Grain Boundary Engineering for Reversible Zn Anodes in Rechargeable Aqueous Zn-Ion Batteries
Abstract
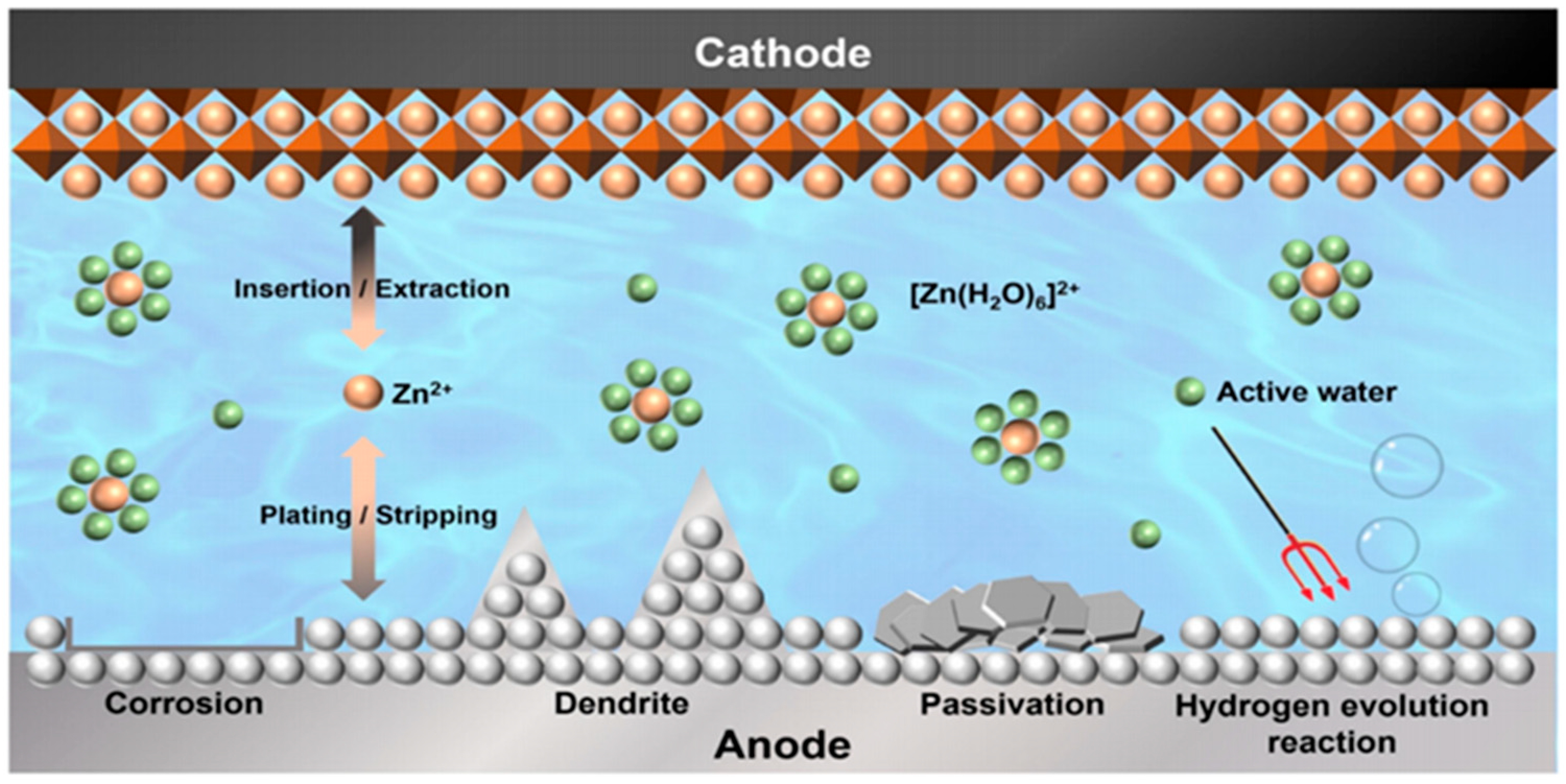
1. Introduction
2. Fundamentals of Grain Boundary Engineering
2.1. Basic Concepts of Grain Boundaries
2.2. Overview of Grain Boundary Engineering
2.3. Strategies and Methods of Grain Boundary Engineering
3. Application of Grain Boundary Engineering in Zinc Anodes
3.1. Grain Boundary Engineering in Pure Zinc Electrodes
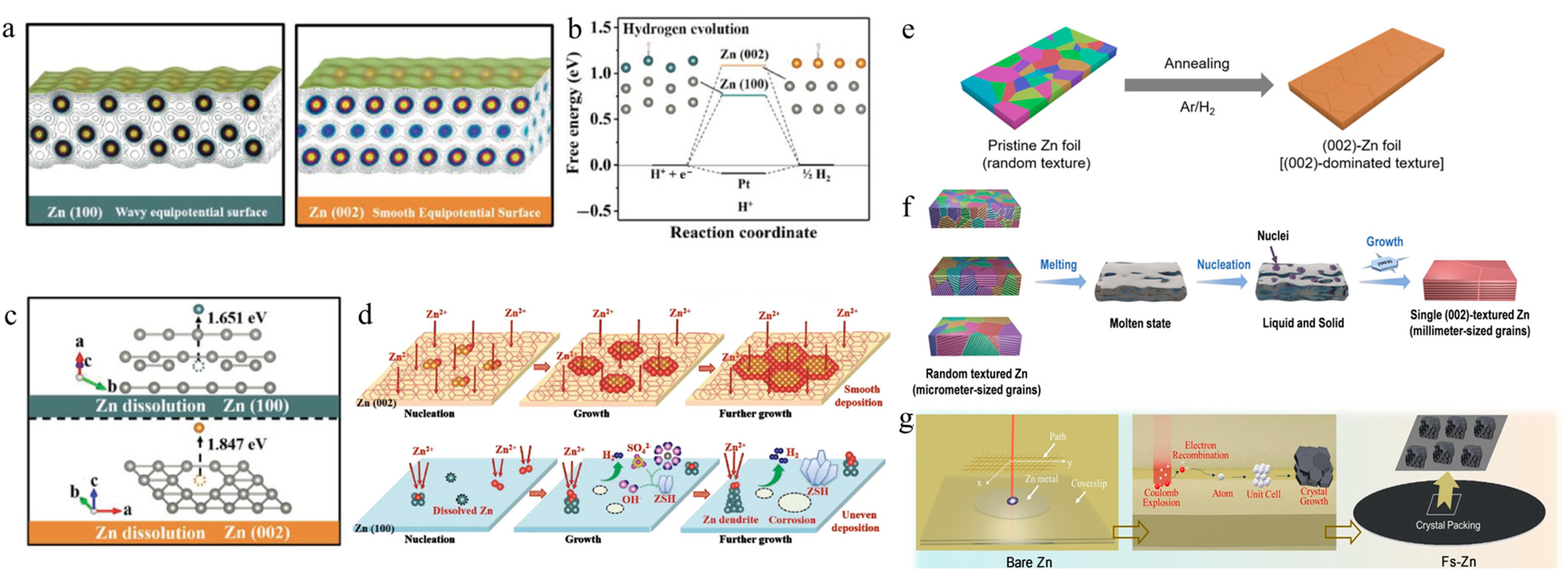
3.2. Application of Grain Boundary Engineering in Zinc Alloy Electrodes
3.3. Synergistic Integration of Grain Boundary Engineering with Multi-Factor Strategies
| Strategy | Methodology | Key Performance Metrics | Effectiveness Evaluation | Refs. |
|---|---|---|---|---|
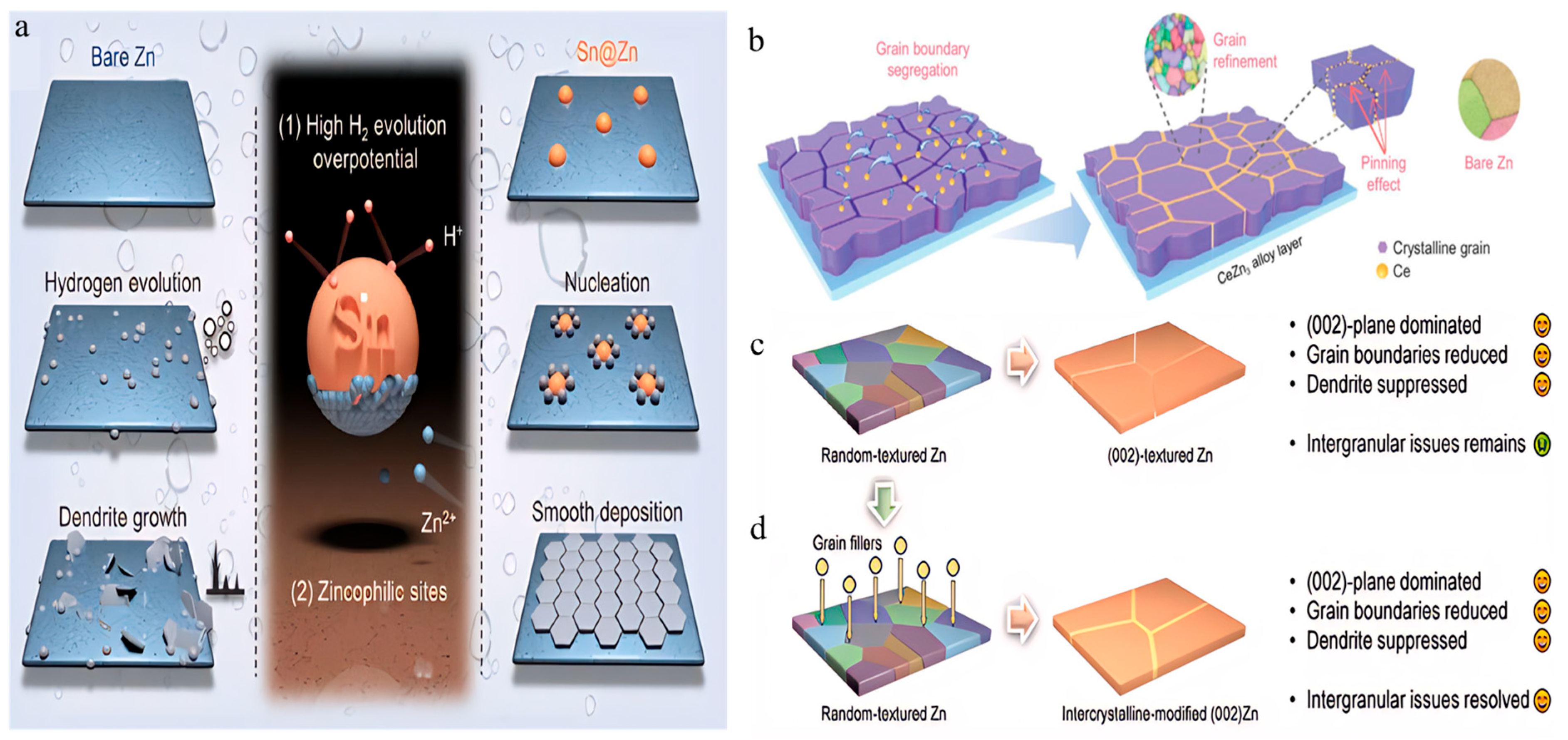
| Grain Boundary Engineering in Pure Zinc Electrodes | Annealing commercial Zn foil at 300 °C for 60 min under 5% Ar/H2 atmosphere. | Symmetric cell cycling: >7000 cycles; Full-cell capacity retention: 92.7% (MnO2 cathode); Coulombic efficiency: 99.9%. | Effectively suppresses dendrite growth and HER corrosion, significantly enhancing cycling stability. | [68] |
| Melting Zn foil at 600 °C for 1 min followed by natural cooling. | Symmetric cell cycling: 3280 h (1 mA cm−2); High-rate performance: 830 h (10 mA cm−2); 75% DOD cycling: 180 h. | Reduces grain boundary density, mitigates side reactions, and improves deep cycling capability. | [70] | |
| Mechanical grinding to control crystallographic orientation. | 75.2% DOD cycling: 250 h (symmetric cell); Full-cell capacity retention: 80% (450 cycles). | Low-cost, scalable method achieving high reversibility. | [73] | |
| Bridgman method to grow single-crystalline Zn with [0001] orientation. | Symmetric cell cycling: >2800 h (1 mA cm−2); - Coulombic efficiency: 99.92% (500 cycles). | Perfect atomic arrangement inhibits dendrites and side reactions, outperforming polycrystalline Zn. | [74] | |
| Coulomb explosion via femtosecond laser (500 fs pulse width) to create UP-GBs. | Cumulative plating capacity: 6.5 Ah cm−2 (vs. 1.2 Ah cm−2 for commercial foil); Stable cycling: 0.548 Ah cm−2 (1 mA cm−2). | Provides abundant nucleation sites for uniform deposition, effectively suppressing dendrites. | [75] | |
| Grain Boundary Engineering in Zinc Alloy Electrodes | Zn-Ti Alloy (TiZn16 at GBs): Melting Zn with 0.5 wt% Ti to form TiZn16 intermetallic compounds at GBs. | Symmetric cell cycling: 1100 h (2 mA cm−2); Full-cell capacity retention: 85% (500 cycles). | Reduces GB reactivity, suppresses corrosion, and promotes uniform nucleation. | [82] |
| Zn-Sn Alloy (Sn@Zn): Chemical displacement to create Sn-based heterogeneous nucleation sites on Zn surfaces | Symmetric cell cycling: 1500 h (1 mA cm−2); Full-cell capacity: 212 mAh g−1 (5 A g−1). | Sn increases HER overpotential, prevents battery swelling, and enables planar deposition. | [83] | |
| Zn-Ce Alloy (Ce Doping): Electrodeposition-assisted Zener pinning to introduce Ce, forming URAL. | Symmetric cell cycling: 4000 h (2 mA cm−2); Full-cell capacity retention: 96% (6000 cycles). | Ce refines grains to ~1 μm, suppresses HER, and enhances cycling stability. | [84] | |
| Zn-In Alloy (In Infiltration): Thermal treatment at 450 °C to infiltrate liquid In into (002)-textured Zn GBs. | DFT calculations confirm high Zn adsorption energy on In, enabling uniform nucleation. | In stabilizes GBs against corrosion and dendrite formation. | [85] | |
