Abstract
This study aims to determine the optimal low-temperature stress relieving heat treatment that minimizes residual stresses while preserving corrosion resistance in Laser Powder Bed Fusion (L-PBF) processed Ti6Al4V alloy. Specifically, it investigates the effects of stress relieving at 400 °C, 600 °C, and 800 °C on microstructure, residual stress, and electrochemical performance. Specimens were characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), and electrochemical techniques. A novel microcapillary electrochemical method was employed to precisely assess passive layer stability and corrosion behaviour under simulated oral conditions, including fluoride contamination and tensile loading. Results show that heat treatments up to 600 °C effectively reduce residual stress with minimal impact on corrosion resistance. However, 800 °C treatment leads to a phase transformation from α′ martensite to a dual-phase α + β structure, significantly compromising passive film integrity. The findings establish 600 °C as the optimal stress-relieving temperature for balancing mechanical stability and electrochemical performance in biomedical and aerospace components.
1. Introduction
Additive Manufacturing (AM) is increasingly utilised in industry for the production of complex metallic components, with Laser Powder Bed Fusion (L-PBF) offering significant advantages in precision and fabrication speed over conventional methods [1]. The application of L-PBF fabricate Ti6Al4V parts has gained significant attention due to the alloy’s excellent corrosion resistance, superior mechanical properties, and biocompatibility, making it highly suitable for aerospace and biomedical uses [2,3]. Comprising 6% aluminium and 4% vanadium, conventionally fabricated Ti6Al4V exhibits an α + β phase microstructure at room temperature, which is highly sensitive to thermal history and cooling rates [4]. The rapid cooling inherent to AM processes often leads to the formation of martensitic α′ phases, altering the alloy’s microstructure [5,6].
However, residual stresses (RS) generated during AM due to the extremely fast cooling rates necessitate post-processing heat treatments (HT) for stress relief [7]. Current literature largely focuses on high-temperature treatments or solution annealing, which significantly alter the microstructure. Jamhari et al. [8] highlighted a lack of data on low-temperature stress relieving, with most studies favouring higher temperatures to facilitate α phase transformation. Haubrich et al. [9] investigated the microstructural evolution of the material under various heat treatment conditions. They reported that treatment at 400 °C did not significantly alter the microstructure, whereas higher temperatures led to noticeable grain coarsening. Syed et al. [10] observed that while stress relieving at 680 °C for 3 h reduced RS, it also partially decomposed the microstructure. Gaiani et al. [11] employed high-temperature HTs to achieve an α + β microstructure similar to conventionally processed Ti grade 5, enhancing ductility. Similar outcomes were reported by Pathania et al. [12], Bologna et al. [13], Ebrahimi et al. [14], Sabban et al. [15] and Mohd Foudzi et al. [16] for treatments above 750 °C, where martensitic α′ phases transformed into the more stable α + β structure. Zhao et al. [17] further demonstrated improved ductility, from 2.2% to 8%, through high-temperature HTs, albeit with notable microstructural changes. It is evident that the optimum heat treatment that can efficiently reduce the residual stress magnitude within a short time frame (considering also the cost effectiveness) is not well understood.
Another critical aspect concerns the corrosion behaviour of L-PBF Ti6Al4V compared to conventionally produced counterparts after stress relieving. Existing literature offers conflicting findings. In authors previous investigation [18], superior corrosion resistance and a more stable passive layer was reported for L-PBF samples in artificial saliva and simulated body fluid (SBF), while Zhan et al. [19] found conventional Ti6Al4V to perform better in 3.5% NaCl, due to a passive layer less thick and more unstable in additive manufactured Ti6Al4V. This instability was caused by the high density of pores in L-PBF alloy, which compromised the integrity of the passive layer. Luo et al. [20] linked higher L-PBF scanning rates to finer grain structures and improved corrosion resistance under SBF. Similarly, Cabrini et al. [21] observed negligible increases in current density during long-term testing of L-PBF samples in SBF, suggesting comparable performance to traditionally manufactured alloys. Chiu et al. [22] highlighted the influence of grain size on the tribo-electrochemical passivity of L-PBF in SBF. This correlation could be attributed to a faster formation of the passive layer at surface crystalline defects, such as grain boundaries and dislocations. Conversely, Chen et al. [23] found L-PBF Ti6Al4V to exhibit inferior corrosion resistance in SBF, 3.5% and 15% NaCl, and phosphate-buffered saline (PBS), attributing this to its martensitic microstructure and more defective passive layer. Zhang et al. [24] also reported poorer corrosion performance of L-PBF alloys in NaF-containing artificial saliva, due to the presence of the martensitic phases and the defects in the passive film.
In the context of heat treatment effects on L-PBF processed Ti6Al4V alloys, several studies have reported varying trends in corrosion behaviour depending on the applied temperature and resulting microstructural transformations. Seo and Lee [25] observed a decrease in corrosion resistance following heat treatments at 650 °C and 750 °C, attributing this to the formation of precipitates such as α2 (Ti3Al). However, at temperatures above 850 °C, corrosion performance improved significantly due to the complete transformation to a stable α + β microstructure. Similarly, Usha Rani et al. [26] reported a reduction in corrosion resistance at 750 °C, with improvements seen at 850 °C and 950 °C, again linked to the stabilisation of the α + β phase structure. Conversely, Yeo et al. [27] found that heat treatment at 950 °C led to a decline in corrosion performance compared to the as-built condition, which they attributed to excessive grain growth and reduced surface area of the passive film. Cecchel et al. [28,29] also investigated high-temperature treatments (730 °C, 800 °C, 940 °C, and 1015 °C) and noted that solution annealing above the β-transus temperature enhanced corrosion resistance, due to the development of a more stable and isotropic microstructure. Other heat treatments in their study also demonstrated corrosion improvement, though to a lesser extent. Interestingly, Ettefagh et al. [30] reported that heat treatments at 600 °C and 800 °C could potentially improve corrosion resistance compared to the as-built condition, highlighting the complexity and variability of the corrosion response based on processing conditions. Despite these efforts, the electrochemical behaviour of L-PBF Ti6Al4V subjected to short-duration, low-temperature stress relieving treatments remains largely underexplored. More importantly, no systematic study to date has investigated the combined influence of heat treatment, residual stress magnitude, and mechanical loading on both the corrosion and semiconductive properties of the alloy. The present work addresses this critical gap, offering new insights through high-resolution characterisation methods and providing practical guidance for optimising post-processing treatments in corrosion-sensitive applications.
This study investigates the impact of low-temperature stress relieving on the stress-assisted corrosion behaviour and semiconductive properties of the native oxide layer on L-PBF Ti6Al4V. Low-temperature heat treatments are favoured to retain the alloy’s fine-grained martensitic microstructure, which is crucial for maintaining corrosion resistance. A novel aspect of this work is the use of the microcapillary electrochemical technique, which enables precise, localised analysis of electrochemical performance. This method, combined with conventional techniques, offers enhanced accuracy and repeatability. Complementary characterisation tools, including SEM for microstructural evaluation and XRD for residual stress analysis, were employed. Electrochemical behaviour was assessed in borate buffer solution and artificial saliva containing fluoride under tensile loading state, simulating physiological conditions. The insights gained hold significant relevance for applications in the aerospace, marine, and biomedical sectors.
2. Materials and Methods
2.1. L-PBF Processing and Sample Preparation
The samples, with dimensions of 30 mm × 30 mm× 10 mm, were fabricated using an EOS M 100 L-PBF system, equipped with a single-mode ytterbium fibre laser (wavelength: 1070 nm, maximum power: 200 W), and a nominal laser spot diameter of approximately 40 µm. Gas-atomised Ti6Al4V powder with a particle size distribution of 15–45 µm was used as the feedstock material. Specimens were built directly on the build platform without the use of support structures. A layer thickness of 0.05 mm was employed. The processing parameters followed the standard settings recommended by the machine manufacturer; however, specific details cannot be disclosed due to industrial confidentiality. Following fabrication, samples were sectioned to a thickness of 0.8 mm, then ground and polished to a final finish using a 0.2 µm colloidal silica suspension. A final cleaning step was performed via ultrasonic agitation for one minute in a 70% ethanol solution to remove surface contaminants and ensure a high-quality surface finish.
Heat treatments were carried out using a Carbolite tubular electric furnace (Carbolite Ltd., Seattle, WA, USA) at temperatures of 400 °C, 600 °C, and 800 °C for 1 h. A controlled atmosphere was maintained by continuous argon gas flow throughout the treatment to prevent unwanted surface oxidation. The heating rate was set to 5 °C/min, followed by furnace cooling to room temperature. After heat treatment, samples were ground, polished, and ultrasonically cleaned and kept untouched for 72 h. It is worth mentioning that preventing oxidation during heat treatment is particularly critical for titanium alloys such as Ti6Al4V, as surface oxidation can result in the formation of a brittle and oxygen-enriched α-case layer. This layer not only weakens mechanical properties, such as fatigue resistance and ductility, but also alters the electrochemical behaviour, negatively affecting corrosion resistance, especially in biomedical and aerospace applications where surface integrity is essential.
