Vacuum Brazing of 6061 Aluminum Using Al-Si-Ge Filler Metals with Different Si Contents
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
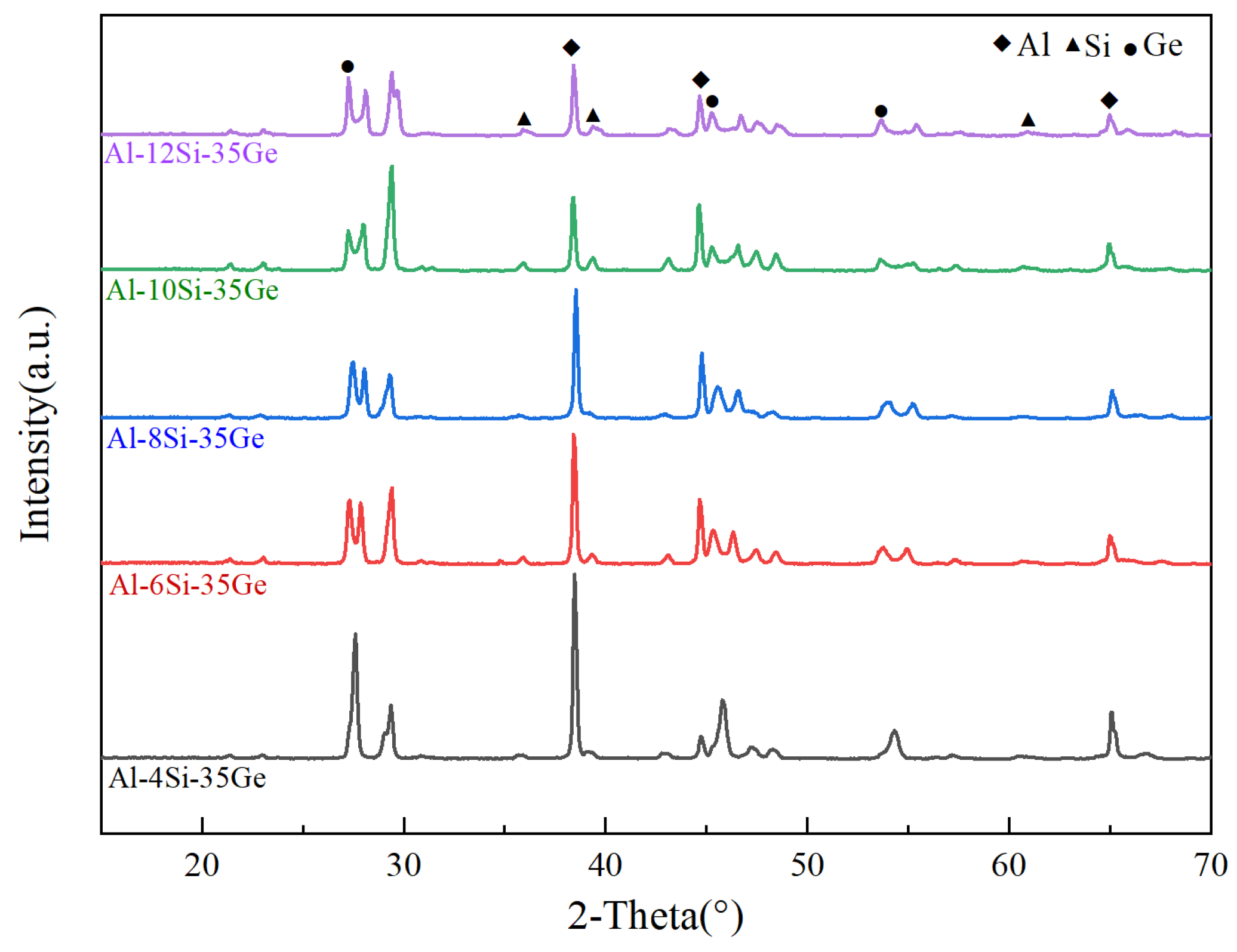
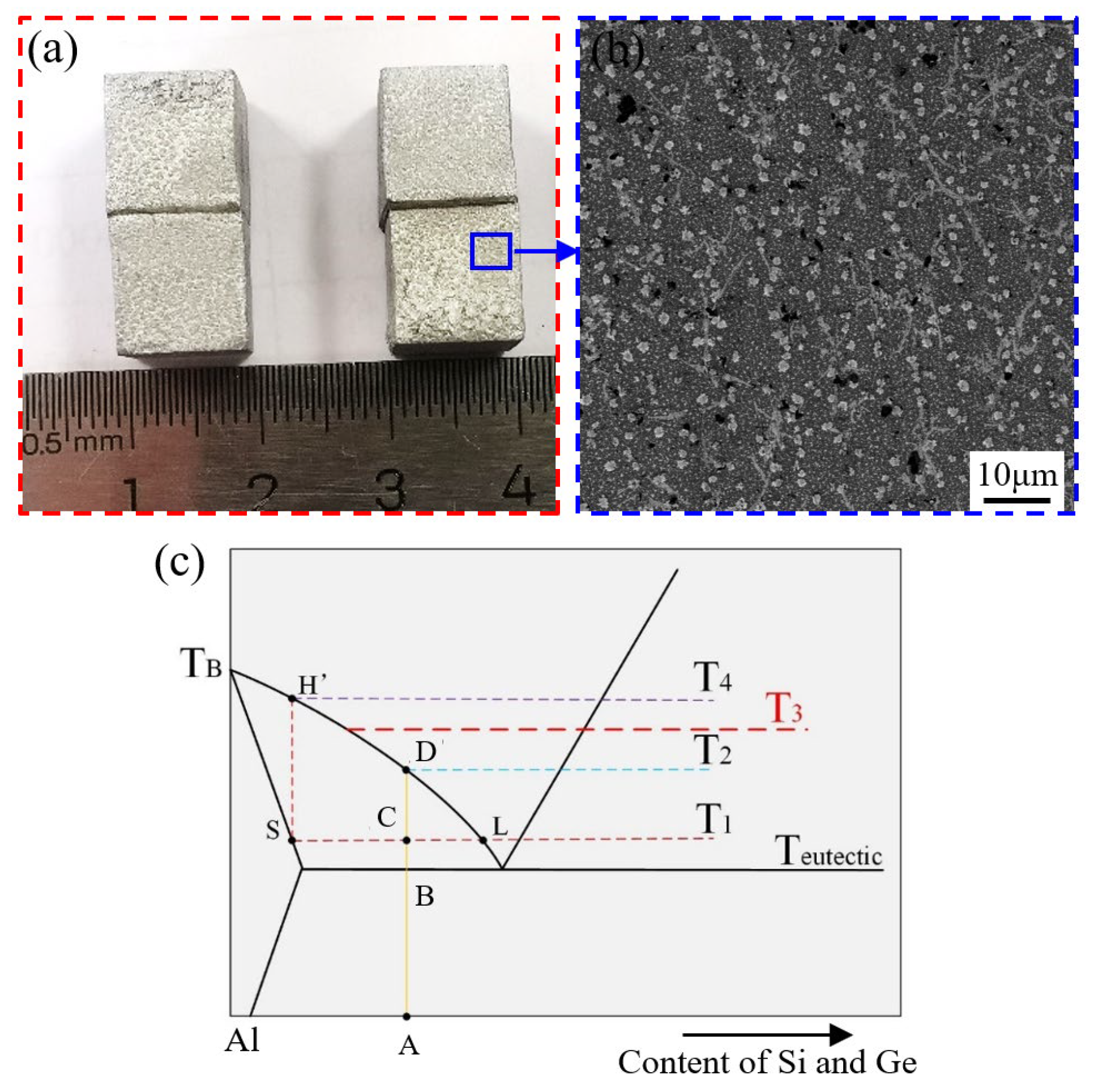
3.1. Microstructures of Al-Si-Ge Filler Metals
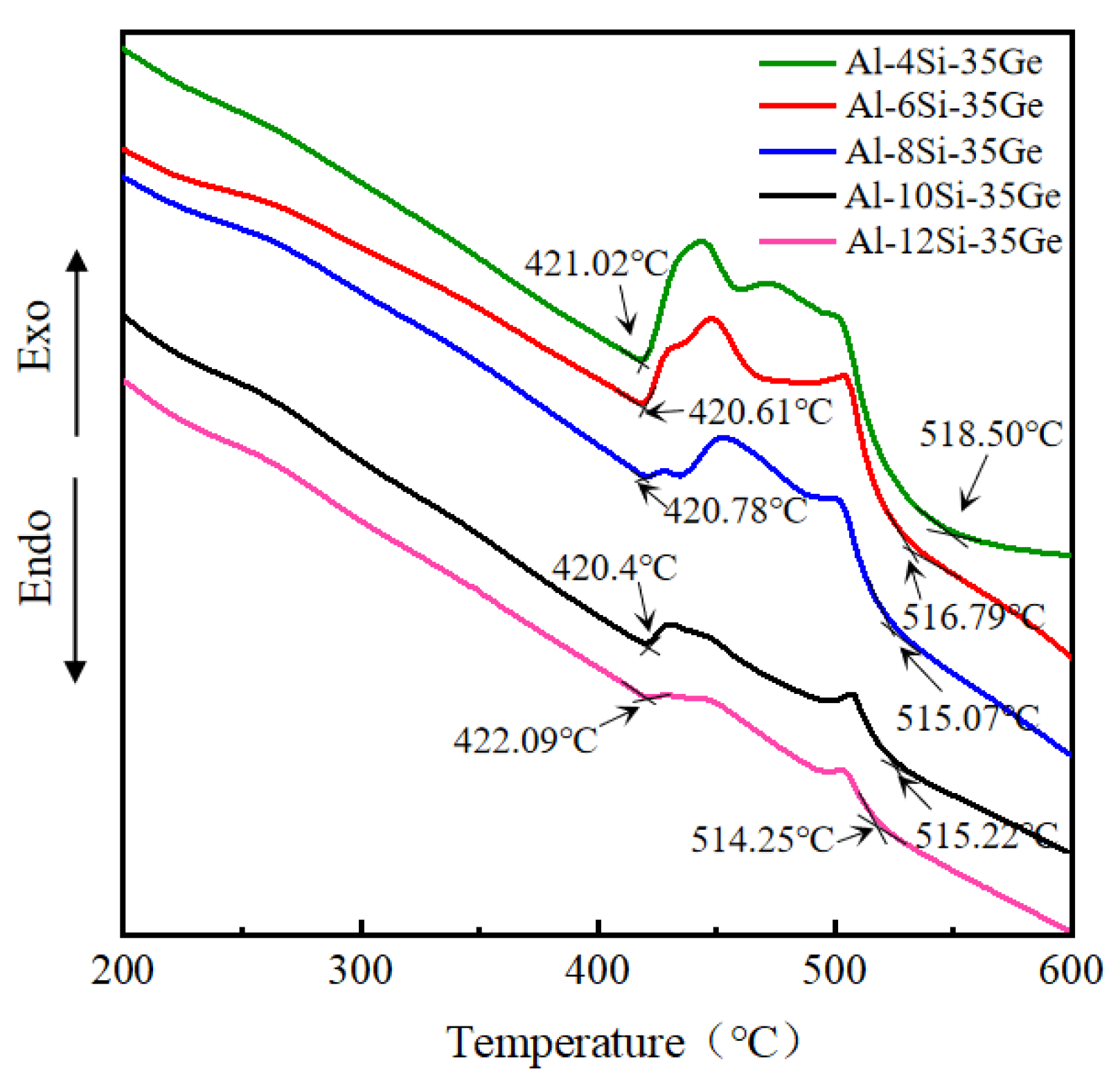
