Thermodynamic Modeling of Microstructural Design of Lightweight Ferritic Steels
Abstract
1. Introduction
2. Materials and Methods
3. Results
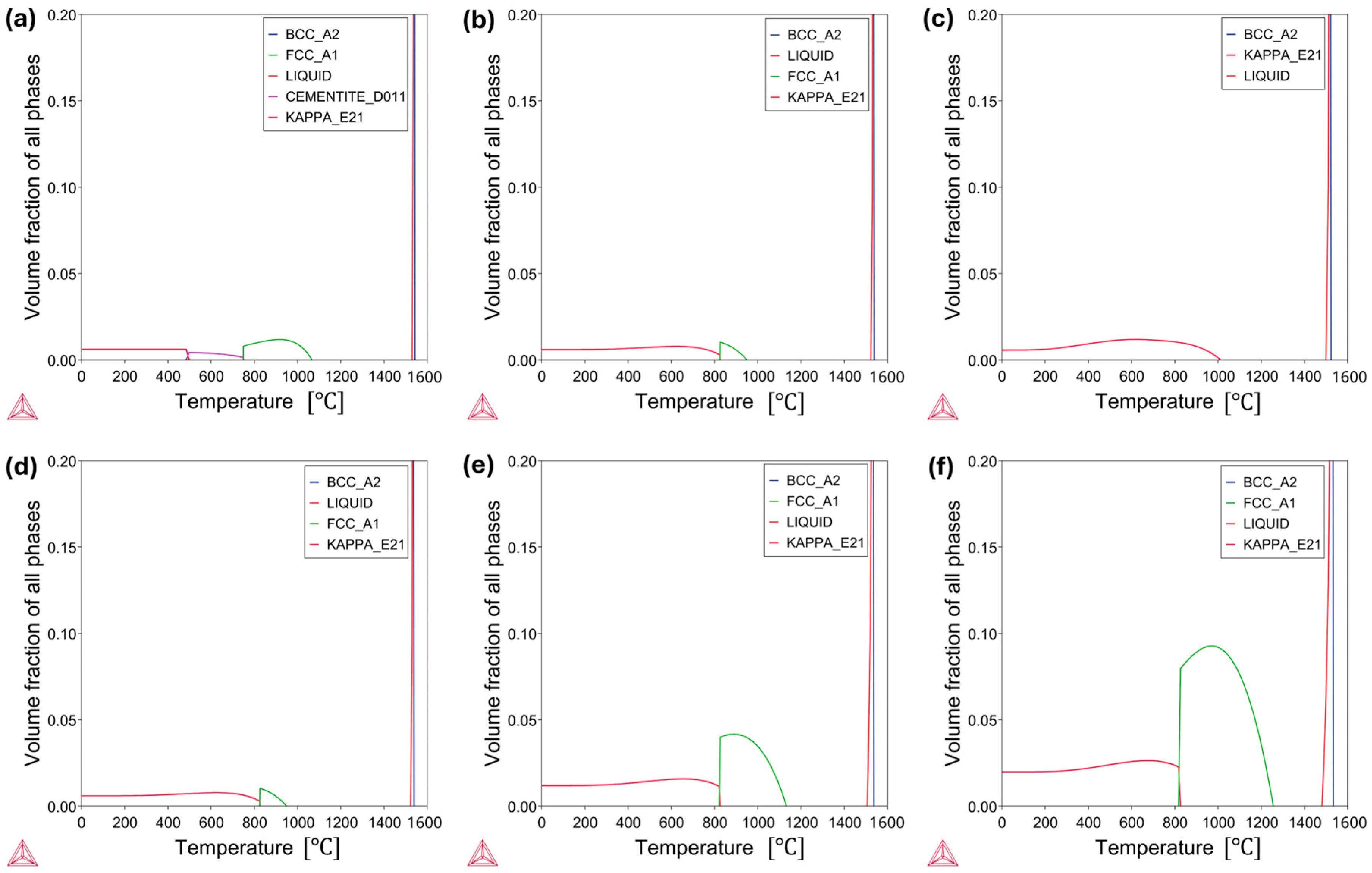
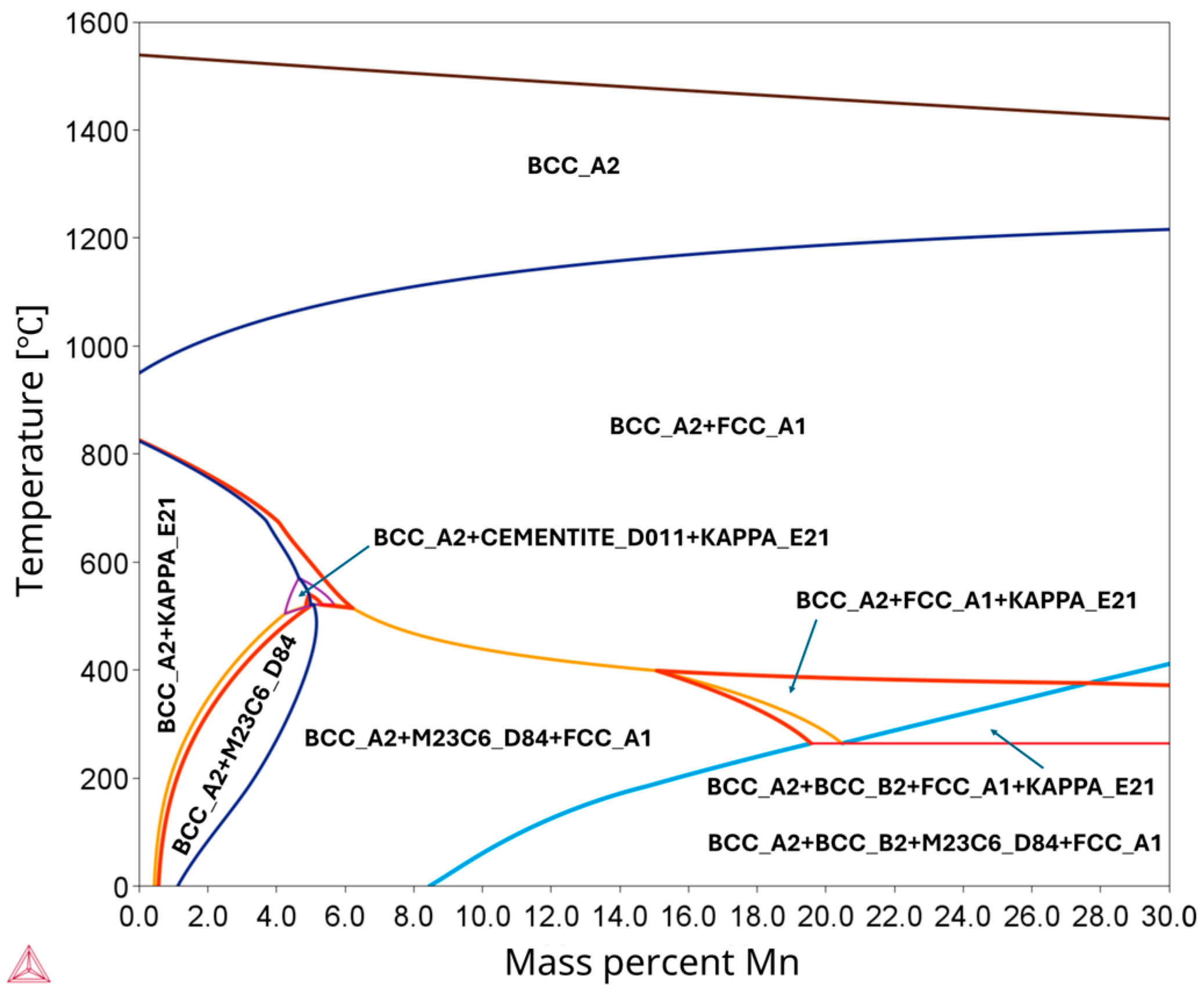
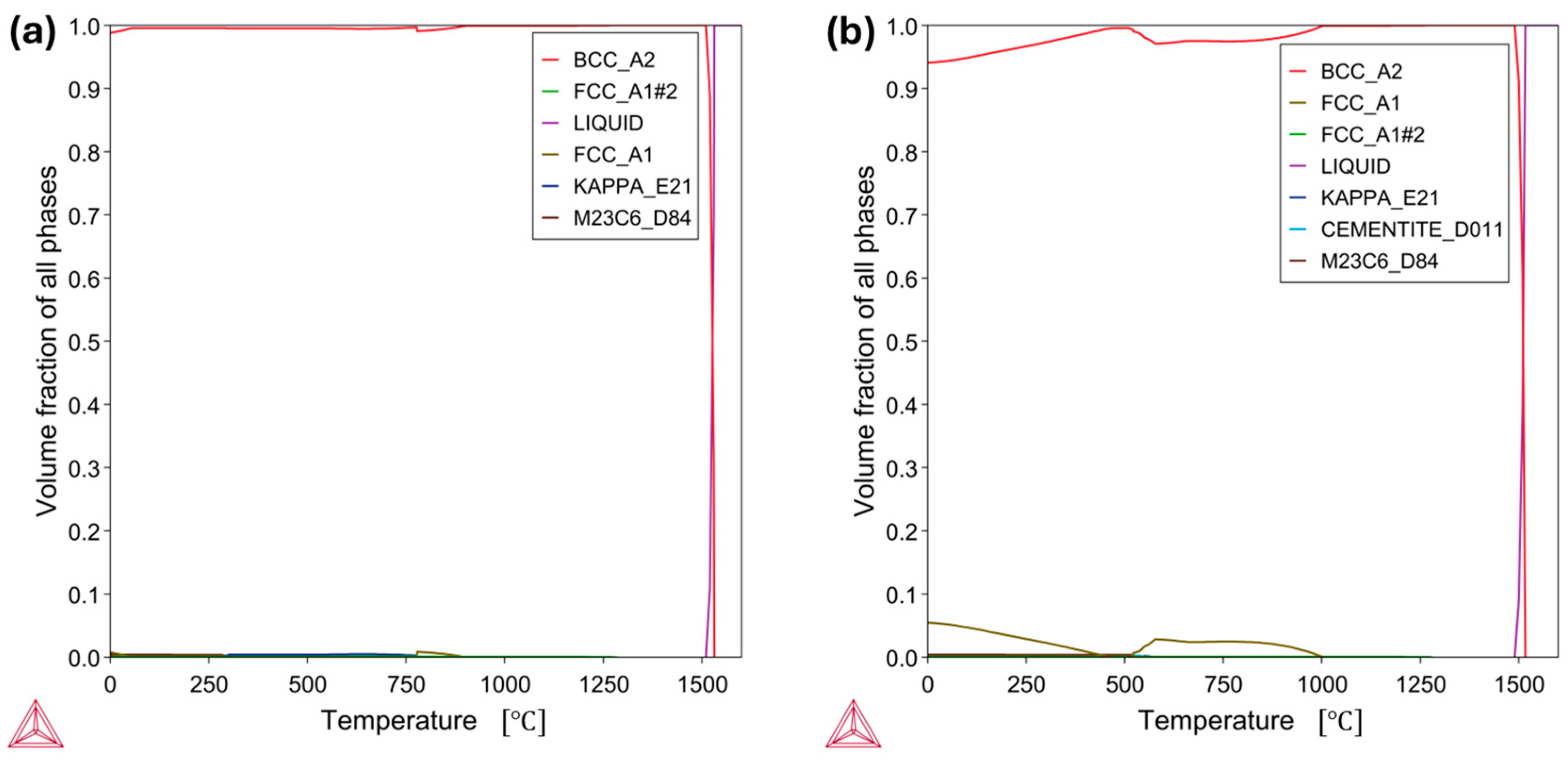
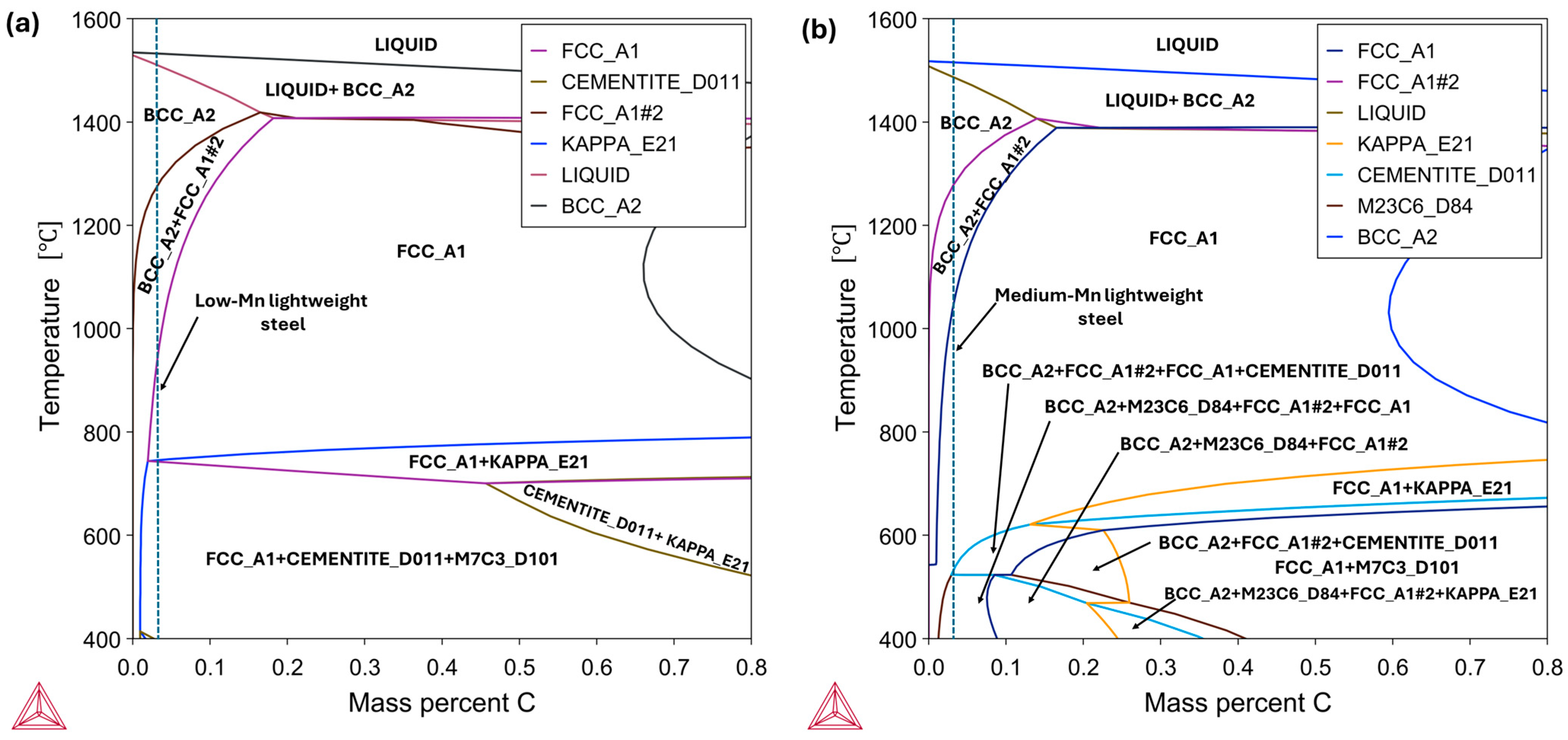
3.1. High-Temperature Equilibrium Phase Diagrams
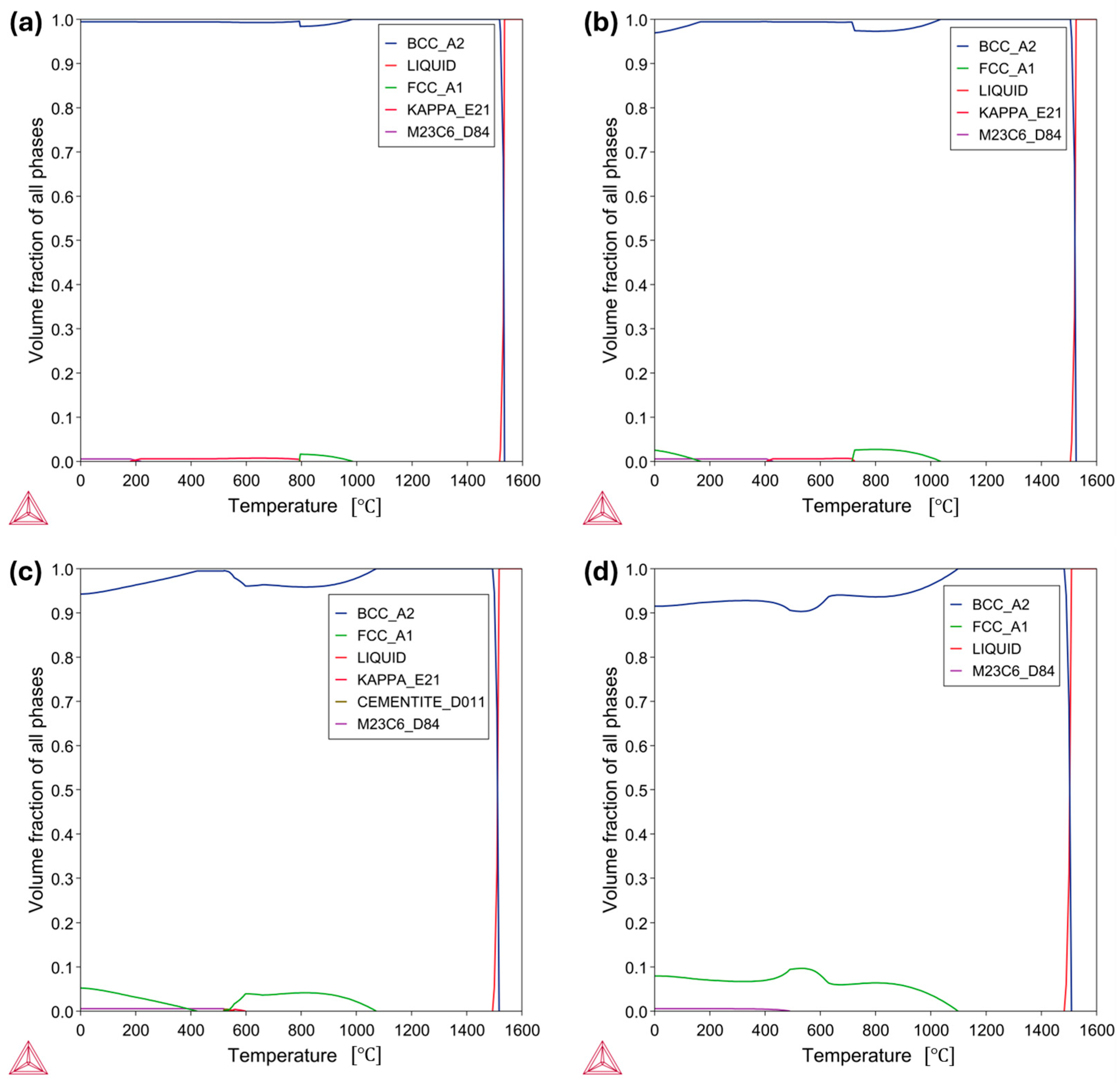
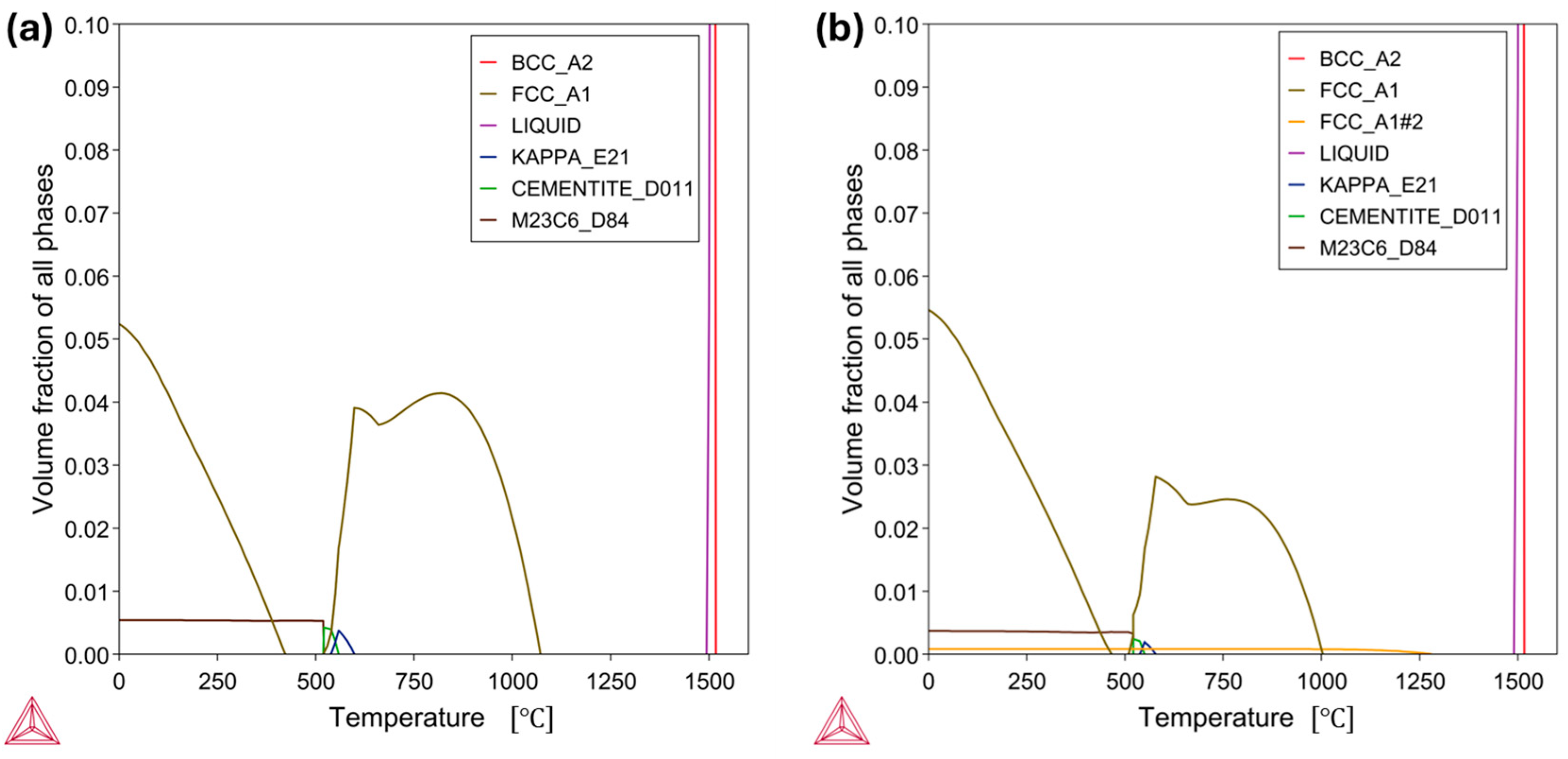
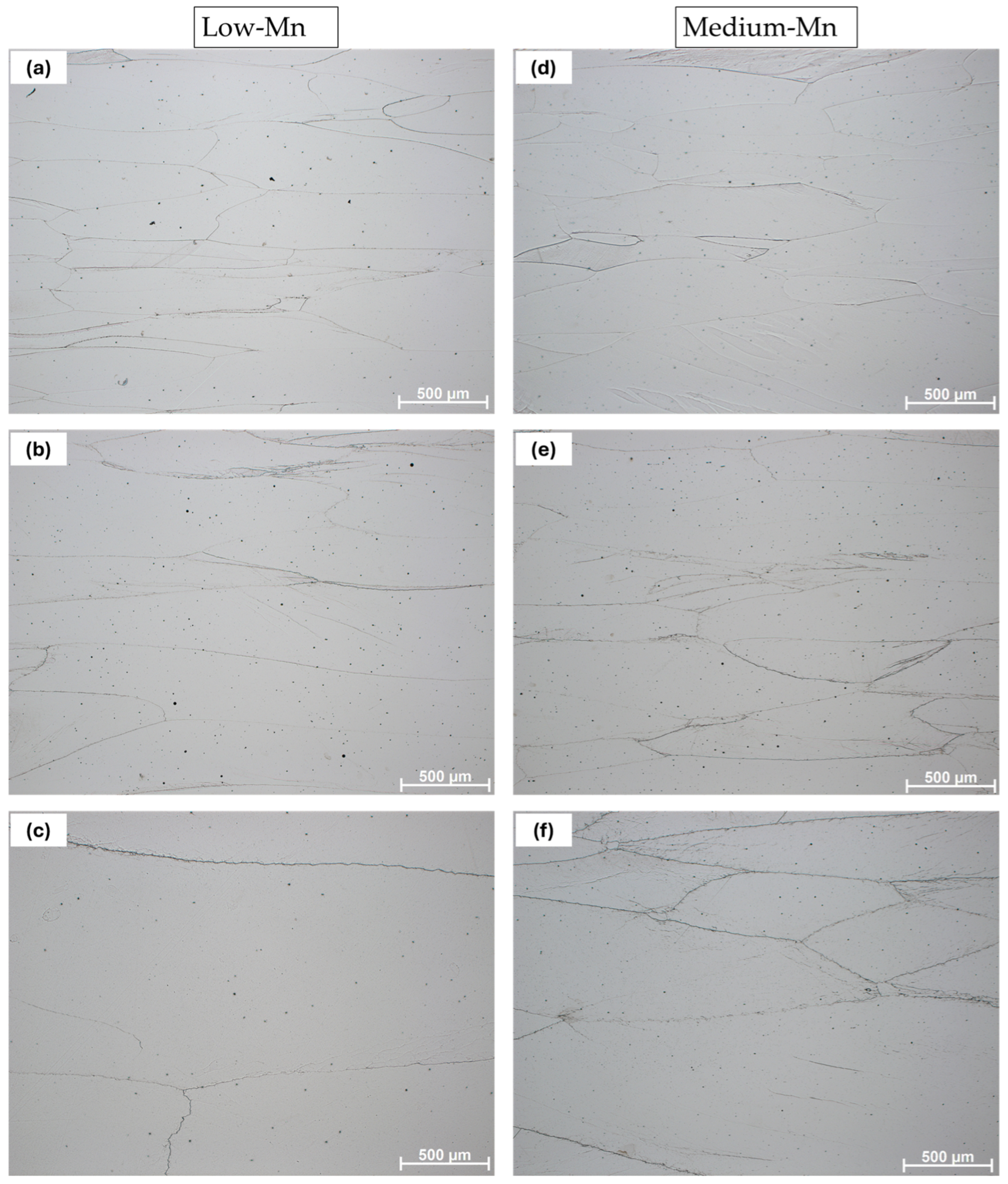
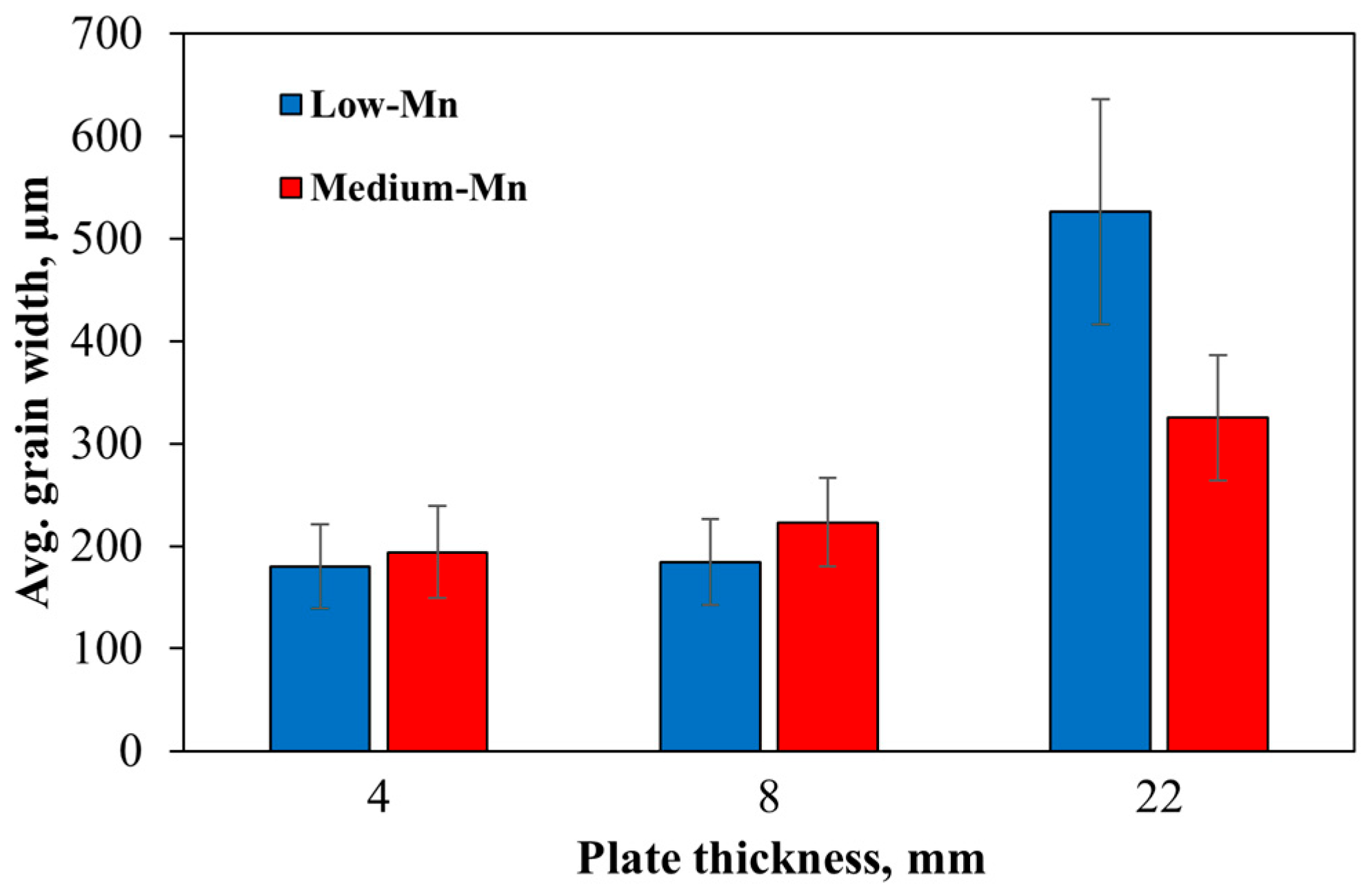
