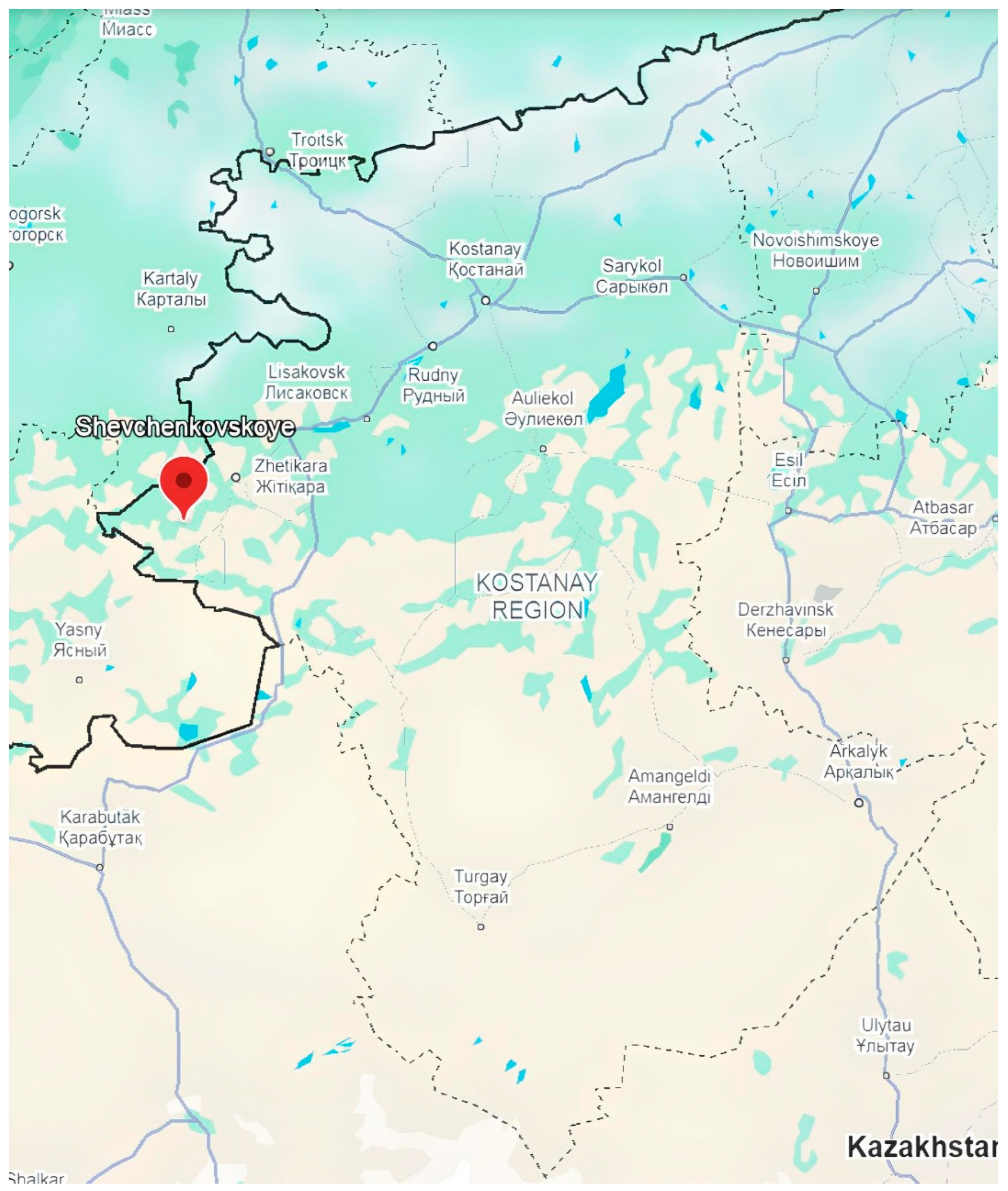
Treatment of Refractory Oxidized Nickel Ores (ONOs) from the Shevchenkovskoye Ore Deposit
Abstract
1. Introduction
- -
- Classification and characteristics of nickel ore types.
- -
- Global reserves and resource distribution.
- -
- Major nickel-bearing countries and deposits.
- -
- Genesis and distribution.
- -
- Industrial types and chemical composition.
- -
- Mineralogical features and processing challenges.
- -
- History and status of ONO mining in Kazakhstan.
- -
- Key deposits and exploration regions.
- -
- Pyrometallurgical methods.
- -
- Shaft and electric furnace smelting.
- -
- Ferronickel and matte production.
- -
- Ausmelt process.
- -
- Hydrometallurgical methods.
- -
- HPAL (high-pressure acid leaching).
- -
- Heap and underground leaching.
- -
- Pyro-hydrometallurgical methods.
- -
- Caron process.
- -
- Combined flowsheets and ammonia leaching.
2. Materials and Methods
Raw Material Base
- Supergene (silicate) deposits.
- Sulfide copper–nickel deposits.
- Deep-sea iron–manganese nodules formed on the ocean floor.
- Supergene silicate ores, where the main nickel-bearing minerals are nickeliferous limonite and garnierite;
- Magmatic sulfide copper–nickel ores, where pyrrhotite (iron–nickel sulfide) is predominant.
