ISE of Precious Metals: Au, Ag, Pd, and Pt
Abstract
1. Introduction
2. Materials and Methods
2.1. Gold (Au)
2.1.1. Physical and Chemical Properties
2.1.2. Applications
2.2. Silver (Ag)
2.2.1. Physical and Chemical Properties
2.2.2. Antimicrobial Properties
2.2.3. Applications
2.3. Platinum (Pt)
2.3.1. Physical and Chemical Properties
2.3.2. Catalytic Properties
2.3.3. Applications
2.4. Palladium (Pd)
2.4.1. Physical and Chemical Properties
2.4.2. Catalytic Properties and Hydrogen Applications
2.4.3. Applications
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Verhoeven, G. Resolving some spatial resolution issues—Part 1: Between line pairs and sampling distance. AARGnews 2018, 57, 25–35. [Google Scholar] [CrossRef]
- Westbrook, H.; Conrad, H. The Science of Hardness Testing and Its Research Application; American Society for Metals: Metals Park, Novelty, OH, USA, 1973. [Google Scholar]
- Smedskjaer, M.M. Indentation size effect and the plastic compressibility of glass. Appl. Phys. Lett. 2014, 104, 251906. [Google Scholar] [CrossRef]
- Kazembeyki, M.; Bauchy, M.; Hoover, C.G. New insights into the indentation size effect in silicate glasses. J. Non-Cryst. Solids 2019, 521, 119494. [Google Scholar] [CrossRef]
- Kazembeyki, M.; Yang, K.; Mauro, J.C.; Smedskjaer, M.M.; Bauchy, M.; Hoover, C.G. Decoupling of indentation modulus and hardness in silicate glasses: Evidence of ashear- to densification-dominated transition. J. Non-Cryst. Solids 2021, 553, 120518. [Google Scholar] [CrossRef]
- Quinn, G.D.; Green, P.; Xu, K. Cracking and the indentation size effect for knoop hardness of glasses. J. Am. Ceram. Soc. 2003, 86, 441–448. [Google Scholar] [CrossRef]
- Jang, J.; Yoo, B.-G.; Kim, Y.-J.; Oh, J.-H.; Choi, I.-C.; Bei, H. Indentation size effect in bulk metallic glass. Scr. Mater. 2011, 64, 753–756. [Google Scholar] [CrossRef]
- Li, H.; Bradt, R.C. The indentation load/size effect and the measurement of the hardness of vitreous silica. J. Non-Cryst. Solids 1992, 146, 197–212. [Google Scholar] [CrossRef]
- Bull, S.J.; Page, T.F.; Yoffe, E.H. An explanation of the indentation size effect in ceramics. Philos. Mag. Lett. 1989, 59, 281–288. [Google Scholar] [CrossRef]
- Gong, J.; Wu, J.; Guan, Z. Examination of the Indentation Size Effect in Low-Load Vickers Hardness Testing of Ceramics. J. Eur. Ceram. Soc. 1999, 19, 2625–2631. [Google Scholar] [CrossRef]
- Elmustafa, A.A.; Stone, D.S. Indentation size effect in polycrystalline F.C.C. metals. Acta Mater. 2002, 50, 3641–3650. [Google Scholar] [CrossRef]
- Zong, Z.; Lou, J.; Adewoye, O.O.; Elmustafa, H.F.; Soboyejo, W.O. Indentation size effects in the nano- and micro-hardness of fcc single crystal metals. Mater. Sci. Eng. 2006, 434, 178–187. [Google Scholar] [CrossRef]