3.2. Microstructural Results
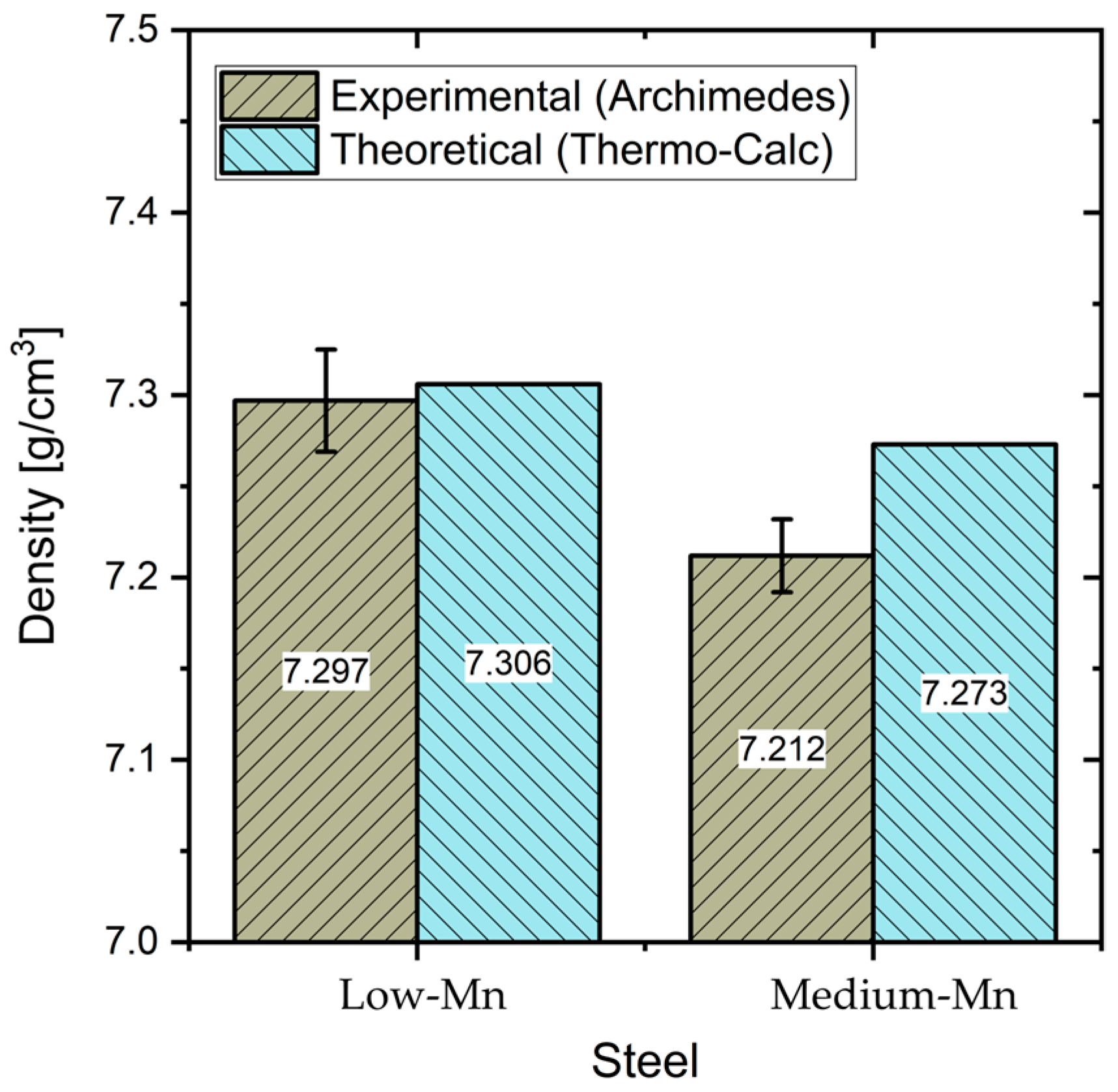
3.3. Density
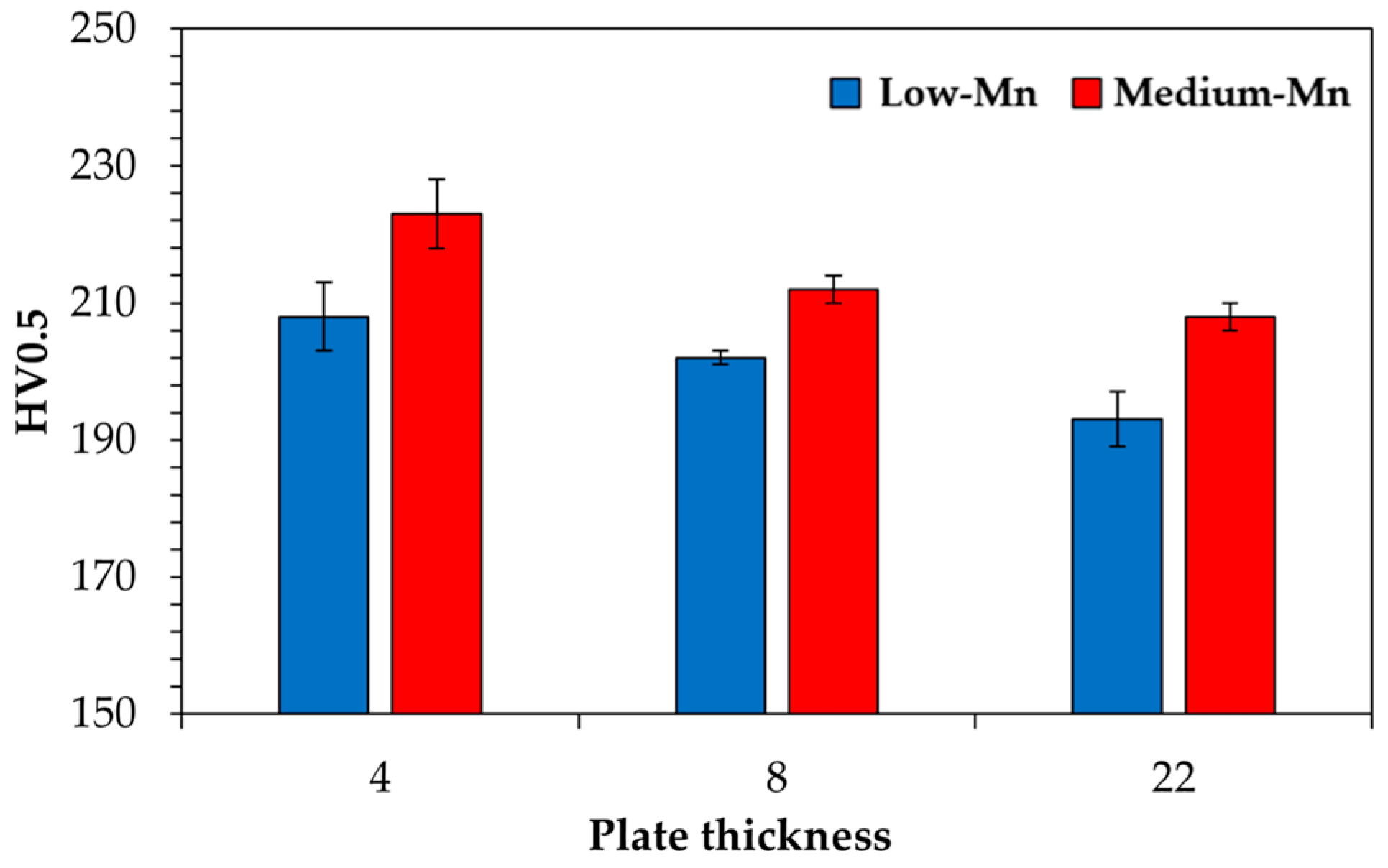
3.4. Hardness
4. Discussion
5. Conclusions
- (1)
- The thermodynamic calculations implied increasing the concentration of Al and C stabilizes the precipitation of harmful κ-carbides. Exceptionally, Al increases the κ-carbide phase stability temperature. Conversely, the increment in Mn content hampered the κ-carbide formation and stabilized the austenitic phase.
- (2)
- In the case of the low-Mn lightweight steel, the thermodynamic calculations’ prediction on the phase constituents was consistent with the microstructural results. On the other hand, for the medium-Mn lightweight steel, the calculation showed a small amount of austenite, but the microstructural results showed only the ferrite phase. This may imply that the existing databases are not precisely aligned with the real complex Fe-Mn-Al-C system. Additionally, it may be related to limited diffusion and hindered phase kinetics due to insufficient heat treatment longevity.
- (3)
- The analysis of pseudo-binary Fe-C phase equilibrium systems for the low-Mn and medium-Mn lightweight steels revealed a significant influence of the increased manganese content on the expansion of the austenite field, the stabilization of and shift in carbides (M23C6, M7C3), and the suppression or displacement of traditional phase fields involving ferrite and cementite. The high aluminum content in both steels promotes the presence of δ-ferrite at elevated temperatures, with a broader stability range observed in the medium-Mn lightweight steel. The three-phase field (Liquid + δ + γ), which is critical in casting processes, is more pronounced in the medium-Mn lightweight steel, potentially increasing the risk of casting defects. The medium-Mn lightweight steel also exhibits a more complex phase transformation sequence at lower temperatures, which may significantly affect the final microstructure and in-service performance.
