Facile Approach for Fabrication of Hydrophobic Aluminum Alloy Surfaces Using Fatty Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Hydrophobic Coatings
2.3. Characterization
3. Results and Discussions
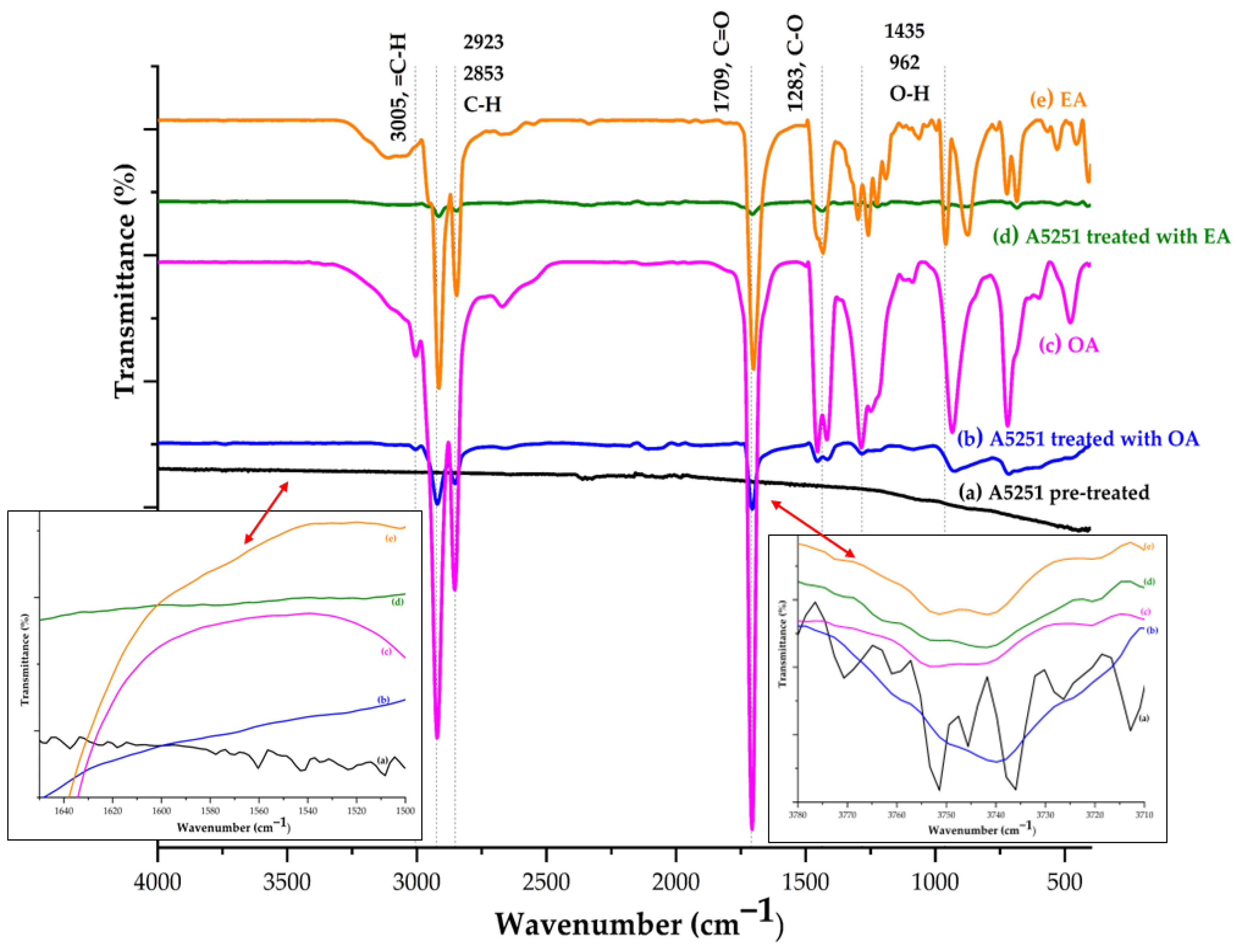
3.1. Functional Group Analysis (FTIR)
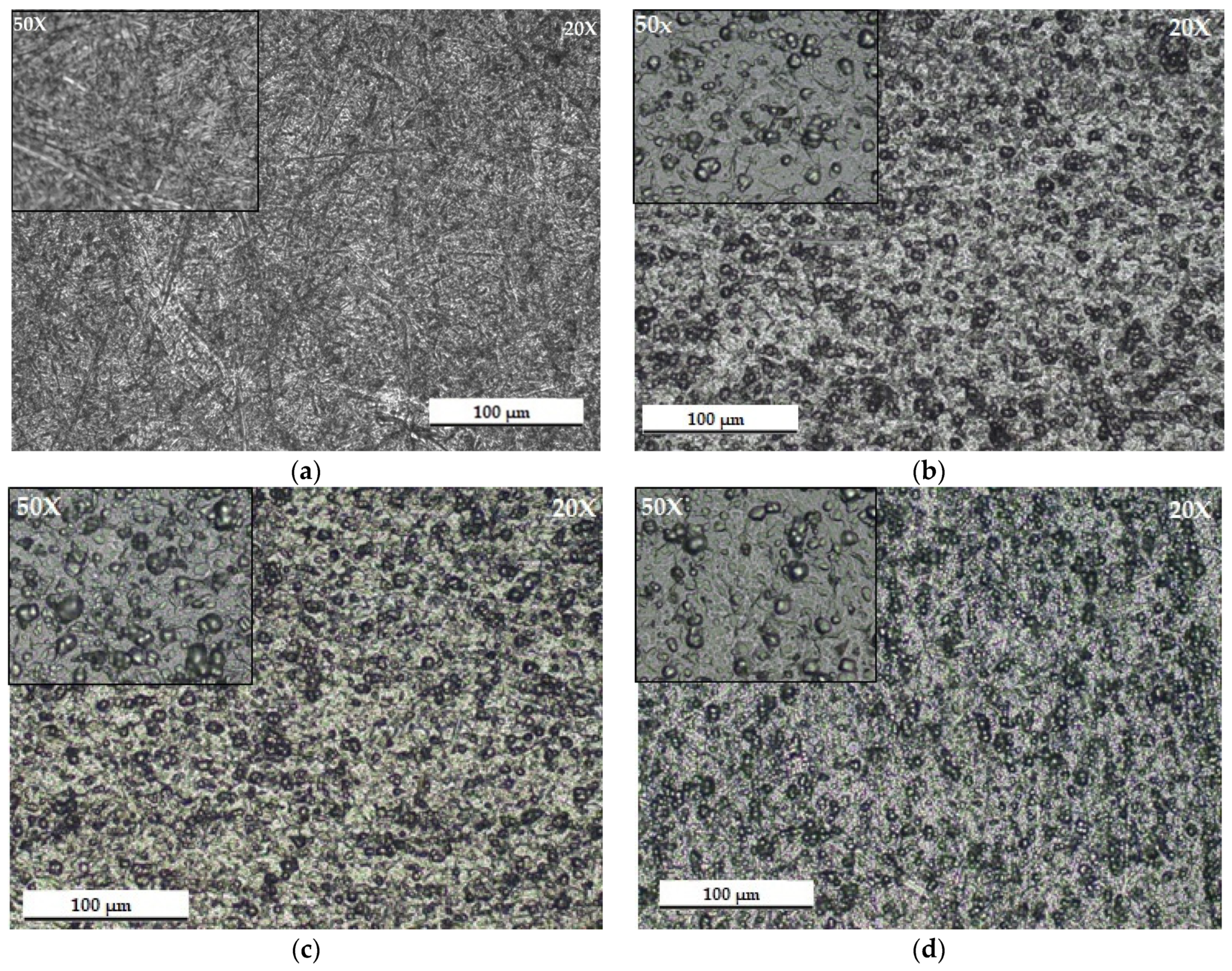
3.2. Optical Microscopy (MO)
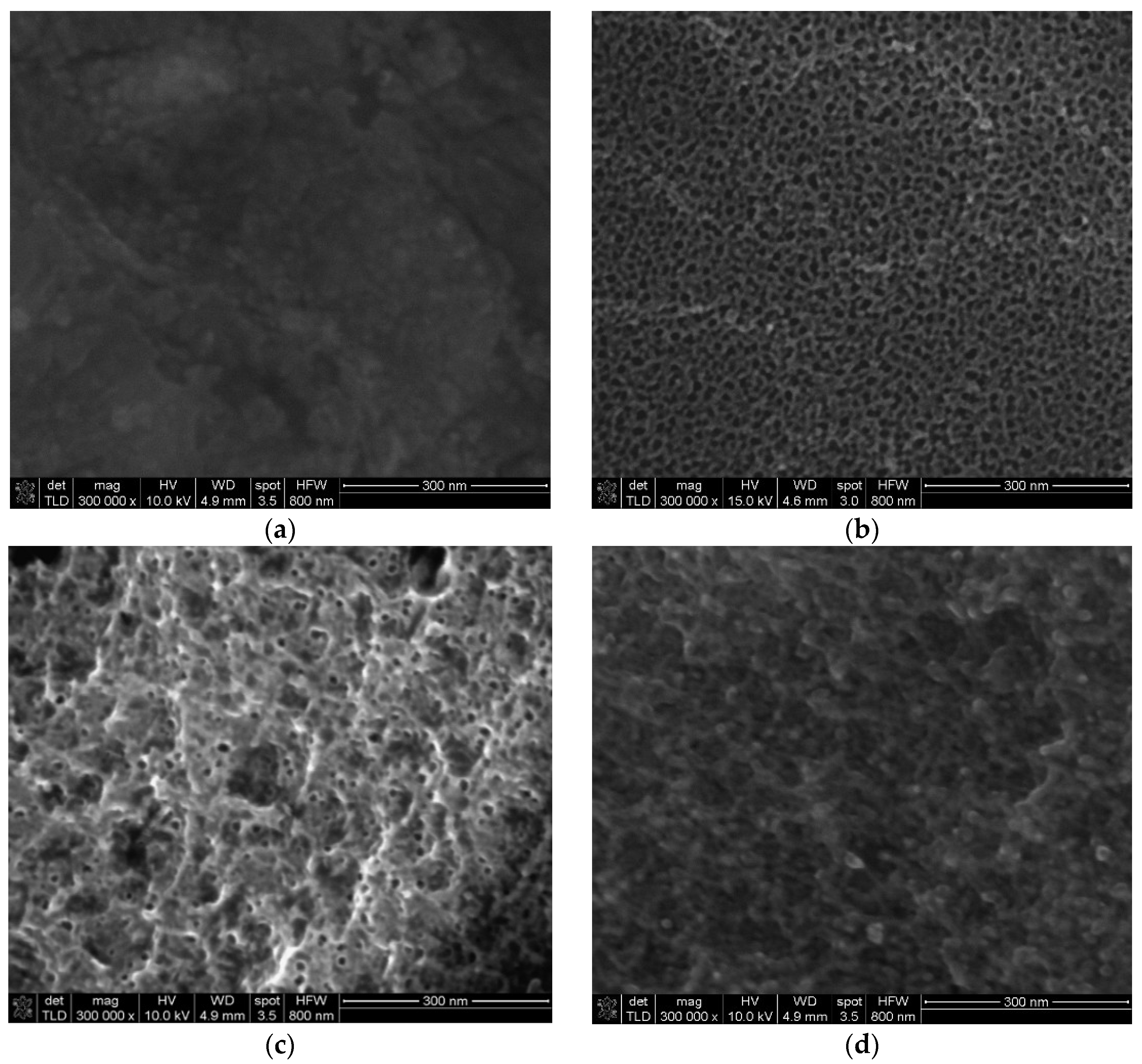
3.3. SEM-EDX Analysis
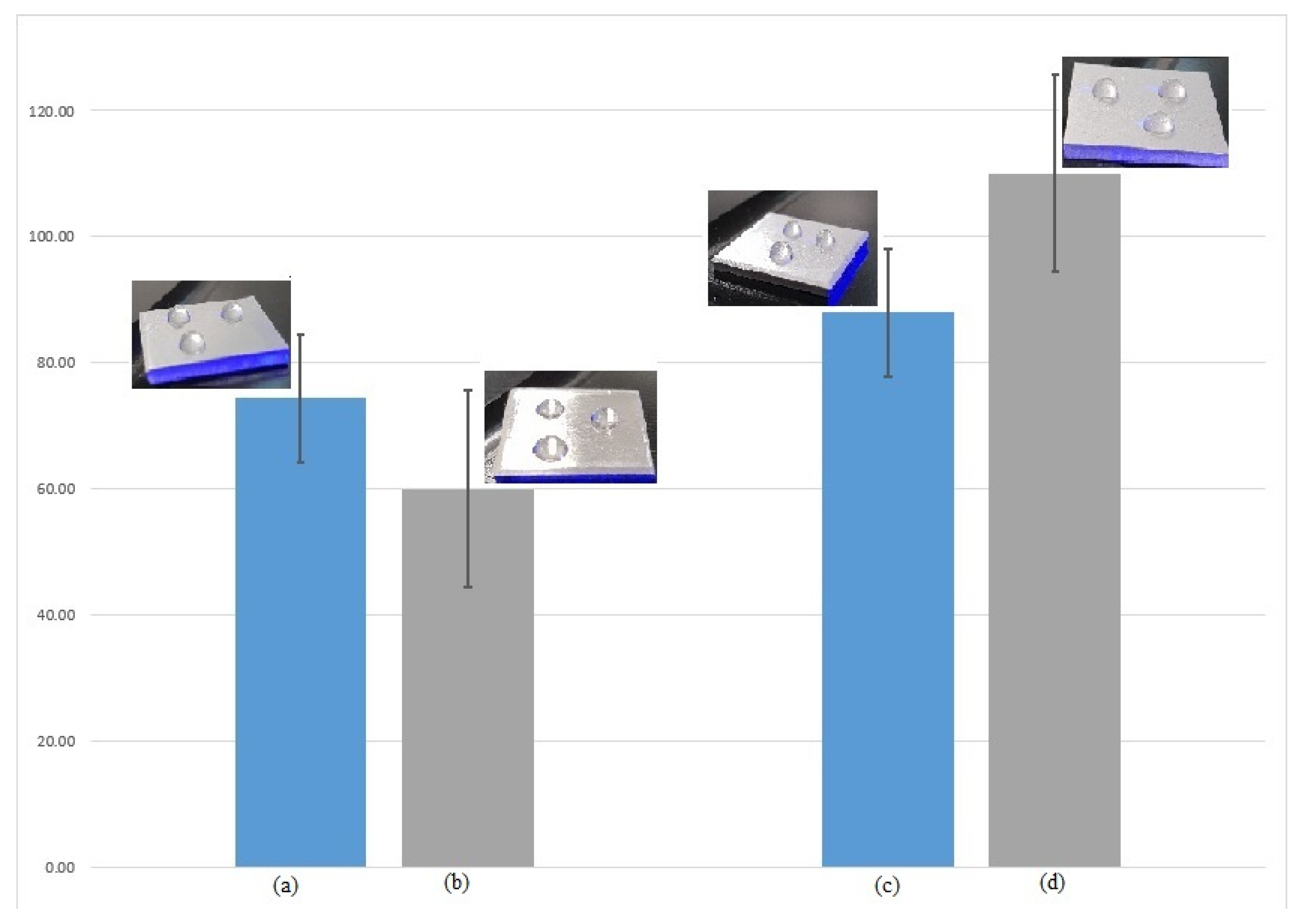
3.4. Wetting Capacity (Contact Angle)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DMF | N, N-dimethylformamide |
| OA | Oleic acid |
| EA | Elaidic acid |
| SA | Stearic acid |
| FTIR | Fourier transform infrared spectroscopy |
| MO | Optical microscopy |
| SEM | Scanning electron microscopy |
| EDX | Element energy dispersive spectroscopy |
| CA | Contact angle |
References
- Zhu, J. A novel fabrication of superhydrophobic surfaces on aluminum substrate. Appl. Surf. Sci. 2018, 447, 363–367. [Google Scholar] [CrossRef]
- Bi, P.; Li, H.; Zhao, G.; Ran, M.; Cao, L.; Guo, H. Robust super-hydrophobic coating prepared by electrochemical surface engineering for corrosion protection. Coatings 2019, 9, 452. [Google Scholar] [CrossRef]
- Panda, B. Corrosion Resistant Superhydrophobic Aluminum Alloy: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1017, 012008. [Google Scholar] [CrossRef]
- Say, Y.; Aslan, N.; Alla, A.M.A.; Ozmen, H. Influence of chemical etchings on surface properties, in-vitro degradation and ion releases of 316l stainless steel alloy for biomedical applications. Mater. Chem. Phys. 2023, 295, 127139. [Google Scholar] [CrossRef]
