Effects of Deoxidation Processes on Inclusions in Environmentally Friendly Free-Cutting Steel
Abstract
1. Introduction
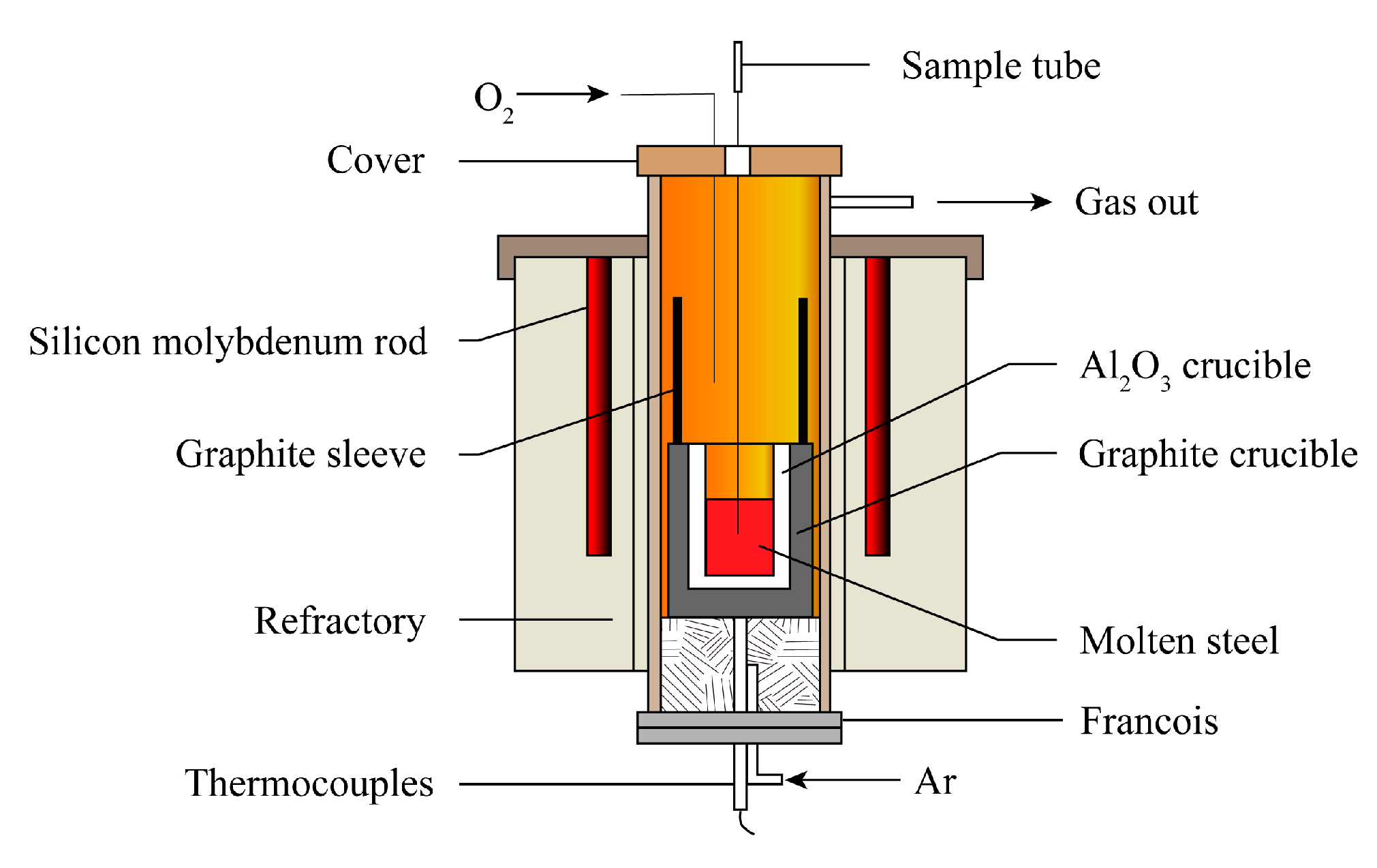
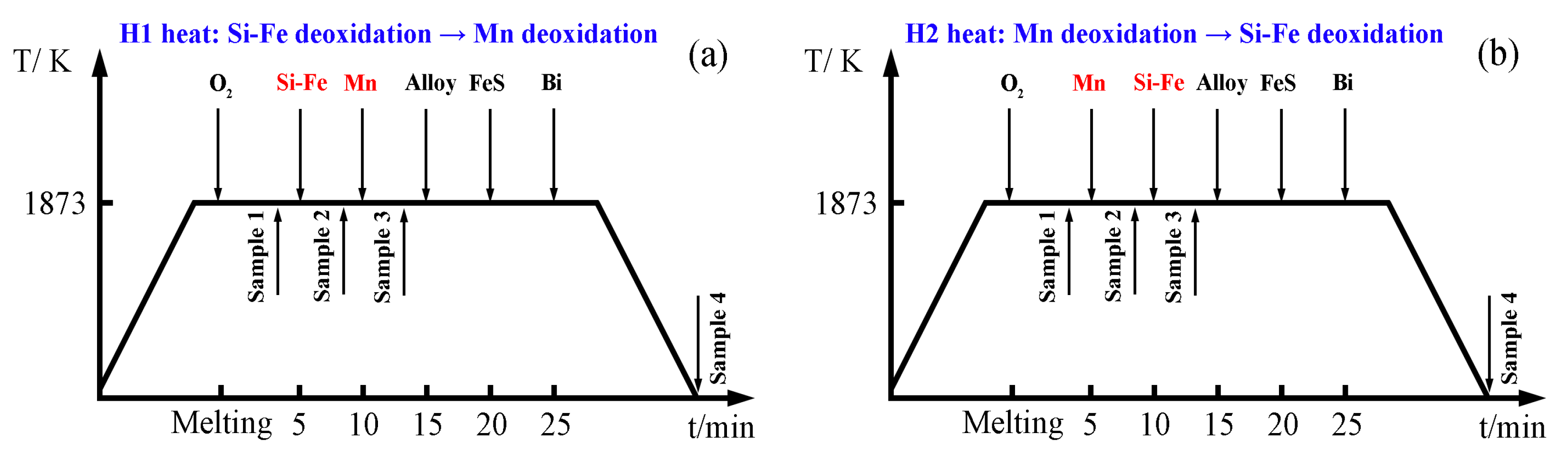
2. Materials and Methods
3. Results
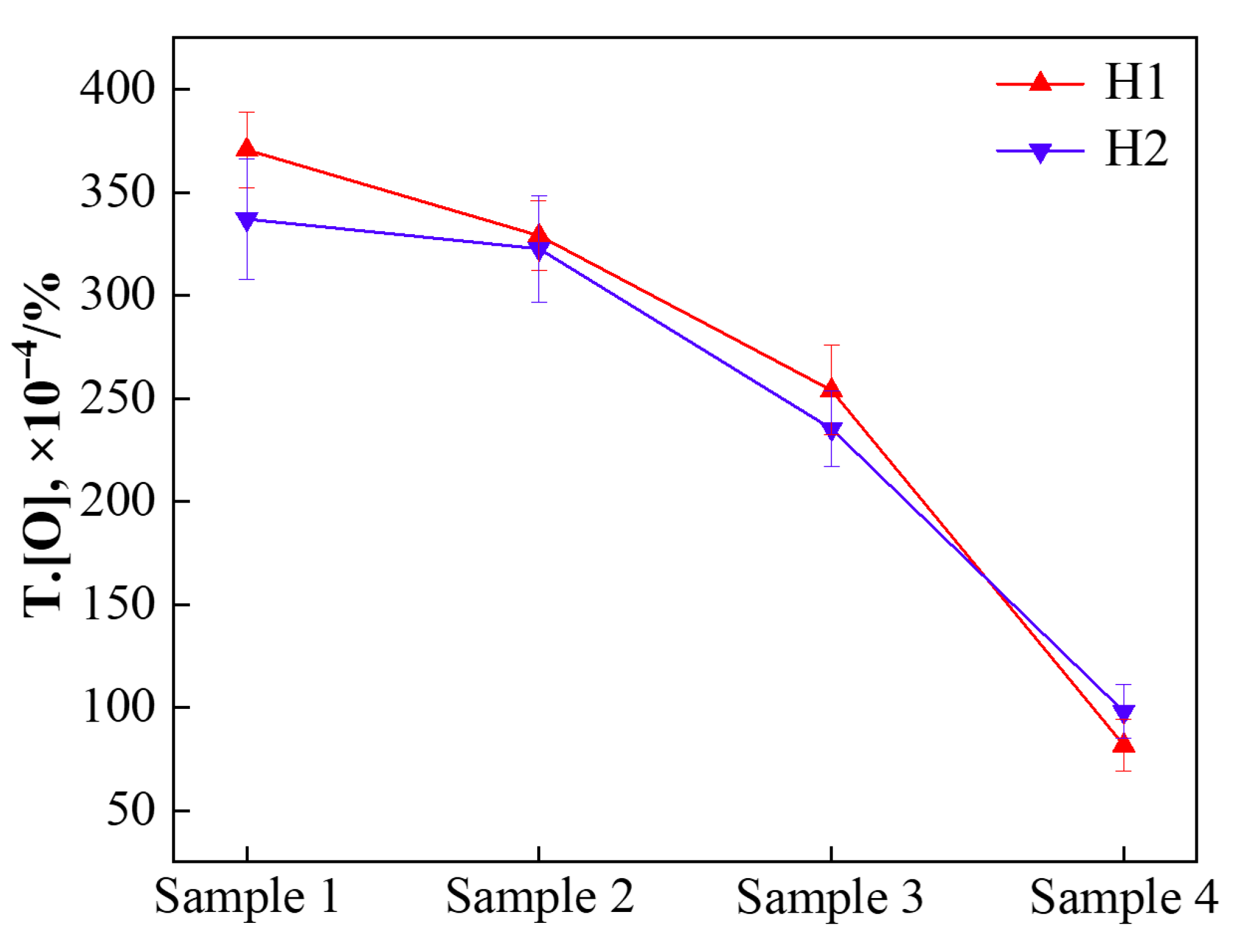
3.1. Change in Molten Steel Composition During Deoxidation
3.2. Inclusion Characteristics
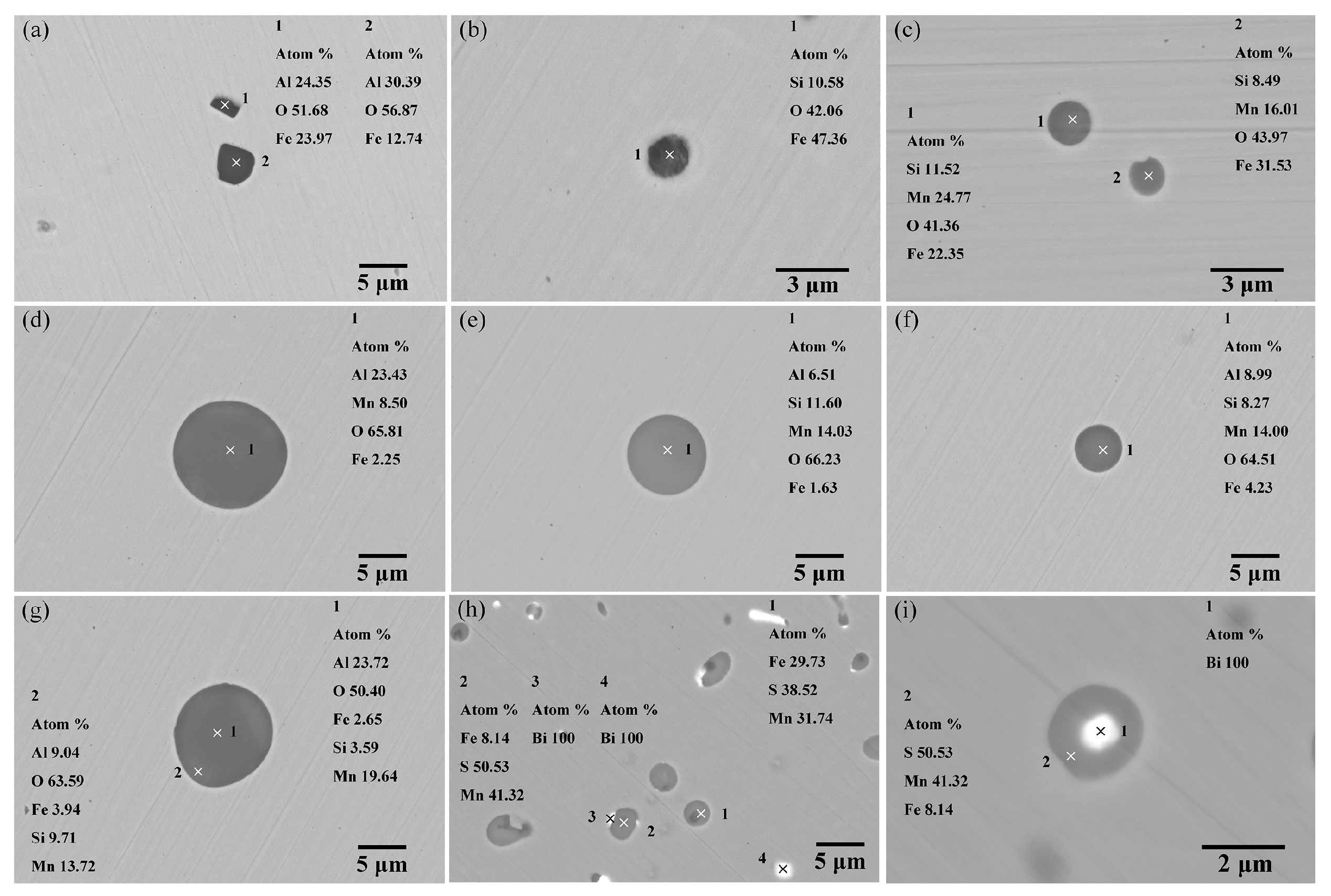
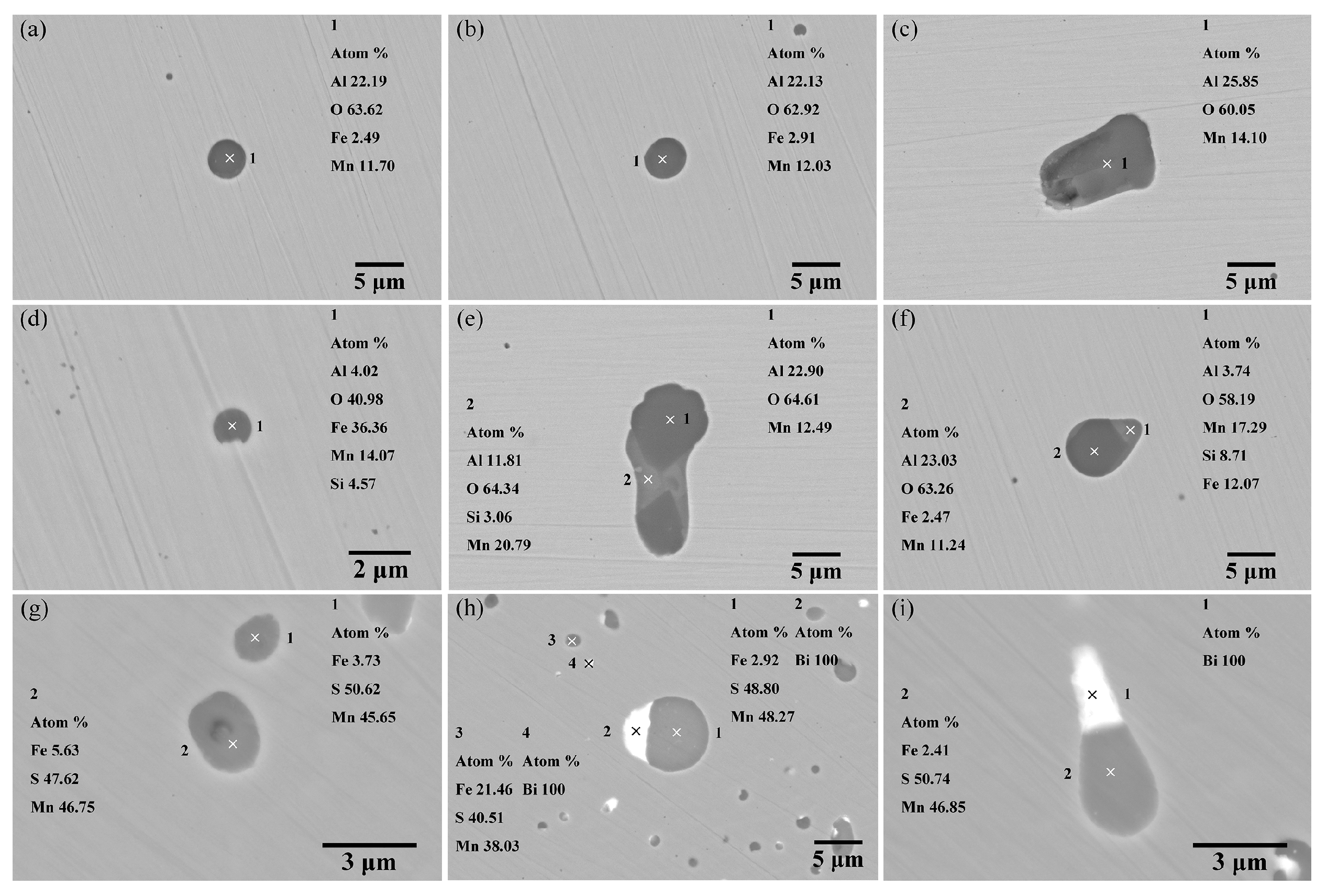
3.2.1. Morphology and Composition of Inclusions
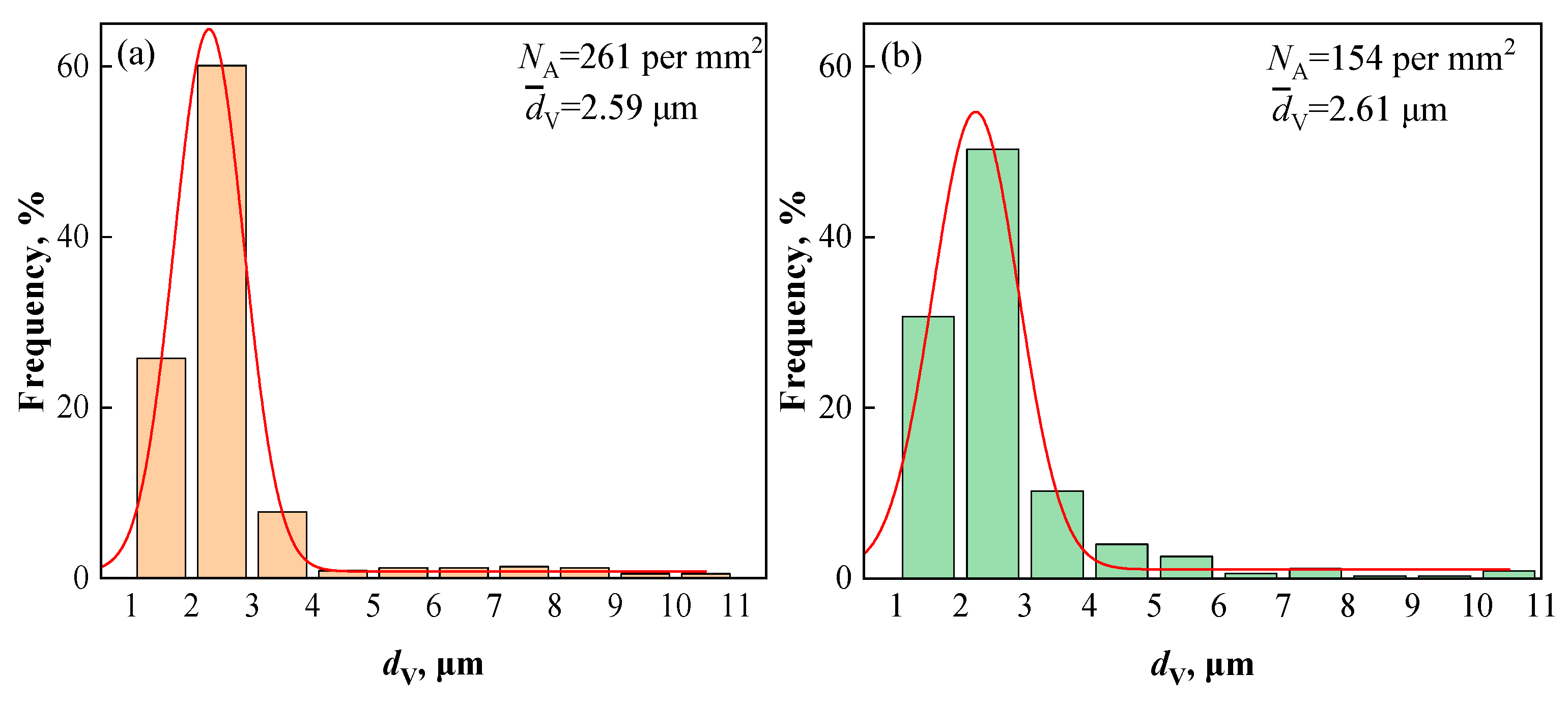
3.2.2. Quantity, Density, and Size Distribution of Inclusions
4. Discussion
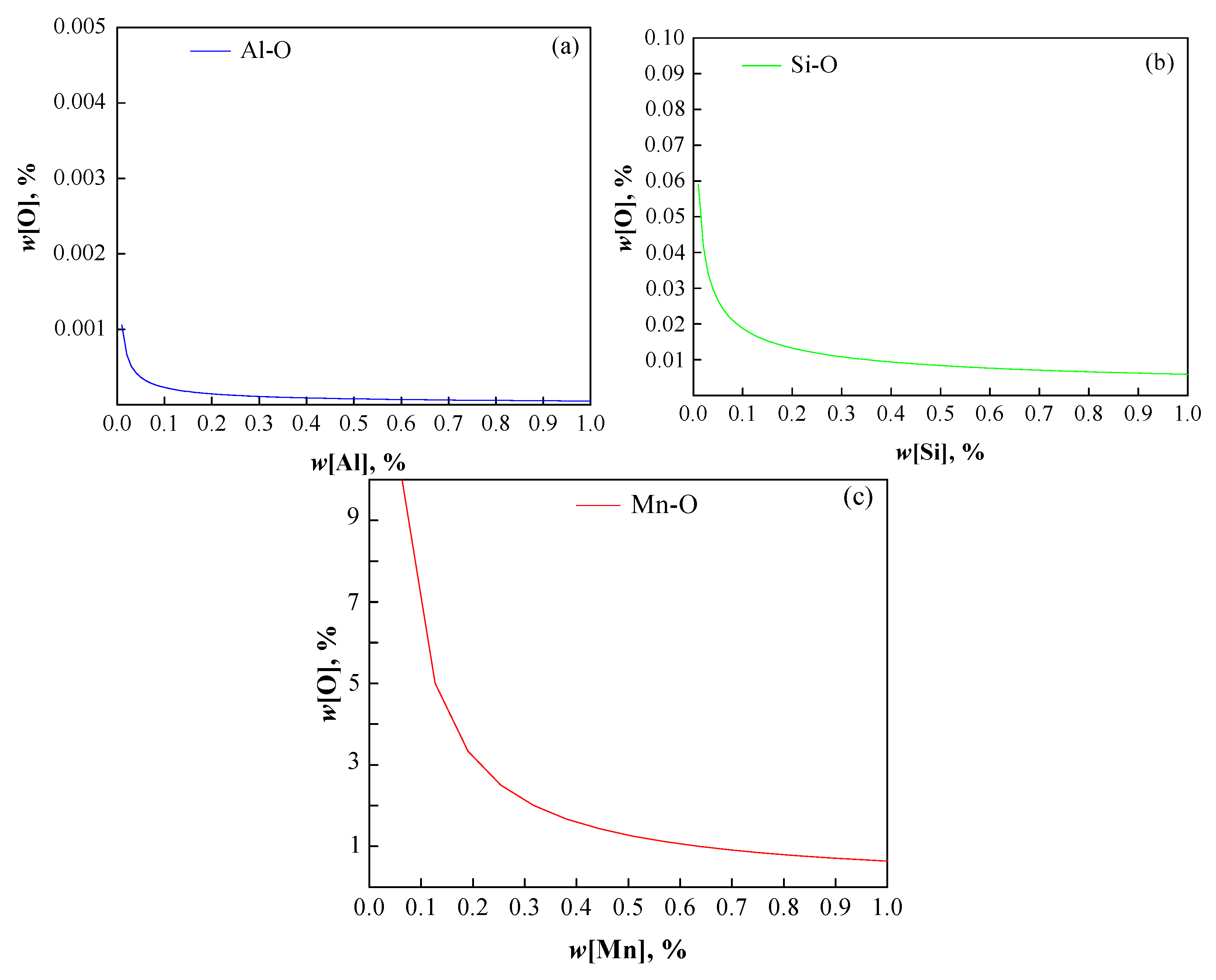
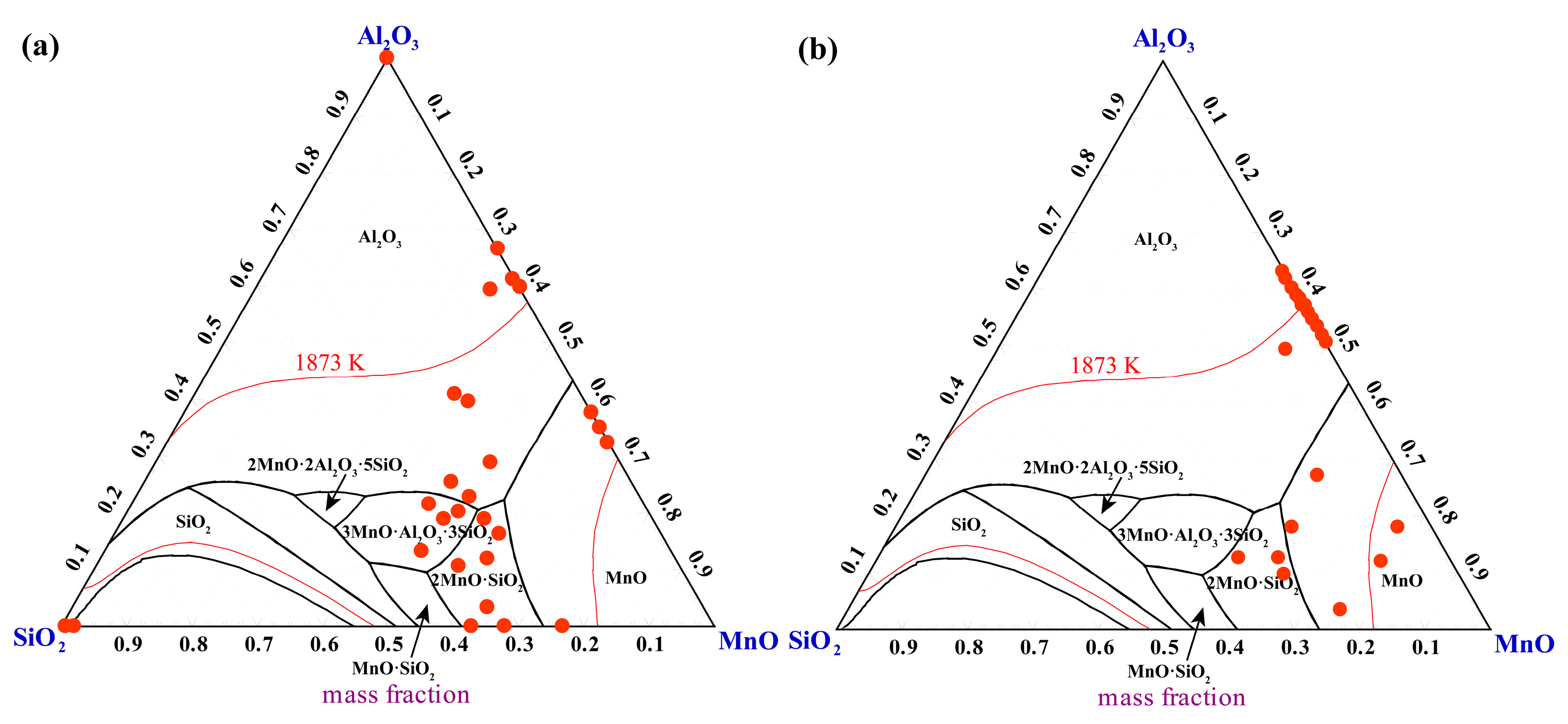
4.1. Effect of Deoxidation Processes on Oxide Inclusions in Steel
4.2. Effect of Deoxidation Processes on Non-Oxide Inclusions in Steel
5. Conclusions
- The oxygen content exhibits a similar decreasing trend in both deoxidation processes, and there is no significant difference in total oxygen content between the final samples, indicating comparable deoxidation efficiencies for the two methods;
- In the process where the Si-Fe alloy is added first, followed by manganese, the inclusions in the molten steel are predominantly liquid-phase Al2O3-SiO2-MnO composite inclusions. In contrast, when manganese is added first, followed by the Si-Fe alloy, in addition to liquid-phase Al2O3-SiO2-MnO composite inclusions in the MnO rich region, there are also some solid-phase Al2O3-MnO inclusions with higher Al2O3 content;
