Fabrication of a Porous TiNi3 Intermetallic Compound to Enhance Anti-Corrosion Performance in 1 M KOH
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Materials
2.3. Characterization Techniques
2.4. Electrochemical Measurements
3. Results and Discussion
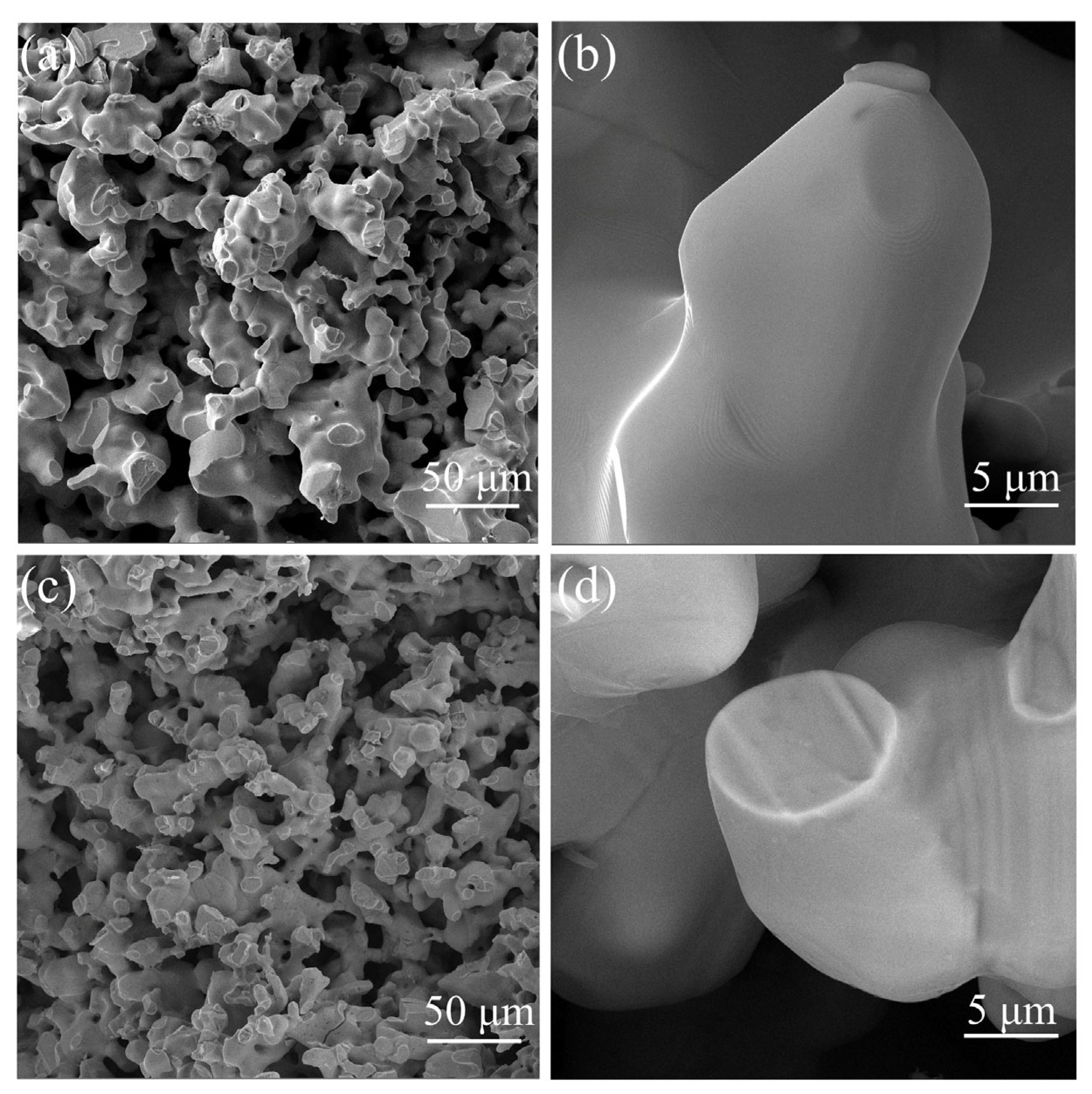
3.1. Characteristics of Phase and Microstructure
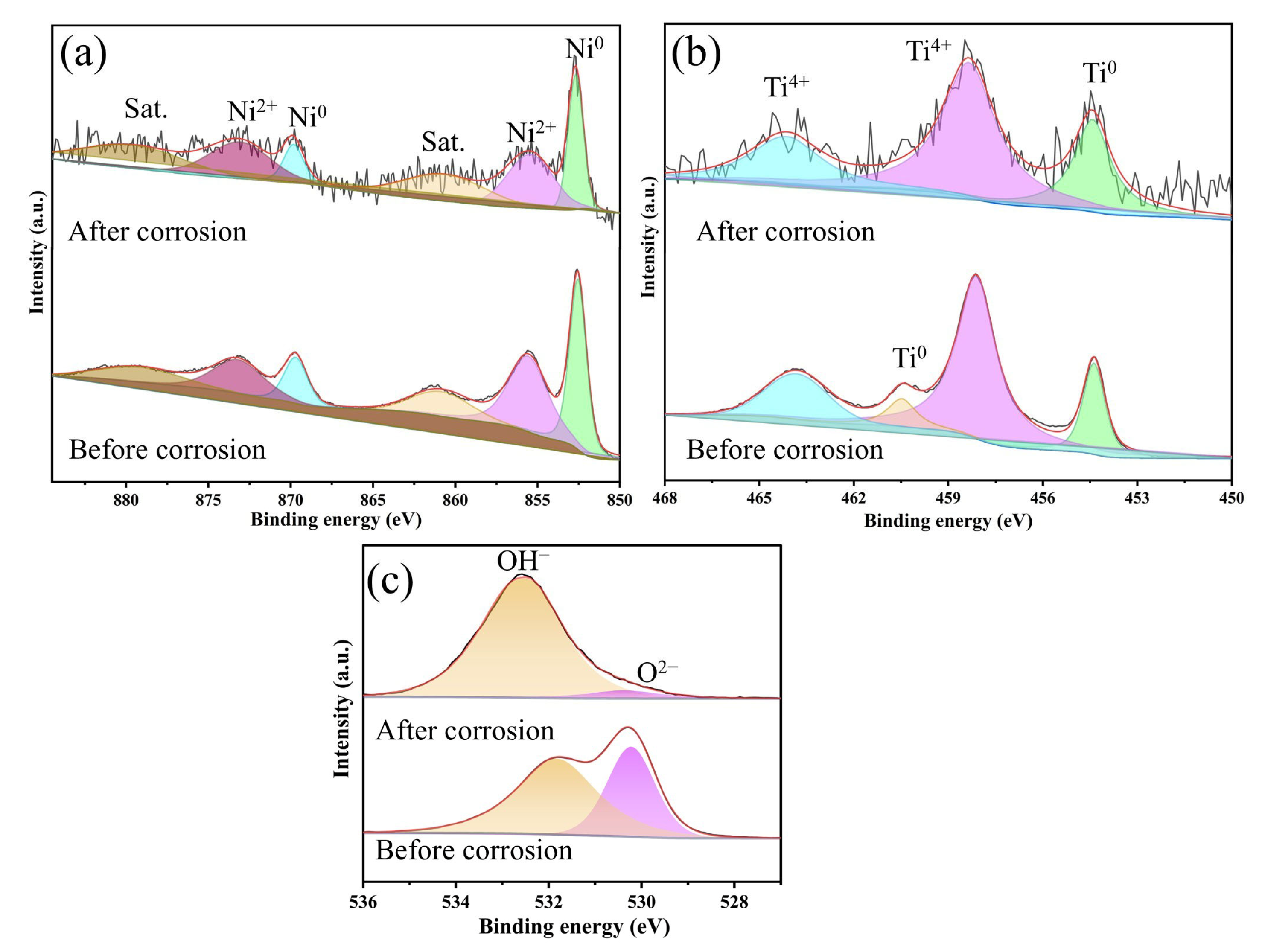
3.2. Corrosion Resistance Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yue, M.; Wang, Y.R.; Weng, J.Y.; Zhang, J.L.; Chi, D.Y.; Shi, M.; Hu, X.G.; Chen, Y.; Li, S.L.; Lan, Y.Q. Multi-metal porous crystalline materials for electrocatalysis applications. Chin. Chem. Lett. 2025, 36, 110049. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, Q.; Zhang, Y.; He, Y.; Gong, W.; Liu, W.; Liu, X.; Li, R. Architecting Gradient Hierarchically Porous Catalyst via Negative Mixing Enthalpy High-Entropy Alloy for Durable Water Splitting at Ampere-Level Current Density. Adv. Funct. Mater. 2025, 35, 2414446. [Google Scholar] [CrossRef]
- Chen, X.; Mu, Y.; Jin, C.; Wei, Y.; Hao, J.; Wang, H.; Caro, J.; Huang, A. Ultrathin Two-Dimensional Porous Fullerene Membranes for Ultimate Organic Solvent Separation. Angew. Chem. 2024, 136, e202401747. [Google Scholar]
- Zheng, D.; Zhu, R.; Hu, Y.; Liang, S.; Sun, H.; Wang, Z. Fabrication and characterization of mullite fiber-based porous ceramics with mixed fiber lengths for high-temperature gas filtration. Ceram. Int. 2025, 51, 23214–23223. [Google Scholar] [CrossRef]
- Tang, Y.; Varyambath, A.; Ding, Y.; Chen, B.; Huang, X.; Zhang, Y.; Yu, D.-g.; Kim, I.; Song, W. Porous organic polymers for drug delivery: Hierarchical pore structures, variable morphologies, and biological properties. Biomater. Sci. 2022, 10, 5369–5390. [Google Scholar] [CrossRef]