- Lin, H.-K.; Cheng, Y.-H.; Li, G.-Y.; Chen, Y.-C.; Bazarnik, P.; Muzy, J.; Huang, Y.; Langdon, T.G. Study on the Surface Modification of Nanostructured Ti Alloys and Coarse-Grained Ti Alloys. Metals 2022, 12, 948. [Google Scholar] [CrossRef]
- Liang, M.; Wei, Y.; Hou, L.; Wang, H.; Li, Y.; Guo, C. Fabrication of a super-hydrophobic surface on a magnesium alloy by a simple method. J. Alloys Compd. 2015, 656, 311–317. [Google Scholar] [CrossRef]
- Yeganeh, M.; Mohammadi, N. Superhydrophobic surface of Mg alloys: A review. J. Magnes. Alloys 2018, 6, 59–70. [Google Scholar] [CrossRef]
- Wu, R.; Liang, S.; Chen, H.; Pan, A.; Jiang, H.; Deng, J.; Yu, Y.; Yuan, Z.; Liu, Q. Fabrication of a super-hydrophobic micro-nanoporous aluminum surface by anodic oxidation. Appl. Mech. Mat. 2012, 200, 190–193. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.W.; Zhang, H.F.; Zhou, Z.P. Fabrication of self-healing super-hydrophobic surfaces on aluminum alloy substrates. AIP Adv. 2015, 5, 41314. [Google Scholar]
- Khaskhoussi, A.; Calabrese, L.; Proverbio, E. Anticorrosion Superhydrophobic Surfaces on AA6082 Aluminum Alloy by HF/HCl Texturing and Self-Assembling of Silane Monolayer. Materials 2022, 15, 8549. [Google Scholar] [CrossRef]
- Yang, E.; Yang, R.; Wei, W.; Mo, Q.; Liang, F.; Li, D.; Li, W. Corrosion resistance and antibacterial properties of hydrophobic modified Ce-doped micro-arc oxidation coating. J. Mater. Res. Technol. 2024, 29, 3303–3316. [Google Scholar] [CrossRef]
- Li, X.; Shi, T.; Zhang, C.; Zhong, B.; Lv, Y.; Zhang, Q. One-Step Preparation of Super-Hydrophobic Micro-Nano Dendrites on Al Alloy for Enhanced Corrosion Resistance. Metals 2018, 8, 960. [Google Scholar] [CrossRef]