- Durst, K.; Backes, B.; Franke, O.; Göoken, M. Indentation size effect in metallic materials: Modeling strength from pop-in to macroscopic hardness using geometrically necessary dislocations. Acta Mater. 2006, 54, 2547–2555. [Google Scholar] [CrossRef]
- Nix, W.D.; Gao, H. Indentation size effects in crystalline: A law for strain gradient plasticity. J. Mech. Phys. Solids 1998, 46, 411–425. [Google Scholar] [CrossRef]
- Bernhardt, E.O. Über die Mikrohärte der Feststoffe im Grenzbereich des Kick’schen Ahnlichkeitssatzes. Int. J. Mater. Res. 1941, 33, 135–144. [Google Scholar] [CrossRef]
- Shi, Y.; Deng, B.; Neuefeind, J.; Zhou, Q.; Smedskjaer, M.M.; Elliott, S.R.; Bauchy, M. Revealing the effect of medium-range structure on silicate glass hardness. Phys. Rev. Mater. 2023, 7, 013602. [Google Scholar] [CrossRef]
- Li, H.; Ghosh, A.; Han, Y.H.; Bradt, R.C. The frictional component of the indentation size effect in low load microhardness testing. J. Mater. Res. 1993, 8, 1028–1032. [Google Scholar] [CrossRef]
- Swain, M.V.; Wittling, M. Indentation Size Effects for Ceramics: Is There a Fracture Mechanics Explanation? Fracture Mechanics of Ceramics; Plenum: New York, NY, USA, 1996; Volume 11, pp. 379–387. [Google Scholar]
- Shrestha, P.; Smedskjaer, M.M.; Bauchy, M.; Hoover, G. Impact of strain rate on the Indentation Size Effect: Evidence of an intimate link between Size effect, Strain Rate and Ductility in Soda-lime Silica glass. J. Non-Cryst. Solids 2024, 637, 123031. [Google Scholar] [CrossRef]
- Sangwal, K.; Surowska, B.; Błaziak, P. Analysis of the Indentation Size Effect in the Microhardness Measurement of Some Cobalt-Based Alloys. Mater. Chem. Phys. 2003, 77, 511–520. [Google Scholar] [CrossRef]
- ISO 6507-1; Metallic materials—Vickers hardness test Part 1—Test method. International Organization for Standardization ISO: Geneva, Switzerland, 2018.
- ISO 14577-1; Metallic materials—Instrumented indentation test for hardness and materials parameters Part 1—Test method. International Organization for Standardization ISO: Geneva, Switzerland, 2015.
- Ma, Q.; Clarke, D.R. Size dependent hardness of silver single crystals. J. Mater. Res. 1995, 10, 853–863. [Google Scholar] [CrossRef]
- Ren, X.J.; Hooper, R.M.; Griffiths, C.; Henshall, J.L. Indentation Size Effect in Ceramics: Correlation with H/E. J. Mater. Sci. Lett. 2003, 22, 1105–1106. [Google Scholar] [CrossRef]
- Sangwal, K. On the Reverse Indentation Size Effect and Microhardness Measurement of Solids. Mater. Chem. Phys. 2000, 63, 145–152. [Google Scholar] [CrossRef]
- Navrátil, V.; Novotná, J. Some Problems Of Microhardness Of Metals. J. Appl. Math. 2009, 2, 241–244. Available online: https://www.researchgate.net/publication/268268064_SOME_PROBLEMS_OF_MICRO-HARDNESS_OF_METALS (accessed on 29 June 2025).
- Gold Nanoparticules and Uses in Cancer. Acta Pharmaceutica Turcica. 2021. Available online: https://dergipark.org.tr/tr/doi/10.33483/jfpau.773430 (accessed on 12 March 2023).