2.2. Microstructural Characterization and XRD Analysis
To reveal the microstructure, samples were etched in Kroll’s reagent for 20 s and rinsed with deionised water. Microstructural analysis was conducted using a Zeiss EVO MA10 (Zeiss, Oberkochen, Germany) scanning electron microscope (SEM) equipped with energy-dispersive X-ray spectroscopy (EDS). To assess changes in the thickness of the α′ martensitic phase, four SEM micrographs were captured for each sample in order to have statistically significant analysis. From each image, 30 individual measurements of α′ thickness were taken to ensure statistical relevance. EDS was used in a qualitative capacity to support microstructural analysis by identifying different phases based on elemental distribution. Spot and area analyses were performed on selected regions, targeting Ti, Al, and V to distinguish α and β phases. No quantitative or full-field elemental mapping was conducted.
Phase evolution in the specimens was analysed using X-ray diffraction (XRD) with a Bruker D8 Advance diffractometer (Karlsruhe, Germany), operating with Cu Kα radiation (λ = 0.15418 nm) at 40 kV and 40 mA. Scans were performed over a 2θ range of 30° to 80°, with a step size of 0.02° and a counting time of 1 s per step. The samples were positioned longitudinally relative to the X-ray beam during measurement. Phase identification was conducted using the Match! software package (Crystal Impact GbR, Bonn, Germany), with reference patterns obtained from the PDF-2 database (ICDD—International Centre for Diffraction Data, Newtown Square, PA, USA).
Furthermore, residual stress measurements were performed using X-ray diffraction (XRD) with Spider TM X GNR diffractometer (GNR, Agrate Conturbia, Italy). The guideline followed for RS measurement using XRD analysis was NPL good practice No. 52 for all the samples [31]. Scans were conducted with a source of Copper with a kβ of Nickel, and the sin2ψ method with the measurement of 9 angles between 0° and 38°. For the titanium alloy samples, the (213) diffraction peak was selected for analysis, as recommended for hexagonal titanium alloys. This peak corresponds to a 2θ angle of approximately 139.4° when using Cu K-α radiation (λ = 1.5406 Å). The sin2ψ method was employed for stress calculation, using an isotropic Young’s modulus of 113.8 GPa and a Poisson’s ratio of 0.342 for Ti-6Al-4V, based on widely accepted literature values for polycrystalline titanium alloys. These parameters ensure accurate determination of residual stress in the near-surface region. The RS was measured along the longitudinal direction of the samples and subsequently repeated transversely to ensure consistency and enable cross-validation of the results. The raw diffraction data were processed using SpiderX software to calculate residual stress values. Residual stress measurements were conducted on the same surface used for electrochemical testing (the final built layer of each specimen, carefully polished to ensure surface uniformity). As the electrochemical response is governed by the surface layer in contact with the electrolyte, surface residual stress was deemed most relevant to the objectives of this study. It is worth noting that the residual stress values reported herein represent macroscopic stresses. No corrections were made for microstresses, as the objective of the study was to assess long-range stress distributions introduced by processing at the macroscale.
2.3. Electrochemical Analysis
2.3.1. Electrochemical Response in Borate Buffer Solution Without Applied Load
Electrochemical characterisation was conducted using a microcapillary setup, which offers high spatial resolution and precision due to the localised testing area [18,32,33,34,35,36,37,38]. This configuration also provides enhanced repeatability compared to conventional methods. The electrochemical cell employed a classic three-electrode arrangement [39]. Potentiodynamic polarisation analyses were carried out in a borate buffer solution (0.0075 M Na2B4O7·10H2O and 0.05 M H3BO3) [40] to investigate the properties of the native oxide layer in a non-aggressive environment. The potentiodynamic polarisation measurements were conducted over a potential range of −1000 mVSCE to +1500 mVSCE at a scan rate of 5 mV/s. Electrochemical impedance spectroscopy (EIS) was conducted on a circular surface area of 1 mm diameter, isolated using adhesive tape, in a 40 mL volume of borate buffer solution. Open Circuit Potential (OCP) was recorded for 1 h, and the stabilised potential value was used as the DC bias for the subsequent electrochemical impedance spectroscopy (EIS) measurement. The EIS test was performed using a 10 mV AC perturbation over a frequency range from 1 MHz to 0.01 Hz. It is worth noting that each experiment was repeated at least three times to ensure the reliability and reproducibility of the reported results.
2.3.2. Stress-Assisted Electrochemical Response in Saliva Solution with NaF Contamination
In addition, the corrosion behaviour of the material was evaluated under tensile loading using the microcapillary electrochemical setup in a simulated oral environment. The testing medium consisted of artificial saliva enriched with 5% sodium fluoride to replicate the potential fluoride contamination that may occur in clinical settings [41]. Fluoride is commonly present in dental care products and prophylactic treatments, and its interaction with titanium alloys is known to significantly compromise the stability of the passive oxide layer, thereby accelerating corrosion processes—particularly in the presence of mechanical stress [42]. Although daily oral care products typically contain low fluoride concentrations, professional dental varnishes frequently used in clinical practice contain up to 5% sodium fluoride (NaF), corresponding to approximately 22,600 ppm fluoride. These are commonly applied during routine dental procedures, particularly in patients with gum recession or exposed implant surfaces. As such, the selected fluoride concentration in this study was chosen to replicate a clinically relevant, high-risk exposure scenario. This enables the assessment of the passive film stability and corrosion resistance of the implant material under aggressive but realistic service conditions
To simulate the mechanical stresses encountered during the service life of dental implants (considering the critical scenario), specimens were subjected to tensile straining corresponding to the 0.2% proof stress, falling within the material’s yield range [43]. The potentiodynamic polarisation analyses were performed after imposing a uniaxial strain perpendicular to the microcapillary cell. The potentiodynamic polarisation tests were conducted over a potential range of −2000 mVSCE to +1500 mVSCE at a scan rate of 2 mV/s. Furthermore, potentiostatic polarisation at +1500 mVSCE for 30 min was then applied to study the kinetics of corrosion occurrence under tensile loading state.
To ensure the reliability and reproducibility of the microcapillary electrochemical measurements, each test was repeated at least three times on different surface locations. Due to the uniform microstructure of L-PBF Ti6Al4V and the small, well-defined contact area (500 µm diameter), the method offers high spatial resolution and minimal variability. The observed standard deviation in electrochemical parameters remained below 3%, confirming excellent reproducibility and validating the robustness of the technique.
3. Results and Discussion
3.1. Microstructural Analysis
Microstructural analysis of the heat-treated specimens as shown in Figure 1 reveals a clear evolution of phase morphology as a function of temperature. In the as-built and low-temperature-treated samples (up to 600 °C), the microstructure was predominantly composed of a metastable martensitic α′ phase. This phase formation is characteristic of the L-PBF process, where extremely high cooling rates, resulting from steep thermal gradients generated by the laser, suppress diffusion-driven phase transformations [20,44]. As a result, the stable equilibrium α + β phases observed in conventionally processed Ti6Al4V are bypassed, and the microstructure instead solidifies into fine, acicular α′ martensite. These α′ structures exhibit as small, needle-like features often [45,46].
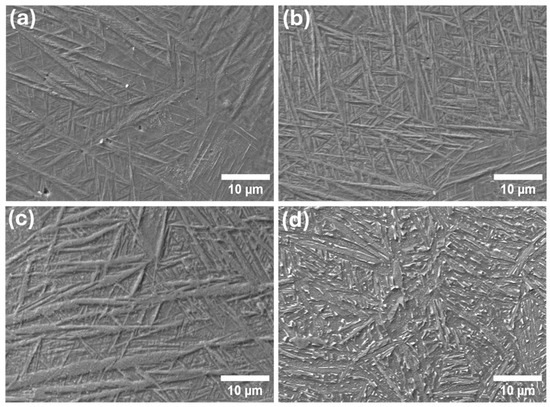
Figure 1.
SEM images of L-PBF Ti6Al4V at different heat treatment temperatures: (a) As-built, (b) 400 °C, (c) 600 °C and (d) 800 °C.
Upon increasing the heat treatment temperature to 800 °C, a significant phase transformation was observed. The metastable α′ phase decomposed, giving rise to a more thermodynamically stable microstructure comprising equiaxed α-phase grains dispersed within a retained β-phase matrix [28,47]. This transformation is driven by enhanced atomic mobility at elevated temperatures, which allows diffusion-controlled processes to occur, promoting the breakdown of the supersaturated α′ martensite and the nucleation and growth of equilibrium phases [12]. Notably, the β-phase, stabilised by vanadium, persists at room temperature following high-temperature exposure due to its increased stability at elevated temperatures [48]. The resulting α + β microstructure [11] observed after treatment at 800 °C closely resembles that of conventionally heat-treated Ti6Al4V, which is known for its improved ductility and balanced mechanical properties [49]. However, microstructural analysis clearly indicates that the size of the metastable α′ phase increases progressively with rising heat treatment temperature. This observation underscores the significant influence of thermal exposure on the growth and coarsening of the α′ phase, as elevated temperatures promote increased atomic mobility and diffusion, facilitating phase thickening even within the metastable regime [17].