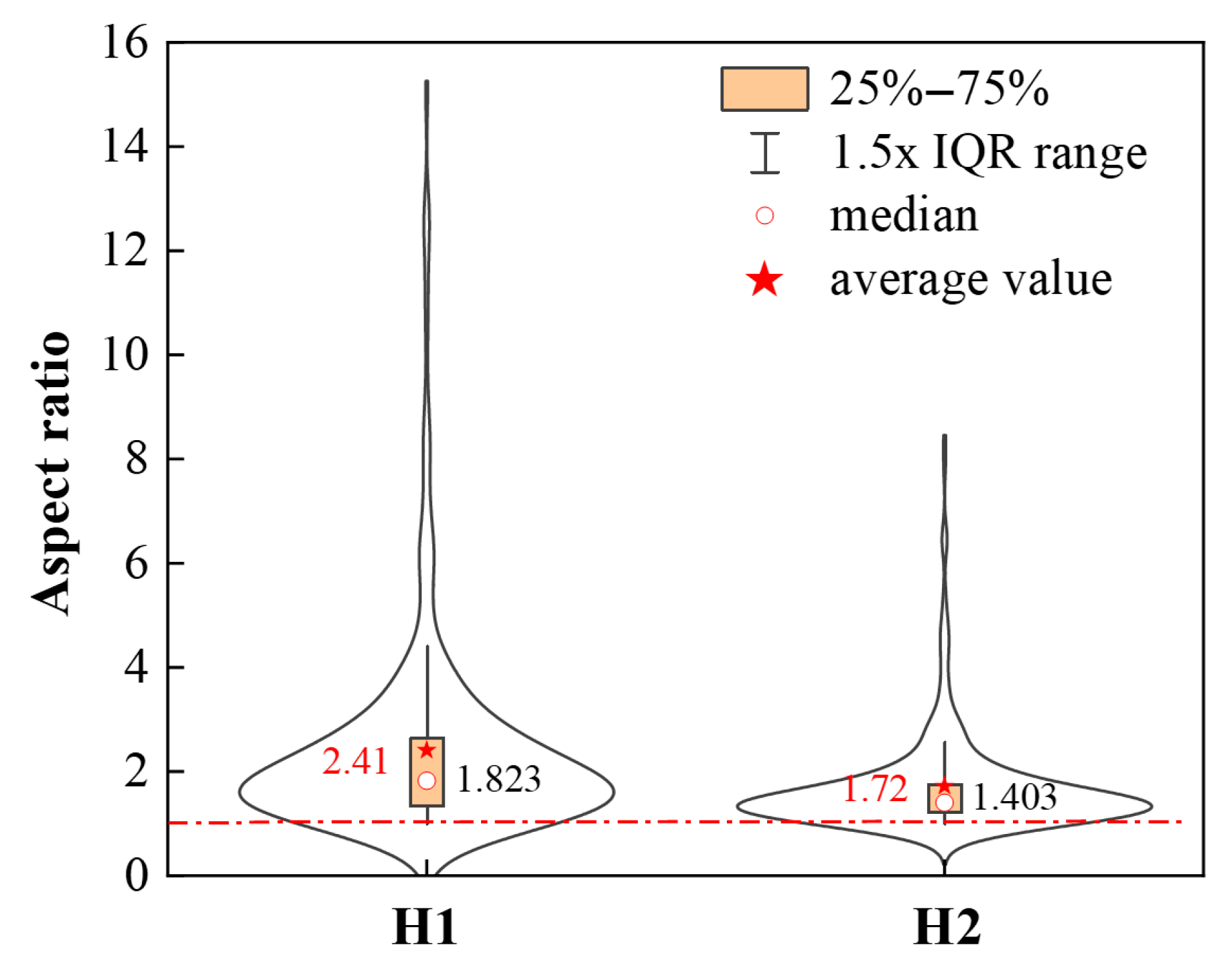
- In both deoxidation furnaces, Bi particles are mainly present adhering to MnS inclusions. The process involving adding the Si-Fe alloy followed by adding manganese results in higher average and median aspect ratios. In the process where manganese is added first followed by the Si-Fe alloy, the spheroidization degree of MnS inclusions is relatively better.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kurka, V.; Kuboň, Z.; Kander, L.; Jonšta, P.; Kotásek, O. The effect of bismuth on technological and material characteristics of low-alloyed automotive steels with a good machinability. Metals 2022, 12, 301. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Wang, J.; Zhu, R.; Zhu, Y.; Lu, F.; Li, J.; Jiang, Z. Effect of bismuth and telluride on the inclusions of sulfur free-cutting steel. Metals 2023, 13, 486–499. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, H.; Zhao, G.; Li, X.; Li, H.; Yu, G.; Jiang, Z. Effect of bismuth content and heating rate on MnS inclusions in free-cutting steel. Metals 2024, 14, 713. [Google Scholar] [CrossRef]