- Lei, H.; Zhou, Z.; Liu, J.; Cao, H.; Wu, L.; Song, P.; Yang, B.; Zhou, W.; Liu, Y.; Kong, Q.; et al. Structural Optimization of 3D-Printed Porous Titanium Implants Promotes Bone Regeneration for Enhanced Biological Fixation. ACS Appl. Mater. Interfaces 2025, 17, 18059–18073. [Google Scholar] [CrossRef]
- Liu, P.S.; Chen, G.F. Porous Materials: Processing and Applications; Elsevier: Oxford, UK, 2014. [Google Scholar]
- Tang, B.; Shang, Z.; Guo, W.; Pang, Z.; Akhtar, F.; Wang, J.; Zhang, B.; Li, S.; Feng, P. Effect of Al variation on microstructure and properties of porous FeCoNiCrAlx high-entropy alloys synthesized via thermal explosion. Intermetallics 2025, 180, 108687. [Google Scholar] [CrossRef]
- Cheng, B.; Li, D.; Yu, M.; Xing, J.; Guo, D.; Li, Y.; Chai, D.-F.; Li, J. Liquid nitrogen quenching-induced grain boundary-rich intermetallic compound/metal/carbon composites for efficient capacitive deionization. Sep. Purif. Technol. 2025, 357, 130043. [Google Scholar] [CrossRef]
- Sharma, A.; Zadorozhnyy, V.Y.; Shahzad, A.; Korol, A.A.; Kaloshkin, S.D.; Qiao, J.C. Investigating the diffusion behavior in the Ti-Fe, Ni-Ti, Ti-Al, and Ni-Al binary systems during solid-state synthesis of intermetallic compounds via mechanical alloying. J. Alloys Compd. 2025, 1022, 179964. [Google Scholar] [CrossRef]
- Zhao, Q.; He, Z.; He, Y.; Qiu, Y.; Wang, Z.; Jiang, Y. Porous TiFe2 intermetallic compound fabricated via elemental powder reactive synthesis. Int. J. Miner. Metall. Mater. 2024, 31, 764–772. [Google Scholar] [CrossRef]
- Yasenchuk, Y.; Marchenko, E.; Gunther, V.; Radkevich, A.; Kokorev, O.; Gunther, S.; Baigonakova, G.; Hodorenko, V.; Chekalkin, T.; Kang, J.-h.; et al. Biocompatibility and Clinical Application of Porous TiNi Alloys Made by Self-Propagating High-Temperature Synthesis (SHS). Materials 2019, 12, 2405. [Google Scholar] [CrossRef]