3.2. Melting Characteristics of Al-Si-Ge Filler Metals
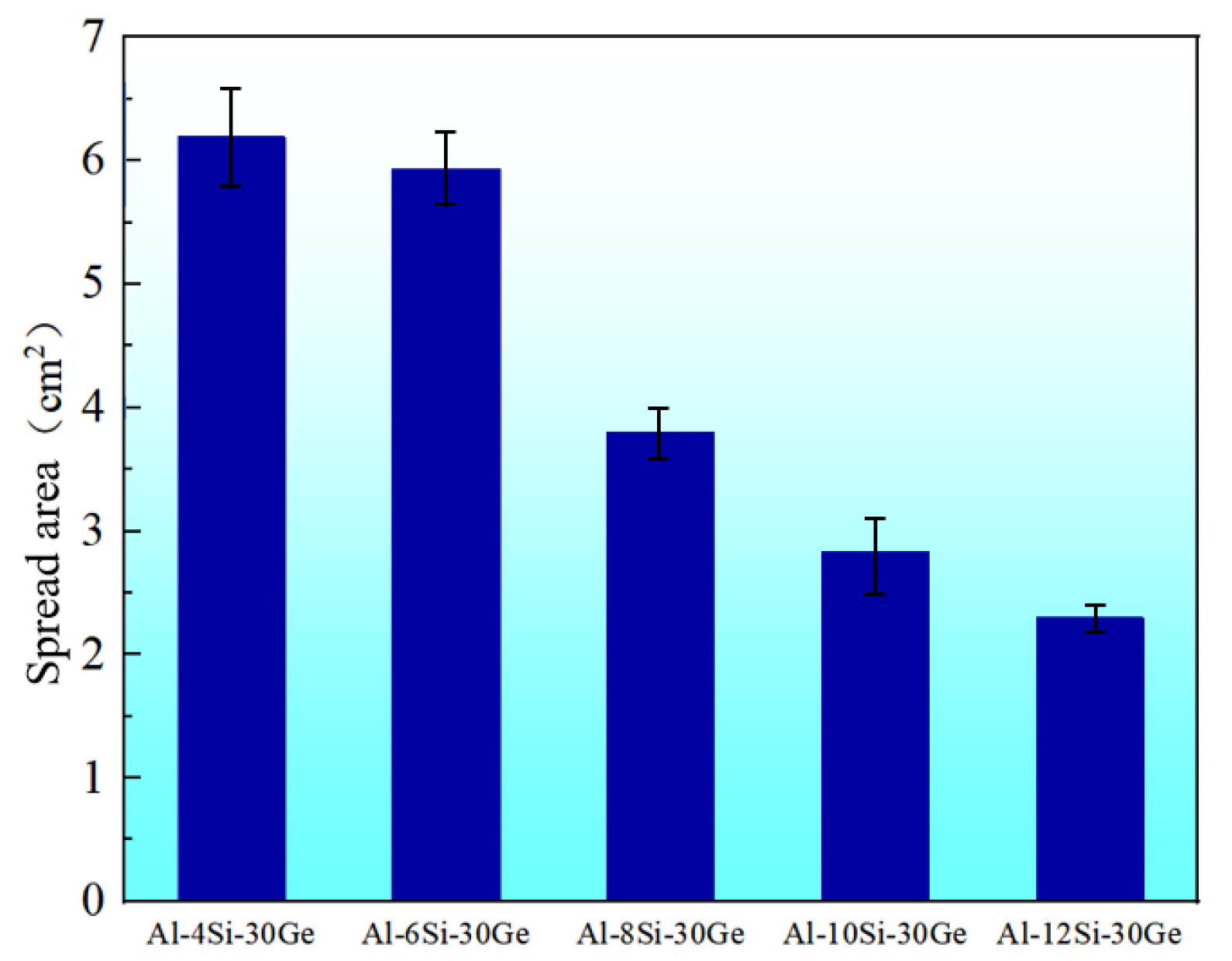
3.3. The Wettability of Al-Si-Ge Filler Metals
3.4. Microstructure of Vacuum-Brazed 6061 Joints
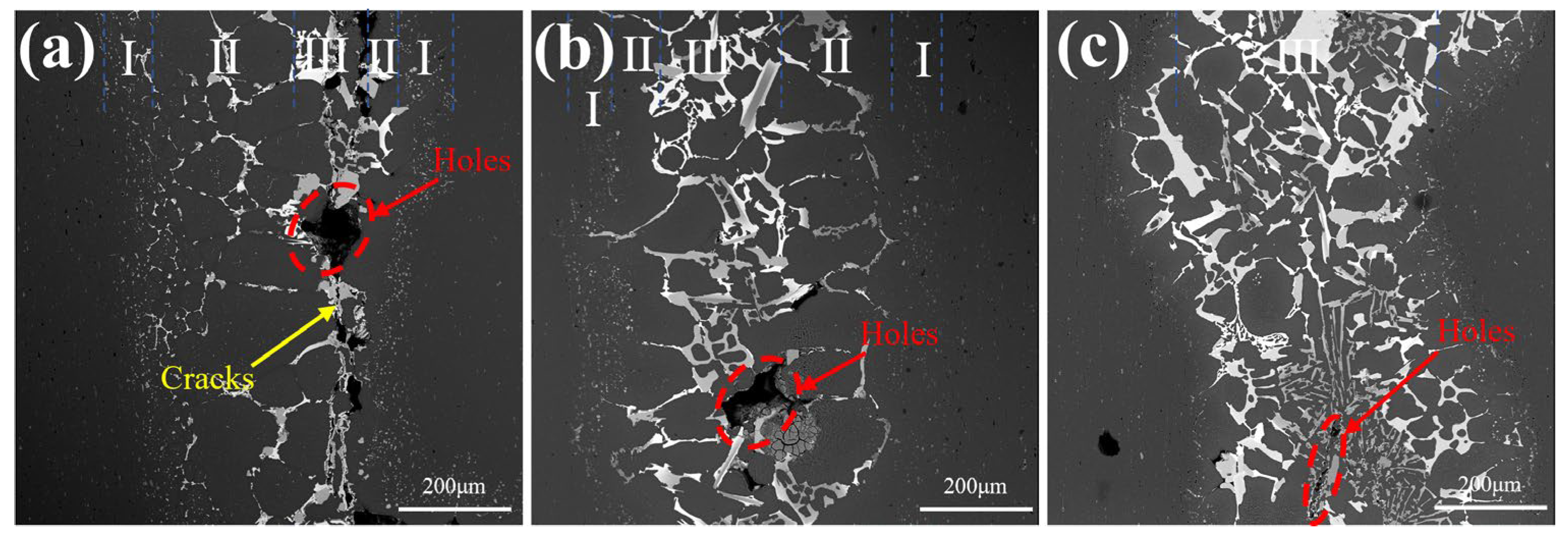
3.5. The Mechanism and Influence of Si in the Microstructure of Brazed Joint