- Faricha, A.; Chakraborty, P.; Chang, T.-F.M.; Sone, M.; Nakamoto, T. New Trend of Amperometric Gas Sensors Using Atomic Gold-Decorated Platinum/Polyaniline Composites. Chemosensors 2024, 12, 27. [Google Scholar] [CrossRef]
- Akh, P.A. Vernonia amygdalina Del (Bitter leaf) a traditional anti-diabetic gold mine—Mini Review. MDPI. J. Ayurveda Integr. Med. Sci. 2024, 10, 170–175. [Google Scholar] [CrossRef]
- Ray, S.K.; Ghosh, N.; Vitkin, A. Diattenuation and retardance signature of plasmonic gold nanorods in turbid media revealed by Mueller matrix polarimetry. Sci. Rep. 2021, 11, 20376. [Google Scholar] [CrossRef]
- Lee, C.H.; Chae, J.; Yang, H.R. Scientific Characterization of Coloration Pigments and Deterioration Diagnosis on the Surface for the Gilt-bronze Seated Bodhisattva at Cheongyang Unjangam Hermitage. J. Conserv. Sci. 2023, 39, 137–151. [Google Scholar] [CrossRef]
- Haueis, P. A generalized patchwork approach to scientific concepts. Philos. Sci. 2021, 88, 716179. [Google Scholar] [CrossRef]
- Daggumati, P.; Kurtulus, O.; Chapman, C.A.R.; Dimlioglu, D.; Seker, E. Microfabrication of nanoporous gold patterns for cell-material interaction studies. J. Vis. Exp. 2013, 78, 50678. [Google Scholar] [CrossRef]
- Benesova, E.; Vidova, V.; Spacil, Z. A comparative study of synthetic winged peptides for absolute protein quantification. Sci. Rep. 2021, 11, 90087. [Google Scholar] [CrossRef]
- Gold Properties and Uses. Energy & Mining. 2019. Available online: https://www.energymining.sa.gov.au/home/events/discover_gold/gold_properties_and_uses (accessed on 12 August 2023).
- Gold: Element Properties and Uses. Stanford Advanced Materials. 2025. Available online: https://www.samaterials.com/blog/gold-element-properties-and-uses.html (accessed on 29 June 2025).
- Oldenburg, S.J. Silver Nanomaterials: Properties & Applications. Sigma-Aldrich. 2025. Available online: https://www.sigmaaldrich.com/SK/en/technical-documents/technical-article/materials-science-and-engineering/biosensors-and-imaging/silver-nanomaterials-properties (accessed on 29 June 2025).
- Platinum: Element Properties and Uses. Stanford Advanced Materials. 2025. Available online: https://www.samaterials.com/blog/platinum-element-properties-and-uses.html (accessed on 29 June 2025).
- Shults, R. Palladium: Properties and Uses. 2024. Available online: https://borro.com/the-basics-of-palladium-a-comprehensive-guide-to-its-properties-and-uses/ (accessed on 21 May 2025).
- Study.com. Gold|Definition, Properties & Uses—Lesson. Available online: https://study.com/learn/lesson/what-is-gold-properties-uses.html (accessed on 29 June 2025).
- Stanford Advanced Materials. Silver: Element Properties and Uses. 2025. Available online: https://www.samaterials.com/blog/silver-element-properties-and-uses.html (accessed on 29 June 2025).
- Gopalakrishnan, U. Frontiers in Bioengineering and Biotechnology. Assessing the Potential Association Between Microbes and Corrosion of Intra-Oral Metallic Alloy-Based Dental Appliances Through a Systematic Review of the Literature. Front. Bioeng. Biotechnol. 2021, 9, 631103. [Google Scholar] [CrossRef]
- Michael, B.; Mooiman, M.B.; Kathryn, C.; Sole, K.C.; Dinham, N. The Precious Metals Industry. Wiley Online Library. 2016. Available online: https://onlinelibrary.wiley.com/doi/10.1002/9781119009115.ch16 (accessed on 29 June 2025).
- Precious Metals, vs. Base Metals: Nature and Experiment. Minerals. 2023. Available online: https://www.mdpi.com/journal/minerals/special_issues/PMBMNE (accessed on 29 June 2025).
- Scientific Properties of Precious Metals. APMEX. 2024. Available online: https://learn.apmex.com/learning-guide/science/scientific-properties-of-precious-metals/ (accessed on 20 January 2024).
- Mohamed, D.S.; Abd El-Baky, R.M.; Sandle, T.; Mandour, S.A.; Ahmed, E.F. Antimicrobial Activity of Silver-Treated Bacteria against Other Multi-Drug Resistant Pathogens in Their Environment. Antibiotics 2020, 9, 181. [Google Scholar] [CrossRef]
- Precious Metals. Total Materia. 2011. Available online: https://www.totalmateria.com/en-us/articles/precious-metals/ (accessed on 20 January 2024).