- Pezzato, L.; Rigon, M.; Martucci, A.; Brunelli, K.; Dadala, M. Plasma Electrolytic Oxidation (PEO) as pre-treatment for sol-gel coating on aluminum and magnesium alloys. Surf. Coat. Technol. 2019, 336, 114–123. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Khorsand, S.; Sokhanvar, S.; Kaboli, A. Science and engineering of superhydrophobic surfaces: Review of corrosion resistance, chemical and mechanical stability. Arab. J. Chem. 2020, 13, 1763–1802. [Google Scholar] [CrossRef]
- Jin, Z.; Cai, C.; Yuan, Y.; Kang, D.; Hunter, J.; Zhou, X. The behaviour of AA5754 and AA5052 aluminium alloys in alkaline etching solution: Similarity and difference. Mater. Charact. 2021, 171, 110768. [Google Scholar] [CrossRef]
- Yashpal, K.; Jawalkar, C.S.; Kant, S. A review of used of aluminum alloys in aircraft components. I-Manager’s. J. Mater. Sci. 2015, 3, 33–38. [Google Scholar]
- Varshney, P.; Mohapatra, S.S.; Kumar, A. Superhydrophobic coatings for aluminum surfaces synthesized by chemical etching process. Int. J. Smart Nano Mat. 2016, 7, 248–264. [Google Scholar] [CrossRef]
- Li, Q.; Li, M.; Lu, G.; Guan, S.; Zhng, E.; Xu, C. Effect of trace elements on the crystallization temperature interval and properties of 5xxx series aluminum alloys. Metals 2020, 10, 483. [Google Scholar] [CrossRef]
- Matei, A.; Tutunaru, O.; Tucureanu, V. Surface pre-treatment of aluminum alloys for the deposition of composite. Mat. Sci. Eng. B 2021, 263, 114874. [Google Scholar] [CrossRef]
- Niedźwiedź, M.; Skoneczny, W.; Bara, M. The influence of anodic alumina coating nanostructure produced on EN AW-5251 alloy on type of tribological wear process. Coatings 2020, 10, 105. [Google Scholar] [CrossRef]