Further statistical analysis of the metastable α′ phase thickness, as presented in Figure 2, reveals a marked increase in α′ thickness as the heat treatment temperature rises from 400 °C to 600 °C. This trend reflects the enhanced atomic diffusion at elevated temperatures, which promotes coarsening of the martensitic structure [16]. However, at 800 °C, the growth of the α′ phase becomes less pronounced. This deviation is attributed to the onset of a thermodynamically driven phase transformation, where the metastable α′ phase begins to decompose into the equilibrium α + β microstructure [13]. Therefore, while some coarsening of the α′ phase may still occur at 800 °C, the dominant microstructural evolution at this temperature is the transformation into a stable dual-phase system. This suggests that, under these thermal conditions, phase transformation is energetically more favourable than continued growth of the non-equilibrium α′ phase [10].
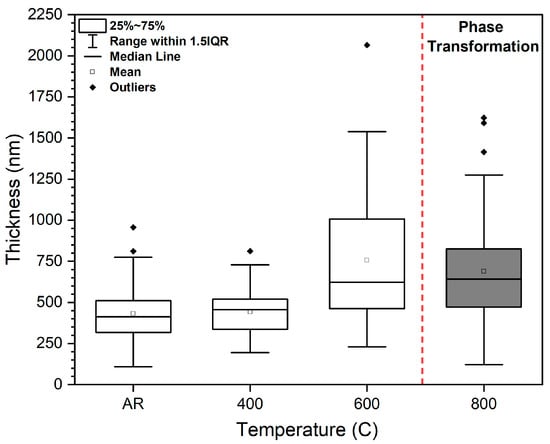
Figure 2.
Statistical analysis of the thickness of the α’ martensitic phases.
3.2. XRD Analysis
Figure 3 presents the XRD patterns of the four specimens, illustrating the characteristic diffraction peaks associated with the α′/α and β phases of the Ti6Al4V alloy produced by L-PBF process. The as-built sample exhibits broad peaks, particularly those corresponding to the α′ phase, which is a metastable martensitic structure formed during the rapid solidification inherent to the L-PBF process. The observed peak broadening is consistent with previous studies and can be attributed to distortions in the α′ crystal lattice, caused by a combination of high internal residual stresses and the supersaturation of alloying elements such as aluminium and vanadium within the phase [50].
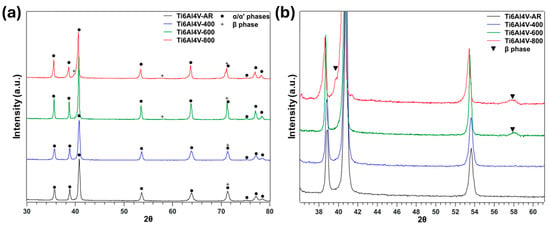
Figure 3.
(a) X-ray diffraction pattern of LPBF Ti6Al4V alloys in the as-built condition and after thermal treatment at 400/600/800 °C; (b) details of the β-phase formation at 600 and 800 °C.
Following heat treatment at 400 °C, the XRD pattern shows no significant alteration in peak positions or shapes, indicating that the microstructure and crystal structure remain largely unchanged at this temperature. However, clear changes are observed in the specimens treated at 600 °C and 800 °C, as shown in Figure 3b. These changes reflect a gradual transformation of the metastable α′ phase into the equilibrium α and β phases. This transformation is driven by the diffusion of alloying elements out of the supersaturated α′ phase. In particular, vanadium, known for its role as a β-phase stabiliser, promotes the formation of the β phase during thermal exposure.
This phase transformation is further supported by the sharpening of the α′/α-related peaks, indicating a reduction in lattice strain and residual stresses. Additionally, the emergence of distinct peaks at 2θ = 39.6° and 57.5°, associated with the β phase, is clearly visible in the 600 °C and 800 °C samples (Figure 3b), confirming findings reported by Xu et al. [51] and Yeo et al. [27]. These results confirm that elevated temperatures not only facilitate stress relaxation but also promote significant phase evolution, which plays a critical role in determining the alloy’s microstructural and electrochemical properties. To ensure the reliability of the XRD measurements, each pattern was recorded multiple times to confirm reproducibility and eliminate potential misalignment effects. Additionally, measurements were repeated after polishing and ultrasonic cleaning of the sample surfaces to avoid interference from surface contaminants or oxidation. Although the differences between the 400 °C and 600 °C heat-treated samples appear subtle, careful analysis reveals noticeable peak sharpening at 600 °C, indicating reduced lattice strain and residual stress. Emerging features at 57.5° and 39.6° 2θ suggest the initial formation of the β-phase, further confirming microstructural changes induced by the heat treatment.
Furthermore, XRD residual stress measurements were carried out to assess the extent to which thermal treatments influence the residual stress state in L-PBF processed Ti6Al4V. As shown in Figure 4 and summarised in Table 1, residual stresses decreased progressively with increasing heat treatment temperature in both the longitudinal and transverse directions. The most substantial reduction occurred between the as-built condition and the 600 °C treatment, with comparatively smaller changes observed beyond this temperature.
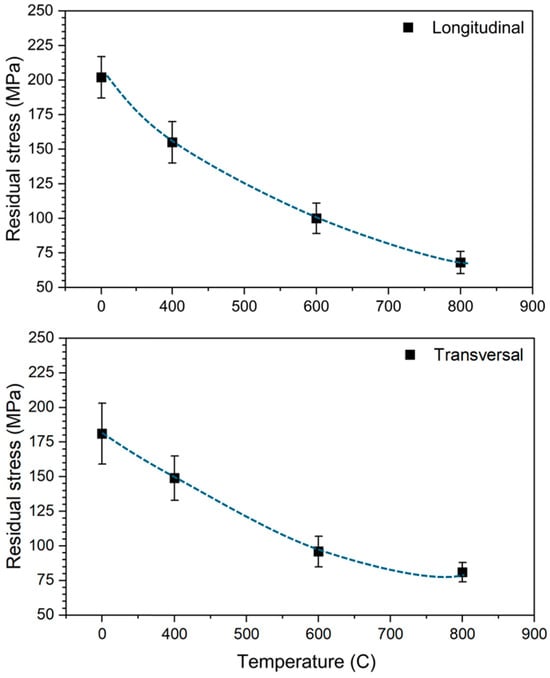
Figure 4.
XRD residual stress variation at different temperatures for L-PBF processed Ti6Al4V.

Table 1.
XRD residual stress magnitude with the corresponding error of measurements.
The highest residual stress was recorded in the as-built condition, with a value of 202 MPa. This is attributed to the intrinsic characteristics of the L-PBF process, which involves rapid localised melting and solidification [7]. The high thermal gradients and uneven cooling generate significant thermal contractions and non-uniform plastic deformation, resulting in the accumulation of tensile and compressive residual stresses within the material [52]. At elevated temperatures, particularly at and above 600 °C, atomic mobility increases sufficiently to enable stress relaxation through recovery and partial recrystallisation mechanisms. These processes allow the redistribution and annihilation of dislocations, thereby reducing the internal stress fields [53]. The lowest residual stress, 68 MPa, was measured for specimens treated at 800 °C, indicating that substantial stress relief had occurred. Based on these findings, a heat treatment temperature of 600 °C appears to be the most effective compromise for relieving residual stresses in L-PBF Ti6Al4V while maintaining the integrity of the microstructure. To ensure the reliability of the XRD-based residual stress measurements, each experiment was repeated multiple times on independently prepared sample surfaces. This included repeated polishing and alignment to minimize surface artifacts and geometric errors. The resulting measurements demonstrated a standard deviation of approximately 3%, indicating excellent repeatability and confirming the consistency of the stress values reported across the different heat treatment conditions.
3.3. Electrochemical Analysis
3.3.1. Electrochemical Impedance Spectroscopy (EIS) Analysis in Borate Buffer Solution
EIS was employed to characterise the electrochemical behaviour of the native oxide layer on L-PBF Ti6Al4V samples, and the results were interpreted through Bode plots as shown in Figure 5. The different electrochemical processes occurring at various frequency ranges could be further revealed, enabling a detailed understanding of surface and interfacial phenomena [54].
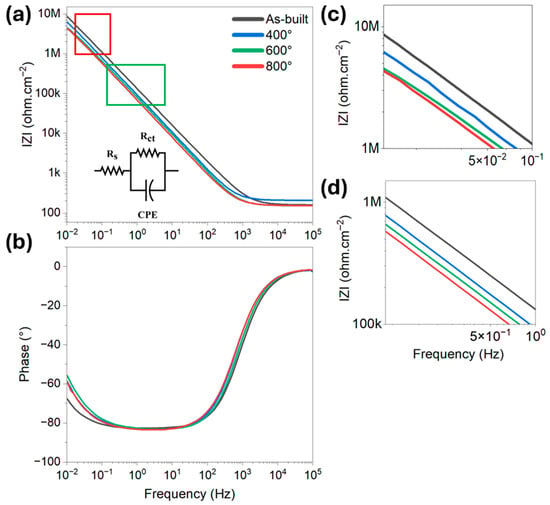
Figure 5.