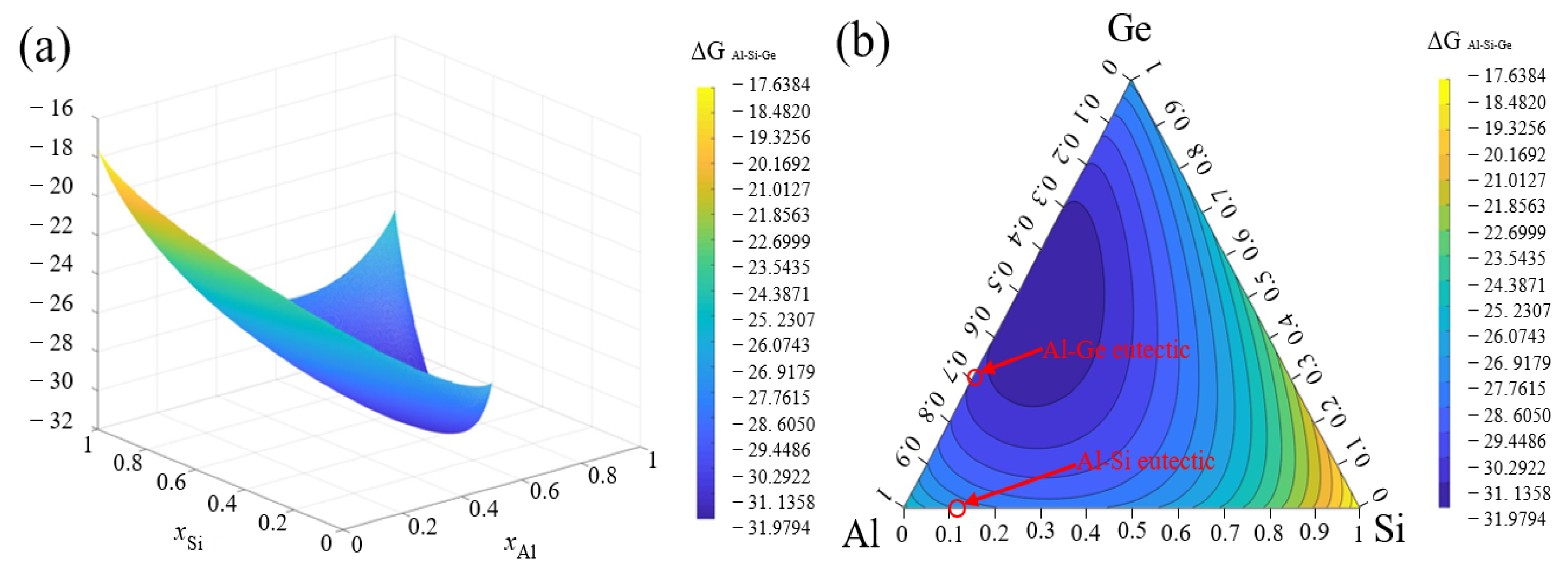
3.5.1. Thermodynamics Model
3.5.2. Thermodynamics Analysis
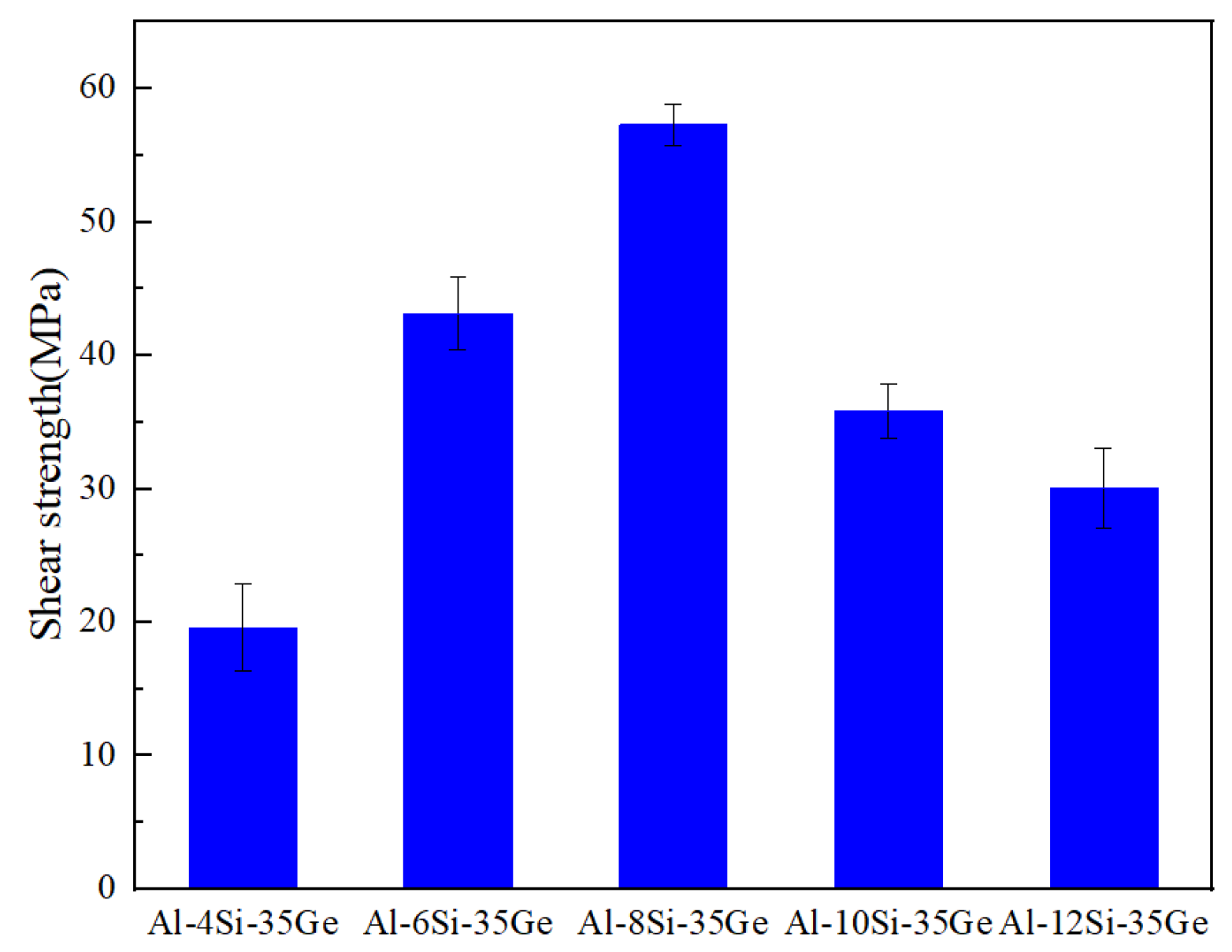
3.6. Mechanical Properties
4. Conclusions
- (1)
- The melting point of Al-xSi-35Ge filler metals decreased from 518.5 °C to 515.22 °C when the Si content increased from 4 wt.% to 12 wt.%. The primary Si phase appeared in the microstructure of filler metals when the Si content was 6 wt.%.
- (2)
- The microstructure of the joint was characterized by a multilayer structure that was primarily composed of three zones: two transition regions (Zone I) and a filler residual region (Zone II).
- (3)
- Liquidation of filler metal occurred during brazing, resulting in the formation of defects such as holes and cracks in the microstructure of 6061 joints. Si can hinder this phenomenon by reducing the difference of μGe in fillers and 6061 substrates.