- Das, S.; Kumar, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. A review on superhydrophobic polymer nanocoatings: Recent development, application. Ind. Eng. Chem. Res. 2018, 57, 2727–2745. [Google Scholar] [CrossRef]
- Pandit, S.K.; Yadav, K.; Chauhan, P.; Kumar, A. Accessing the corrosion resistance for metallic surfaces using long-chain fatty acids. RSC Appl. Interfaces 2025. [Google Scholar] [CrossRef]
- Calabrese, L.; Khaskhoussi, A.; Patane, A.; Proverbio, E. Assessment of super-hydrophobic textured coatings on AA6082 aluminum alloy. Coatings 2019, 9, 352. [Google Scholar] [CrossRef]
- Fallah, M.; Rabiee, A.; Ghashghaee, M.; Ershad-Langroudi, A. Enhanced procedure for fabrication of an ultrahydrophobic aluminum alloy surface using fatty acid modifiers. Phys. Chem. Res. 2017, 5, 339–357. [Google Scholar]
- Malta, M.I.C.; Silva, R.G.C.; Silva, H.A.C.; Silva Filho, W.L.C.; Oliviera, S.H.; Araujo, E.G.; Filho, S.L.U.; Vieira, M.R.S. Influence of different fatty acids on the wettability, self-cleaning and anti-corrosion behavior of superhydrophobic surfaces obtained with LDH on aluminum. Surf. Coat. Technol. 2024, 488, 131039. [Google Scholar] [CrossRef]
- Huang, Z.; Yong, Q.; Xie, Z.H. Stearic acid modified porous nickel-based coating on magnesium alloy AZ31 for high superhydrophobicity and corrosion resistance. Corros. Sci. 2023, 10, 38–47. [Google Scholar] [CrossRef]
- Sacilotto, D.G.; Kunst, S.R.; Soares, L.G.; Pandolfo Carone, C.L.; Metz Arnold, D.C.; Oliveira, C.T.; Ferreira, J.Z. Evaluation of corrosion of 5052 aluminum alloy using superhydrophobic coatings based on stearic acid. Matéria 2025, 30, e20240824. [Google Scholar] [CrossRef]