- Hosni, B.; Khaldi, C.; ElKedim, O.; Fenineche, N.; Lamloumi, J. Electrochemical properties of Ti2Ni hydrogen storage alloy. Int. J. Hydrogen Energy 2017, 42, 1420–1428. [Google Scholar] [CrossRef]
- Qiu, Y.; He, Z.; He, Y.; Zhao, Q.; Wang, Z.; Jiang, Y. Porous TiNi3-based intermetallics as active and robust monolith catalysts for hydrogen evolution. Chem. Commun. 2022, 58, 13943–13946. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ramirez, M.J.; Lopez-Sesenes, R.; Rosales-Cadena, I.; Gonzalez-Rodriguez, J.G. Corrosion behaviour of Ti–Ni–Al alloys in a simulated human body solution. J. Mater. Res. Technol. 2018, 7, 223–230. [Google Scholar] [CrossRef]
- El-Sayed, A.R.; Mohran, H.S.; Abd El-Lateef, H.M. Corrosion Study of Zinc, Nickel, and Zinc-Nickel Alloys in Alkaline Solutions by Tafel Plot and Impedance Techniques. Metall. Mater. Trans. A 2012, 43, 619–632. [Google Scholar] [CrossRef]
- Safavi, M.S.; Bordbar-Khiabani, A.; Khalil-Allafi, J.; Mozafari, M.; Visai, L. Additive Manufacturing: An Opportunity for the Fabrication of Near-Net-Shape NiTi Implants. J. Manuf. Mater. Process. 2022, 6, 65. [Google Scholar] [CrossRef]
- Chmielewska, A.; Dobkowska, A.; Kijeńska-Gawrońska, E.; Jakubczak, M.; Krawczyńska, A.; Choińska, E.; Jastrzębska, A.; Dean, D.; Wysocki, B.; Święszkowski, W. Biological and Corrosion Evaluation of In Situ Alloyed NiTi Fabricated through Laser Powder Bed Fusion (LPBF). Int. J. Mol. Sci. 2021, 22, 13209. [Google Scholar] [CrossRef]
- Safavi, M.S.; Bordbar-Khiabani, A.; Walsh, F.C.; Mozafari, M.; Khalil-Allafi, J. Surface modified NiTi smart biomaterials: Surface engineering and biological compatibility. Curr. Opin. Biomed. Eng. 2023, 25, 100429. [Google Scholar] [CrossRef]