- (4)
- A prominent density reduction was achieved in both steels. The low-Mn steel has a density of 7.30 g/cm3, while the medium-Mn steel achieved a relatively higher density reduction, with a density of 7.21 g/cm3. About a 7.2% and 8.3% density reduction was achieved, in comparison to DP980 automotive steel, respectively.
- (5)
- The low-Mn steel shows a hardness between 193 and 203 HV, whereas it increases to a range of 208–223 for the medium-Mn steel, clearly showing the solid solution strengthening effect of Mn. Higher hardness values in both steels are received for thinner plates due to faster cooling rates but the effect is only minor.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCC | Body-centered cube |
| FCC | Face-centered cube |
| LDS | Low-density steel |
| SFE | Stacking fault energy |
References
- Kim, H.; Suh, D.-W.; Kim, N.J. Fe–Al–Mn–C Lightweight Structural Alloys: A Review on the Microstructures and Mechanical Properties. Sci. Technol. Adv. Mater. 2013, 14, 014205. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Song, R.; Li, Y.; Sun, T.; Wang, K. Tensile Deformation of Low Density Duplex Fe–Mn–Al–C Steel. Mater. Des. 2015, 76, 32–39. [Google Scholar] [CrossRef]
- Lee, H.-J.; Sohn, S.S.; Lee, S.; Kwak, J.-H.; Lee, B.-J. Thermodynamic Analysis of the Effect of C, Mn and Al on Microstructural Evolution of Lightweight Steels. Scr. Mater. 2013, 68, 339–342. [Google Scholar] [CrossRef]
- Kozłowska, A.; Grajcar, A.; Opara, J.; Kaczmarczyk, J.; Janik, A.; Radwański, K. Mechanical Behaviour and Micromechanical Modelling of Medium-Mn Steel Microstructure Evolution. Int. J. Mech. Sci. 2022, 220, 107151. [Google Scholar] [CrossRef]
- Yu, L.; Gu, X.; Qian, L.; Jiang, P.; Wang, W.; Yu, M. Application of Tailor Rolled Blanks in Optimum Design of Pure Electric Vehicle Crashworthiness and Lightweight. Thin-Walled Struct. 2021, 161, 107410. [Google Scholar] [CrossRef]
- Chen, S.; Rana, R.; Haldar, A.; Ray, R.K. Current State of Fe-Mn-Al-C Low Density Steels. Prog. Mater. Sci. 2017, 89, 345–391. [Google Scholar] [CrossRef]
- Ding, H.; Liu, D.; Cai, M.; Zhang, Y. Austenite-Based Fe-Mn-Al-C Lightweight Steels: Research and Prospective. Metals 2022, 12, 1572. [Google Scholar] [CrossRef]
- Zargaran, A.; Kim, H.S.; Kwak, J.H.; Kim, N.J. Effects of Nb and C Additions on the Microstructure and Tensile Properties of Lightweight Ferritic Fe–8Al–5Mn Alloy. Scr. Mater. 2014, 89, 37–40. [Google Scholar] [CrossRef]
- Khaple, S.; Golla, B.R.; Prasad, V.V.S. A Review on the Current Status of Fe–Al Based Ferritic Lightweight Steel. Def. Technol. 2023, 26, 1–22. [Google Scholar] [CrossRef]
- Rana, R.; Lahaye, C.; Ray, R.K. Overview of Lightweight Ferrous Materials: Strategies and Promises. JOM 2014, 66, 1734–1746. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, L.; Yang, W.; Shen, P. Effect of Various Aluminum Content on the Formation of Inclusion. In TMS 2017 146th Annual Meeting & Exhibition Supplemental Proceedings; The Minerals, Metals & Materials Society TMS, Ed.; The Minerals, Metals & Materials Series; Springer International Publishing: Cham, Switzerland, 2017; pp. 483–489. ISBN 978-3-319-51492-5. [Google Scholar]
- Grajcar, A.; Skrzypczyk, P.; Kozłowska, A. Effects of Temperature and Time of Isothermal Holding on Retained Austenite Stability in Medium-Mn Steels. Appl. Sci. 2018, 8, 2156. [Google Scholar] [CrossRef]
- Zhang, B.-G.; Zhang, X.-M.; Liu, H.-T. Precipitation Behavior of B2 and κ-Carbide during Aging and Its Effect on Mechanical Properties in Al-Containing High Strength Steel. Mater. Charact. 2021, 178, 111291. [Google Scholar] [CrossRef]
- Brüx, U.; Frommeyer, G.; Jimenez, J. Light-Weight Steels Based on Iron-Aluminium-Influence of Micro Alloying Elements (B, Ti, Nb) on Microstructures, Textures and Mechanical Properties. Steel Res. 2002, 73, 543–548. [Google Scholar] [CrossRef]
- Lu, W.J.; Qin, R.S. Influence of κ-Carbide Interface Structure on the Formability of Lightweight Steels. Mater. Des. 2016, 104, 211–216. [Google Scholar] [CrossRef]
- Sohn, S.S.; Lee, B.-J.; Lee, S.; Kwak, J.-H. Effect of Mn Addition on Microstructural Modification and Cracking Behavior of Ferritic Light-Weight Steels. Metall. Mater. Trans. A 2014, 45, 5469–5485. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, S.; Liu, S.; Guo, Y.; Liang, S.; Zhang, S.; Zhang, X.; Zhang, J.; Liu, R. Effect of Annealing Treatment on Microstructure and Mechanical Properties of Lightweight Steels Micro-Alloyed with Vanadium and Niobium. Mater. Sci. Eng. A 2023, 886, 145700. [Google Scholar] [CrossRef]
- Opiela, M.; Fojt-Dymara, G.; Grajcar, A.; Borek, W. Effect of Grain Size on the Microstructure and Strain Hardening Behavior of Solution Heat-Treated Low-C High-Mn Steel. Materials 2020, 13, 1489. [Google Scholar] [CrossRef]
- Xiong, W.; Olson, G.B. Cybermaterials: Materials by Design and Accelerated Insertion of Materials. npj Comput. Mater. 2016, 2, 15009. [Google Scholar] [CrossRef]
- Van De Walle, A.; Asta, M. High-Throughput Calculations in the Context of Alloy Design. MRS Bull. 2019, 44, 252–256. [Google Scholar] [CrossRef]
- Klancnik, G.; Petrovic, S.; Medved, J. Thermodynamic Calculation of Phase Equilibria in Stainless Steels. J. Min. Metall. Sect. B Metall. 2012, 48, 383–390. [Google Scholar] [CrossRef]
- Chin, K.-G.; Lee, H.-J.; Kwak, J.-H.; Kang, J.-Y.; Lee, B.-J. Thermodynamic Calculation on the Stability of (Fe, Mn) 3AlC Carbide in High Aluminum Steels. J. Alloys Compd. 2010, 505, 217–223. [Google Scholar] [CrossRef]
- Burja, J.; Šetina Batič, B.; Balaško, T. Kappa Carbide Precipitation in Duplex Fe-Al-Mn-Ni-C Low-Density Steel. Crystals 2021, 11, 1261. [Google Scholar] [CrossRef]
- Dykas, J.; Samek, L.; Grajcar, A.; Kozłowska, A. Modelling of Phase Diagrams and Continuous Cooling Transformation Diagrams of Medium Manganese Steels. Symmetry 2023, 15, 381. [Google Scholar] [CrossRef]
- Chen, P.; Li, X.; Yi, H. The κ-Carbides in Low-Density Fe-Mn-Al-C Steels: A Review on Their Structure, Precipitation and Deformation Mechanism. Metals 2020, 10, 1021. [Google Scholar] [CrossRef]
- Scherbring, S.; Chen, G.; Veltel, B.; Bartzsch, G.; Richter, J.; Vollmer, M.; Blankenburg, M.; Shyamal, S.; Volkova, O.; Niendorf, T.; et al. Microstructural Constituents and Mechanical Properties of Low-Density Fe-Cr-Ni-Mn-Al-C Stainless Steels. Materials 2022, 15, 5121. [Google Scholar] [CrossRef]
- Rana, R.; Liu, C.; Ray, R.K. Low-Density Low-Carbon Fe–Al Ferritic Steels. Scr. Mater. 2013, 68, 354–359. [Google Scholar] [CrossRef]
- Frutos, E.; Morris, D.G.; Muñoz-Morris, M.A. Evaluation of Elastic Modulus and Hardness of Fe–Al Base Intermetallics by Nano-Indentation Techniques. Intermetallics 2013, 38, 1–3. [Google Scholar] [CrossRef]