- Zhang, D.; Wan, Y.; Nagayama, G. Superhydrophobicity of thermally annealed aluminum surfaces and its effect on corrosion resistance. Appl. Phys. Lett. 2023, 123, 091603. [Google Scholar] [CrossRef]
- Semiletov, A.M.; Kudelina, A.A.; Kuznetsov, Y.I. On Increasing the Stability of Superhydrophobic Layers of Oleic Acid on the Surface of D16 Aluminum Alloy. Prot. Met. Phys. Chem. Surf. 2022, 58, 1278–1283. [Google Scholar] [CrossRef]
- Wang, H.; Wei, Y.; Liang, M.; Hou, L.; Li, Y.; Guo, C. Fabrication of stable and corrosion-resisted super-hydrophobic film on Mg alloy. Colloids Surf. A 2016, 509, 351–358. [Google Scholar] [CrossRef]
- Huang, Y.; Sarkar, D.K.; Grant Chen, X. Superhydrophobic aluminum alloy surfaces prepared by chemical etching process and their corrosion resistance properties. Appl. Surf. Sci. 2015, 356, 1012–1024. [Google Scholar] [CrossRef]
- Wei, Z.; Jiang, D.; Chen, J.; Ren, S.; Ji, L. Fabrication of mechanically robust superhydrophobic aluminum surface by acid etching and stearic acid modification. J. Adhes. Sci. Technol. 2017, 31, 2380–2397. [Google Scholar] [CrossRef]
- Tudu, B.K.; Kumar, A.; Bhushan, B. Facile approach to develop anti-corrosive superhydrophobic aluminum with high mechanical, chemical and thermal durability. Philos. Trans. R. Soc. A 2019, 377, 20180272. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Zhang, C.; Song, L.; Zeng, R.; Li, S. Fabrication of the Superhydrophobic surface on magnesium alloy and its corrosion resistance. J. Mater. Sci. Technol. 2015, 31, 1139–1143. [Google Scholar] [CrossRef]