- Unique—more than 1 million tonnes of nickel.
- Very large—from 500 thousand to 1 million tonnes.
- Large—from 250 thousand to 500 thousand tonnes.
- Medium—from 100 thousand to 250 thousand tonnes.
- Small—less than 100 thousand tonnes.
3. Characteristics of Oxidized Nickel Ores (ONOs)
- Iron-rich ores (including ochreous, leptochloritic, and hematitic varieties);
- Magnesium-rich ores (predominantly serpentinites enriched with nickel-bearing silicates).
- Limonitic ores consist mainly of iron oxides and hydroxides;
- Serpentinite ores consist of iron–magnesium silicates.
4. Kazakhstani Deposits of Oxidized Nickel Ores
5. Processing Methods for Oxidized Nickel Ores (ONOs)
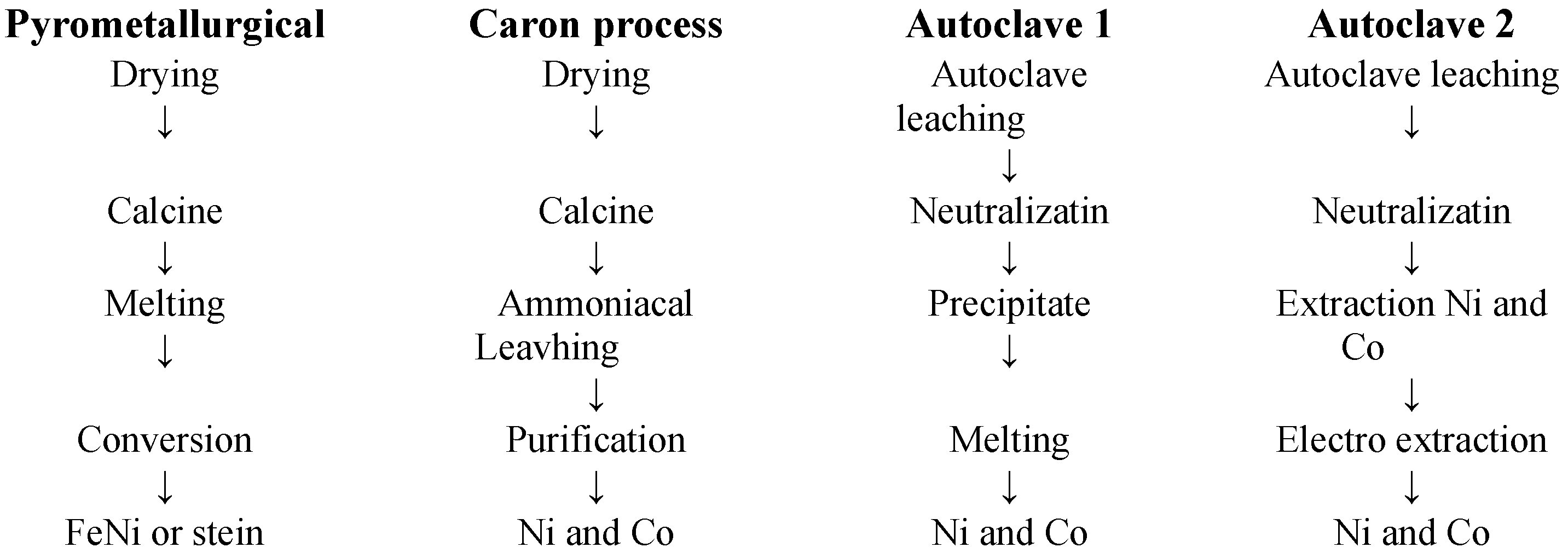
5.1. Pyrometallurgical Methods for Processing Oxidized Nickel Ores
5.2. Hydrometallurgical Methods for Processing Oxidized Nickel Ores (ONOs)
5.3. Pyro-Hydrometallurgical Methods for Processing ONOs
6. Discussion
- No need for elevated pressures;
- Improved filterability of precipitates;
- The potential to produce valuable by-products.