| Ga-In-Zn Ternary Alloy Coating: Phase diagram-guided design of liquid alloy coating. | Extends cycling life and improves anode stability. | Synergistically optimizes GB and surface properties for enhanced performance. | [86] | |
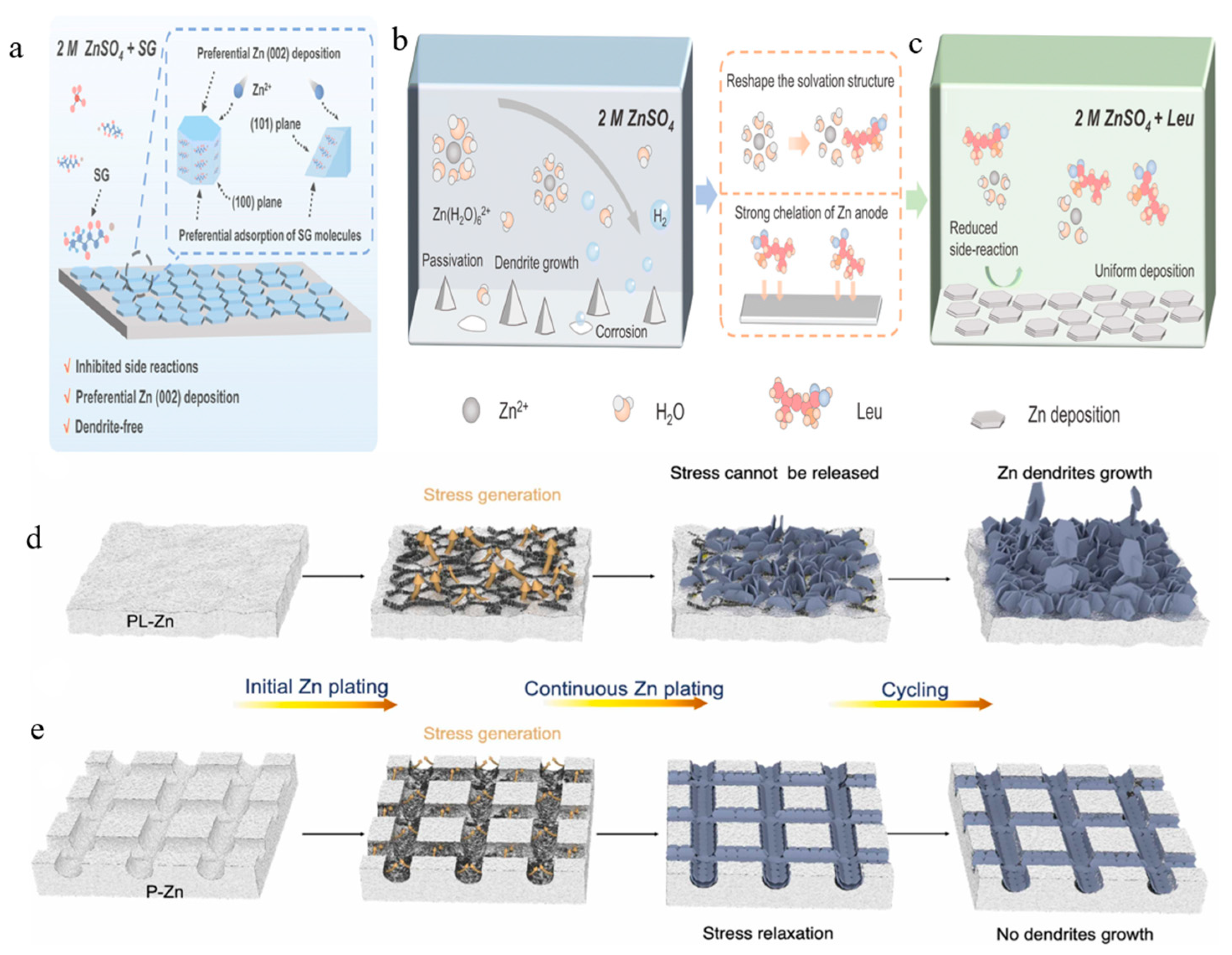
| Multi-Factor Coupling Strategies | Electrolyte Additive: Adding SG (20 mM) to 2 M ZnSO4 to induce (002)-oriented deposition. | Symmetric cell cycling: 2000 h (1 mA cm−2); Full-cell capacity retention: 95% (2000 cycles). | SG selectively passivates non-(002) facets, suppressing dendrites while maintaining high-rate capability. | [87] |
| Electrolyte Additive: Adding Leu (20 mM) to 2 M ZnSO4 to inhibit side reactions. | No ZHS byproducts detected post-cycling; High Coulombic efficiency. | Leu preferentially adsorbs on high-energy facets, reducing corrosion and passivation. | [88] | |
| Coating Zn anode with PVA layer. | Cycling life: >5000 h; Enhanced corrosion resistance. | PVA isolates Zn from electrolyte while promoting (002)-oriented deposition. | [89] | |
| Fabricating microgrooves (30 μm width/25 μm depth) via mesh-assisted calendaring, followed by Nafion coating. | Full-cell capacity: 186 mAh g−1 (MnO2 cathode); Excellent flexibility. | Microgrooves relieve plating stress; Nafion minimizes electrolyte contact, suitable for flexible devices. | [90] |
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H. Progress and perspectives of flow battery technologies. Curr. Opin. Electrochem. 2019, 18, 123–125. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, J.; Wang, X.; Chen, L.; Cao, G.; Wang, J.; Zhang, H.; He, X. Insights for understanding multiscale degradation of LiFePO4 cathodes. eScience 2022, 2, 125–137. [Google Scholar] [CrossRef]
- Lu, W.; Li, X.; Zhang, H. The next generation vanadium flow batteries with high power density—A perspective. Phys. Chem. Chem. Phys. 2017, 20, 23–35. [Google Scholar] [CrossRef]
- Al-Amin, M.; Islam, S.; Shibly, S.U.A.; Iffat, S. Comparative Review on the Aqueous Zinc-Ion Batteries (AZIBs) and Flexible Zinc-Ion Batteries (FZIBs). Nanomaterials 2022, 12, 3997. [Google Scholar] [CrossRef]
- Li, C.; Jin, S.; Archer, L.A.; Nazar, L.F. Toward practical aqueous zinc-ion batteries for electrochemical energy storage. Joule 2022, 6, 1733–1738. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Y.; Gao, J.; Guo, Z.; Chen, A.; Liu, N.; Lu, X.; Zhang, X.; Zhang, N. Zincophilic Design and the Electrode/Electrolyte Interface for Aqueous Zinc-Ion Batteries: A Review. Batter. Supercaps 2023, 6, e202200478. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, R.; Lu, Q.; Liu, P.; Hu, L.; Zhang, K.; Bai, P.; Xu, R.; Cao, X.; Sun, Z.; et al. Recent progress and perspectives on highly utilized Zn metal anode-towards marketable aqueous Zn-ion batteries. Energy Storage Mater. 2024, 72, 103689. [Google Scholar] [CrossRef]
- Al-Abbasi, M.; Zhao, Y.; He, H.; Liu, H.; Xia, H.; Zhu, T.; Wang, K.; Xu, Z.; Wang, H.; Zhang, W.; et al. Challenges and protective strategies on zinc anode toward practical aqueous zinc-ion batteries. Carbon. Neutralization 2024, 3, 108–141. [Google Scholar] [CrossRef]
- Xie, W.; Zhu, K.; Jiang, W.; Yang, H.; Ma, M.; Zhao, L.; Yang, W. Highly 002-Oriented Dendrite-Free Anode Achieved by Enhanced Interfacial Electrostatic Adsorption for Aqueous Zinc-Ion Batteries. ACS Nano 2024, 18, 21184–21197. [Google Scholar] [CrossRef]