- Ng, W.F.; Wong, M.H.; Cheng, F.T. Stearic acid coating on magnesium for enhancing corrosion resistance in Hanks’ solution. Surf. Coat. Technol. 2010, 204, 1823–1830. [Google Scholar] [CrossRef]
- Li, C.; Ma, R.; Du, A.; Fan, Y.; Zhao, X.; Cao, X. Superhydrophobic film on hot-dip galvanized steel with corrosion resistance and self-cleaning properties. Metals 2018, 8, 687. [Google Scholar] [CrossRef]
- Sharifi Golru, S.; Attaa, M.M.; Ramezanzadeh, B. Effects of different surface cleaning procedures on the superficial morphology and the adhesive strength of epoxy coating on aluminum alloy 1050. Prog. Org. Coat. 2015, 87, 52–60. [Google Scholar] [CrossRef]
- Huynh, V.; Ngo, N.K.; Golden, T.D. Surface activation and pretreatments for biocompatible metals and alloys used in biomedical application. Int. J. Biomater. 2019, 2019, 3806504. [Google Scholar] [CrossRef]
- Salman, S.A.; Okido, M. Self-assembled monolayers formed on AZ31 Mg alloy. J. Phys. Chem. Solids 2012, 73, 863–866. [Google Scholar] [CrossRef]
- Kamde, M.A.; Mahton, Y.; Saha, P. A stearic acid/polypyrrole-based superhydrophobic coating on squeeze-cast Mg-Sr-Y-Ca-Zn alloys for improved salt-water corrosion. Surf. Coat. Techn. 2022, 448, 128890. [Google Scholar] [CrossRef]
- Chankitmunkong, S.; Eskin, D.; Limmaneevichitr, C.; Kengkla, N.; Diewwanit, O. Characterization of the anodic film and corrosion resistance of an A535 aluminum alloy after intermetallics removal by different etching time. Metals 2022, 12, 1140. [Google Scholar] [CrossRef]
- Escobar, A.M.; Llorca-Isern, N. Superhydrophobic coating deposited directly on aluminum. Appl. Surf. Sci. 2014, 305, 774–782. [Google Scholar] [CrossRef]
