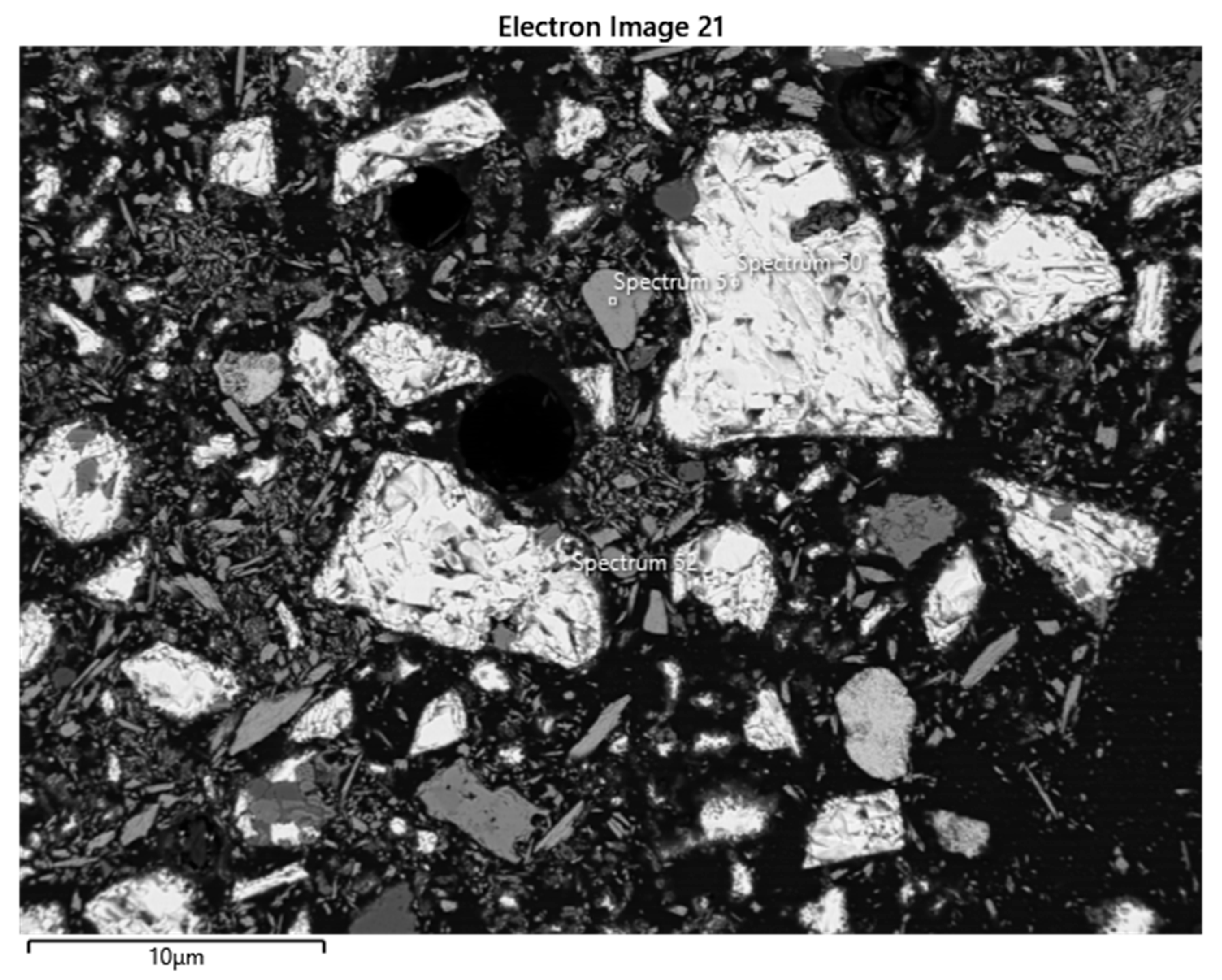

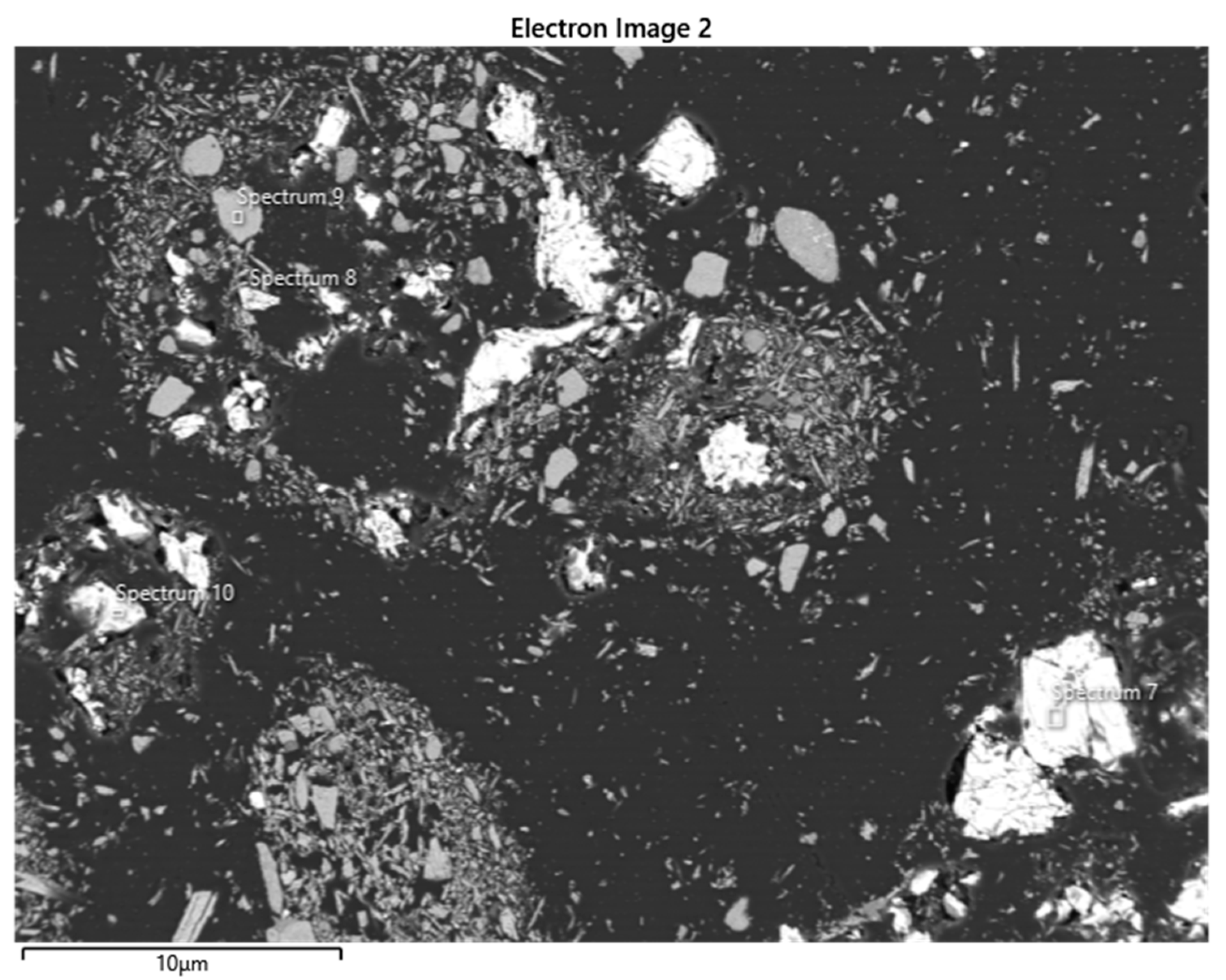

| Element | Spectrum 50 (Sphalerite) | Spectrum 51 (Pyrolusite) | Spectrum 52 (Chalcopyrite) | |||
|---|---|---|---|---|---|---|
| Weight% | Atomic% | Weight% | Weight% | Weight% | Atomic% | |
| O | 30.83 | 60.19 | ||||
| Al | 0.22 | 0.25 | ||||
| Si | 0.60 | 0.67 | ||||
| S | 33.38 | 49.81 | 0.28 | 0.27 | 35.18 | 50.28 |
| Mn | 0.42 | 0.37 | 66.89 | 38.04 | 0.41 | 0.34 |
| Fe | 12.05 | 10.33 | 29.69 | 24.36 | ||
| Cu | 0.36 | 0.27 | 33.02 | 23.82 | ||
| Ni | 0.23 | 0.12 | ||||
| Zn | 53.30 | 39.02 | 0.95 | 0.45 | 1.71 | 1.20 |
| Cd | 0.48 | 0.20 | ||||
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Element | Spectrum 7 (Sphalerite) | Spectrum 8 (Chalcopyrite) | Spectrum 9 (Pyrolusite) | Spectrum 10 (Marcasite) | ||||
|---|---|---|---|---|---|---|---|---|
| Weight % | Atomic % | Weight % | Weight % | Weight % | Atomic % | Weight % | Atomic % | |
| O | 30.39 | 59.90 | ||||||
| S | 32.47 | 48.68 | 34.27 | 49.19 | 0.33 | 0.33 | 38.88 | 52.56 |
| Mn | 0.83 | 0.73 | 2.29 | 1.92 | 69.28 | 39.77 | 0.66 | 0.52 |
| Fe | 11.91 | 10.26 | 29.71 | 24.48 | 60.46 | 46.92 | ||