- Li, G.; Wang, F.; Chen, Z.; Fan, Y.; Li, P.; Liu, M.; Wu, H. Effects of bismuth particle inclusions on surface and internal wear of single crystal iron: A molecular dynamics simulation. Micro Nanostructures 2024, 193, 207912. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.; Hu, T.; Xu, X.; Liu, N.; Fu, J. The forms and evolution of free-cutting phase in Bi-Te-S free-cutting steel. Steel Res. Int. 2024, 95, 2300608. [Google Scholar] [CrossRef]
- Xu, Q.; Zhan, D.; Xu, W.; Fan, F.; Li, H.; Li, H.; Wang, S. Effect of different N and Si contents microstructures and properties of HRB400e steel containing vanadium. J. Iron Steel Res. Int. 2025, 32, 452–465. [Google Scholar] [CrossRef]
- Chaus, A. Application of bismuth for solidification structure refinement and properties enhancement in as-cast high-speed steels. ISIJ Int. 2005, 45, 1297–1306. [Google Scholar] [CrossRef]
- Liu, B.; Xin, R.; Xie, J.; Fu, J. Behaviors of novel manufacturing 1215MS+Bi steel in casting thermal processes. Mater. Manuf. Process 2024, 39, 374–385. [Google Scholar] [CrossRef]
- Reynolds, P.; Block, V.; Essel, I.; Klocke, F. Alternatives to lead as a machinability enhancer in free cutting steels. Steel Res. Int. 2007, 78, 908–914. [Google Scholar] [CrossRef]
- Xie, J.B.; Li, J.; Li, Z.W.; Wu, L.P.; Zhang, P.P.; Fu, J.X. Forms of Bi-sulphide in 1215MS steel related to machining and mechanical performance. Ironmak. Steelmak. 2021, 48, 927–935. [Google Scholar] [CrossRef]