- Matei, A.; Stoian, M.; Craciun, G.; Tucureanu, V. Chemical synthesis and characterization of fatty acid-capped ZnO nanoparticles. Compos. Sci. 2024, 8, 429. [Google Scholar] [CrossRef]
- Mussa, M.H.; Takita, S.; Deeba, F.Z.; Lewis, O.; Farmilo, N. Study the effect of adding oleic acid to reduce the medium diffusion in pores of hybrid silica-based sol-gel coatings. Mater. Proc. 2021, 3, 1–10. [Google Scholar] [CrossRef]
- Kakinuma, H.; Muto, I.; Oya, Y.; Momii, T.; Sugawara, Y.; Hara, N. Improving the pitting corrosion resistance of AA1050 Aluminum by removing intermetallic particles during conversion treatments. Mater. Trans. 2021, 8, 1160–1167. [Google Scholar] [CrossRef]
- Araoyinbo, A.O.; Rahmat, A.; Derman, M.N.; Ahmad, K.R. Room temperature anodization of aluminum and the effect of the electrochemical cell in the formation of porous alumina films from acid and alkaline electrolytes. Adv. Mater. Lett. 2012, 3, 273–278. [Google Scholar] [CrossRef]
- Zhu, T.; Yuan, Y.; Xiang, H.; Liu, G.; Dai, X.; Song, L.; Liao, R. A composite pore-structured superhydrophobic aluminum surface for durable anti-icing. J. Mater. Res. Technol. 2023, 27, 3151–3163. [Google Scholar] [CrossRef]
- Prabhu, D.; Rao, P. Corrosion behavior of 6063 aluminum alloy in acidic and in alkaline media. Arab. J. Chem. 2017, 10, S2234–S2244. [Google Scholar]
- Goja, F.; Loulakis, M.; Papoutsaki, L.; Tzortzakis, S.; Chrissopoulou, K.; Anastasoadis, S.A. Altering the Surface Properties of Metal Alloys Utilizing Facile and Ecological Methods. Langmuir 2022, 38, 4826–4838. [Google Scholar] [CrossRef] [PubMed]