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kalungi, P.; Yao, Z.; Huang, H. Aspects of Nickel, Cobalt and Lithium, the Three Key Elements for Li-Ion Batteries: An Overview on Resources, Demands, and Production. Materials 2024, 17, 4389. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B. Cobalt and Nickel Production. In Encyclopedia of Materials, Science and Technology, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2001; pp. 1288–1294. [Google Scholar]
- Thorne, R.L.; Roberts, S.; Herrington, R. Climate Change and the Formation of Nickel Laterites. Geology 2012, 40, 331–334. [Google Scholar] [CrossRef]
- Butt, C.R.M.; Cluzel, D. Nickel Laterite Ore Deposits: Weathered Serpentinites. Elements 2013, 9, 123–128. [Google Scholar] [CrossRef]
- Savina, A.A.; Boev, A.O.; Orlova, E.D.; Morozov, A.V.; Abakumov, A.M. Nickel as a key element in the future energy. Russ. Chem. Rev. 2023, 92, RCR5086. [Google Scholar] [CrossRef]
- Lv, W.; Xin, Y.; Elliott, R.; Song, J.; Lv, X.; Barati, M. Drying kinetics of a Philippine nickel laterite ore by microwave heating. Miner. Process. Extr. Metall. Rev. 2020, 42, 46–52. [Google Scholar] [CrossRef]
- Gudivada, G.; Pandey, A.K. Recent Developments in Nickel-Based Superalloys for Gas Turbine Applications. J. Alloys Compd. 2023, 963, 171128. [Google Scholar] [CrossRef]
- Wu, R.; Zhao, Y.; Yin, Q.; Wang, J.; Ai, X.; Wen, Z. Atomistic Simulation Studies of Ni-Based Superalloys. J. Alloys Compd. 2021, 855, 157355. [Google Scholar] [CrossRef]
- Long, H.; Mao, S.; Liu, Y.; Zhang, Z.; Han, X. Microstructural and compositional design of Ni-based single crystalline superalloys—A review. J. Alloys Compd. 2018, 743, 203–220. [Google Scholar] [CrossRef]
- Cempel, M.; Nikel, G. Nickel: A Review of Its Sources and Environmental Toxicology. Pol. J. Environ. Stud. 2006, 15, 375–382. [Google Scholar]
- Aheiev, M.I.; Sanin, V.V.; Shvindina, N.V.; Kaplanskii, Y.Y.; Levashov, E.A. Oxidation kinetics and mechanism of nickel alloys. Poroshkovaya Metall. I Funktsional’nye Pokrytiya 2022, 16, 3. [Google Scholar] [CrossRef]
- Galimov, V.R.; Mansurov, D.I. The possibility of using modern additive technologies for growing products made of heat-resistant nickel alloys. Mater. Technol. Des. 2022, 4, 14–21. [Google Scholar] [CrossRef]
- Processing of Cobalt-Nickel Ore. Available online: https://invest.gov.kz/doing-business-here/invest-projects/8326/ (accessed on 3 March 2025).
- Mukhamediyarov, N.; Sabitova, A.; Nurgaliev, N.; Ualikhanov, A.; Aitkazin, N. The Current State of Waste from Metallurgical Production in South Kazakhstan and the Prospects for Their Processing; News of the Academy of Sciences of the Republic of Kazakhstan, Chemistry and Technology Series; National Academy of Science Republic of Kazakhstan: Almaty, Kazakhstan, 2025; pp. 183–197. [Google Scholar]
- Orazymbetova, A.O.; Sagitova, G.F.; Sidikov, A.S.; Sakibayeva, S.A.; Suigenbayeva, A.Z. Physical and Chemical Modification of a Natural Mineral of the Changkanai Deposit; Technical Sciences Series; Bulletin of Shakarim University: Semey, Kazakhstan, 2025; pp. 459–469. [Google Scholar] [CrossRef]
- In 2025, the Bugetkol Nickel-Cobalt Deposit Will Be Launched in Kazakhstan. Available online: https://dprom.kz/novosti/v-rk-zapustyat-neekyel-kobaltovoye-bugyetkol/ (accessed on 5 March 2025).
- QazNickel. Geography. Available online: https://www.fincraftresources.kz/activity/kaznickel_llp?language=en (accessed on 5 March 2025).
- Antonenko, A.A.; Miniskul, S.D.; Kazhan, I.M. Copper-nickel mineralization of Kazakhstan. In Proceedings of the International Scientific and Practical Conference: K.I. Satpayev and Earth Sciences, Almaty, Kazakhstan, 11–12 April 2024; pp. 39–43. [Google Scholar]
- Wani, O.B.; Khan, S.; Shoaib, M.; Gonçalves, C.D.C.; Chen, Z.; Zeng, H.; Bobicki, E.R. Processing of low-grade ultramafic nickel ores: A critical review. Miner. Eng. 2024, 218, 108976. [Google Scholar] [CrossRef]
- Nickel Statistics and Information. Available online: https://www.usgs.gov/centers/national-minerals-information-center/nickel-statistics-and-information#:~:text=The%20bulk%20of%20the%20nickel%20mined%20comes,the%20principal%20ore%20mineral%20is%20pentlandite%20%5B(Ni%2CFe)9S8%5D (accessed on 7 March 2025).
- Boyarko, G.Y.; Lapteva, A.M.; Bolsunovskaya, L.M. Mineral resource base of Russia’s copper: Current state and development prospects. Min. Sci. Tecnol. Russ. 2024, 9, 4. [Google Scholar] [CrossRef]
- Vershinin, A. The Ural Nickel Belt. Ore Subformations of Supergene Nickel Deposits in the Urals and Their Characteristics. Min. J. 1996, 8, 5–16. [Google Scholar]
- Sobol, S. Autoclave Methods for Processing Oxidized Nickel Ores; Central Research Institute of Economics and Information of Non-Ferrous Metallurgy (TsNII E&I NFM): Moscow, Russia, 1980; p. 13. [Google Scholar]
- Baturin, G. Ore potential of the ocean. Nature 2002, 5, 27–38. [Google Scholar]
- Glumov, I.; Zadornov, M.; Kuznecov, K. The World Ocean is a storehouse of metallurgical raw materials. Met. Eurasia 1998, 3, 40–45. [Google Scholar]
- Yakovlev, P. Industrial Types of Ore Deposits: A Textbook for Universities; Nedra: Moscow, Russia, 1986. [Google Scholar]
- U.S. Geological Survey, Nickel Statistics and Information, 2006. Available online: http://minerals.usgs.gov/minerals/pubs/commodity/nickel/index.html (accessed on 10 March 2025).