- Dai, H.; Sun, T.; Zhou, J.; Wang, J.; Chen, Z.; Zhang, G.; Sun, S. Unraveling chemical origins of dendrite formation in zinc-ion batteries via in situ/operando X-ray spectroscopy and imaging. Nat. Commun. 2024, 15, 8577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mao, L.; Xia, Z.; Fan, M.; Deng, Y.; Chen, Z. Zincophilic Design for Highly Stable and Dendrite-Free Zinc Metal Anodes in Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2025, 35, 2412547. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.-P.; Fu, N.; Zhou, W.-B.; Liu, B.; Deng, Q.; Wu, X.-W. Comprehensive review on zinc-ion battery anode: Challenges and strategies. InfoMat 2022, 4, e12306. [Google Scholar] [CrossRef]
- Hu, Y.; Du, H.; Lu, J.; Li, Z.; Ma, Y.; Song, S. Achieving dendrite-free and long cyclic stability of zinc anode via CeO2 doped PEO interface modification. Fuel 2023, 354, 129417. [Google Scholar] [CrossRef]
- Li, F.; Zhang, H.; Liu, X.; Xu, B.; Wang, C. Dendrite-Free Zn Anode Modified by Organic Coating for Stable Aqueous Zinc Ion Batteries. Batteries 2024, 10, 420. [Google Scholar] [CrossRef]
- Zhang, Z.; Said, S.; Smith, K.; Zhang, Y.S.; He, G.; Jervis, R.; Shearing, P.R.; Miller, T.S.; Brett, D.J.L. Dendrite suppression by anode polishing in zinc-ion batteries. J. Mater. Chem. A 2021, 9, 15355–15362. [Google Scholar] [CrossRef]
- Gao, Q.; He, W.; Liu, C.; You, Y.; Zhang, P.; Liu, L.; Xu, G.; Gong, K.; Zhang, A.; Sun, Z. Dendrite-Free Zinc Anodes via a Three-Dimensional Ti2AlC Coating for High-Performance Zinc-Ion Batteries. ACS Appl. Energy Mater. 2025, 8, 1526–1534. [Google Scholar] [CrossRef]
- Das, A.; Balakrishnan, N.T.M.; Sreeram, P.; Fatima, M.J.J.; Joyner, J.D.; Thakur, V.K.; Pullanchiyodan, A.; Ahn, J.-H.; Raghavan, P. Prospects for magnesium ion batteries: A compreshensive materials review. Coord. Chem. Rev. 2024, 502, 215593. [Google Scholar] [CrossRef]
- Das, S.K.; Mahapatra, S.; Lahan, H. Aluminium-ion batteries: Developments and challenges. J. Mater. Chem. A 2017, 5, 6347–6367. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.-G.; Liu, H.; You, Z.; Wei, C.; Kang, F. Electrochemical activation of commercial MnO microsized particles for high-performance aqueous zinc-ion batteries. J. Power Sources 2019, 438, 226951. [Google Scholar] [CrossRef]
- Zhang, C.; Holoubek, J.; Wu, X.; Daniyar, A.; Zhu, L.; Chen, C.; Leonard, D.P.; Rodríguez-Pérez, I.A.; Jiang, J.-X.; Fang, C.; et al. A ZnCl2 water-in-salt electrolyte for a reversible Zn metal anode. Chem. Commun. 2018, 54, 14097–14099. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-H.; Liu, H.-B. Research Progress of Zinc Anode Materials for Aqueous Zinc Ion Recharge Battery. Chin. J. Inorg. Chem. 2019, 35, 1999–2012. [Google Scholar] [CrossRef]
- Chen, L.-N.; Yan, M.-Y.; Mei, Z.-W.; Mai, L.-Q. Research Progress and Prospect of Aqueous Zinc Ion Battery. J. Inorg. Mater. 2017, 32, 225–234. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Wang, H.; Cui, F.; Zhu, G. Integrated pyrazine-based porous aromatic frameworks/carbon nanotube composite as cathode materials for aqueous zinc ion batteries. Chem. Eng. J. 2022, 450, 138051. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.; Tang, Y.; Ji, X.; Wang, H. Interfacial Design of Dendrite-Free Zinc Anodes for Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 13180–13191. [Google Scholar] [CrossRef]
- Yufit, V.; Tariq, F.; Eastwood, D.S.; Biton, M.; Wu, B.; Lee, P.D.; Brandon, N.P. Operando Visualization and Multi-scale Tomography Studies of Dendrite Formation and Dissolution in Zinc Batteries. Joule 2019, 3, 485–502. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, X.; Luo, M.; Cao, K.; Lu, Y.; Xu, B.B.; Pan, H.; Tao, K.; Jiang, Y. Amino Acid-Induced Interface Charge Engineering Enables Highly Reversible Zn Anode. Adv. Funct. Mater. 2021, 31, 2103514. [Google Scholar] [CrossRef]