- Xie, J.B. Forms and roles of bismuth in steel: A review. Steel Res. Int. 2023, 94, 2200625. [Google Scholar] [CrossRef]
- Fukumoto, S.; Sakaizawa, Y. Formation of Monotectic Sulfide in Free-Machining Ferritic Stainless Steels during Solidification. ISIJ Int. 2020, 60, 1829–1831. [Google Scholar] [CrossRef]
- Liu, H.T.; Chen, W.Q. Effect of total oxygen content on the machinability of low carbon resulfurized free cutting steel. Steel Res. Int. 2012, 83, 1172–1179. [Google Scholar] [CrossRef]
- Sui, H.; Wang, L.J.; Wang, Q.; Wang, H.M.; Che, D.H.; Li, J.M.; Chou, K.C. The Formation and Growth of Sulfides in Free-Cutting Stainless Steel. Steel Res. Int. 2018, 89, 1800179. [Google Scholar] [CrossRef]
- Deng, Z.Y.; Zhu, M.Y.; Zhong, B.J.; Gao, X.L. Effect of deoxidation methods on inclusions in steel. J. Univ. Sci. Technol. 2012, 34, 1256–1261. (In Chinese) [Google Scholar]
- Liu, J.W.; Tang, H.Y.; Guo, L.Z.; Zhang, J.Q. Effect of deoxidizing and alloying routes on the evolution of non-metallic inclusions in 55SiCr spring steel. Metals 2022, 12, 1531. [Google Scholar] [CrossRef]
- Li, B.; Zhu, H.Y.; Zheng, Z.H.; Chen, J.; Song, M.M. Effect of deoxidation methods on non-metallic inclusions in high-Al steel. J. Iron Steel Res. 2022, 34, 1240–1249. (In Chinese) [Google Scholar]
