Highly Selective Recovery of Pt(IV) from HCl Solutions by Precipitation Using 1,4-Bis(aminomethyl)cyclohexane as a Precipitating Agent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Metal Precipitation Experiments from Metal-Containing HCl Solutions
2.3. Purification of Pt(IV)-Containing Precipitate
2.4. Metal Precipitation from HCl Leachate of Spent Catalysts
2.5. Reduction of Pt(IV)-Containing Precipitate
2.6. Single Crystal Preparation
2.7. Measurements
3. Results
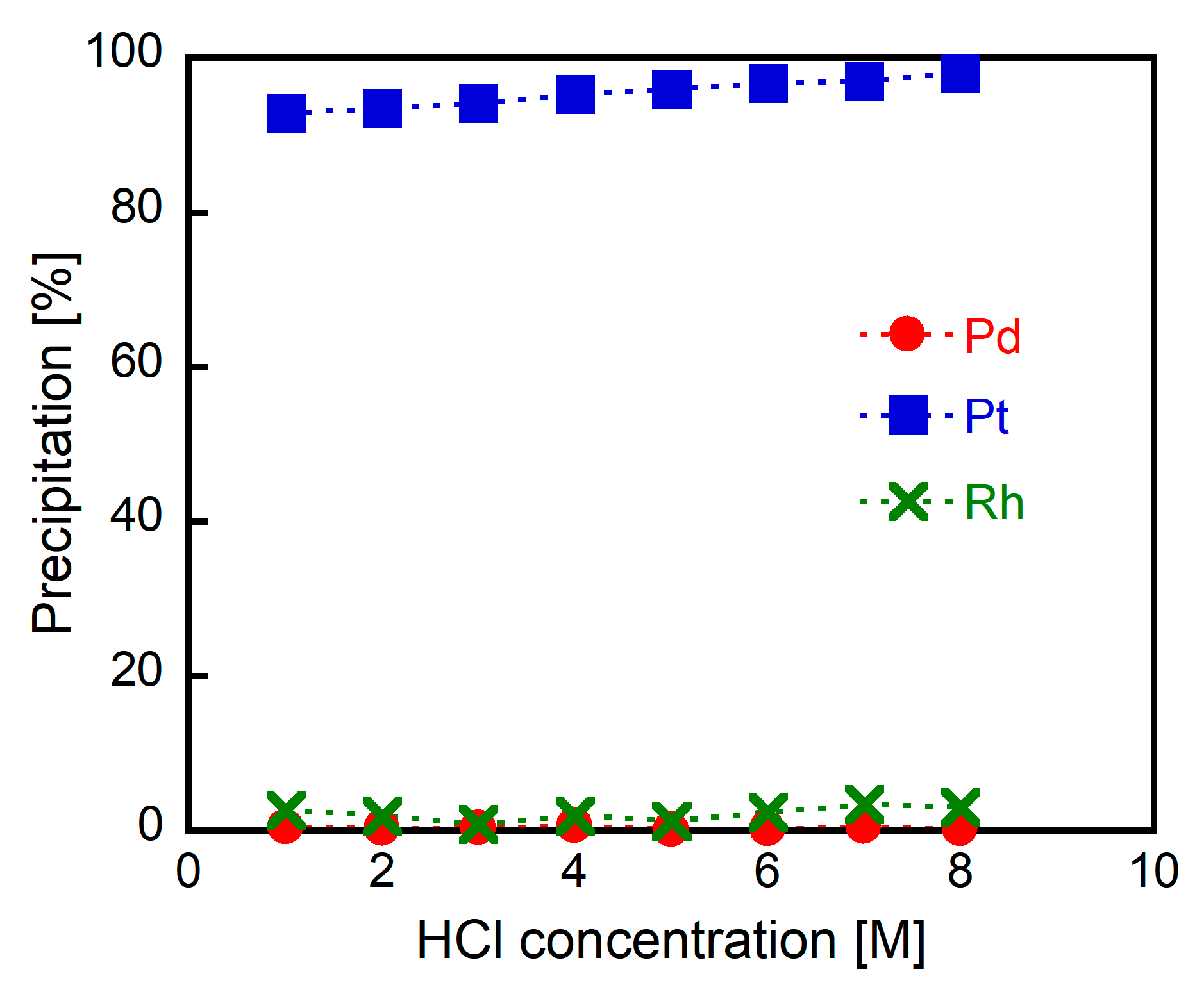
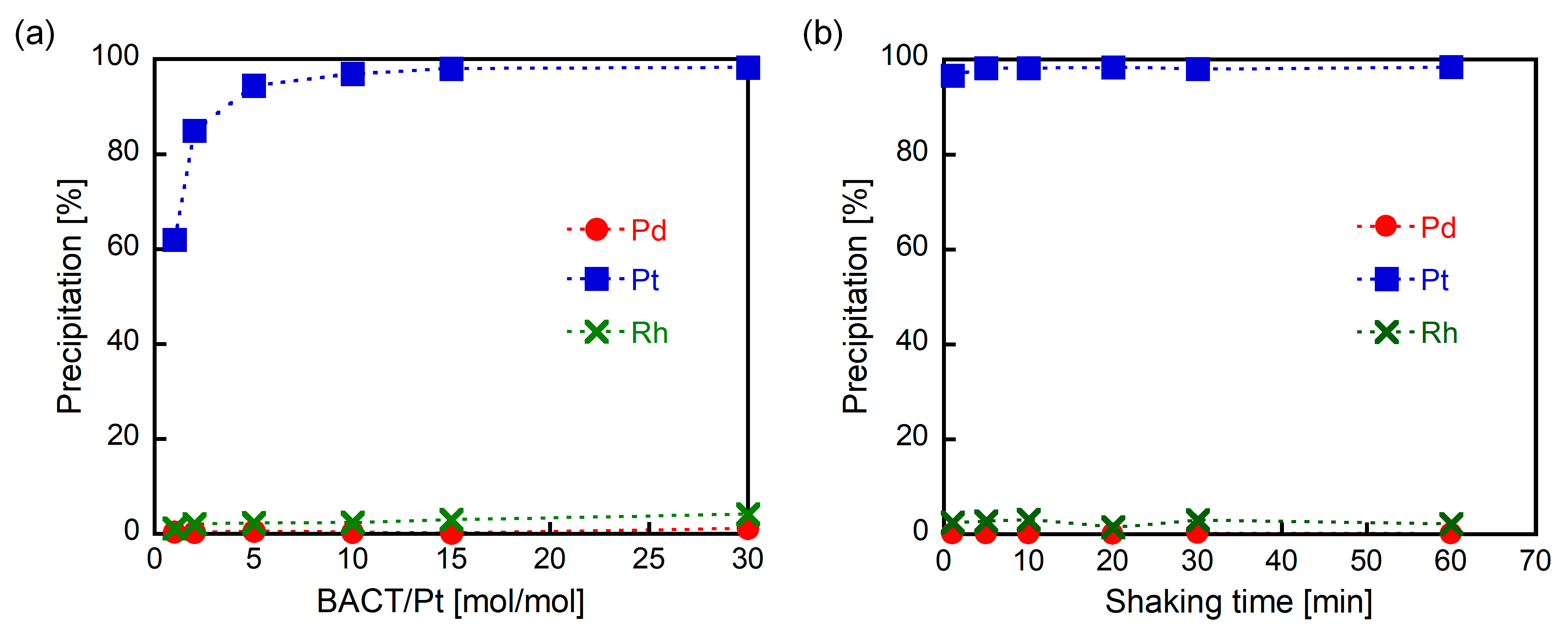
3.1. Precipitation Experiments Using BACT