EIS analysis of the L-PBF processed alloy Ti6Al4V treated at different temperatures, and the equivalent electrical circuit used for the fitting of the data (a) modulus, (b) phase angle, higher magnification of (c) low frequency region (red box), and (d) mid frequency region (green box).
At high frequencies, the impedance response is primarily governed by the solution resistance (Rs). Changes in this region typically reflect variations in the electrolyte composition or surface roughness, but are less sensitive to changes within the oxide layer itself [55]. In the mid-frequency region, the impedance is influenced by the charge transfer resistance (Rct) and the dielectric properties of the passive film. This region is particularly significant for evaluating the integrity and compactness of the oxide layer [56], and it is usually characterized by a mixed capacitive and resistive response, as clearly shown through a single time constant by the phase angle between −80° and −90°. A higher modulus in this frequency range indicates a more resistive and protective oxide barrier, suggesting improved corrosion resistance. At low frequencies, the impedance behaviour is associated with slower processes such as diffusion of ionic species and long-term stability of the passive film [57]. A higher impedance modulus in this range generally corresponds to enhanced corrosion resistance and stability of the oxide film over time, as it reflects reduced ionic transport and minimal degradation at the metal–electrolyte interface [58].
As illustrated in Figure 5, the differences in impedance response across specimens subjected to various heat treatment temperatures clearly demonstrate the impact of thermal exposure on the properties of the passive oxide layer. Notably, the impedance modulus decreased significantly in both the low- and mid-frequency regions for all heat-treated samples when compared to the as-built condition. The most pronounced reduction was observed in specimens treated at 800 °C, whereas the variation in modulus between the 400 °C and 600 °C treatments was comparatively minor. These trends suggest a strong correlation between the impedance response and the evolving microstructural characteristics and residual stress state of the L-PBF processed material [25,59].
To enable a quantitative comparison of the EIS results across different heat treatment conditions, an equivalent electrical circuit model was employed to fit the impedance data [60]. The selected model consisted of a solution resistance (Rs) in series with a parallel combination of charge transfer resistance (Rct) and a constant phase element (CPE) [61]. This configuration is widely used to simulate the electrochemical response of passive films on metallic surfaces [62]. The Rs represents the resistance of the electrolyte between the working and reference electrodes and is primarily influenced by the conductivity and geometry of the testing medium. The Rct is associated with the resistance to electrochemical reactions occurring at the metal–electrolyte interface and serves as a key indicator of corrosion resistance. Higher Rct values typically reflect a more stable and protective passive layer. A CPE was used in place of an ideal capacitance to account for non-ideal capacitive behaviour frequently observed in real electrochemical systems [61,63]. Such non-ideal behaviour arises due to surface roughness, inhomogeneities in the oxide layer, or variations in current distribution across the interface. The impedance of the CPE is defined by the expression:
where Q is the CPE constant (with units of S·sn.cm−2), ω is the angular frequency, j is the imaginary unit, and n is the phase shift exponent (0 ≤ n ≤ 1). When n equals 1, the CPE behaves as an ideal capacitor; when n equals 0, it behaves as a pure resistor. The value of n thus provides insight into the degree of deviation from ideal capacitive behaviour, values closer to 1 indicate a more homogeneous and uniform passive layer [61,64]. The fitted data with the corresponding error is summarized in Table 2.

Table 2.
Summary of equivalent circuit fitted on the EIS results.
As expected, the Rct exhibited a marked decrease in samples subjected to stress-relieving heat treatments compared to the as-built condition. This reduction became more pronounced with increasing temperature, particularly in the specimen treated at 800 °C, indicating a substantial decline in the protective properties of the passive oxide layer. In contrast, samples treated at 400 °C and 600 °C showed only moderate reductions in Rct, suggesting a less significant impact on the electrochemical barrier at these lower temperatures. Additionally, the n parameter of the CPE decreased with increasing temperature, reflecting a greater deviation from ideal capacitive behaviour. This trend is indicative of increased surface heterogeneity or degradation in the uniformity of the passive film at higher temperatures. Notably, the Q parameter, which relates to the capacitive response of the interface, remained relatively unchanged across all heat-treated specimens, suggesting that while the film’s homogeneity and charge transfer resistance were affected, its overall capacitive characteristics were less sensitive to the heat treatment conditions highlighting the presence of a robust passive layer on the surface which is expected for Ti6Al4V alloy.
To further evaluate the electrochemical properties of the passive oxide layer, the values obtained from the equivalent electrical circuit (EEC) fitting were used to calculate the effective capacitance (Ceff). This calculation assumes a normal (through-thickness) distribution of capacitance within the oxide film and was performed using the following expression [65].
Ceff,norm = Q1/nRct(1−n)/n
Unlike the widely used Brug formula, which accounts for lateral surface inhomogeneities, this approach was deemed more appropriate for the present study. All specimen surfaces were mirror-polished to achieve high topographical uniformity, minimising surface roughness effects. As such, the primary variation in capacitance arises in the direction normal to the surface, consistent with the layered structure of the oxide film. This method therefore offers a more accurate representation of the dielectric behaviour across the film thickness, yielding a more reliable interpretation of the oxide layer’s electrochemical performance.
The Ceff,norm parameter exhibited a consistent increasing trend across the heat-treated samples, reflecting a progressive shift in the capacitive behaviour of the passive layer as the surface characteristics evolved. This increase, when considered alongside the concurrent decrease in charge transfer resistance (Rct), indicates a deterioration in the protective quality of the oxide film, particularly in the specimens treated at 600 °C and 800 °C. These samples demonstrated a more conductive and less resistive passive layer, which may be attributed to increased heterogeneity or structural defects within the oxide, likely introduced through microstructural changes at elevated temperatures. In contrast, the as-built and 400 °C-treated specimens maintained higher Rct values and lower Ceff,norm, signifying a more uniform and protective passive film, and thereby superior corrosion resistance. To ensure the robustness of these results, each EIS measurement was repeated a minimum of three times. The data consistently followed the same trend across all conditions, with a standard deviation of less than 3% in the fitted values, confirming the high repeatability and reliability of the electrochemical analysis.
3.3.2. Potentiodynamic Polarisation in Borate Buffer Solution
To further investigate the changes in passive layer properties under varying heat treatment conditions, the potentiodynamic polarisation tests were conducted. This technique provides critical insights into the electrochemical behaviour of materials by measuring their response to an applied potential [66]. Key parameters derived from the potentiodynamic polarisation curves include the corrosion potential (Ecorr), corrosion current density (icorr), and passive current density (ipass). The corrosion potential (Ecorr) represents the open-circuit potential at which the anodic and cathodic reactions are in dynamic equilibrium. However, for passive materials such as Ti6Al4V, where active corrosion does not typically occur due to the formation of a stable oxide layer, Ecorr does not accurately reflect the material’s corrosion resistance. Instead, it serves as an approximate indication of the thermodynamic tendency for oxidation or reduction but not the kinetics of degradation. The corrosion current density (icorr), often obtained through Tafel extrapolation, provides a more meaningful metric for assessing corrosion behaviour, as it reflects the rate of electrochemical reactions at the surface. Lower icorr values generally indicate better corrosion resistance. The passive current density, measured within the passive region of the potentiodynamic polarisation curve, offers insight into the stability and protectiveness of the passive film. A low and stable passive current density suggests the presence of a compact, adherent, and self-healing oxide layer that effectively limits metal dissolution.
As illustrated in Figure 6, the potentiodynamic polarisation behaviour of the native oxide layer indicates minimal changes in passive film performance for heat treatments up to 600 °C. The corrosion behaviour remains largely unchanged in this temperature range, suggesting that low- to mid-temperature stress relieving does not significantly compromise the integrity of the passive layer. Notably, a slight improvement in passive current density was observed in specimens treated at 600 °C, implying a marginal enhancement in the compactness and stability of the oxide film. In contrast, a marked increase in passive current density was recorded for samples treated at 800 °C, indicating a substantial reduction in the protective capacity of the passive layer and a greater susceptibility to ionic release. This degradation highlights that while high-temperature stress relieving effectively reduces residual stresses, it also negatively impacts the electrochemical stability of the surface oxide. The findings suggest a trade-off at elevated temperatures, where microstructural changes and oxide layer destabilisation outweigh the benefits of stress relief with respect to corrosion resistance. the summary of the electrochemical magnitudes obtained from potentiodynamic polarization analysis is presented in Table 3.
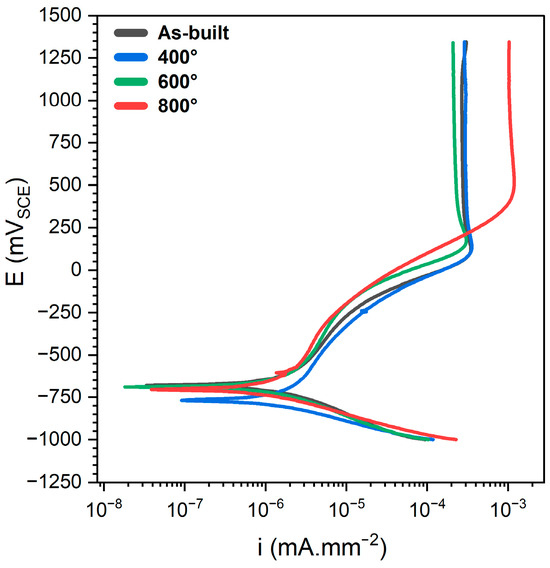
Figure 6.