- Zhan, D.P.; Zhang, H.S.; Li, S.C.; Jiang, Z.H. Steel deoxidation and non-metallic inclusion control using AlMnCa alloy. J. Northeast. Univ. Nat. Sci. 2006, 27, 1118–1121. (In Chinese) [Google Scholar]
- Gu, C.; Bao, Y.; Gan, P.; Lian, J.; Münstermann, S. An experimental study on the impact of deoxidation methods on the fatigue properties of bearing steels. Steel Res. Int. 2018, 89, 1800129. [Google Scholar] [CrossRef]
- Xie, J.B.; Liu, B.B.; Wu, X.Y.; Fu, J.X. Distribution of Bi-MnS inclusions existing in 1215MS steels: Correlation with thermal physical coefficient. Met. Mater. Int. 2022, 28, 1306–1313. [Google Scholar] [CrossRef]
- Hu, S.H.; Li, Z.W.; Fan, T.; Fu, J.X. Effect of bismuth on sulfide in high sulfur free-cutting steel. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Wuhan, China, 19–21 July 2019. [Google Scholar]
- Zhang, X.B.; Roelofs, H.; Lemgen, S.; Urlau, U.; Subramanian, S.V. Application of thermodynamic model for inclusion control in steelmaking to improve the machinability of low carbon free cutting steels. Steel Res. Int. 2004, 75, 314–321. [Google Scholar] [CrossRef]
- Sigworth, G.K.; Elliott, J.F. The thermodynamics of liquid dilute iron alloys. Met. Sci. 1974, 8, 298–310. [Google Scholar] [CrossRef]
- Ohta, H.; Suito, H. Activities in CaO-SiO2-Al2O3 slags and deoxidation equilibria of Si and Al. Metall. Mater. Trans. B 1996, 27, 943–953. [Google Scholar] [CrossRef]