- Roorda, H.J.; Queneau, P.E. Recovery of Nickel and Cobalt from Ilmenites by Aqueous Chlorination in Sea Water. Trans. Inst. Min. Metal. 1973, 82, 79–87. [Google Scholar]
- World Bureau of Metal Statistics Outlook 2024. Available online: https://www.lseg.com/en/data-analytics/trading-solutions/world-bureau-metal-statistics (accessed on 11 March 2025).
- Krivtsov, A.; Ostapenko, P. Mineral raw materials. In Nickel and Cobalt: Handbook; GEInformmark CJSC: Moscow, Russia, 1997. [Google Scholar]
- Kolmachikhina, O.B.; Polygalov, S.E.; Lobanov, V.G. Research of Possibility of Processing of Oxidized Nickel Ore by Chloride Sublimation Roasting Technology RusMetal. IOP Conf. Ser. Mater. Sci. Eng. 2020, 969, 012050. [Google Scholar] [CrossRef]
- Ivanov, N.S.; Malimbayev, M.S.; Abilmagzhanov, A.Z.; Kholkin, O.S.; Adelbayev, I.Y.; Brodskiy, A.R. Processing of oxidized nickel ores using sintering, roasting and leaching processes. Miner. Eng. 2022, 181, 107498. [Google Scholar] [CrossRef]
- Reznik, I.; Ermakov, G.; Shneerson, Y. Nickel (Vol. 3, in 3 Vols.); Nauka i Tekhnologii: Moscow, Russia, 2003. [Google Scholar]
- Weizager, M.; Kormilitsyn, S. Modern methods of processing oxidized nickel ores abroad. Non-Ferr. Met. 1992, 6, 11–16. [Google Scholar]
- Nickel Market Size and Share Analysis—Growth Trends and Forecasts (2024–2029). Available online: https://www.mordorintelligence.com/ru/industry-reports/nickel-market (accessed on 24 February 2025).
- Prishletsov, D. Development of nickel production from oxidized nickel ores in the USSR. In Proceedings of the “Gipronickel” Institute (Issue 35: Technological Issues); USSR: St. Petersburg, Russia, 1967. [Google Scholar]
- Tarasov, A.; Bocharov, V. Combined Technologies of Non-Ferrous Metallurgy; FSUE Gintsvetmet Institute: Moscow, Russia, 2001. [Google Scholar]
- Konovalova, L.; Borodina, K.; Vokhmjanina, N. The Serov hypergene nickel deposit. In Ore-Bearing Weathering Crusts, Ore-Bearing Weathering Crusts; Nauka: Moscow, Russia, 1974; pp. 272–284. [Google Scholar]
- Pavlov, N. Nickel-Bearing Weathering Crusts of the Urals; Nauka: Moscow, Russia, 1970; p. 228. [Google Scholar]
- Elias, M. Nickel Laterites in SE Asia. In Proceedings of the Presentation at ‘Bali 2013’. East Asia: Geology, Exploration Technologies and Mines, Bali, Indonesia, 27 May 2013; p. 48. [Google Scholar]
- Reuter Markus, J.; Stephen, H.; Robert, M.; Kaye, A.; Ausmelt Technology. Developments in Lead and Zinc Processing. In Proceedings of the Zinc and Lead Metallurgy, 47th Annual Conference of Metallurgists of CIM, Winnipeg, MB, Canada, 24–27 August 2008; pp. 63–75. [Google Scholar]
- Kulvitsky, L.; Matveev, P.; Tseinert, V. Integrated use of raw materials in the nickel-cobalt industry. Bull. Non-Ferr. Metall. CIIN 1958, 13, 54–56. [Google Scholar]
- Simons, C.S. The Production of Nickel: Extractive Metallurgy—Past, Present and Future. In Proceedings of the Extractive Metallurgy of Nickel and Cobalt: Proceedings of a Symposium, The Metallurgical Society, Proceedings of the 117th TMS Annual Meeting, Phoenix, AZ, USA, 25–28 January 1988; pp. 91–134. [Google Scholar]
- Taylor, A. Review of Nickel-Cobalt Laterite Processes, Nickel-Cobalt-6. In Technical Sessions Proceedings; Alta Metallurgical Services: Perth, Australia, 2000. [Google Scholar]
- Bergman, R.A. Nickel Production from Low-Iron Laterite Ores: Process Descriptions. C. Bull 2003, 96, 127–138. [Google Scholar]
- Diaz, C.M.; Landolt, C.A.; Vahed, A.; Warner, A.E.M.; Taylor, J.C. Extractive Metallurgy of Nickel and Cobalt: Proceedings of a Symposium, The Metallurgical Society. In Proceedings of the 117th TMS Annual Meeting, Phoenix, AZ, USA, 25–28 January 1988. [Google Scholar]
- Ozberk, E.; Gendron, A.S.; Kaiura, G.H. Review of Nickel Smelters, Proceedings-Nickel Metallurgy, Volume I: Extraction and Refining of Nickel; Ozberk, E., Marcuson, S.W., Eds.; The Metallurgical Society of the Canadian Institute of Mining, Metallurgy and Petroleum and Nickel Development Institute: Montreal, QC, Canada, 1986; Volume 3, pp. 304–313. [Google Scholar]