- Wu, F.; Chen, H.; Yang, Z.; Qiao, J.; Hou, Y.; Yan, R.; Bai, H. Investigation on the electronic structures, elastic and thermodynamic properties of TiNi, Ti2Ni and TiNi3 intermetallic compound. Mater. Today Commun. 2023, 34, 105273. [Google Scholar] [CrossRef]
- Solmaz, R.; Döner, A.; Kardaş, G. The stability of hydrogen evolution activity and corrosion behavior of NiCu coatings with long-term electrolysis in alkaline solution. Int. J. Hydrogen Energy 2009, 34, 2089–2094. [Google Scholar] [CrossRef]
- Jiang, Y.; He, Y.-h.; Gao, H.-y. Pore constitution and tortuosity factor of reactively synthesized porous TiAl intermetallic compound. Trans. Nonferrous Met. Soc. China 2021, 31, 726–733. [Google Scholar] [CrossRef]
- Liang, J.T.; Lin, H.E. Unveiling the Corrosion Mechanism of the NixSey Intermetallic Compound in Water-Based Solution. ACS Appl. Mater. Interfaces 2024, 16, 68247–68261. [Google Scholar] [CrossRef]
- Saedy, S.; Palagin, D.; Safonova, O.; van Bokhoven, J.A.; Khodadadi, A.; Mortazavi, Y. Understanding the mechanism of synthesis of Pt3Co intermetallic nanoparticles via preferential chemical vapor deposition. J. Mater. Chem. A 2017, 5, 24396–24406. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, X.; Zhang, X.; Zhang, J.; Shang, Z.; Yu, Y. Reaction Process and Pore Structure Control of Porous NiAl Intermetallic Compounds via Thermal Explosion. J. Mater. Eng. Perform. 2024, 33, 10377–10385. [Google Scholar] [CrossRef]
- Shen, B.; He, Y.; Wang, Z.; Yu, L.; Jiang, Y.; Gao, H. Reactive synthesis of porous FeSi intermetallic compound. J. Alloys Compd. 2020, 826, 154227. [Google Scholar] [CrossRef]