- (4)
- The maximum shear strength of the joint was 57.3 MPa, using Al-8Si-35Ge filler metal. Lower Si content in fillers resulted in defects in joints, and higher Si content introduced much more brittle Si(Ge).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erturun, V.; Odabas, D.C. Fretting wear behaviour in 6061-T6 aluminium alloy. Aeronaut. J. 2024, 128, 178–190. [Google Scholar] [CrossRef]
- Zhu, Z.; Hu, Z.; Ng, F.L.; Seet, H.L.; Nai, S.M.L. Extending the mechanical property regime of laser powder bed fusion Sc- and Zr-modified Al6061 alloy by manipulating process parameters and heat treatment. Addit. Manuf. 2024, 85, 104164. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, Z.; Wang, K.; Li, X. Brazing of Al2O3-6061 aluminum alloy based on femtosecond laser surface groove structure. Ceram. Int. 2022, 48, 36953–36960. [Google Scholar] [CrossRef]
- Xie, J.; Cai, C.; Zhang, B.; Yu, J.; Liu, Y.; Chen, H. Microstructure evolution and fracture behavior of rotating laser welded-brazed 6061 aluminum alloys/304 SS dissimilar joint. Mater. Charact. 2023, 195, 112543. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Yi, R.; Ouyang, Y. Special brazing and soldering. J. Manuf. Process. 2020, 60, 608–635. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Liu, K.; Wang, Z.; Qing, G.; Wang, Y.; Yan, C.; He, C.; Zhang, R. Numerical and experimental investigation of an automatic brazing vacuum seal system for Mars soil sample return. Vacuum 2024, 219, 112687. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Ma, L.; Liu, Y.; Zhan, X. Thermal-fluid-solid coupling analysis of vacuum brazing process for a titanium alloy plate-fin structure. Vacuum 2023, 218, 112612. [Google Scholar] [CrossRef]
- Prakashraj, E.; Ghosh, A. Finite element based 3-D modelling of residual stress in high vacuum actively brazed diamond/Ni-Cr filler/C45 steel joint. Diam. Relat. Mater. 2023, 139, 110359. [Google Scholar] [CrossRef]
- Niu, Z.; Huang, J.; Yang, H.; Chen, S.; Zhao, X. Preparation and Properties of a Novel Al-Si-Ge-Zn Filler Metal for Brazing Aluminum. J. Mater. Eng. Perform. 2015, 24, 2327–2334. [Google Scholar] [CrossRef]
- Schwartz, M.; O’Keere, R. Fluxless Brazing of Aluminum. U.S. Patent 3457630A, 29 July 1969. [Google Scholar]
- Zhang, J.; Cheng, J.; Gong, Y. Welding 6061 airborne computer cabinet in high-precision vacuum brazing furnace. Vacuum 2009, 46, 6–8. [Google Scholar]
- Tian, H. Research on Domestic 6061 Vacuum Brazing and Gas Quenching Process. Mod. Weld. 2008, 4, 23–25. [Google Scholar]
- Zhang, Y.; Gao, T.; Liu, X. Influence of Ge content on the microstructure, phase formation and microhardness of hypereutectic Al-Si alloys. J. Alloys Compd. 2014, 585, 442–447. [Google Scholar] [CrossRef]
- Qiang, W.; Wang, K. Double-sided coaxial GTA flat-overhead welding of 5083 aluminum alloy. J. Mater. Process. Technol. 2019, 272, 9–16. [Google Scholar] [CrossRef]
- Chang, S.; Tsao, L.C.; Li, T.; Chuang, T. Joining 6061 aluminum alloy with Al-Si-Cu filler metals. J. Alloys Compd. 2009, 488, 174–180. [Google Scholar] [CrossRef]
- Niu, Z.; Huang, J.; Chen, S.; Zhao, X. Effect of germanium additions on the microstructures and properties of Al-Si filler metals for brazing aluminum. Trans. Nonferrous Met. Soc. China 2016, 26, 775–782. [Google Scholar] [CrossRef]
- Huang, T.; Gan, G.; Liu, C.; Ma, P.; Ma, Y.; Tang, Z.; Cheng, D.; Liu, X.; Tian, K. Properties of Cu/Zn+15%SAC0307+15%Cu/Al joints by different ultrasonic-assisted degrees. Microelectron. Int. 2023, 40, 70–80. [Google Scholar] [CrossRef]
- Long, W.; Lu, Q.; He, P.; Xue, S.; Wu, M.; Xue, P. In situ synthesis of Al-Si-Cu alloy during brazing process and mechanical property of brazing joint. J. Mater. Eng. 2016, 44, 17–23. [Google Scholar]
- Niu, Z.; Huang, J.; Liu, K.; Xu, F. Microstructure and property of 6061 aluminum alloy brazed joint with Al-Si-Ge-Zn filler metal. Trans. China Weld. Inst. 2017, 38, 97–101. [Google Scholar]
- Werner, W.; Tenn, C. Fluxless Aluminum Brazing. U.S. Patent 3844777A, 29 October 1974. [Google Scholar]
- Kayamoto, T.; Jong-Hoon, K.; Saito, S.; Onzawa, T. Brazing of Al-Mg alloy and Al-Mg-Si alloy with Al-Ge based filler metals. Q. J. Jpn. Weld. Soc. 1994, 12, 495–501. [Google Scholar] [CrossRef]
- Kayamoto, T.; Saito, S.; Ogusu, M.; Onzawa, T. Vacuum brazing of 2017 aluminum alloy using Al-Ge-Cu based fillers; Al-Ge-Cu ki ro wo mochiita 2017 aluminium gokin no shinku rozuke. Yosetsu Gakkai Ronbunshu 1995, 33, 20–24. [Google Scholar]
- Ivannikov, A.; Abramov, A.; Popov, N.; Penyaz, M.; Suchkov, A.; Pukhareva, N.; Sevryukov, O. Joining Stainless-Steel AISI 304 and High-Strength Aluminum Alloy AA 6082 by Brazing Using Al-Ge-Si Foils. Metals 2023, 13, 149. [Google Scholar] [CrossRef]
- Fedorov, V.; Uhlig, T.; Wagner, G. Microstructure and Mechanical Properties of AA 6082/AISI 304 Joints Brazed Using Al-Ge-Si Filler Metal. Metals 2023, 13, 1574. [Google Scholar] [CrossRef]
- GB/T 11363-2008; Test Method of the Strength for Brazed and Soldered Joint. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China and China National Standardization Administration: Beijing, China, 2008.