- Reznik, I.; Ermakov, G.; Shneerson, Y. Nickel; Nauka i Tekhnologii: Moscow, Russia, 2000; Volume 1. [Google Scholar]
- Borbat, V.; Lesh, I. New Processes in Nickel and Cobalt Metallurgy; Metallurgiya: Moscow, Russia, 1976. [Google Scholar]
- Naboichenko, S. Autoclave Hydrometallurgy of Non-Ferrous Metals; USTU-UPI State Educational Institution: Yekaterinburg, Russia, 2002. [Google Scholar]
- Laverov, N. Underground Leaching of Polymetallic Ores; Publishing House of the Academy of Mining Sciences: Moscow, Russia, 1998. [Google Scholar]
- Meshram, P.; Pandey, B.D. Advanced Review on Extraction of Nickel from Primary and Secondary Sources. Miner. Process. Extr. Metall. Rev. 2018, 40, 157–193. [Google Scholar] [CrossRef]
- Tsidaev, B.; Golik, V.; Guriev, G. Combination of borehole hydromining and heap leaching methods. Tsvetnaya Metall. 2001, 7, 4–6. [Google Scholar]
- Sviblov, V. Pilot Tests of Underground Leaching of Nickel from Silicate Ores. Subsoil Use—XXI Century 2009, 2, 68–73. [Google Scholar]
- Duyvesteyn, W.P.; Liu, H.; Davis, M.J. Heap Leaching of Nickel Containing Ore. U.S. Patent 6,312,500, 6 November 2001. [Google Scholar]
- European Nickel PLC. 3aldağ Project. 2005. Available online: https://caldagnikel.com.tr/en/ (accessed on 25 February 2025).
- Krylova, L.N.; Kimelena, E.A.; Balantseva, E.B.; Starodubtseva, V.D. A Method for Extracting Metals from Silicate Nickel Ores. Patent RU2478127C1, 26 December 2012. [Google Scholar]
- Grebnev, G.S.; Zabolotsky, A.I.; Saven, N.V.; Sukleta, S.A.; Krinitsyn, A.P.; Zab-olotsky, K.A. Heap. Leaching of Silicate Nickel Ores. Patent RU2006115189, 11 November 2007. [Google Scholar]
- Panova, Y.; Aubakirov, Y.; Arbag, H. Selection of Sorption Materials for the Extraction of Nickel and Cobalt from the Ore of the Gornostaevskoye Deposit; Chemical Bulletin of Kazakh National University: Almaty, Kazakhstan, 2021; Issue 3, pp. 1–12. [Google Scholar] [CrossRef]
- Bailey, J.E.; Bohnet, M.; Brinder, J. (Eds.) Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2002. [Google Scholar]
- Neudorf, D. Atmospheric Leaching of Laterites. In Proceedings of the ALTA Nickel/Cobalt 12, Perth, Australia, 15–17 May 2007. [Google Scholar]
- Verbaan, N.; Sist, F.; Mackie, S.; Todd, I.; Neudorf, D. Development and Piloting of Skye’s Atmospheric Limonite and Saprolite Leach Process (SAL) at SGS Minerals. In Proceedings of the ALTA Nickel/Cobalt 12, Perth, Australia, 15–17 May 2007. [Google Scholar]
- Vardill, W.D.; Collins, M.J. Introduction to the Ambatovy Nickel Project in Madagascar. In Proceedings of the ALTA Nickel-Cobalt, Perth, Australia, 16–18 May 2005. [Google Scholar]
- Bacon, W.G.; Colton, D.F.; Krause, E.; Mihaylov, I.O.; Singhal, A.; Vahed, A.; Duterque, J.P. The Development of the Goro Nickel Process. In Proceedings of the ALTA Nickel-Cobalt, Perth, Australia, 16–18 May 2005. [Google Scholar]
- Lynch, E.; Baillie, M.G.; Steemson, M.; Buhrer, D.A. Recent Advances in the Weda Bay Nickel/Cobalt Laterite Project. In Proceedings of the ALTA Nickel-Cobalt, Perth, Australia, 16–18 May 2005. [Google Scholar]
- Matheson, P. Gladstone Pacific Nickel Ltd A New Lateritic Nickel/Cobalt Project for Central Queesland. In Proceedings of the ALTA Nickel-Cobalt, Perth, Australia, 16–18 May 2005. [Google Scholar]
- Becker, G.; Park, L. The Gladstone Nickel Project—Location, Location, Location. In Proceedings of the ALTA Nickel/Cobalt 11, Perth, Australia, 15–17 May 2006. [Google Scholar]
- Becker, G.S.; Mason, P.G. Gladstone Nickel Project—Benefiting from Regional Resources, Australian Infrastructure, Proven Technology and Chinese Project Implementation. In Proceedings of the ALTA Nickel-Cobalt, Perth, Australia, 16–18 June 2008. [Google Scholar]
- Barnett, B. A new process for nickel laterite leaching: Direct Nickel Process. In Proceedings of the ALTA 2014 Nickel-Cobalt-Copper Conference, ALTA Metallurgical Services, Perth, Australia, 24–31 May 2014. [Google Scholar]
- Direct Nickel Ltd. Technology Overview. 2020. Available online: https://www.directnickel.com/technology (accessed on 17 March 2025).