- Huang, X.; Li, Z.; Yu, Z.; Deng, X.; Xin, Y. Recent Advances in the Synthesis, Properties, and Biological Applications of Platinum Nanoclusters. J. Nanomater. 2019, 2019, 6248725. [Google Scholar] [CrossRef]
- Volostnykh, M.V. Water-soluble platinum and palladium porphyrins with peripheral ethyl phosphonic acid substituents: Synthesis, aggregation in solution, and photocatalytic properties. R. Soc. Chem. 2025, 54, 6. [Google Scholar] [CrossRef]
- Loza, K.; Heggen, M.; Epple, M. Synthesis, Structure, Properties, and Applications of Bimetallic Nanoparticles of Noble Metals. Adv. Funct. Mater. 2020, 30, 1909260. [Google Scholar] [CrossRef]
- Key Advantages of Platinum-Based Catalysts Over Iridium. 2023. Available online: https://www.nikalyte.com/key-advantages-of-platinum-based-catalysts-over-iridium/ (accessed on 21 January 2024).
- Hughes, A.E.; Haque, N.; Northey, S.A.; Giddey, S. Platinum Group Metals: A Review of Resources, Production and Usage with a Focus on Catalysts. Resources 2021, 10, 93. [Google Scholar] [CrossRef]
- Crystal Structures of Metals. Chemistry LibreTexts. 2021. Available online: https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Introduction_to_Inorganic_Chemistry_(Wikibook)/06:_Metals_and_Alloys-_Structure_Bonding_Electronic_and_Magnetic_Properties/6.04:_Crystal_Structures_of_Metals (accessed on 30 June 2022).
- C&CS. Palladium-Platinum Catalysts. 2024. Available online: https://www.candcs.de/en/product-category/palladium-platinum-catalysts/ (accessed on 29 June 2025).
- Takano, S.; Tsukuda, T. Chemically Modified Gold/Silver Superatoms as Artificial Elements at Nanoscale: Design Principles and Synthesis Challenges. J. Am. Chem. Soc. 2021, 143, 11465. [Google Scholar] [CrossRef]
- Wikipedia. Gold. 2001. Available online: https://en.wikipedia.org/wiki/Gold (accessed on 29 June 2025).
- Schmidbaur, H.; Cronje, S.; Djordjevic, B.; Schuster, O. Understanding gold chemistry through relativity. Chem. Phys. 2005, 311, 151–161. [Google Scholar] [CrossRef]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial Silver in Medicinal and Consumer Applications. 2018. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6315945/ (accessed on 29 April 2020).
- ISO 6507-2; Metallic materials—Vickers hardness test Part 2—Verification and calibration of testing machines. International Organization for Standardization ISO: Geneva, Switzerland, 2005.