- Jiang, H.; Ye, S.; Ma, R.; Yu, P. Influences of sintering parameters on shape-retention ability of porous Ni3Al intermetallic fabricated by powder metallurgy. Intermetallics 2019, 105, 48–55. [Google Scholar] [CrossRef]
- Ru, J.; Wang, Y.; Wang, Y.; Xu, X. Microstructure, phase composition and oxidation behavior of porous Ti-Si-Mo intermetallic compounds fabricated by reactive synthesis. High Temp. Mater. Process. 2020, 39, 26–32. [Google Scholar] [CrossRef]
- Laeng, J.; Xiu, Z.; Xu, X.; Sun, X.; Ru, H.; Liu, Y. Phase formation of Ni–Ti via solid state reaction. Phys. Scr. 2007, 2007, 250. [Google Scholar] [CrossRef]
- Li, Z.; Luo, H.; Hou, L.; Zhao, Q.; Wang, X.; Chang, Y. Effect of Mn addition on the corrosion behavior of FeCrNiMnxSi alloys in simulated seawater environment. Corros. Sci. 2025, 252, 112978. [Google Scholar] [CrossRef]
- Hou, X.; Ren, Q.; Yang, Y.; Cao, X.; Hu, J.; Zhang, C.; Deng, H.; Yu, D.; Li, K.; Lan, W. Effect of temperature on the electrochemical pitting corrosion behavior of 316L stainless steel in chloride-containing MDEA solution. J. Nat. Gas Sci. Eng. 2021, 86, 103718. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, B.; Zhang, M.; Guo, R.P.; Wang, X.J.; Qiao, J.W.; Wang, Z.H. Corrosion Behavior of In-situ Zr-Based Metallic Glass Matrix Composites in Aqueous Environments. J. Mater. Eng. Perform. 2024, 33, 274–282. [Google Scholar] [CrossRef]
- Frenck, J.-M.; Vollmer, M.; Mandel, M.; Krüger, L.; Niendorf, T. On the Influence of Microstructure on the Corrosion Behavior of Fe–Mn–Al–Ni Shape Memory Alloy in 5.0 wt% NaCl Solution. Adv. Eng. Mater. 2021, 23, 2000865. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, B.Z.; Yuan, Y.F.; Guo, S.Y.; Pan, J. Influence of Microstructure and Passive Film on the Corrosion Behavior of CoCrFeMnNi High-Entropy Alloy Prepared by Different Cooling Methods in a Simulated Alkaline Soil Solution. J. Mater. Eng. Perform. 2022, 31, 9244–9261. [Google Scholar] [CrossRef]