Representative potentiodynamic polarization results for specimens treated at different temperatures in borate buffer solution.

Table 3.
Summary of electrochemical magnitudes (corrosion density—Icorr, corrosion potential—Ecorr, passive current—Ipass, and potential at onset of passivity—Epass) obtained from the potentiodynamic polarization analysis with a standard deviation of 5% considering multiple experiments.
3.3.3. Electrochemical Polarization in NaF Containing Solution Under Stress-Assisted Conditions
The role of fluoride in the corrosion behaviour of titanium alloys, particularly in the context of dental implants, is of significant concern. Fluoride-containing compounds are commonly found in oral hygiene products and dental treatment agents, such as gels, rinses, and etchants, frequently used in dental clinics and laboratories [42,67]. These products often contain high concentrations of fluoride ions, which can migrate into the peri-implant environment, especially in cases of incomplete rinsing or residual contamination during prosthetic procedures. When titanium-based implants are exposed to fluoride-rich environments, particularly under acidic or mechanical stress conditions, the passive oxide layer that normally protects the metal can become destabilised. Fluoride ions are known to penetrate and disrupt the titanium oxide film, leading to its breakdown and promoting localised corrosion such as pitting and stress corrosion cracking [42]. This is especially critical in the oral cavity, where implants are subjected to complex interactions involving mechanical loads, salivary constituents, and microbial activity. Therefore, understanding the corrosion behaviour of titanium alloys, such as L-PBF Ti6Al4V, in fluoride-containing environments is essential for predicting the long-term performance of dental implants [68]. Evaluating their electrochemical response under simulated oral conditions provides valuable insights into the durability of the passive film and the potential risk of implant degradation due to fluoride exposure.
Potentiostatic polarisation tests were conducted under constant potential following the application of tensile strain to all specimens. A potential of +1500 mV vs. SCE was selected for each test, based on prior potentiodynamic polarisation results under identical conditions. This ensured a consistent and comparable basis for evaluating the potentiostatic response across all samples. As illustrated in Figure 7 and summarized in Table 4, the potentiodynamic polarisation analysis under aggressive fluoride-contaminated conditions revealed a consistent trend: the as-built and 400 °C heat-treated specimens exhibited the lowest anodic current densities, indicating more effective passivation behaviour. In contrast, samples treated at 600 °C and 800 °C displayed progressively higher anodic current densities, suggesting a deterioration in the stability and protectiveness of the passive film with increasing heat treatment temperature.
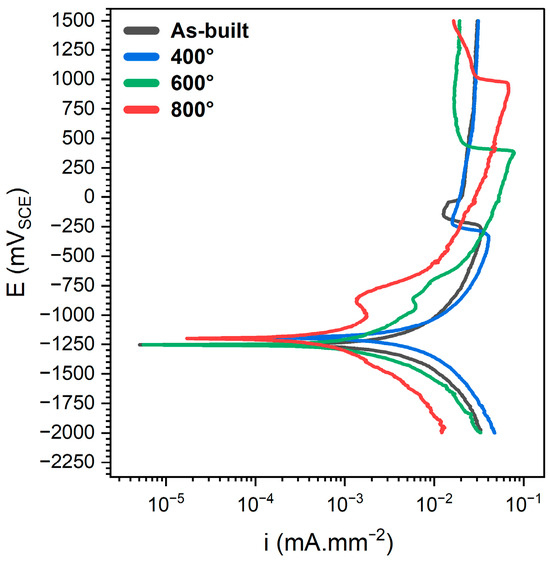
Figure 7.
Potentiodynamic polarization in saliva solution with 5% NaF under tensile stress state corresponding to the 0.2% proof stress.

Table 4.
Summary of electrochemical magnitudes obtained from potentiodynamic polarization in saliva solution with 5% NaF under tensile strain state corresponding to the 0.2% proof stress with a standard deviation of 5% among multiple experiments.
More importantly, the data indicate that at higher temperatures, the alloy remains in an active corrosion state over a broader potential range, requiring significantly higher anodic potentials to transition into the passive region. For the as-built and 400 °C samples, passivation occurred at relatively low potentials, reflecting a more responsive and protective oxide layer. At 600 °C, the passivation potential (Epass) increased noticeably, indicating a reduced tendency for the oxide layer to stabilise. This effect was even more pronounced at 800 °C, where the highest Epass was observed, signifying a substantial compromise in passivation behaviour.
Potentiostatic polarisation results within the passive region as shown in Figure 8 revealed notable differences in the corrosion behaviour of the specimens, particularly highlighting the distinct response of the sample treated at 800 °C. The as-built specimen exhibited the most rapid stabilisation of current density, indicating a swift formation of a stable passive layer. This behaviour was closely followed by the samples treated at 400 °C and 600 °C, which demonstrated similar trends with only slight variations in passive current density and stabilisation time.
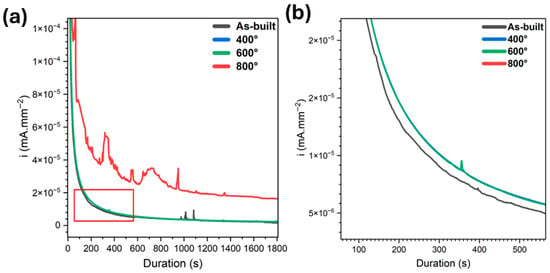
Figure 8.
(a) Potentiostatic polarization (at 1500 mVSCE) of the specimens treated at different temperatures under tensile straining in 5% NaF solution, and (b) the magnified region shown as red box.
In contrast, the 800 °C-treated specimen showed markedly different behaviour. It required a significantly longer time to reach a steady-state current, and the initial phase of polarisation was characterised by frequent spikes and fluctuations in current density. These transients suggest repeated breakdown and repassivation events, pointing to a passive film that is less stable and more susceptible to localised corrosion processes. Even after eventual stabilisation, the passive current density remained substantially higher than that of the other samples, indicating a reduced ability of the passive layer to inhibit faradaic reactions.
This behaviour can be directly linked to the microstructural transformation occurring at 800 °C, where the metastable α′ (martensitic) phase decomposes into a dual-phase α + β microstructure [12,13,16]. While stress relieving at this temperature effectively reduces residual stresses, it also results in a microstructural configuration that appears less favourable for corrosion resistance. In particular, the single-phase α′ martensitic structure present in the as-built and lower-temperature-treated specimens demonstrated superior corrosion performance, likely due to its finer grain structure and greater homogeneity. In contrast, the α + β dual-phase microstructure introduces heterogeneities and phase boundaries that may serve as initiation sites for corrosion, particularly under tensile straining and in aggressive environments such as fluoride-containing media [69]. These findings underscore the complex interplay between heat treatment, microstructure, and corrosion behaviour, and suggest that preserving the martensitic α′ phase through moderate thermal exposure may offer advantages in maintaining passive layer stability and corrosion resistance in critical biomedical applications.
3.3.4. SEM Analysis of Corrosion Morphology
Further SEM analysis was conducted on the corroded surfaces of the specimens following potentiostatic polarisation in fluoride-containing solution under applied tensile strain, with the aim of correlating the observed electrochemical responses, particularly for the 800 °C-treated sample, with the underlying surface degradation mechanisms. As shown in Figure 9, the corrosion morphology of the as-built specimen and the sample treated at 400 °C appears largely similar and the α′ needle-like structures are slightly exposed after corrosion. These observations are consistent with the electrochemical data, indicating relatively stable passive layer performance at lower heat treatment temperatures. In contrast, distinct changes in corrosion morphology were evident in the specimens treated at 600 °C and 800 °C. Both exhibited surface degradation in the form of micropitting and localised corrosion features, suggesting a breakdown in the protective oxide layer. Notably, the sample treated at 800 °C displayed significantly more severe localised corrosion, characterised by deeper and more widespread surface damage.
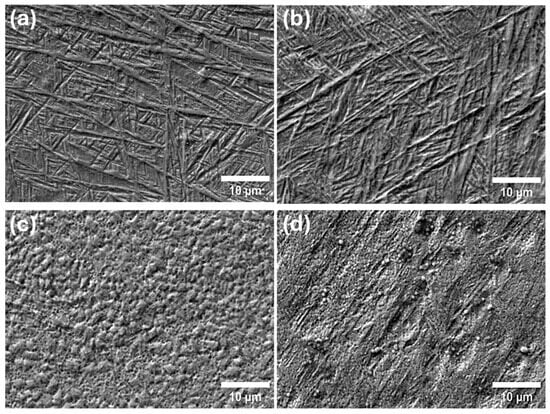
Figure 9.