- Petrík, J.; Blaško, P.; Petryshynets, I.; Mihaliková, M.; Pribulová, A.; Futáš, P. The Influence of the Degree of Tension and Compression of Aluminum on the Indentation Size Effect (ISE). Metals 2022, 12, 2063. [Google Scholar] [CrossRef]
- Petrík, J.; Blaško, P.; Mihaliková, M.; Vasilňáková, A.; Mikloš, V. The Relationship between the Deformation and the Indentation Size Effect (ISE). Metall. Res. Technol. 2019, 116, 622. [Google Scholar] [CrossRef]
- Liu, N.; Yang, X.; Yu, Z.; Zhao, L. Indentation Size Effect of Germanium Single Crystal with Different Crystal Orientations. Trans. Nonferrous Met. Soc. China 2020, 30, 181–190. [Google Scholar] [CrossRef]
- Kim, H.; Kim, T. Measurement of Hardness on Traditional Ceramics. J. Eur. Ceram. Soc. 2002, 22, 1437–1445. [Google Scholar] [CrossRef]
- Machaka, R.; Derry, T.E.; Sigalas, I.; Herrmann, M. Analysis of the Indentation Size Effect in the Microhardness Measurements in B6O. Adv. Mater. Sci. Eng. 2011, 2011, 539252. [Google Scholar] [CrossRef]
- Petrík, J.; Blaško, P.; Markulík, Š.; Šolc, M.; Palfy, P. The Indentation Size Effect (ISE) of Metals. Crystals 2022, 12, 795. [Google Scholar] [CrossRef]
- Šolc, M.; Blaško, P.; Petrík, J.; Palfy, P.; Girmanová, L. The Indentation Size Effect (ISE) of Ag–Cu Alloys. Crystals 2023, 13, 91. [Google Scholar] [CrossRef]