- Willenshofer, P.D.; Coradini, D.S.R.; Renk, O.; Uggowitzer, P.J.; Tunes, M.A.; Pogatscher, S. Comparative analysis of experimental techniques for microstructural characterization of novel nanostructured aluminium alloys. Mater. Charact. 2024, 215, 114154. [Google Scholar] [CrossRef]
- Zhu, Q.; Du, X.; Liu, Y.; Fang, X.; Chen, D.; Zhang, Z. Preparation and Applications of Superhydrophobic Coatings on Aluminum Alloy Surface for Anti-Corrosion and Anti-Fouling: A Mini Review. Coatings 2023, 13, 1881. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Szymczyk, K.; Janczuk, B.; Longwic, R.; Sander, P. Surface, Volumetric, and Wetting Properties of Oleic, Linoleic, and Linolenic Acids with Regards to Application of Canola Oil in Diesel Engines. Appl. Sci. 2019, 9, 3445. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, A.; Brincoveanu, O.; Ţucureanu, V. Facile Approach for Fabrication of Hydrophobic Aluminum Alloy Surfaces Using Fatty Acids. Metals 2025, 15, 884. https://doi.org/10.3390/met15080884
Matei A, Brincoveanu O, Ţucureanu V. Facile Approach for Fabrication of Hydrophobic Aluminum Alloy Surfaces Using Fatty Acids. Metals. 2025; 15(8):884. https://doi.org/10.3390/met15080884
Chicago/Turabian StyleMatei, Alina, Oana Brincoveanu, and Vasilica Ţucureanu. 2025. "Facile Approach for Fabrication of Hydrophobic Aluminum Alloy Surfaces Using Fatty Acids" Metals 15, no. 8: 884. https://doi.org/10.3390/met15080884
APA StyleMatei, A., Brincoveanu, O., & Ţucureanu, V. (2025). Facile Approach for Fabrication of Hydrophobic Aluminum Alloy Surfaces Using Fatty Acids. Metals, 15(8), 884. https://doi.org/10.3390/met15080884