- Zhang, Z.F.; Zhang, W.B.; Zhang, Z.G.; Chen, X.F. Nickel extraction from nickel laterites: Processes, resources, environment and cost. China Geol. 2025, 8, 187–213. [Google Scholar] [CrossRef]
- Reid, J.; Barnett, S. Nickel Laterite Hydrometallurgical Processing Update, Nickel-Cobalt-8. In Technical Sessions Proceedings; Alta Metallurgical Services: Perth, Australia, 2002; p. 27. [Google Scholar]
- Chalkley, M.E.; Toirac, I.L. The Acid Pressure Leach Process for Nickel and Cobalt Laterite. Part 1: Review of Operations at Moa, Nickel Cobalt 97, Volume 1, Hydrometallurgy and Refining of Nickel and Cobalt. In Proceedings of the 27th Annual Hydrometallurgical Meeting of CIM, Sudbury, ON, Canada, 17–20 August 1997; Mihaylov, I., Cooper, W.C., Eds.; CIM Montreal: Montreal, QC, Canada, 1997; p. 341. [Google Scholar]
- Taylor, A. Nickel Processing Technology 10 Years on from Cawse, Bulong and Murrin Murrin. In Proceedings of the ALTA Nickel/Cobalt 12, Perth, Australia, 15–17 May 2007. [Google Scholar]
- Berezowsky, R.M. Laterite: New Life of Limonite. Miner. Ind. Int. 1997, 1034, 46–55. [Google Scholar]
- Motteram, G.; Ryan, M.; Berezowsky, R.M.; Raudsepp, R. Murrin Murrin Nickel-Cobalt Project: Project Development Overview. In Proceedings of the Nickel-Cobalt Pressure Leaching and Hydrometallurgy Forum Held in Perth, Alta Metallurgical Services, Perth, Australia, 13–14 May 1996. [Google Scholar]
- Urbain, D.; Duterque, J.P.; Palanque, P.; Rey, P. Economic Comparison Between the Sulphuric Acid Leach Process and Other Processes for Oxidized Nickel Ores, Proceedings-Nickel Metallurgy; The Metallurgical Society of the Canadian Institute of Mining, Metallurgy and Petroleum (MetSoc of CIM) and the Nickel Development Institute (NiDI): Montreal, QC, Canada, 1986; Volume 1, pp. 578–596. [Google Scholar]
- O’Kane, P.T. Energy Consumption and Economic Trends in the Production of Nickel from Laterites. In Proceedings of the International Laterite Symposium, The Society of Mining Engineers of AIME, New Orleans, LA, USA, 19–21 February 1979; pp. 503–521. [Google Scholar]
- Nesterov, Y.; Kancel, A. Method for Processing Nickel-Cobalt Raw Materials. Patent RU2393251, 30 January 2009. [Google Scholar]
- Drinkard, W.F., Jr. Nickel-Laterite Process. U.S. Patent 2010/0064854, 18 March 2010. [Google Scholar]
- Urazov, G.; Chernomorsky, M. Nickel Metallurgy; Moscow–Leningrad: State Scientific and Technical Publishing House: Moscow, Russia, 1931. [Google Scholar]
- Franklyn, L.; Manchanda, S. Cawse: 10 Years On. In Proceedings of the ALTA Nickel-Cobalt, Perth, Australia, 16–18 June 2008. [Google Scholar]
- Harris, B.; Magee, J.; Valls, R. Beyond PAL: The Chesbar Option, AAL. In Proceedings of the ALTA Nickel-Cobalt-9, Perth, Australia, 18–20 May 2003. [Google Scholar]
- Harris, G.B.; Magee, T.J.; Lakshmanan, V.I.; Sridhar, R. The Jaguar Nickel Inc. Sechol Laterite Project Atmospheric Chloride Leach Process. In Proceedings of the International Laterite Nickel Symposium, TMS Annual Meeting, Charlotte, NC, USA, 14–18 March 2004; p. 219. [Google Scholar]
- Harris, B.; White, C.; Jansen, M.; Pursell, D. A New Approach to High Chloride Leaching of Nickel Laterites. In Proceedings of the ALTA Ni/Co 2006, Perth, Australia, 15–17 May 2006. [Google Scholar]
- Crundwell, F.K.; Moats, M.S.; Ramachandran, V.; Robinson, T.G.; Davenport, W.G. Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals, 1st ed.; Elsevier: Oxford, UK, 2011; ISBN 978-0-08-096809-4. [Google Scholar]