- Luo, C.; Yuan, H. Measurement and modeling of deformation-induced martensitic transformation in a metastable austenitic stainless steel under cyclic loadings. Acta Mater. 2022, 238, 118202. [Google Scholar] [CrossRef]
- Feng, Z.; Feng, Y.; Fan, F.; Deng, D.; Dong, H.; Liu, S.; Kang, L.; Jun, S.C.; Wang, L.; Zhu, J.; et al. Functionalization design of zinc anode for advanced aqueous zinc-ion batteries. SusMat 2024, 4, e184. [Google Scholar] [CrossRef]
- Sajjadnejad, M.; Ghorbani, M.; Afshar, A. Microstructure-corrosion resistance relationship of direct and pulse current electrodeposited Zn-TiO2 nanocomposite coatings. Ceram. Int. 2015, 41, 217–224. [Google Scholar] [CrossRef]
- Jia, X.; Liu, C.; Neale, Z.G.; Yang, J.; Cao, G. Active Materials for Aqueous Zinc Ion Batteries: Synthesis, Crystal Structure, Morphology, and Electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.Y.; Chang, W.J.; Jang, S.; Kim, M.; Kim, Y.; Kim, B.; Park, W.I. Controlling dendrite growth and side reactions in anode-free Zn-ion aqueous batteries with PMMA:Zn coated electrode. J. Energy Storage 2024, 76, 109791. [Google Scholar] [CrossRef]
- Cao, Z.; Zhuang, P.; Zhang, X.; Ye, M.; Shen, J.; Ajayan, P.M. Strategies for Dendrite-Free Anode in Aqueous Rechargeable Zinc Ion Batteries. Adv. Energy Mater. 2020, 10, 2001599. [Google Scholar] [CrossRef]
- Shi, P.; Fu, Z.-H.; Zhou, M.-Y.; Chen, X.; Yao, N.; Hou, L.-P.; Zhao, C.-Z.; Li, B.-Q.; Huang, J.-Q.; Zhang, X.-Q.; et al. Inhibiting intercrystalline reactions of anode with electrolytes for long-cycling lithium batteries. Sci. Adv. 2022, 8, eabq3445. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Ko, S.-T.; Dawson, A.; Agyeman-Budu, D.; Whang, G.; Zhao, Y.; Qin, M.; Dunn, B.S.; Weker, J.N.; Tolbert, S.H.; et al. Thermodynamics-driven interfacial engineering of alloy-type anode materials. Cell Rep. Phys. Sci. 2022, 3, 100694. [Google Scholar] [CrossRef]
- Yule, L.C.; Shkirskiy, V.; Aarons, J.; West, G.; Bentley, C.L.; Shollock, B.A.; Unwin, P.R. Nanoscale Active Sites for the Hydrogen Evolution Reaction on Low Carbon Steel. J. Phys. Chem. C 2019, 123, 24146–24155. [Google Scholar] [CrossRef]
- Huang, S.; Wu, W.; Su, Y.; Qiao, L.; Yan, Y. Insight into the corrosion behaviour and degradation mechanism of pure zinc in simulated body fluid. Corros. Sci. 2021, 178, 109071. [Google Scholar] [CrossRef]
- Cai, Z.; Ou, Y.; Zhang, B.; Wang, J.; Fu, L.; Wan, M.; Li, G.; Wang, W.; Wang, L.; Jiang, J.; et al. A Replacement Reaction Enabled Interdigitated Metal/Solid Electrolyte Architecture for Battery Cycling at 20 mA cm−2 and 20 mAh cm−2. J. Am. Chem. Soc. 2021, 143, 3143–3152. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kobayashi, R.; Watanabe, T. Control of grain boundary connectivity based on fractal analysis for improvement of intergranular corrosion resistance in SUS316L austenitic stainless steel. Acta Mater. 2016, 102, 397–405. [Google Scholar] [CrossRef]
- Shi, P.; Hu, R.; Zhang, T.; Yuan, L.; Li, J. Grain boundary character distribution and its effect on corrosion of Ni-23Cr-16Mo superalloy. Mater. Sci. Technol. 2017, 33, 84–91. [Google Scholar] [CrossRef]
- Liu, T.; Xia, S.; Bai, Q.; Zhou, B.; Zhang, L.; Lu, Y.; Shoji, T. Three-dimensional study of grain boundary engineering effects on intergranular stress corrosion cracking of 316 stainless steel in high temperature water. J. Nucl. Mater. 2018, 498, 290–299. [Google Scholar] [CrossRef]