SEM analysis of the corrosion morphology for the specimens (a) As-built, (b) 400°, (c) 600° and (d) 800° under tensile straining in 5% NaF containing solution.
This increased susceptibility is closely linked to the phase transformation that occurs at higher temperatures. The transition from a single-phase α′ martensitic structure to a dual-phase α + β microstructure introduces compositional and electrochemical heterogeneity at the microstructural level. The coexistence of these two phases can lead to galvanic coupling, where the more anodic phase preferentially corrodes in the presence of an electrolyte. This galvanic interaction accelerates corrosion kinetics locally, promoting selective dissolution and contributing to the formation of pits and other localised defects. These findings reinforce the electrochemical results and highlight the detrimental impact of high-temperature heat treatments on corrosion resistance, driven by phase instability and microstructural heterogeneity in L-PBF Ti6Al4V alloys.
The findings of this study clearly demonstrate that while stress relieving heat treatments are essential for preserving the dimensional stability and preventing distortion in L-PBF processed Ti6Al4V components, they can influence the corrosion behaviour depending on the applied temperature. At moderate temperatures, a slight degradation in corrosion performance was observed, likely due to the coarsening of the metastable α′ martensitic phase. More critically, heat treatments at elevated temperatures (800 °C) led to a transformation from a single-phase α′ structure to a dual-phase α + β microstructure. This phase change was accompanied by a significant deterioration in passive layer stability and corrosion resistance, particularly under the combined effects of tensile loading and a fluoride-rich aggressive environment. Moreover, the reduction in residual stress at 800 °C was only marginal compared to that achieved at 600 °C, further questioning the benefit of high-temperature treatments.
Importantly, the use of the novel microcapillary electrochemical technique in this investigation provided enhanced resolution and sensitivity, enabling precise detection of subtle changes in passive film behaviour and corrosion kinetics across the various treatment conditions. This approach proved highly effective in identifying the electrochemical implications of microstructural and residual stress alterations, which may not be easily discernible using conventional methods.
Overall, this study presents a significant advancement in understanding the corrosion-performance relationship of L-PBF Ti6Al4V under post-processing conditions and establishes 600 °C as the optimum stress relieving temperature (from the heat treatment temperatures tested). This temperature offers an effective balance between residual stress reduction and minimal compromise in corrosion resistance, making it the most favourable condition for preserving the functional integrity of the alloy in demanding applications.
It is worth noting that the electrochemical behaviour was evaluated in a saliva solution containing a specific fluoride concentration, which may not fully replicate the complexity of physiological environments. Additionally, the mechanical testing under stress-assisted conditions was limited to a single tensile load magnitude, which does not capture the full range of stresses that implants may experience in vivo. Moreover, the electrochemical impedance and corrosion responses reported here reflect short-term behaviour, and do not account for long-term degradation mechanisms such as fatigue-assisted corrosion or biological interactions over extended implantation periods. Future work will aim to address these aspects to better approximate in vivo conditions and further validate the findings.
The identification of 600 °C as an optimized stress-relief temperature for L-PBF Ti-6Al-4V carries significant implications for critical engineering applications. Residual stresses introduced during the additive manufacturing process can lead to part distortion, reduced fatigue life, and dimensional inaccuracies, issues particularly detrimental in geometrically sensitive components such as dental implants or precision aerospace parts. Applying a well-calibrated stress-relief treatment at 600 °C effectively mitigates these macrostresses without compromising the alloy’s corrosion resistance. This balance is crucial for components exposed to aggressive physiological or atmospheric environments, where electrochemical stability determines longevity and safety. In the case of dental implants, minimizing residual stress helps maintain precise fit and osseointegration performance, while corrosion resistance ensures biocompatibility. Similarly, in aerospace components, the ability to reduce geometric warping and stress concentrations without degrading surface passivity contributes to longer service life and reduced maintenance intervals. Thus, this study offers actionable insight into thermal post-processing strategies that enhance both manufacturing reliability and in-service durability.
4. Conclusions
This study investigated the effect of low-temperature stress relieving heat treatments on the microstructure, residual stress, and corrosion performance of L-PBF processed Ti6Al4V alloy. A particular emphasis was placed on understanding the stability of the native oxide layer and the electrochemical response under fluoride-rich environments and mechanical strain, using advanced characterisation techniques including XRD, SEM, and a high-precision microcapillary electrochemical setup. The main findings and novelties of the study are summarised as follows:
A stress relieving temperature of 600 °C was identified as optimal among the heat treatment temperatures tested, providing effective residual stress reduction with minimal compromise in corrosion resistance.
Heat treatment at 800 °C resulted in the transformation of the metastable α′ martensitic phase to a dual-phase α + β microstructure, which significantly deteriorated the passive layer integrity and corrosion resistance, especially under tensile loading in fluoride-rich environments.
The single-phase α′ martensitic structure in as-built and lower-temperature-treated specimens offered superior passivation behaviour compared to the heterogeneous α + β structure, highlighting the critical role of microstructure in corrosion resistance.
Potentiostatic polarisation under constant strain revealed that higher heat treatment temperatures delayed passivation, produced unstable current responses, and increased passive current densities, confirming a reduction in passive film performance.
This investigation contributes to a deeper understanding of the interplay between heat treatment, microstructure, and electrochemical behaviour of L-PBF Ti6Al4V alloys, offering practical guidelines for selecting stress relieving parameters that maintain both mechanical integrity and corrosion resistance, especially in critical biomedical and aerospace applications.
Author Contributions
Conceptualization, R.I.R. and A.Y.; Methodology, A.Y.; Software, L.D.; Validation, R.I.R. and A.Y.; Formal analysis, F.C. and A.Y.; Investigation, L.D. and A.Y. and F.C.; Resources, K.B. and R.I.R.; Writing—original draft, L.D.; F.C., R.I.R. and A.Y.; Writing—review & editing, K.B., R.I.R. and A.Y.; Visualization, L.D.; Supervision, A.Y.; Project administration, K.B. and R.I.R.; Funding acquisition, K.B. and R.I.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by OSCARS project, European Commission’s Horizon Europe Research and Innovation programme grant number 101129751.
Data Availability Statement
The raw data required to reproduce these findings will be made publicly available upon final publication of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wong, K.V.; Hernandez, A. A Review of Additive Manufacturing. Int. Sch. Res. Not. 2012, 2012, 208760. [Google Scholar] [CrossRef]
- Dilberoglu, U.M.; Gharehpapagh, B.; Yaman, U.; Dolen, M. The Role of Additive Manufacturing in the Era of Industry 4.0. Procedia Manuf. 2017, 11, 545–554. [Google Scholar] [CrossRef]
- Abdulhameed, O.; Al-Ahmari, A.; Ameen, W.; Mian, S.H. Additive manufacturing: Challenges, trends, and applications. Adv. Mech. Eng. 2019, 11, 1687814018822880. [Google Scholar] [CrossRef]
- Ti-6al-4v Alloy—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/engineering/ti-6al-4v-alloy (accessed on 13 March 2025).