- Moskalyk, R.R.; Alfantazi, A.M. Nickel laterite processing and electrowinning practice. Miner. Eng. 2002, 15, 593–605. [Google Scholar] [CrossRef]
- Morin, D.; D’Hugues, P. Bioleaching of a cobalt-containing pyrite in stirred reactors: A case study from laboratory scale to industrial application. Hydrometallurgy 2007, 83, 121–132. [Google Scholar] [CrossRef]
- Goode, J.R.; Fawcett, J.J. The Intec Nickel Process: Chloride leaching at atmospheric pressure. In Proceedings of the ALTA Nickel/Cobalt Conference Proceedings, Perth, Australia, 19–22 May 2005. [Google Scholar]
- Dalvi, A.D.; Bacon, W.G.; Osborne, R.C. The past and the future of nickel laterites. In Proceedings of the PDAC 2004 International Convention, Toronto, ON, Canada, 7–10 March 2004. [Google Scholar]
- Carlesi, C.; Cortes, E.; Dibernardi, G.; Morales, J.; Muñoz, E. Hydrometallurgical processing of nickel laterite ores: A review. Hydrometallurgy 2016, 165, 142–158. [Google Scholar] [CrossRef]
| Country | Resources | Average | |||
|---|---|---|---|---|---|
| Identified Thousand Tons | Share in the World, % | Reliable Thousands of Tons | Share in the World, % | Content of Ni, % | |
| Russia | 27.900 | 9.8 | 22,100 | 13.7 | 1.32 |
| Finland | 4300 | 1.5 | 2350 | 1.5 | 0.3 |
| Indonesia | 30.500 | 10.7 | 17.000 | 10.5 | 1.76 |
| Kazakhstan | 1900 | 0.7 | 1900 | 1.2 | 1.5 |
| China | 10.100 | 3.5 | 6750 | 4.2 | 1 |
| Philippines | 13,660 | 4.8 | 8330 | 5.2 | 1.42 |
| Madagascar | 1480 | 0.5 | 1395 | 0.9 | 1.04 |
| South Africa | 18.650 | 6.5 | 10.020 | 6.2 | 0.17 |
| Brazil | 18.050 | 6.3 | 9960 | 6.2 | 1.15 |
| Guatemala | 4600 | 1.6 | 2735 | 1.7 | 1.63 |
| Canada | 12.190 | 4.3 | 8200 | 5.1 | 1.07 |
| Cuba | 20.120 | 7.1 | 13.400 | 8.3 | 1.3 |
| USA | 6100 | 2.1 | 1900 | 1.2 | 0.85 |
| Australia | 49.400 | 17.3 | 26.400 | 16.4 | 0.78 |
| New Caledonia | 20.000 | 7 | 7200 | 4.5 | 1.93 |
| Others | 45.975 | 16.3 | 21.704 | 13.2 | |
| Total | 284.925 | 100 | 161.344 | 100 | |
| Type of Ore | Content, % | Metal Content Ratio | Characteristic Deposits | ||||
|---|---|---|---|---|---|---|---|
| MgO | SiO2 | Fe2O3 | Al2O3 | Fe–Ni | Ni–Co | ||
| Magnesian (Serpentinic) | 18 | 43 | 17 | 5 | 2:1 | 27:1 | Kimpersai (Kazakhstan), New Caledonian (Australia), Cuban (Cuba) |
| Alumino-Magnesian | 13.0 | 41.0 | 18.0 | 12.0 | 8:1 | 50:1 | Ufaley (Russia) |
| Ferruginous-magnesian (nontrotitic) | 8.0 | 41.0 | 25.0 | 6.0 | 17:1 | 20:1 | Kimpersai (70%) (Kazakhstan), Pobuzhskoye (Russia) |
| Ferruginous-siliceous (transitional) | 4.0 | 32.0 | 44.0 | 7.0 | 33:1 | 13:1 | Buruktal, Shevchenkivske (Kazakhstan), Yelizavetinskoye |
| Ferruginous (ochreous-chamositic) | 7.0 | 18.0 | 46.0 | 7.0 | 40:1 | 8:1 | Serovskoye (Russia) |
| Ferruginous (ochreous, lateritic) | 2.5 | 10.0 | 63.0 | 5.0 | 50:1 | 8:1 | Kimpersai (Overburden) (Kazakhstan) |
| Deposits | Components, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ni | Co | Fe | Cr | Mn | Al | Mg | SiO2 | CaO | |
| Goro-1 Goro-2 (New Caledonia) | 1.51 | 0.072 | 49.7 | 2.55 | 0.51 | 3.08 | 0.66 | 3.7 | 0.08 |
| 1.65 | 0.12 | 6.1 | 0.52 | 0.25 | 0.44 | 20.2 | 43.7 | 0.17 | |
| Mindanao (Philippines) Surigao (Philippines) Manicani (Philippines) | 2.8 | 0.1 | 17.1 | 0.8 | - | 0.16 | 13.1 | 28.1 | low |
| 0.75 | - | 47.8 | 2.8 | - | 4.4 | - | 1.08 | - | |
| 0.5–1 | - | 50.2 | 2.7 | - | 3.2 | - | 2.6 | - | |
| Rarona (Indonesia) | 0.37 | - | 50.2 | 2.1 | - | 3.7 | - | 0.63 | - |
| Morro do Engenho (Brazil) | 1.37 | 0.1 | 26.7 | - | - | 3.2 | 5.8 | 30 | 0.15 |