- Stratulat, A.; Duff, J.A.; Marrow, T.J. Grain boundary structure and intergranular stress corrosion crack initiation in high temperature water of a thermally sensitised austenitic stainless steel, observed in situ. Corros. Sci. 2014, 85, 428–435. [Google Scholar] [CrossRef]
- Lehockey, E.M.; Palumbo, G. On the creep behaviour of grain boundary engineered nickel. Mater. Sci. Eng.-Struct. Mater. Prop. Microstruct. Process. 1997, 237, 168–172. [Google Scholar] [CrossRef]
- Xu, M.; Chen, J.; Lu, H.; Xu, J.; Yu, C.; Wei, X. Effects of residual stress and grain boundary character on creep cracking in 2.25Cr-1.6W steel. Mater. Sci. Eng.-Struct. Mater. Prop. Microstruct. Process. 2016, 659, 188–197. [Google Scholar] [CrossRef]
- Watanabe, T. A new era of grain boundary design and control for high temperature materials. In Creep and Fracture of Engineering Materials and Structures; Key Engineering Materials; Sakuma, T., Yagi, K., Eds.; Transtec Publications Ltd.: Zurich, Switzerland, 2000; pp. 237–244. [Google Scholar]
- Watanabe, T. Grain boundary engineering: Historical perspective and future prospects. J. Mater. Sci. 2011, 46, 4095–4115. [Google Scholar] [CrossRef]
- Lejček, P.; Paidar, V. Challenges of interfacial classification for grain boundary engineering. Mater. Sci. Technol. 2005, 21, 393–398. [Google Scholar] [CrossRef]
- Shimada, M.; Kokawa, H.; Wang, Z.J.; Sato, Y.S.; Karibe, I. Optimization of grain boundary character distribution for intergranular corrosion resistant 304 stainless steel by twin-induced grain boundary engineering. Acta Mater. 2002, 50, 2331–2341. [Google Scholar] [CrossRef]
- Singh, R.; Chowdhury, S.G.; Kumar, B.R.; Das, S.K.; De, P.K.; Chattoraj, I. The importance of grain size relative to grain boundary character on the sensitization of metastable austenitic stainless steel. Scr. Mater. 2007, 57, 185–188. [Google Scholar] [CrossRef]
- Kurban, M.; Erb, U.; Aust, K.T. A grain boundary characterization study of boron segregation and carbide precipitation in alloy 304 austenitic stainless steel. Scr. Mater. 2006, 54, 1053–1058. [Google Scholar] [CrossRef]
- Schwartz, A.J.; King, W.E.; Kumar, M. Influence of processing method on the network of grain boundaries. Scr. Mater. 2006, 54, 963–968. [Google Scholar] [CrossRef]
- Randle, V.; Coleman, M. A study of low-strain and medium-strain grain boundary engineering. Acta Mater. 2009, 57, 3410–3421. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Li, D.; Guo, S. Relationships Between the Σ9 and Σ27 Boundaries and the Connectivity of Random Boundary in Hastelloy C-276 Alloy. Rare Met. Mater. Eng. 2018, 47, 2318–2321. [Google Scholar]
- Huo, R.; Du, Z.; Cheng, J.; Sun, B.; Gong, T.; Du, X. The Influence of Deformation and Heat Treatment on the Grain Boundary Character Distribution in CoCrFeNi High-Entropy Alloy. J. Mater. Eng. Perform. 2025, 1–12. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Sun, N.; Sun, Y.; Shan, A. Effects of prior deformation and annealing process on microstructure and annealing twin density in a nickel based alloy. Mater. Charact. 2014, 95, 299–306. [Google Scholar] [CrossRef]
- Huang, W.; Chai, L.; Li, Z.; Yang, X.; Guo, N.; Song, B. Evolution of microstructure and grain boundary character distribution of a tin bronze annealed at different temperatures. Mater. Charact. 2016, 114, 204–210. [Google Scholar] [CrossRef]
- Tokita, S.; Kokawa, H.; Sato, Y.S.; Fujii, H.T. In situ EBSD observation of grain boundary character distribution evolution during thermomechanical process used for grain boundary engineering of 304 austenitic stainless steel. Mater. Charact. 2017, 131, 31–38. [Google Scholar] [CrossRef]