- Liu, S.; Shin, Y.C. Additive manufacturing of Ti6Al4V alloy: A review. Mater. Des. 2019, 164, 107552. [Google Scholar] [CrossRef]
- Buhairi, M.A.; Foudzi, F.M.; Jamhari, F.I.; Sulong, A.B.; Radzuan, N.A.M.; Muhamad, N.; Mohamed, I.F.; Azman, A.H.; Harun, W.S.W.; Al-Furjan, M.S.H. Review on volumetric energy density: Influence on morphology and mechanical properties of Ti6Al4V manufactured via laser powder bed fusion. Prog. Addit. Manuf. 2023, 8, 265–283. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.Y.; Fang, X.Y.; Guo, Y.B. Residual Stress in Metal Additive Manufacturing. Procedia CIRP 2018, 71, 348–353. [Google Scholar] [CrossRef]
- Jamhari, F.I.; Foudzi, F.M.; Buhairi, M.A.; Sulong, A.B.; Radzuan, N.A.M.; Muhamad, N.; Mohamed, I.F.; Jamadon, N.H.; Tan, K.S. Influence of heat treatment parameters on microstructure and mechanical performance of titanium alloy in LPBF: A brief review. J. Mater. Res. Technol. 2023, 24, 4091–4110. [Google Scholar] [CrossRef]
- Haubrich, J.; Gussone, J.; Barriobero-Vila, P.; Kürnsteiner, P.; Jägle, E.A.; Raabe, D.; Schell, N.; Requena, G. The role of lattice defects, element partitioning and intrinsic heat effects on the microstructure in selective laser melted Ti-6Al-4V. Acta Mater. 2019, 167, 136–148. [Google Scholar] [CrossRef]
- Syed, A.K.; Ahmad, B.; Guo, H.; Machry, T.; Eatock, D.; Meyer, J.; Fitzpatrick, M.E.; Zhang, X. An experimental study of residual stress and direction-dependence of fatigue crack growth behaviour in as-built and stress-relieved selective-laser-melted Ti6Al4V. Mater. Sci. Eng. A 2019, 755, 246–257. [Google Scholar] [CrossRef]
- Gaiani, S.; Ferrari, E.; Gozzi, M.; Di Giovanni, M.T.; Gualtieri, M.; Colombini, E.; Veronesi, P. Impact of Post-Process Heat Treatments Performed on Ti6Al4V Titanium Alloy Specimens Obtained Using LPBF Technology. Technologies 2023, 11, 100. [Google Scholar] [CrossRef]
- Pathania, A.; Subramaniyan, A.K.; Nagesha, B.K. Influence of post-heat treatments on microstructural and mechanical properties of LPBF-processed Ti6Al4V alloy. Prog. Addit. Manuf. 2022, 7, 1323–1343. [Google Scholar] [CrossRef]
- Bologna, O.; Cecchel, S.; Cornacchia, G.; Avanzini, A.; Sepe, R.; Berto, F.; Razavi, N. Investigating post-processing impact on fatigue performance of LPBF Ti6Al4V with heat treatment, high pressure heat treatment, and dry electropolishing strategies. Int. J. Fatigue 2024, 185, 108365. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Kermanpur, A.; Kharaziha, M. The effects of pore size and heat treatment on compression and corrosion behaviors of Ti–6Al–4V sheet-based gyroid implants fabricated by laser powder-bed fusion process. J. Mater. Res. Technol. 2023, 26, 7707–7721. [Google Scholar] [CrossRef]
- Sabban, R.; Bahl, S.; Chatterjee, K.; Suwas, S. Globularization using heat treatment in additively manufactured Ti-6Al-4V for high strength and toughness. Acta Mater. 2019, 162, 239–254. [Google Scholar] [CrossRef]
- Foudzi, F.M.; Sulong, A.B.; Muhamad, N.; Radzuan, N.A.M.; Mohamed, I.F.; Jamhari, F.I.; Buhairi, M.A.; Lin, N.H.; Hung, L.Y.; Chia, C.C.; et al. Effect of Heat Treatment on Hardness and Microstructure of Titanium Alloy (Ti6Al4V) via Laser Powder Bed Fusion (LPBF). In Proceedings of the Intelligent Manufacturing and Mechatronics, iM3F 2023, Pekan, Malaysia, 7–8 August 2023; Aziz, R.A., Ismail, Z., Iqbal, A.K.M.A., Ahmed, I., Eds.; Springer Nature: Singapore, 2024; pp. 469–478. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, Y.; Deng, W.; Li, J.; Chen, M.; Liu, S.; Li, W.; Liu, Y.; Ji, V. Effects of post-heat treatments on the microstructure and mechanical properties of Ti–6Al–4V alloy fabricated by selective laser melting. J. Mater. Res. Technol. 2024, 33, 1155–1164. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Capuzzo, N.; Khodabakhshi, M.; Dabalà, M. Evaluation of stress-assisted corrosion performance of L-PBF processed Ti6Al4V: A microcapillary electrochemical approach. Eng. Fail. Anal. 2024, 166, 108891. [Google Scholar] [CrossRef]
- Zhan, Z.; Zhang, Q.; Wang, S.; Liu, X.; Zhang, H.; Sun, Z.; Ge, Y.; Du, N. Comparison on the Electrochemical Corrosion Behavior of Ti6Al4V Alloys Fabricated by Laser Powder Bed Fusion and Casting. Materials 2024, 17, 3322. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, M.; Zhu, J.; Tu, J.; Jiao, S. Microstructure and Corrosion Resistance of Ti6Al4V Manufactured by Laser Powder Bed Fusion. Metals 2023, 13, 496. [Google Scholar] [CrossRef]
- Cabrini, M.; Carrozza, A.; Lorenzi, S.; Pastore, T.; Testa, C.; Manfredi, D.; Fino, P.; Scenini, F. Influence of surface finishing and heat treatments on the corrosion resistance of LPBF-produced Ti-6Al-4V alloy for biomedical applications. J. Mater. Process. Technol. 2022, 308, 117730. [Google Scholar] [CrossRef]
- Chiu, T.-M.; Mahmoudi, M.; Dai, W.; Elwany, A.; Liang, H.; Castaneda, H. Corrosion assessment of Ti-6Al-4V fabricated using laser powder-bed fusion additive manufacturing. Electrochim. Acta 2018, 279, 143–151. [Google Scholar] [CrossRef]
- Chen, X.; Liao, Q.; Gong, M.; Fu, Q. Corrosion Performances of Selective Laser Melting Ti6Al4V Alloy in Different Solutions. Metals 2023, 13, 192. [Google Scholar] [CrossRef]
- Zhang, H.; Man, C.; Dong, C.; Wang, L.; Li, W.; Kong, D.; Wang, L.; Wang, X. The corrosion behavior of Ti6Al4V fabricated by selective laser melting in the artificial saliva with different fluoride concentrations and pH values. Corros. Sci. 2021, 179, 109097. [Google Scholar] [CrossRef]
- Seo, D.-I.; Lee, J.-B. Influence of Heat Treatment Parameters on the Corrosion Resistance of Additively Manufactured Ti–6Al–4V Alloy. J. Electrochem. Soc. 2020, 167, 101509. [Google Scholar] [CrossRef]
- Rani, S.U.; Thampi, V.V.A.; Kesavan, D.; Ramanathan, S.; Kamaraj, M. An integrated investigation of the effect of sub-transus treatment on the microstructure and corrosion behaviour of the LPBF Ti–6Al–4V alloy. Mater. Chem. Phys. 2024, 322, 129555. [Google Scholar] [CrossRef]
- Yeo, I.; Bae, S.; Amanov, A.; Jeong, S. Effect of Laser Shock Peening on Properties of Heat-Treated Ti–6Al–4V Manufactured by Laser Powder Bed Fusion. Int. J. Precis. Eng. Manuf.-Green Technol. 2021, 8, 1137–1150. [Google Scholar] [CrossRef]
- Cecchel, S.; Ferrario, D.; Cornacchia, G.; Gelfi, M. Correction to [Development of Heat Treatments for Selective Laser Melting Ti6Al4V Alloy: Effect on Microstructure, Mechanical Properties, and Corrosion Resistance]. Adv. Eng. Mater. 2024, 26, 2400901. [Google Scholar] [CrossRef]
- Cecchel, S.; Ferrario, D.; Cornacchia, G.; Gelfi, M. Development of Heat Treatments for Selective Laser Melting Ti6Al4V Alloy: Effect on Microstructure, Mechanical Properties, and Corrosion Resistance. Adv. Eng. Mater. 2020, 22, 2000359. [Google Scholar] [CrossRef]
- Ettefagh, A.H.; Zeng, C.; Guo, S.; Raush, J. Corrosion behavior of additively manufactured Ti-6Al-4V parts and the effect of post annealing. Addit. Manuf. 2019, 28, 252–258. [Google Scholar] [CrossRef]
- Fitzpatrick, M.; Fry, A.; Holdway, P.; Kandil, F.; Shackleton, J.; Suominen, L. Determination of Residual Stresses by X-ray Diffraction. In A National Measurement Good Practice Guide No. 52; National Physical Laboratory (NPL): Middlesex, UK, 2002. [Google Scholar]
- Yazdanpanah, A.; Pietri, A.D.; Hjal, A.B.; Khodabakhshi, M.; Biasiolo, L.; Dabalà, M. Electrochemical and localized corrosion characteristics of kolsterised and DLC-coated 316LVM stainless steel for biomedical applications. Appl. Surf. Sci. 2025, 693, 162808. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Revilla, R.I.; Franceschi, M.; Pagot, G.; Khodabakhshi, M.; De Graeve, I.; Di Noto, V.; Dabalà, M.; Lozano-Perez, S. Exploring the mechanism of stress-induced passive layer degradation in additively manufactured Ni-Fe-Cr-based alloy 718. Corros. Sci. 2024, 241, 112523. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Pagot, G.; Franceschi, M.; Rebesan, P.; Venturin, M.; Botinha, J.; Gerhmann, B.; De Graeve, I.; Noto, V.D.; Revilla, R.I.; et al. Mechanism of alteration in passivity of additively manufactured Ni-Fe-Cr Alloy 718 caused by minor carbon variation. Electrochim. Acta 2024, 503, 144925. [Google Scholar] [CrossRef]