- Navi, A.S.; Haghighi, S.E.; Haghpanahi, M.; Momeni, A. Investigation of Microstructure and Corrosion of TiNbTaZrMo High-Entropy Alloy in the Simulated Body Fluid. J. Bionic Eng. 2021, 18, 118–127. [Google Scholar] [CrossRef]
- Guo, X.J.; Yang, X.; Yuan, X.Y.; Zhou, D.; Lu, Y.; Liu, J.K. Oxygen Vacancy Defects and a Field Effect-Mediated ZnO/WO2.92 Heterojunction for Enhanced Corrosion Resistance. Inorg. Chem. 2021, 60, 15390–15403. [Google Scholar] [CrossRef]
- Luo, H.; Su, H.; Dong, C.; Li, X. Passivation and electrochemical behavior of 316L stainless steel in chlorinated simulated concrete pore solution. Appl. Surf. Sci. 2017, 400, 38–48. [Google Scholar] [CrossRef]
- Zou, P.; Li, J.; Zhang, Y.; Liang, C.; Yang, C.; Fan, H.J. Magnetic-field-induced rapid synthesis of defect-enriched Ni-Co nanowire membrane as highly efficient hydrogen evolution electrocatalyst. Nano Energy 2018, 51, 349–357. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, M.; Han, C.; Zhao, Z.; Jia, X.; Li, S.; Liu, J. Effect of hydrogen on corrosion resistance and fatigue properties of near-β Ti-5Al-5Mo-5V-3Cr-1Zr alloy. Corros. Sci. 2025, 244, 112637. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Li, Y.; He, Z.; Zhang, G.; Wang, Z.; Wang, S.; Hu, F.; Zhou, Q. Corrosion and Interfacial Contact Resistance of NiTi Alloy as a Promising Bipolar Plate for PEMFC. Molecules 2024, 29, 3696. [Google Scholar] [CrossRef]
- Shtefan, V.; Navas, N.F.; Kaban, I.; Hantusch, M.; Gebert, A. Pitting corrosion mechanisms of Ti-Cu-(Pd-) based metallic glasses in simulated physiological solution. Corros. Sci. 2025, 251, 112913. [Google Scholar] [CrossRef]