- Zhang, Q.; Zhuang, H. Atlas of Ternary Alloy Phase Diagrams. In China Machine Press26; China Machine Press: Beijing, China, 2022; pp. 157–158. ISSN 978-7-122-40940-9. [Google Scholar]
- Cheng, Y.; Wu, X.; Zhou, Y.; Chen, Y. Study on brazability of Al–Cu–Si–Ni filler metal at medium-temperature on different parent metal. Electr. Weld. Mach. 2008, 38, 37–41. [Google Scholar]
- Zhang, Q.; Zhuang, H. Handbook of Brazing and Soldering, 3rd ed.; China Machine Press: Beijing, China, 2008; pp. 102–103. ISSN 978-7-111-57708-9. [Google Scholar]
- Liu, Z.; Li, Y.; Ruan, F.; Zhang, G.; Zhao, M.; Liu, Z.; Zhang, J. Enthalpy analysis of Ce–Mg–Ni–H formation based on extended miedema theory: Investigation of selected Ce2MgNi2–H2. Int. J. Hydrogen Energy 2021, 46, 4201–4210. [Google Scholar] [CrossRef]
- Witusiewicz, V.T. Thermodynamics of liquid binary alloys of the 3d transition metals with metalloids: Generalization. J. Alloys Compd. 1995, 221, 74–85. [Google Scholar] [CrossRef]
- Buschow, K.H.J.; Bouten, P.C.P.; Miedema, A.R. Hydrides formed from intermetallic compounds of two transition metals: A special class of ternary alloys. Rep. Prog. Phys. 1982, 45, 937–1039. [Google Scholar] [CrossRef]
- Mou, G.; Hua, X.; Wu, D.; Huang, Y.; Lin, W.; Xu, P. Microstructure and mechanical properties of cold metal transfer welding-brazing of titanium alloy (TC4) to stainless steel (304L) using V-shaped groove joints. J. Mater. Process. Technol. 2019, 266, 696–706. [Google Scholar] [CrossRef]
- Tan, C.; Zang, C.; Xia, H.; Zhao, X.; Zhang, K.; Meng, S.; Chen, B.; Song, X.; Li, L. Influence of Al additions in Zn–based filler metals on laser welding–brazing of Al/steel. J. Manuf. Process. 2018, 34, 251–263. [Google Scholar] [CrossRef]


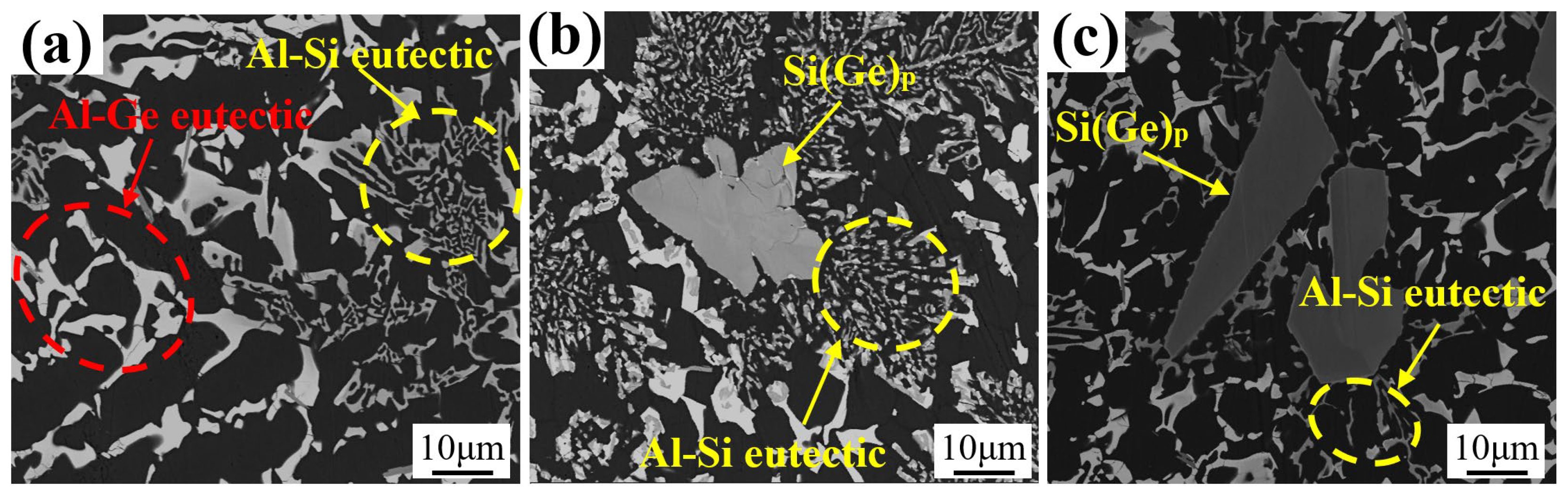

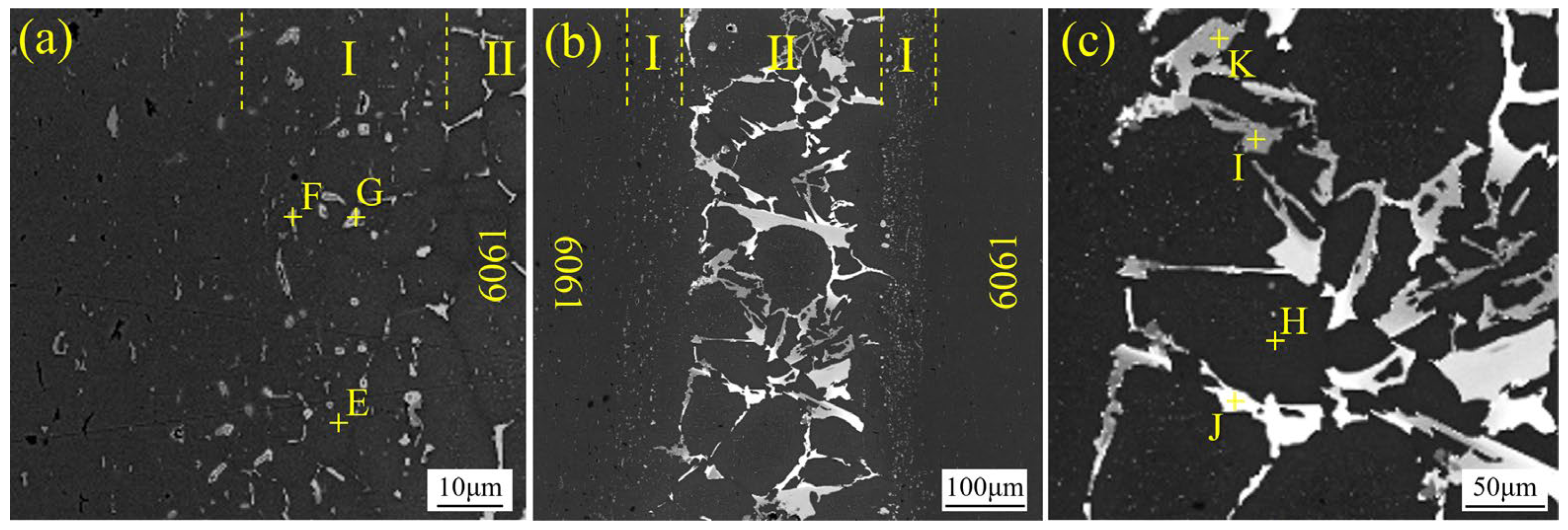