- Zhang, J.X.; Zhang, J.Y.; Xiao, W.C.; Hou, J.X.; Li, Q.; Xiao, B.; Yang, S.Y.; Wu, J.L.; Zhang, T.L.; Yang, T. Grain refinement induced unusually large shape memory effect in lightweight titanium alloy. Acta Mater. 2024, 272, 119936. [Google Scholar] [CrossRef]
- Shi, F.; Tian, P.C.; Jia, N.; Ye, Z.H.; Qi, Y.; Liu, C.M.; Li, X.W. Improving intergranular corrosion resistance in a nickel-free and manganese-bearing high-nitrogen austenitic stainless steel through grain boundary character distribution optimization. Corros. Sci. 2016, 107, 49–59. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, F.; Liu, L.; Li, Q.; Liu, L.; Liu, F.; Huang, C. Effect of grain size and distribution on the corrosion behavior of Y2O3 dispersion-strengthened 304 stainless steel. Mater. Today Commun. 2022, 31, 103723. [Google Scholar] [CrossRef]
- Randle, V.; Jones, R. Grain boundary plane distributions and single-step versus multiple-step grain boundary engineering. Mater. Sci. Eng.-Struct. Mater. Prop. Microstruct. Process. 2009, 524, 134–142. [Google Scholar] [CrossRef]
- Seita, M.; Gao, S. Broadening the design space of engineering materials through “additive grain boundary engineering”. J. Mater. Sci. 2022, 57, 9530–9540. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, Q.; Tang, T.; Yin, J.; Quilty, C.D.; Renderos, G.D.; Liu, X.; Deng, Y.; Wang, L.; Bock, D.C.; et al. Reversible epitaxial electrodeposition of metals in battery anodes. Science 2019, 366, 645–648. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, S.; Li, J.; Luo, X.; Liu, Z.; Zhang, T.; Cao, X.; Long, M.; Lu, B.; Pan, A.; et al. Surface-Preferred Crystal Plane for a Stable and Reversible Zinc Anode. Adv. Mater. 2021, 33, 2100187. [Google Scholar] [CrossRef]
- Wen, Q.; Fu, H.; Cui, R.; Chen, H.-Z.; Ji, R.-H.; Tang, L.-B.; Yan, C.; Mao, J.; Dai, K.-H.; Zhang, X.-H.; et al. Recent advances in interfacial modification of zinc anode for aqueous rechargeable zinc ion batteries. J. Energy Chem. 2023, 83, 287–303. [Google Scholar] [CrossRef]
- Zhang, Z.; Xia, S.; Dong, A.; Li, X.; Wang, F.; Yang, J.; Ruan, J.; Li, Q.; Sun, D.; Fang, F.; et al. Mechanical Grinding Formation of Highly Reversible (002)-Textured Zinc Metal Anodes. Adv. Energy Mater. 2025, 15, 2403598. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhong, X.; Zhang, Q.; Zhang, M.; Dai, L.; Xiao, X.; Xu, J.; Jiao, M.; Wang, B.; Li, H.; et al. An extended substrate screening strategy enabling a low lattice mismatch for highly reversible zinc anodes. Nat. Commun. 2024, 15, 753. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Cai, Z.; Zhan, R.; Wang, W.; Fu, L.; Wan, M.; Xiao, R.; Ou, Y.; Wang, L.; et al. Stable interphase chemistry of textured Zn anode for rechargeable aqueous batteries. Sci. Bull. 2022, 67, 716–724. [Google Scholar] [CrossRef]
- Yi, Z.; Chen, G.; Hou, F.; Wang, L.; Liang, J. Strategies for the Stabilization of Zn Metal Anodes for Zn-Ion Batteries. Adv. Energy Mater. 2021, 11, 2003065. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Q.; Han, X.; Wang, C.; Chen, J.; Hu, T.; He, Q.; Zhu, X.; Yuan, D.; Chen, J.; et al. Converting Commercial Zn Foils into Single (002)-Textured Zn with Millimeter-Sized Grains for Highly Reversible Aqueous Zinc Batteries. Angew. Chem.-Int. Ed. 2024, 63, e202401507. [Google Scholar] [CrossRef]