- Mertens, A.; Bellhouse, E.M.; McDermid, J.R. Microstructure and Mechanical Properties of a Mixed Si–Al TRIP-Assisted Steel Subjected to Continuous Galvanizing Heat Treatments. Mater. Sci. Eng. A 2014, 608, 249–257. [Google Scholar] [CrossRef]
- Heo, Y.-U.; Song, Y.-Y.; Park, S.-J.; Bhadeshia, H.K.D.H.; Suh, D.-W. Influence of Silicon in Low Density Fe-C-Mn-Al Steel. Metall. Mater. Trans. A 2012, 43, 1731–1735. [Google Scholar] [CrossRef]
- Khaple, S.; Baligidad, R.G.; Satya Prasad, V.V.; Satyanarayana, D.V.V. Microstructure and Mechanical Properties of Fe–7Al Based Lightweight Steel Containing Carbon. Mater. Sci. Technol. 2015, 31, 1408–1416. [Google Scholar] [CrossRef]
- Torabi, S.A.; Amini, K.; Naseri, M. Investigating the Effect of Manganese Content on the Properties of High Manganese Austenitic Steels. Int. J. Adv. Des. Manuf. Technol. 2017, 10, 75–83. [Google Scholar]
- Cao, W.; Wang, H.; Wang, C.; Liang, J.; Godfrey, A.; Wang, Y.; Weng, Y. Effect of Alloying Content on Microstructure and Mechanical Properties of Fe-Mn-Al-C Low-Density Steels. Mater. Sci. Eng. A 2023, 886, 145675. [Google Scholar] [CrossRef]
- Wang, L.; Parker, S.; Rose, A.; West, G.; Thomson, R. Effects of Solute Nb Atoms and Nb Precipitates on Isothermal Transformation Kinetics from Austenite to Ferrite. Metall. Mater. Trans. A 2016, 47, 3387–3396. [Google Scholar] [CrossRef]
- Huo, L.; Ma, T.; Gao, W.; Li, Y.; Zhang, H.; Gao, J. Study on the Austenite Grain Growth Behavior of Fe-Mn-Al-C Low-Density Steel Containing Niobium. Metals 2025, 15, 576. [Google Scholar] [CrossRef]
- Shen, Y.F.; Wang, C.M.; Sun, X. A Micro-Alloyed Ferritic Steel Strengthened by Nanoscale Precipitates. Mater. Sci. Eng. A 2011, 528, 8150–8156. [Google Scholar] [CrossRef]
- Khaple, S.; Prakash, U.; Golla, B.R.; Satya Prasad, V.V. Effect of Niobium Addition on Microstructure and Mechanical Properties of Fe–7Al–0.35C Low-Density Steel. Metallogr. Microstruct. Anal. 2020, 9, 127–139. [Google Scholar] [CrossRef]
- Skowronek, A.; Grajcar, A.; Sozańska-Jędrasik, L.; Radwański, K.; Matus, K.; Opara, J. Retained Austenite Grain Size as a Strength-Plasticity Relationship Indicator in Al-Alloyed Medium-Mn Steel Processed by Intercritical Annealing. J. Mater. Res. Technol. 2023, 26, 3201–3213. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Jimenez, J.A.; Lopez-Ezquerra, B.; Rementeria, R.; Morales-Rivas, L.; Kuntz, M.; Caballero, F.G. Analyzing the Scale of the Bainitic Ferrite Plates by XRD, SEM and TEM. Mater. Charact. 2016, 122, 83–89. [Google Scholar] [CrossRef]
- Chang, L.C.; Bhadeshia, H.K.D.H. Austenite Films in Bainitic Microstructures. Mater. Sci. Technol. 1995, 11, 874–882. [Google Scholar] [CrossRef]
- Bruce, D.; Paradise, P.; Saxena, A.; Temes, S.; Clark, R.; Noe, C.; Benedict, M.; Broderick, T.; Bhate, D. A Critical Assessment of the Archimedes Density Method for Thin-Wall Specimens in Laser Powder Bed Fusion: Measurement Capability, Process Sensitivity and Property Correlation. J. Manuf. Process. 2022, 79, 185–192. [Google Scholar] [CrossRef]
- Suh, D.-W.; Park, S.-J.; Lee, T.-H.; Oh, C.-S.; Kim, S.-J. Influence of Al on the Microstructural Evolution and Mechanical Behavior of Low-Carbon, Manganese Transformation-Induced-Plasticity Steel. Metall. Mater. Trans. A 2010, 41, 397–408. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, F.; Zhang, Q.C.; Du, J.H.; Shi, F.; Li, X.W. Precipitation Behavior of κ-Carbides and Its Relationship with Mechanical Properties of Fe–Mn–Al–C Lightweight Austenitic Steel. J. Mater. Res. Technol. 2023, 25, 3780–3788. [Google Scholar] [CrossRef]
- Han, S.Y.; Shin, S.Y.; Lee, S.; Kim, N.J.; Kwak, J.-H.; Chin, K.-G. Effect of Carbon Content on Cracking Phenomenon Occurring during Cold Rolling of Three Light-Weight Steel Plates. Metall. Mater. Trans. A 2011, 42, 138–146. [Google Scholar] [CrossRef]
- Park, G.; Nam, C.H.; Zargaran, A.; Kim, N.J. Effect of B2 Morphology on the Mechanical Properties of B2-Strengthened Lightweight Steels. Scr. Mater. 2019, 165, 68–72. [Google Scholar] [CrossRef]
- Mishra, B.; Sarkar, R.; Singh, V.; Mukhopadhyay, A.; Mathew, R.T.; Madhu, V.; Prasad, M.J.N.V. Microstructure and Deformation Behaviour of Austenitic Low-Density Steels: The Defining Role of B2 Intermetallic Phase. SSRN Electron. J. 2021, 20, 101198. [Google Scholar] [CrossRef]
- Park, S.-W.; Park, J.Y.; Cho, K.M.; Jang, J.H.; Park, S.-J.; Moon, J.; Lee, T.-H.; Shin, J.-H. Effect of Mn and C on Age Hardening of Fe–Mn–Al–C Lightweight Steels. Met. Mater. Int. 2019, 25, 683–696. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Liu, M.; Ma, Z.; Yang, Y.; Xu, Z.; Song, C. Relationship between Austenite-Stabilizing Elements and Austenite Fraction in Near-Rapidly Solidified Fe–Mn–Al–C Lightweight Steel. Steel Res. Int. 2022, 93, 2200422. [Google Scholar] [CrossRef]
- Zhao, T.; Hao, X.; Wang, Y.; Chen, C.; Wang, T. Influence of Thermo-Mechanical Process and Nb-V Microalloying on Microstructure and Mechanical Properties of Fe–Mn–Al–C Austenitic Steel. Coatings 2023, 13, 1513. [Google Scholar] [CrossRef]
- Bai, Y.; Jiao, D.; Li, J.; Yang, Z. Effect of Nb Content on the Stacking Fault Energy, Microstructure and Mechanical Properties of Fe-25Mn-9Al-8Ni-1C Alloy. Mater. Today Commun. 2022, 31, 103554. [Google Scholar] [CrossRef]
- Cai, F.; Zhou, M.; Tian, J.; Xu, G. Comparative Study of the Role of Niobium in Low-Carbon Ferritic and Bainitic Steels. Mater. Sci. Eng. A 2022, 851, 143579. [Google Scholar] [CrossRef]
- Huo, W.; Song, R.; Zhang, Z.; Wang, Y.; Zhou, N.; Zhao, S.; Zhang, Y.; Sun, J. Effect of Nb Contents on Microstructure Characteristics and Yielding Behavior of Fe–4Mn–2Al-0.2C Steel. Mater. Sci. Eng. A 2021, 819, 141457. [Google Scholar] [CrossRef]
- Buckholz, S.A. The Influence of Aluminum and Carbon on the Abrasion Resistance of High Manganese Steels. Master’s Thesis, Missouri University of Science and Technology, Rolla, MO, USA, 2018. [Google Scholar]
- Sohn, S.S.; Lee, S.; Lee, B.-J.; Kwak, J.-H. Microstructural Developments and Tensile Properties of Lean Fe-Mn-Al-C Lightweight Steels. JOM 2014, 66, 1857–1867. [Google Scholar] [CrossRef]
- De Moor, E.; Matlock, D.K.; Speer, J.G.; Merwin, M.J. Austenite Stabilization through Manganese Enrichment. Scr. Mater. 2011, 64, 185–188. [Google Scholar] [CrossRef]
- ArcelorMittal. NAFTA_Data Sheet-Dual Phase. ArcelorMittal North America, Chicago, USA. 2016. Available online: https://fce.arcelormittal.com/repository2/About/Automotive/201609_NAFTA_DataSheet-DualPhase.pdf (accessed on 11 May 2025).