- Gasparrini, C.; Douglas, J.O.; Yazdanpanah, A.; Stroud, R.; Divitini, G.; Dabalà, M.; Scatigno, G.G.; Pedrazzini, S.; Wenman, M.R.; Badocco, D.; et al. Corrosion of 316L exposed to highly concentrated borated water used as shield in nuclear fusion experimental reactors cooling circuits. Corros. Sci. 2024, 230, 111902. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Revilla, R.I.; Franceschi, M.; Fabrizi, A.; Khademzadeh, S.; Khodabakhshi, M.; De Graeve, I.; Dabalà, M. Unveiling the impact of laser power variations on microstructure, corrosion, and stress-assisted surface crack initiation in laser powder bed fusion-processed Ni-Fe-Cr alloy 718. Electrochim. Acta 2024, 476, 143723. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Revilla, R.; Graeve, I.D.; Dabalà, M. Correlation of Microstructural Events with Stress Corrosion Cracking Initiation Behaviour in Additively Manufactured Ni-Based Alloy 718: Microcapillary Electrochemical Technique Implementation. ECS Meet. Abstr. 2023, MA2023-02, 1057. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Franceschi, M.; Pagot, G.; Khodabakhshi, M.; Fassinato, E.; Zotta, L.; Hanemann, T.; Shaikh, A.S.; Di Noto, V.; De Graeve, I.; et al. Passivity and breakdown mechanisms in laser powder bed fusion processed Ni-based Alloy 625: Influence of scan strategy. Corros. Sci. 2025, 255, 113129. [Google Scholar] [CrossRef]
- Newman, J.; Balsara, N.P. Electrochemical Systems; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Heakal, F.; Shehata, O. Insight into the Electrochemical and Semiconducting Properties of Native Oxide Films on Ti Metal and Its Ti–6Al–4V Alloy in Borate Buffer Solutions. Prot. Met. Phys. Chem. Surf. 2020, 56, 333–342. [Google Scholar] [CrossRef]
- Duffo, G.; Castillo, E. Development of an Artificial Saliva Solution for Studying the Corrosion Behavior of Dental Alloys. Corrosion 2004, 60, 594–602. [Google Scholar] [CrossRef]
- Robin, A.; Meirelis, J.P. Influence of fluoride concentration and pH on corrosion behavior of Ti-6Al-4V and Ti-23Ta alloys in artificial saliva. Mater. Corros. 2007, 58, 173–180. [Google Scholar] [CrossRef]
- Jones, R.H. Stress-Corrosion Cracking. In Corrosion: Fundamentals, Testing, and Protection; ASM International: Almere, The Netherlands, 2003. [Google Scholar] [CrossRef]
- Ni, C.; Zhu, J.; Zhang, B.; An, K.; Wang, Y.; Liu, D.; Lu, W.; Zhu, L.; Liu, C. Recent advance in laser powder bed fusion of Ti–6Al–4V alloys: Microstructure, mechanical properties and machinability. Virtual Phys. Prototyp. 2025, 20, e2446952. [Google Scholar] [CrossRef]
- Cao, S.; Chen, Z.; Lim, C.V.S.; Yang, K.; Jia, Q.; Jarvis, T.; Tomus, D.; Wu, X. Defect, Microstructure, and Mechanical Property of Ti-6Al-4V Alloy Fabricated by High-Power Selective Laser Melting. JOM 2017, 69, 2684–2692. [Google Scholar] [CrossRef]
- Krakhmalev, P.; Fredriksson, G.; Yadroitsava, I.; Kazantseva, N.; Plessis, A.D.; Yadroitsev, I. Deformation Behavior and Microstructure of Ti6Al4V Manufactured by SLM. Phys. Procedia 2016, 83, 778–788. [Google Scholar] [CrossRef]
- Song, Z.; Liu, Y.; Wang, J.; Zhu, G.; Wang, L.; Zeng, X.; Knezevic, M. Advanced phenomenological models guided heat treating processes for LPBF Ti-6Al-4V alloy. Mater. Today Commun. 2025, 42, 111186. [Google Scholar] [CrossRef]
- Zhang, W.; Xing, L.; Zhang, S.; Wang, K.; Chen, J.; Chen, J.; Fang, G.; Liu, W. The reverse transformation mechanism of β phase and its stability of Ti-6Al-4V alloy fabricated via laser powder bed fusion. Mater. Des. 2024, 241, 112926. [Google Scholar] [CrossRef]
- Joshi, V.A. Titanium Alloys: An Atlas of Structures and Fracture Featuresl; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Sallica-Leva, E.; Caram, R.; Jardini, A.L.; Fogagnolo, J.B. Ductility improvement due to martensite α′ decomposition in porous Ti–6Al–4V parts produced by selective laser melting for orthopedic implants. J. Mech. Behav. Biomed. Mater. 2016, 54, 149–158. [Google Scholar] [CrossRef]
- Xu, W.; Lui, E.W.; Pateras, A.; Qian, M.; Brandt, M. In situ tailoring microstructure in additively manufactured Ti-6Al-4V for superior mechanical performance. Acta Mater. 2017, 125, 390–400. [Google Scholar] [CrossRef]
- Xie, D.; Lv, F.; Yang, Y.; Shen, L.; Tian, Z.; Shuai, C.; Chen, B.; Zhao, J. A Review on Distortion and Residual Stress in Additive Manufacturing. Chin. J. Mech. Eng. Addit. Manuf. Front. 2022, 1, 100039. [Google Scholar] [CrossRef]
- Megahed, M.; Mindt, H.-W.; N’Dri, N.; Duan, H.; Desmaison, O. Metal additive-manufacturing process and residual stress modeling. Integr. Mater. Manuf. Innov. 2016, 5, 61–93. [Google Scholar] [CrossRef]
- Mansfeld, F.; Shih, H.; Greene, H.; Tsai, C.H. Analysis of EIS Data for Common Corrosion Processes. In Electrochemical Impedance: Analysis and Interpretation; ASTM Selected Technical Papers; ASM International: Almere, The Netherlands, 1993. [Google Scholar] [CrossRef]
- Boudinar, Y.; Belmokre, K.; Touzet, M.; Devos, O.; Puiggali, M. Investigation of the passivation process of plastically deformed 316L stainless steel using the high frequency capacitance obtained by EIS. Mater. Corros. 2019, 70, 206–215. [Google Scholar] [CrossRef]
- Zuo, Y.; Pang, R.; Li, W.; Xiong, J.P.; Tang, Y.M. The evaluation of coating performance by the variations of phase angles in middle and high frequency domains of EIS. Corros. Sci. 2008, 50, 3322–3328. [Google Scholar] [CrossRef]
- Martinez, S.; Hudec, B.; Šoić, I. Low-frequency EIS interpretation with the potential to predict the durability of protective coatings. Prog. Org. Coat. 2024, 197, 108811. [Google Scholar] [CrossRef]
- Ibriş, N.; Rosca, J.C.M. EIS study of Ti and its alloys in biological media. J. Electroanal. Chem. 2002, 526, 53–62. [Google Scholar] [CrossRef]
- Sofuoğlu, M.A.; Haydarlar, G.; Sheikhi, M.R.; Hopali, C.; Tekkalmaz, M. Detection of residual stresses with impedance spectroscopy: A novel approach. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2024, 09544089241285084. [Google Scholar] [CrossRef]
- Yuan, X.-Z.; Song, C.; Wang, H.; Zhang, J. (Eds.) EIS Equivalent Circuits. In Electrochemical Impedance Spectroscopy in PEM Fuel Cells; Springer: London, UK, 2010; pp. 139–192. [Google Scholar] [CrossRef]
- Yuan, X.-Z.; Song, C.; Wang, H.; Zhang, J. (Eds.) Impedance and its Corresponding Electrochemical Processes. In Electrochemical Impedance Spectroscopy in PEM Fuel Cells; Springer: London, UK, 2010; pp. 95–138. [Google Scholar] [CrossRef]
- Orazem, M.E. Measurement model for analysis of electrochemical impedance data. J. Solid State Electrochem. 2024, 28, 1273–1289. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. An integrated approach to electrochemical impedance spectroscopy. Electrochim. Acta 2008, 53, 7360–7366. [Google Scholar] [CrossRef]
- Ruiz, A.; Hernández, H.; Hernández, J.; Orozco-Cruz, R.; Reynoso, A.R.; González, C.; Miranda-Hernández, J. Electrochemical Impedance Spectroscopy (EIS): A Review Study of Basic Aspects of the Corrosion Mechanism Applied to Steels; Springer: London, UK, 2020. [Google Scholar] [CrossRef]
- Orazem, M.E.; Frateur, I.; Tribollet, B.; Vivier, V.; Marcelin, S.; Pébère, N.; Bunge, A.L.; White, E.A.; Riemer, D.P.; Musiani, M. Dielectric Properties of Materials Showing Constant-Phase-Element (CPE) Impedance Response. J. Electrochem. Soc. 2013, 160, C215. [Google Scholar] [CrossRef]
- Aslam, R. Chapter 2—Potentiodynamic polarization methods for corrosion measurement. In Electrochemical and Analytical Techniques for Sustainable Corrosion Monitoring; Aslam, J., Verma, C., Hussain, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 25–37. [Google Scholar] [CrossRef]
- Medjedovic, E.; Medjedovic, S.; Deljo, D.; Sukalo, A. Impact of Fluoride on Dental Health Quality. Mater. Socio-Medica 2015, 27, 395–398. [Google Scholar] [CrossRef]
- Schiff, N.; Grosgogeat, B.; Lissac, M.; Dalard, F. Influence of fluoride content and pH on the corrosion resistance of titanium and its alloys. Biomaterials 2002, 23, 1995–2002. [Google Scholar] [CrossRef]
- Licausi, M.P.; Muñoz, A.I.; Borrás, V.A. Influence of the fabrication process and fluoride content on the tribocorrosion behaviour of Ti6Al4V biomedical alloy in artificial saliva. J. Mech. Behav. Biomed. Mater. 2013, 20, 137–148. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).