| Cu | 1.98 | 1.50 | 33.29 | 24.11 | ||||
| Zn | 52.81 | 38.84 | 0.43 | 0.31 | ||||
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanović, A.; Anđić, D.; Bugarčić, M.; Jelić, I.; Vujović, N.; Anderson, C.; Sokić, M. Recovery of Cu and Fe from a Sphalerite Concentrate by the MnO2–KI Leaching Oxidation System. Metals 2025, 15, 1039. https://doi.org/10.3390/met15091039
Jovanović A, Anđić D, Bugarčić M, Jelić I, Vujović N, Anderson C, Sokić M. Recovery of Cu and Fe from a Sphalerite Concentrate by the MnO2–KI Leaching Oxidation System. Metals. 2025; 15(9):1039. https://doi.org/10.3390/met15091039
Chicago/Turabian StyleJovanović, Aleksandar, Dimitrije Anđić, Mladen Bugarčić, Ivana Jelić, Nela Vujović, Corby Anderson, and Miroslav Sokić. 2025. "Recovery of Cu and Fe from a Sphalerite Concentrate by the MnO2–KI Leaching Oxidation System" Metals 15, no. 9: 1039. https://doi.org/10.3390/met15091039
APA StyleJovanović, A., Anđić, D., Bugarčić, M., Jelić, I., Vujović, N., Anderson, C., & Sokić, M. (2025). Recovery of Cu and Fe from a Sphalerite Concentrate by the MnO2–KI Leaching Oxidation System. Metals, 15(9), 1039. https://doi.org/10.3390/met15091039