- Bøving, K.G. Hardness Testing. In NDE Handbook; Elsevier: Amsterdam, The Netherlands, 1989; pp. 103–119. ISBN 978-0-408-04392-2. [Google Scholar]
- Rahnama, A.; Kotadia, H.; Clark, S.; Janik, V.; Sridhar, S. Nano-Mechanical Properties of Fe-Mn-Al-C Lightweight Steels. Sci. Rep. 2018, 8, 9065. [Google Scholar] [CrossRef]
- Farabi, N.; Chen, D.L.; Zhou, Y. Microstructure and Mechanical Properties of Laser Welded Dissimilar DP600/DP980 Dual-Phase Steel Joints. J. Alloys Compd. 2011, 509, 982–989. [Google Scholar] [CrossRef]
- Oh, S.-J.; Kim, B.-C.; Suh, M.-C.; Shon, I.-J.; Lee, S.-J. Influence of Carbon Content on Austenite Stability and Strain-induced Transformation of Nanocrystalline FeNiC Alloy by Spark Plasma Sintering. Arch. Metall. Mater. 2019, 3, 863–867. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Guo, H.; Jiang, J.; Bai, Y.; Zhang, D. Effect of Carbon Content on Microstructural Evolution of 8Cr Steel in Austenitizing Process. Mater. Sci. Eng. A 2020, 796, 140026. [Google Scholar] [CrossRef]
- Grajcar, A.; Kciuk, M.; Topolska, S.; Płachcińska, A. Microstructure and Corrosion Behavior of Hot-Deformed and Cold-Strained High-Mn Steels. J. Mater. Eng. Perform. 2016, 25, 2245–2254. [Google Scholar] [CrossRef]
- Banis, A.; Gomez, A.; Bliznuk, V.; Dutta, A.; Sabirov, I.; Petrov, R.H. Microstructure Evolution and Mechanical Behavior of Fe–Mn–Al–C Low-Density Steel upon Aging. Mater. Sci. Eng. A 2023, 875, 145109. [Google Scholar] [CrossRef]
- Yang, L.; Huang, F.; Guo, Z.; Rong, Y.; Chen, N. Investigation on the Formation Mechanism of Ordered Carbide (FeMn)3AlC in the Al Added Twinning-Induced Plasticity Steels. J. Shanghai Jiaotong Univ. (Sci.) 2016, 21, 406–410. [Google Scholar] [CrossRef]
- Kwon, M.H.; Kim, J.-K.; Bian, J.; Mohrbacher, H.; Song, T.; Kim, S.K.; De Cooman, B.C. Solidification Microsegregation and Hot Ductility of Fe-Mn-C-Al-xNb TWIP Steels. Metall. Mater. Trans. A 2018, 49, 5509–5523. [Google Scholar] [CrossRef]
- Tsai, K.-Y.; Tsai, M.-H.; Yeh, J.-W. Sluggish Diffusion in Co–Cr–Fe–Mn–Ni High-Entropy Alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Mapelli, C.; Barella, S.; Gruttadauria, A.; Mombelli, D.; Bizzozero, M.; Veys, X. γ Decomposition in Fe–Mn–Al–C Lightweight Steels. J. Mater. Res. Technol. 2020, 9, 4604–4616. [Google Scholar] [CrossRef]
- Zhu, C.; Lu, Z.P.; Nieh, T.G. Incipient Plasticity and Dislocation Nucleation of FeCoCrNiMn High-Entropy Alloy. Acta Mater. 2013, 61, 2993–3001. [Google Scholar] [CrossRef]
- Xing, J.; Wei, Y.; Hou, L. An Overview of the Effects of Alloying Elements on the Properties of Lightweight Fe-(15–35) Mn-(5–12) Al-(0.3–1.2) C Steel. JOM 2018, 70, 929–937. [Google Scholar] [CrossRef]
- Rawat, P.; Prakash, U.; Prasad, V.V.S. Phase Transformation and Hot Working Studies on High-Al Fe-Al-Mn-C Ferritic Low-Density Steels. J. Mater. Eng. Perform. 2021, 30, 6297–6308. [Google Scholar] [CrossRef]
- Tipalin, S.A.; Belousov, V.B.; Lyubetskaya, S.I. Testing the Cross-Sectional Microhardness in Sheets with A 0.08% Carbon Concentration. Solid State Phenom. 2021, 316, 269–275. [Google Scholar] [CrossRef]
| Chemical Composition/wt% | C | Al | Mn | Nb | Pmax | Smax | O, ppm | N, ppm |
|---|---|---|---|---|---|---|---|---|
| Low-Mn lightweight steel | 0.04 | 5.5 | 1.6 | 0.075 | <0.010 | <0.010 | 16 | 25 |
| Medium-Mn lightweight steel | 0.04 | 5.6 | 5.5 | 0.080 | <0.010 | <0.010 | 6 | 20 |
| Approach | Element (wt%) | Density (g/cm3) | Density Reduction (%) | ||||
|---|---|---|---|---|---|---|---|
| C | Al | Mn | Nb | Archimedes’ (A) | Thermo-Calc (TC) | ||
| Theoretical | 0.03 | 6.0 | 1.6 | 0.080 | - | 7.306 | 7.00 |
| Experimental | 0.04 | 5.5 | 1.6 | 0.075 | 7.297 | - | 7.23 |
| Theoretical | 0.03 | 6.0 | 5.0 | 0.080 | 7.273 | 7.00 | |
| Experimental | 0.04 | 5.6 | 5.5 | 0.080 | 7.212 | - | 8.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kori, T.H.; Skowronek, A.; Opara, J.; Cardoso, A.P.D.; Grajcar, A. Thermodynamic Modeling of Microstructural Design of Lightweight Ferritic Steels. Metals 2025, 15, 912. https://doi.org/10.3390/met15080912
Kori TH, Skowronek A, Opara J, Cardoso APD, Grajcar A. Thermodynamic Modeling of Microstructural Design of Lightweight Ferritic Steels. Metals. 2025; 15(8):912. https://doi.org/10.3390/met15080912
Chicago/Turabian StyleKori, Tamiru Hailu, Adam Skowronek, Jarosław Opara, Ana Paula Domingos Cardoso, and Adam Grajcar. 2025. "Thermodynamic Modeling of Microstructural Design of Lightweight Ferritic Steels" Metals 15, no. 8: 912. https://doi.org/10.3390/met15080912
APA StyleKori, T. H., Skowronek, A., Opara, J., Cardoso, A. P. D., & Grajcar, A. (2025). Thermodynamic Modeling of Microstructural Design of Lightweight Ferritic Steels. Metals, 15(8), 912. https://doi.org/10.3390/met15080912