- El-Sayed, A.R.; Mohran, H.S.; Abd El-Lateef, H.M. Effect of minor nickel alloying with zinc on the electrochemical and corrosion behavior of zinc in alkaline solution. J. Power Sources 2010, 195, 6924–6936. [Google Scholar] [CrossRef]
- Lin, D.; Hu, J.; Xi, X.; Liu, Z.; Wen, J.; Wang, Z.; Song, X.; Bian, H.; Tang, Z.; Fu, W.; et al. Influence of pH on the corrosion behavior of biomedical Ti/Au/ZrO2 brazing joints. Mater. Chem. Phys. 2023, 295, 127079. [Google Scholar] [CrossRef]




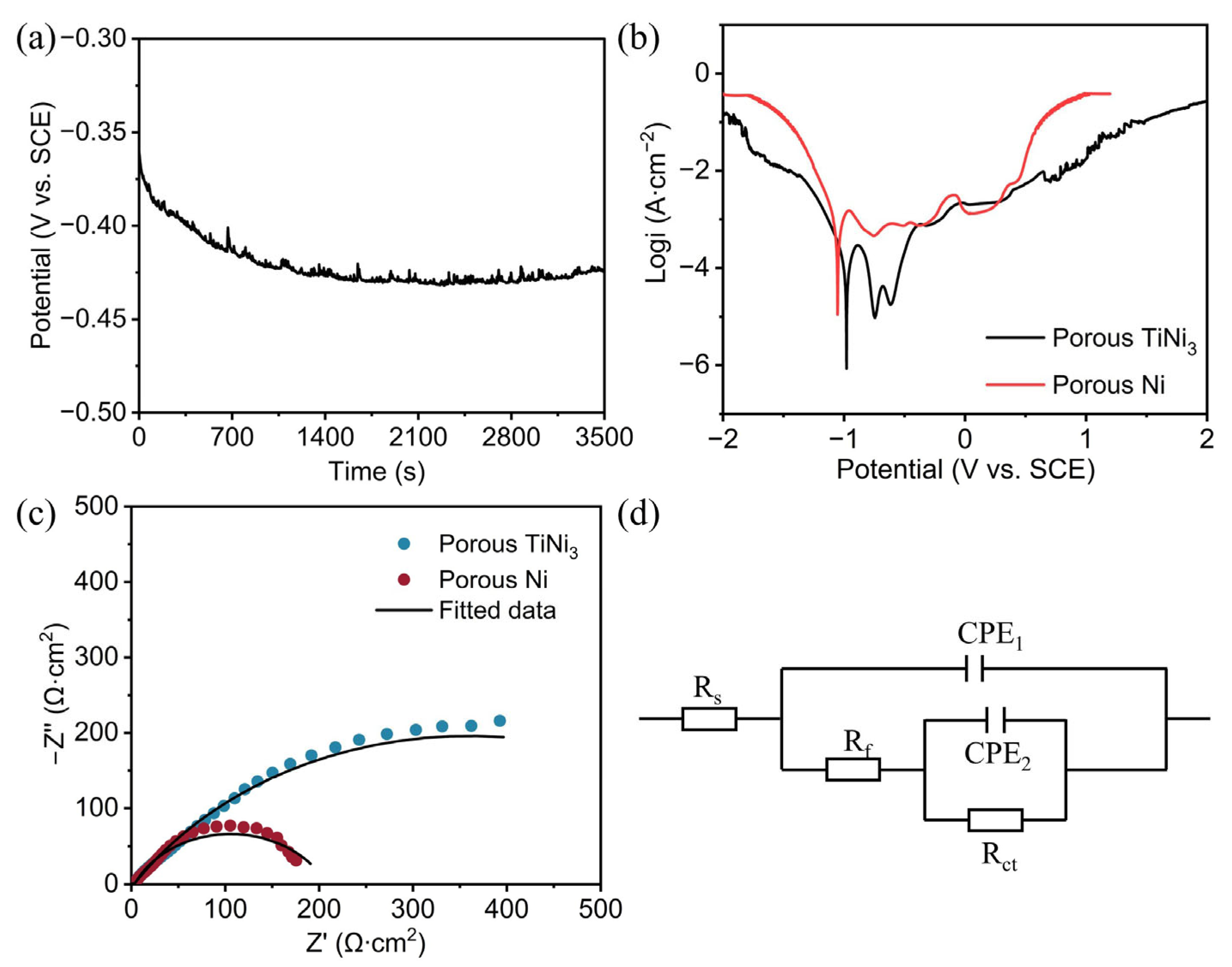
| Samples | Rs (Ω cm2) | CPE1 (F/cm2) | Rf (Ω cm2) | CPE2 (F/cm2) | Rct (Ω cm2) |
|---|---|---|---|---|---|
| TiNi3 | 0.62 | 6.453 × 10−7 | 2.26 | 6.998 × 10−3 | 713.9 |
| Ni | 2.76 | 5.621 × 10−5 | 0.77 | 6.191 × 10−3 | 204.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Qiu, Y.; He, Y.; Zhao, Q.; Wang, Z.; Jiang, Y. Fabrication of a Porous TiNi3 Intermetallic Compound to Enhance Anti-Corrosion Performance in 1 M KOH. Metals 2025, 15, 865. https://doi.org/10.3390/met15080865
He Z, Qiu Y, He Y, Zhao Q, Wang Z, Jiang Y. Fabrication of a Porous TiNi3 Intermetallic Compound to Enhance Anti-Corrosion Performance in 1 M KOH. Metals. 2025; 15(8):865. https://doi.org/10.3390/met15080865
Chicago/Turabian StyleHe, Zhenli, Yue Qiu, Yuehui He, Qian Zhao, Zhonghe Wang, and Yao Jiang. 2025. "Fabrication of a Porous TiNi3 Intermetallic Compound to Enhance Anti-Corrosion Performance in 1 M KOH" Metals 15, no. 8: 865. https://doi.org/10.3390/met15080865
APA StyleHe, Z., Qiu, Y., He, Y., Zhao, Q., Wang, Z., & Jiang, Y. (2025). Fabrication of a Porous TiNi3 Intermetallic Compound to Enhance Anti-Corrosion Performance in 1 M KOH. Metals, 15(8), 865. https://doi.org/10.3390/met15080865