- Greil, S.; Edtmaier, C.; Haubner, R.; Lauter, L. Metallographic investigations of silver alloys used for minting. Mater. Sci. Forum. 2017, 891, 89–94. [Google Scholar] [CrossRef]
- Maughan, M.R.; Zbib, H.M.; Bahr, D.F. Variation in the nanoindentation hardness of platinum. J. Mater. Res. 2013, 28, 2819–2828. [Google Scholar] [CrossRef]
- Kim, Y.C.; Gwak, E.J.; Ahn, S.M.; Jang, J.I.; Han, H.N.; Kim, J.Y. Indentation size effect in nanoporous gold. Acta Mater. 2017, 138, 52–60. [Google Scholar] [CrossRef]

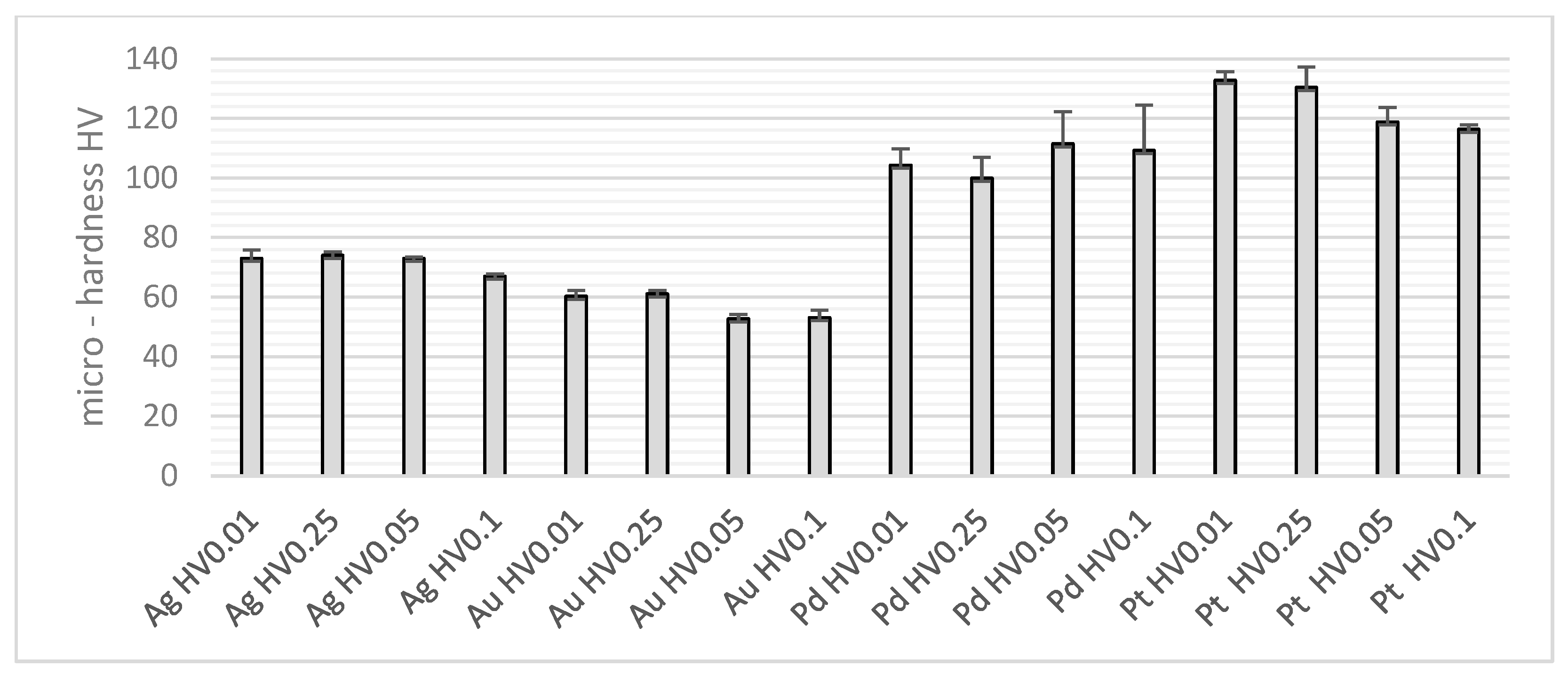
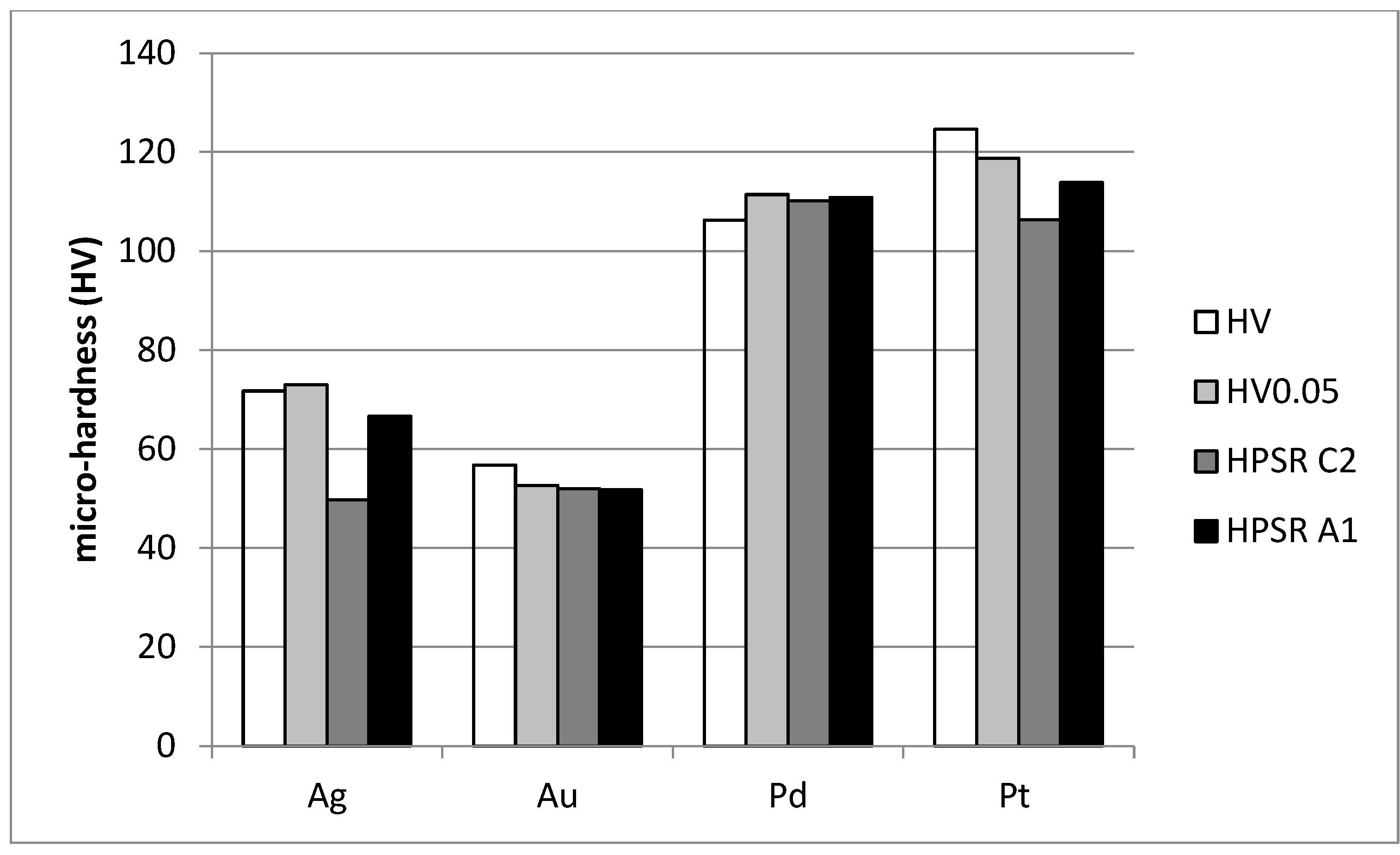
| HV0.01 | HV0.025 | HV0.05 | HV0.1 | |
|---|---|---|---|---|
| Ag | 1.427 | 1.239 | 1.385 | 1.286 |
| Au | 1.220 | 1.341 | 1.204 | 1.392 |
| Pd | 1.080 | 0.931 | 1.011 | 0.869 |
| Pt | 0.751 | 0.838 | 0.989 | 0.991 |
| HV | SD (HV) | p-Normality Test | T (°C) | RH (%) | p-ANOVA | α | |
|---|---|---|---|---|---|---|---|
| Ag | 71.73 | 3.12 | 0.0056 | 19.2 | 33.5 | 1.97∙10−5 | 77.5 |
| Au | 56.78 | 4.36 | 0.05487 | 18.6 | 33.8 | 7.57∙10−7 | 85.1 |
| Pd | 106.23 | 10.55 | 0.82977 | 20.9 | 36.7 | 0.3313 | 18.7 |
| Pt | 124.58 | 8.45 | 0.08984 | 20.4 | 39.5 | 3.54∙10−5 | 75.7 |