| Point | Al | Si | Ge | Possible Phase |
|---|---|---|---|---|
| A | 97.96 | 0 | 2.04 | α-Al |
| B | 0.62 | 1.31 | 98.07 | Ge |
| C | 4.26 | 63.93 | 31.81 | Si(Ge)P |
| D | 0 | 54.21 | 45.41 | Si(Ge)E |
| Point | Al | Si | Ge | Possible Phase |
|---|---|---|---|---|
| E | 97.4 | 1.7 | 0.9 | α-Al |
| F | 90 | 1.9 | 9.1 | (Al, Ge) |
| G | 46.1 | 0.6 | 53.3 | (Al, Ge) |
| H | 96.9 | 0 | 3.1 | α-Al |
| I | 1.2 | 59.2 | 39.6 | Si(Ge) |
| J | 5.2 | 43.9 | 50.9 | Si(Ge) |
| K | 50.3 | 0.4 | 49.7 | (Al, Ge) |
| Point | Al | Si | Ge | O | Possible Phase |
|---|---|---|---|---|---|
| 1 | 76.8 | 7.5 | 1.6 | 14.1 | α-Al |
| 2 | 1.9 | 0.8 | 91.3 | 6 | Ge |
| 3 | 0.3 | 52.5 | 46.1 | 1.1 | Si(Ge) |
| 4 | 95.4 | 0.8 | 1.4 | 2.4 | α-Al |
| 5 | 1.6 | 0 | 97.1 | 1.3 | Ge |
| 6 | 0 | 56.1 | 42.3 | 1.6 | Si(Ge) |
| 7 | 1.2 | 40.4 | 55.3 | 3.1 | Si(Ge) |
| 8 | 2.8 | 0.7 | 93.8 | 2.5 | Ge |
| 9 | 0 | 48 | 50.1 | 1.9 | Si(Ge) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Shan, J.; Qin, J.; Shen, Y.; Jiang, C.; Jing, P. Vacuum Brazing of 6061 Aluminum Using Al-Si-Ge Filler Metals with Different Si Contents. Metals 2025, 15, 857. https://doi.org/10.3390/met15080857
Huang S, Shan J, Qin J, Shen Y, Jiang C, Jing P. Vacuum Brazing of 6061 Aluminum Using Al-Si-Ge Filler Metals with Different Si Contents. Metals. 2025; 15(8):857. https://doi.org/10.3390/met15080857
Chicago/Turabian StyleHuang, Sen, Jiguo Shan, Jian Qin, Yuanxun Shen, Chao Jiang, and Peiyao Jing. 2025. "Vacuum Brazing of 6061 Aluminum Using Al-Si-Ge Filler Metals with Different Si Contents" Metals 15, no. 8: 857. https://doi.org/10.3390/met15080857
APA StyleHuang, S., Shan, J., Qin, J., Shen, Y., Jiang, C., & Jing, P. (2025). Vacuum Brazing of 6061 Aluminum Using Al-Si-Ge Filler Metals with Different Si Contents. Metals, 15(8), 857. https://doi.org/10.3390/met15080857