- Ohta, H.; Suito, H. Activities of SiO2 and Al2O3 and activity coefficients of FetO and MnO in CaO-SiO2-Al2O3-MgO slag. Metall. Mater. Trans. B 1998, 29, 119–129. [Google Scholar] [CrossRef]
- Andersson, M.A.T.; Jonsson, L.T.I.; Jönsson, P.G. A thermodynamic and kinetic model of reoxidation and desulphurization in the ladle furnace. ISIJ Int. 2000, 40, 1080–1088. [Google Scholar] [CrossRef]
- Hino, M.; Ito, K. Thermodynamic Data for Steelmaking; Tokoku University Press: Sendai, Japan, 2010; pp. 259–264. [Google Scholar]
- Xu, J.L.; Song, B.; Chen, J.K.; Han, Q.Y.; Jiang, G.C. Dissolution equilibrium of Bi vapour in liquid Fe and influence of Ni and Mn. Acta Met. Sin. 1993, 29, 65–68. (In Chinese) [Google Scholar]
- Song, B.; Zhao, B.D.; Han, Q.Y.; Liu, Y. Dissolution equilibrium of bismuth vapor in liquid iron and the interaction effect of third element. J. Univ. Sci. Technol. 1997, 19, 213–217. (In Chinese) [Google Scholar]
- Ohta, H.; Suito, H. Activities in MnO-SiO2-Al2O3 slags and deoxidation equilibria of Mn and Si. Metall. Mater. Trans. B 1996, 27, 263–270. [Google Scholar] [CrossRef]
- Fujisawa, T.; Sakao, H. Equilibrium between MnO-SiO2-Al2O3-FeO slags and liquid steel. Tetsu-to-Hagané 1977, 63, 1504–1511. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, K.P.; Jiang, M.; Zhao, H.Q.; Wang, X.H.; Wang, Y. Characterization on changes of oxide inclusions in production process of tire cord steel. Iron Steel 2016, 51, 31–37. (In Chinese) [Google Scholar][Green Version]