| n | Amoc (N mm−2) | c0 (N) | c1 (N mm−1) | c2 (N mm−2) | W (g) | A1 (N mm−2) | c1/c2 (mm) | |
|---|---|---|---|---|---|---|---|---|
| Ag | 1.9447 | 312.30 | −0.0730 | 6.351 | 263.03 | 2.28 | 352.47 | 0.02415 |
| Au | 1.8696 | 192.04 | 0.0226 | −0.095 | 275.01 | 2.22 | 273.80 | −0.00035 |
| Pd | 2.0606 | 703.29 | −0.0134 | 0.193 | 582.55 | −1.12 | 585.96 | 0.00033 |
| Pt | 1.8795 | 415.86 | −0.0025 | 2.136 | 562.25 | 2.19 | 602.14 | 0.00380 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girmanová, L.; Petrík, J.; Šolc, M.; Blaško, P.; Pribulová, A.; Futáš, P. ISE of Precious Metals: Au, Ag, Pd, and Pt. Metals 2025, 15, 1038. https://doi.org/10.3390/met15091038
Girmanová L, Petrík J, Šolc M, Blaško P, Pribulová A, Futáš P. ISE of Precious Metals: Au, Ag, Pd, and Pt. Metals. 2025; 15(9):1038. https://doi.org/10.3390/met15091038
Chicago/Turabian StyleGirmanová, Lenka, Jozef Petrík, Marek Šolc, Peter Blaško, Alena Pribulová, and Peter Futáš. 2025. "ISE of Precious Metals: Au, Ag, Pd, and Pt" Metals 15, no. 9: 1038. https://doi.org/10.3390/met15091038
APA StyleGirmanová, L., Petrík, J., Šolc, M., Blaško, P., Pribulová, A., & Futáš, P. (2025). ISE of Precious Metals: Au, Ag, Pd, and Pt. Metals, 15(9), 1038. https://doi.org/10.3390/met15091038