- Yu, X.; Li, Z.; Wu, X.; Zhang, H.; Zhao, Q.; Liang, H.; Wang, H.; Chao, D.; Wang, F.; Qiao, Y.; et al. Ten concerns of Zn metal anode for rechargeable aqueous zinc batteries. Joule 2023, 7, 1145–1175. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, J.; He, Q.; Li, M.; Feng, S.; Wang, Y.; Yuan, D.; Chen, J.; Alshareef, H.N.; Ma, Y. Texture Control of Commercial Zn Foils Prolongs Their Reversibility as Aqueous Battery Anodes. ACS Energy Lett. 2022, 7, 3564–3571. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Liu, Y.; Lu, B.; Liang, S.; Zhou, J. Single [0001]-oriented zinc metal anode enables sustainable zinc batteries. Nat. Commun. 2024, 15, 2735. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Wei, T.; Zhang, S.; Liu, H.; Li, C.; Li, Z.; Yang, M.; Liu, C.; Pei, Y. Single-Crystalline Zn(002) Facet Enables Ultrastable Anode–Electrolyte Interface. Small Struct. 2025, 6, 2400325. [Google Scholar] [CrossRef]
- Lian, S.; Cai, Z.; Yan, M.; Sun, C.; Chai, N.; Zhang, B.; Yu, K.; Xu, M.; Zhu, J.; Pan, X.; et al. Ultra-High Proportion of Grain Boundaries in Zinc Metal Anode Spontaneously Inhibiting Dendrites Growth. Angew. Chem.-Int. Ed. 2024, 63, e202406292. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Liang, Z.; Wang, W.; Dong, D.; Lu, Y.-C. A nuclei-rich strategy for highly reversible dendrite-free zinc metal anodes. Energy Environ. Sci. 2023, 16, 6026–6034. [Google Scholar] [CrossRef]
- Li, Y.; Wu, P.; Zhong, W.; Xie, C.; Xie, Y.; Zhang, Q.; Sun, D.; Tang, Y.; Wang, H. A progressive nucleation mechanism enables stable zinc stripping–plating behavior. Energy Environ. Sci. 2021, 14, 5563–5571. [Google Scholar] [CrossRef]
- Zhou, L.-F.; Du, T.; Li, J.-Y.; Wang, Y.-S.; Gong, H.; Yang, Q.-R.; Chen, H.; Luo, W.-B.; Wang, J.-Z. A strategy for anode modification for future zinc-based battery application. Mater. Horiz. 2022, 9, 2722–2751. [Google Scholar] [CrossRef]
- Huang, W.; Huang, Y.; Huang, X.; Shao, F.; Liu, W.; Kang, F. 3D Leaf-Like Copper-Zinc Alloy Enables Dendrite-Free Zinc Anode for Ultra-Long Life Aqueous Zinc Batteries. Small 2024, 20, 2404294. [Google Scholar] [CrossRef]
- Li, J.; Liang, H.; Long, Y.; Yu, X.; Li, J.; Li, N.; Han, J.; Wang, J.; Yang, Z. Dual-function protective layer for highly reversible Zn anode. J. Energy Chem. 2024, 98, 12–23. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, J.; Ren, H.; Chen, Y.; Chua, R.; Jie, E.T.J.; Cai, Y.; Edison, E.; Manalastas, W.; Wong, M.W.; et al. Anion Texturing Towards Dendrite-Free Zn Anode for Aqueous Rechargeable Batteries. Angew. Chem. 2021, 60, 7213–7219. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, S.; Chen, M.; Lu, B.; Zhang, X.; Liang, S.; Zhou, J. Tailoring grain boundary stability of zinc-titanium alloy for long-lasting aqueous zinc batteries. Nat. Commun. 2023, 14, 7080. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Han, S.; Zhou, P.; Lu, B.; Zhou, J.; Zeng, Z.; Chen, Z.; Zhou, J. Hetero Nucleus Growth Stabilizing Zinc Anode for High-Biosecurity Zinc-Ion Batteries. Nano-Micro Lett. 2023, 15, 237. [Google Scholar] [CrossRef]
- Chen, M.; Gong, Y.; Zhao, Y.; Song, Y.; Tang, Y.; Zeng, Z.; Liang, S.; Zhou, P.; Lu, B.; Zhang, X.; et al. Spontaneous grain refinement effect of rare earth zinc alloy anodes enables stable zinc batteries. Natl. Sci. Rev. 2024, 11, nwae205. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Wu, Q.; Wang, C.; He, Q.; Hu, T.; Han, X.; Chen, J.; Zhang, Y.; Chen, J.; et al. Grain Boundary Filling Empowers (002)-Textured Zn Metal Anodes with Superior Stability. Adv. Mater. 2024, 36, 2411004. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Luo, Z.; Deng, W.; Wei, W.; Chen, L.; Pan, A.; Ma, J.; Wang, C.; Zhu, L.; Xie, L.; et al. Liquid Alloy Interlayer for Aqueous Zinc-Ion Battery. ACS Energy Lett. 2021, 6, 675–683. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Ruan, P.; Hu, X.; Lu, B.; Yuan, X.; Tian, S.; Zhou, J. Inducing preferential growth of the Zn. (002) plane by using a multifunctional chelator for achieving highly reversible Zn anodes. Nanoscale 2024, 16, 2923–2930. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Z.; Ruan, P.; Hu, X.; Yuan, X.; Lu, B.; Qin, L.; Zhou, J. Guiding uniform Zn deposition with a multifunctional additive for highly utilized Zn anodes. Nanoscale 2024, 16, 18835–18842. [Google Scholar] [CrossRef]