- Shibata, H.; Kimura, K.; Tanaka, T.; Kitamura, S. Mechanism of change in chemical composition of oxide inclusions in Fe-Cr alloys deoxidized with Mn and Si by heat treatment at 1473K. ISIJ Int. 2011, 51, 1944–1950. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.; Shibata, H.; Kitamura, S. Reaction between MnO-SiO2-FeO oxide and Fe-Mn-Si solid alloy during heat treatment. ISIJ Int. 2014, 54, 2144–2153. [Google Scholar] [CrossRef]
- Ohta, H.; Suito, H. Precipitation and dispersion control of MnS by deoxidation products of ZrO2, Al2O3, MgO and MnO-SiO2 particles in Fe-10mass%Ni alloy. ISIJ Int. 2006, 46, 480–489. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, F.; Yang, S.F.; Liu, J.H.; Li, J.S. Sulfide transformation with tellurium treatment for Y15 free-cutting steel. Metall. Mater. Trans. B 2019, 50, 2284–2295. [Google Scholar] [CrossRef]
- Zheng, L.C.; Malflift, A.; Wollants, P.; Blanpain, B.; Guo, M.X. Effect of surfactant Te on the formation of MnS inclusion in steel. Metall. Mater. Trans. B 2017, 48, 2447–2458. [Google Scholar] [CrossRef]
| Alloy | C | Si | Mn | S | Al | Bi | P | Fe |
|---|---|---|---|---|---|---|---|---|
| Si-Fe | 0.03 | 72.2 | - | 0.003 | 1.2 | - | 0.008 | 25.6 |