| Moa, Cuba | 1.4 | 0.14 | 47.5 | 2.5 | 0.8 | 4.5 | 1 | 2.5 | 0.02 |
| Name | Chemical Formula | Ni, % |
|---|---|---|
| Garnierite | (Ni, Mg)4[Si4O10] (OH)4·4H2O | 16–35 |
| Revdinskite | (Ni, Mg)8[Si4O10] (OH)8 | 16–35 |
| Nickel-bearing kerolite | (Mg, Ni)4[Si4O10] (OH)4·4H2O | 10–15 |
| Nonotronite | m{Mg3[Si4O10](OH)2}·p{(Al,Fe)2·[Si4O10] (OH)2} | 0.5–2.0 |
| Nickel-bearing serpophite | (Mg, Ni, Fe)6[Si4O10] (OH)8 | 4–5 |
| Nickel-bearing hydrochlorite | (Mg, Al, Fe)6 [(Si, Al)4O10]·(OH)8·nH2O | 2–6 |
| Asbolane, psilomelane varieties | m(Co, Ni)O·MnO2·nH2O | 0.8–2.0 |
| Producer | Start-Up Year | Ni Content in Initial Ore, % | Product | Ni Content in Product, % | Production Capacity, Thousand t/Year |
|---|---|---|---|---|---|
| Doniambo, New Caledonia | 1958 | 2.7 | Finestein | 25 | 49 |
| Fe-Ni | 78 | 11 | |||
| P.T. Inco, Indonesia | 1977 | 1.97 | Finestein | 79 | 43 |
| Pacific Metals, Japan | 1966 | 2.4 | Fe-Ni | 14–22 | 48 |
| Falconbridge, Dominican Republic | 1971 | 1.75 | Fe-Ni | 38 | 30 |
| Cerro Matoso, Colombia | 1982 | 2.9 | Fe-Ni | 45 | 27 |
| Larco, Greece | 1966 | 1.25 | Fe-Ni | 24–30 | 19.5 |
| Hyuga Smelter, Japan | 1956 | 2.4 | Fe-Ni | 20–25 | 22 |
| Anglo American, Venezuela | 2000 | 2.1 | Fe-Ni | 20 | 17 |
| P.T. Aneka Tambang, Indonesia | 1975 | 2.25 | Fe-Ni | 25 | 11 |
| Company | Designed Capacity, T Ni/Year | Content in Ore,% | Final Product | |
|---|---|---|---|---|
| Ni | Co | |||
| Moa Bay, Cuba | 25,000 | 1.4 | 0.13 | NiS-CoS |
| Murrin Murrin, Australia | 40,000 | 0.99–1.3 | 0.07 | NiS-CoS |
| Coral Bay, Philippines | 20,000 | 1.26 | 0.12 | NiS-CoS |
| Goro, New Caledonia | 60,000 | 2 | 0.1 | NiO |
| Ambatovy, Madagascar | 60,000 | 1.1 | 0.1 | Ni refined |
| Company | Designed Capacity, T Ni/Year | Content in Ore, % | Final Product | |
|---|---|---|---|---|
| Ni | Co | |||
| Nicaro, Cuba | 22.000 | 1.4 | 0.1 | NiO sinter |
| Queensland Nickel, Australia | 18.000 | 1.35 | 0.11 | NiO granules, NiS-CoS |
| Nonoc, Philippines | 30.000 | 1.22 | 0.1 | NiO powder, NiS-CoS |
| Tocantins, Brazil | 5000 | 1.6 | 0.14 | Ni cathode |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tauakelov, C.A.; Rakhimbayev, B.S.; Yskak, A.; Valiev, K.K.; Tastanov, Y.A.; Ibrayev, M.K.; Bulaev, A.G.; Daribayeva, S.A.; Kazbekova, K.A.; Joldassov, A.A. Treatment of Refractory Oxidized Nickel Ores (ONOs) from the Shevchenkovskoye Ore Deposit. Metals 2025, 15, 876. https://doi.org/10.3390/met15080876
Tauakelov CA, Rakhimbayev BS, Yskak A, Valiev KK, Tastanov YA, Ibrayev MK, Bulaev AG, Daribayeva SA, Kazbekova KA, Joldassov AA. Treatment of Refractory Oxidized Nickel Ores (ONOs) from the Shevchenkovskoye Ore Deposit. Metals. 2025; 15(8):876. https://doi.org/10.3390/met15080876
Chicago/Turabian StyleTauakelov, Chingis A., Berik S. Rakhimbayev, Aliya Yskak, Khusain Kh. Valiev, Yerbulat A. Tastanov, Marat K. Ibrayev, Alexander G. Bulaev, Sevara A. Daribayeva, Karina A. Kazbekova, and Aidos A. Joldassov. 2025. "Treatment of Refractory Oxidized Nickel Ores (ONOs) from the Shevchenkovskoye Ore Deposit" Metals 15, no. 8: 876. https://doi.org/10.3390/met15080876
APA StyleTauakelov, C. A., Rakhimbayev, B. S., Yskak, A., Valiev, K. K., Tastanov, Y. A., Ibrayev, M. K., Bulaev, A. G., Daribayeva, S. A., Kazbekova, K. A., & Joldassov, A. A. (2025). Treatment of Refractory Oxidized Nickel Ores (ONOs) from the Shevchenkovskoye Ore Deposit. Metals, 15(8), 876. https://doi.org/10.3390/met15080876