- Chen, X.; Li, W.; Hu, S.; Akhmedov, N.G.; Reed, D.; Li, X.; Liu, X. Polyvinyl alcohol coating induced preferred crystallographic orientation in aqueous zinc battery anodes. Nano Energy 2022, 98, 107269. [Google Scholar] [CrossRef]
- Chen, J.; Qiao, X.; Han, X.; Zhang, J.; Wu, H.; He, Q.; Chen, Z.; Shi, L.; Wang, Y.; Xie, Y.; et al. Releasing plating-induced stress for highly reversible aqueous Zn metal anodes. Nano Energy 2022, 103, 107814. [Google Scholar] [CrossRef]


| Properties | AZIBs | LIBs |
|---|---|---|
| Energy Density | 50–135 Wh/kg | 160 Wh/kg |
| Safety | Excellent: Non-flammable aqueous electrolyte; no dendritic explosion risks | Poor: Flammable organic electrolyte; lithium dendrites may trigger thermal runaway |
| Cost | Low: Abundant zinc resources | High: Dependent on scarce resources (e.g., lithium, cobalt) |
| Cycle Life | Moderate: 100–1500 cycles | Long: 3000 cycles |
| Environmental Friendliness | High: Non-toxic components | Low: Pollution risks from organic solvents |
| Rate Capability | Superior: Fast Zn2+ diffusion enables rapid charging | Moderate: Li+ mobility limited by organic electrolytes |
| Key Challenges | Zinc dendrites, hydrogen evolution reaction (HER), side reactions | Lithium dendrites, thermal runaway, electrolyte decomposition |
| Properties | Zn | Li | Na | Mg | Al |
|---|---|---|---|---|---|
| Ionic radius (Å) | 0.74 (Zn2+) | 0.76 (Li+) | 1.02 (Na+) | 0.72 (Mg2+) | 0.54 (Al3+) |
| Volumetric capacity (mAh/cm3) | 5855 | 2046 | 1129 | 3833 | 8046 |
| Electrode potential (V vs. SHE) | −0.76 | −3.04 | −2.71 | −2.37 | −1.66 |
| Battery system | Aqueous zinc-ion batteries | Organic lithium-ion batteries | Sodium-ion batteries | Magnesium-ion batteries | Aluminum-ion batteries |
| Key advantages | Safe with low cost and excellent rate capability | Energy-dense with low self-discharge and mature technology | Resource-abundant with cost-effectiveness and good low-temperature performance | Divalent-enabled with dendrite-free nature and high volumetric energy density | Trivalent-advantaged with corrosion-resistant and lightweight properties |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-X.; Wang, J.-Z.; Cao, L.; Wang, H.; Cheng, Z.-Y.; Zhou, L.-F.; Du, T. Grain Boundary Engineering for Reversible Zn Anodes in Rechargeable Aqueous Zn-Ion Batteries. Metals 2025, 15, 784. https://doi.org/10.3390/met15070784
Liu Y-X, Wang J-Z, Cao L, Wang H, Cheng Z-Y, Zhou L-F, Du T. Grain Boundary Engineering for Reversible Zn Anodes in Rechargeable Aqueous Zn-Ion Batteries. Metals. 2025; 15(7):784. https://doi.org/10.3390/met15070784
Chicago/Turabian StyleLiu, Yu-Xuan, Jun-Zhe Wang, Lei Cao, Hao Wang, Zhen-Yu Cheng, Li-Feng Zhou, and Tao Du. 2025. "Grain Boundary Engineering for Reversible Zn Anodes in Rechargeable Aqueous Zn-Ion Batteries" Metals 15, no. 7: 784. https://doi.org/10.3390/met15070784
APA StyleLiu, Y.-X., Wang, J.-Z., Cao, L., Wang, H., Cheng, Z.-Y., Zhou, L.-F., & Du, T. (2025). Grain Boundary Engineering for Reversible Zn Anodes in Rechargeable Aqueous Zn-Ion Batteries. Metals, 15(7), 784. https://doi.org/10.3390/met15070784