| Mn | 0.04 | 0.1 | 99.6 | 0.05 | - | - | 0.005 | 0.15 |
| FeS | 0.03 | 0.3 | 0.08 | 27.3 | - | - | 0.03 | 72 |
| Bi | 0.002 | - | - | - | - | 99.99 | - | 0.005 |
| No. | C | Si | Mn | S | Al | Bi | Fe |
|---|---|---|---|---|---|---|---|
| H1 | 0.052 | 0.047 | 1.21 | 0.34 | 0.0034 | 0.068 | Bal. |
| H2 | 0.061 | 0.049 | 1.20 | 0.32 | 0.0028 | 0.077 | Bal. |
| fC | fSi | fMn | fS | fAl | fBi | fO |
|---|---|---|---|---|---|---|
| 1.03 | 1.09 | 0.95 | 0.93 | 0.91 | 0.61 | 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Li, B.; Huang, L.; Zhan, D.; Gao, H. Effects of Deoxidation Processes on Inclusions in Environmentally Friendly Free-Cutting Steel. Metals 2025, 15, 1018. https://doi.org/10.3390/met15091018
Wang D, Li B, Huang L, Zhan D, Gao H. Effects of Deoxidation Processes on Inclusions in Environmentally Friendly Free-Cutting Steel. Metals. 2025; 15(9):1018. https://doi.org/10.3390/met15091018
Chicago/Turabian StyleWang, Dong, Binzhou Li, Luoyi Huang, Dongping Zhan, and Hang Gao. 2025. "Effects of Deoxidation Processes on Inclusions in Environmentally Friendly Free-Cutting Steel" Metals 15, no. 9: 1018. https://doi.org/10.3390/met15091018
APA StyleWang, D., Li, B., Huang, L., Zhan, D., & Gao, H. (2025). Effects of Deoxidation Processes on Inclusions in Environmentally Friendly Free-Cutting Steel. Metals, 15(9), 1018. https://doi.org/10.3390/met15091018