3.2. Purification of Pt(IV)-Contaning Precipitate
3.3. Selective Precipitation of Pt(IV) from HCl Leachate of Spent Catalysts
3.4. Reduction of Pt(IV)-Containing Precipitate
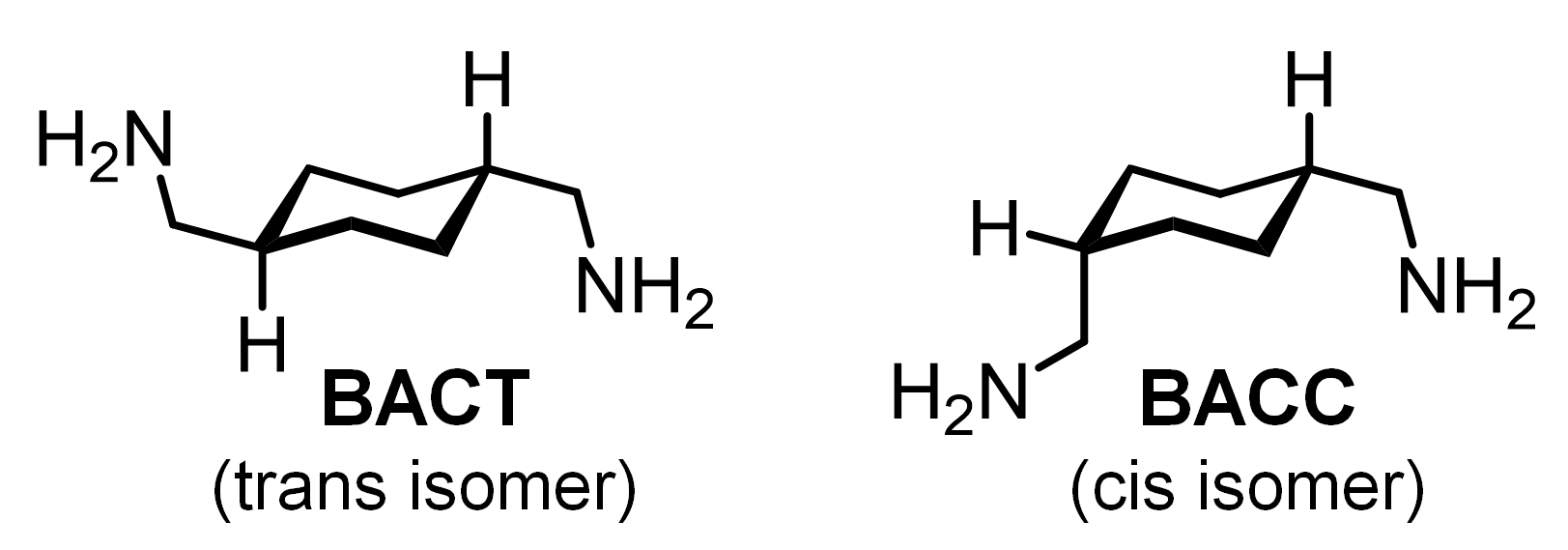
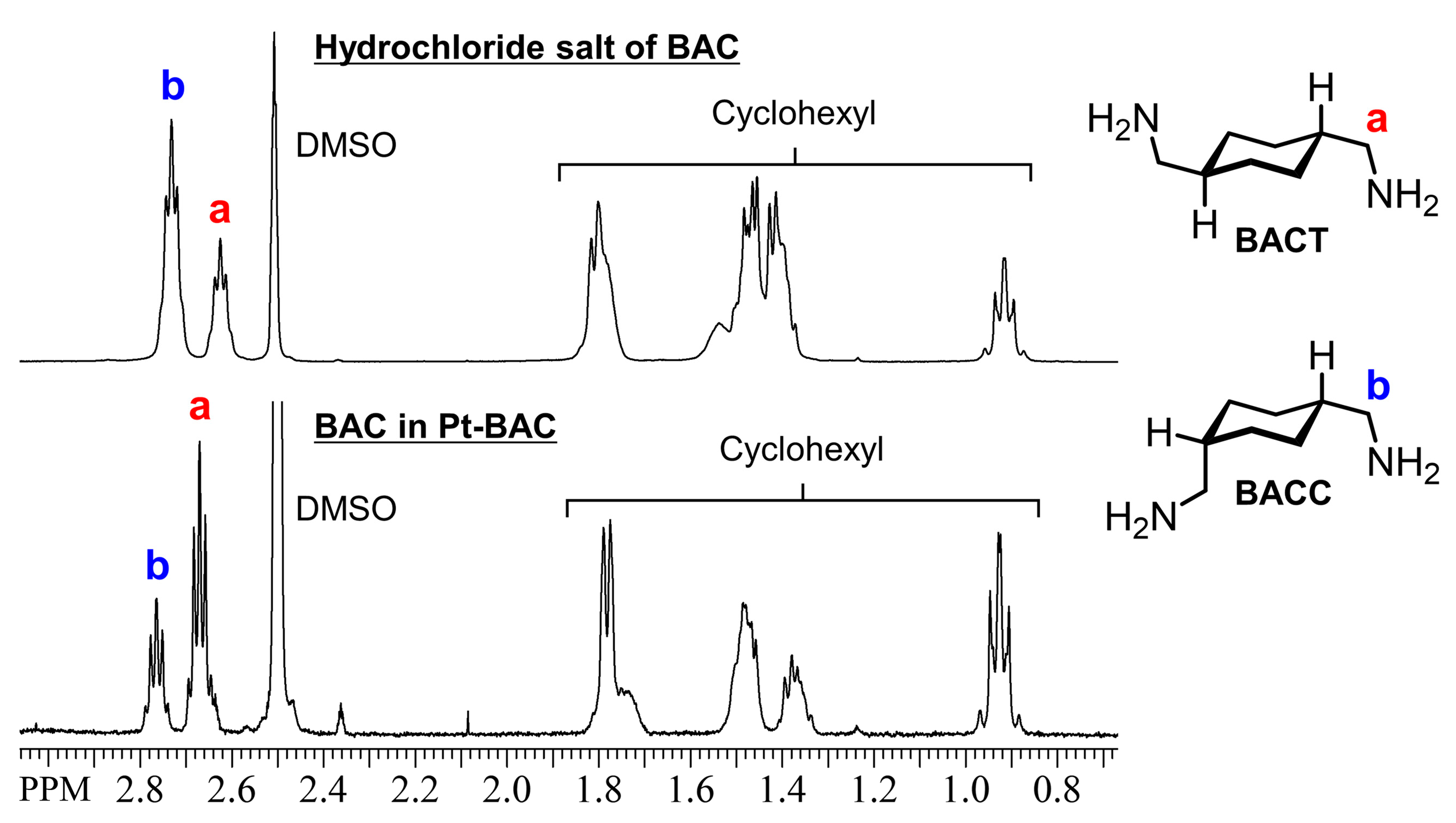
3.5. Effect of Isomers of Precipitating Agent on Metal Precipitation
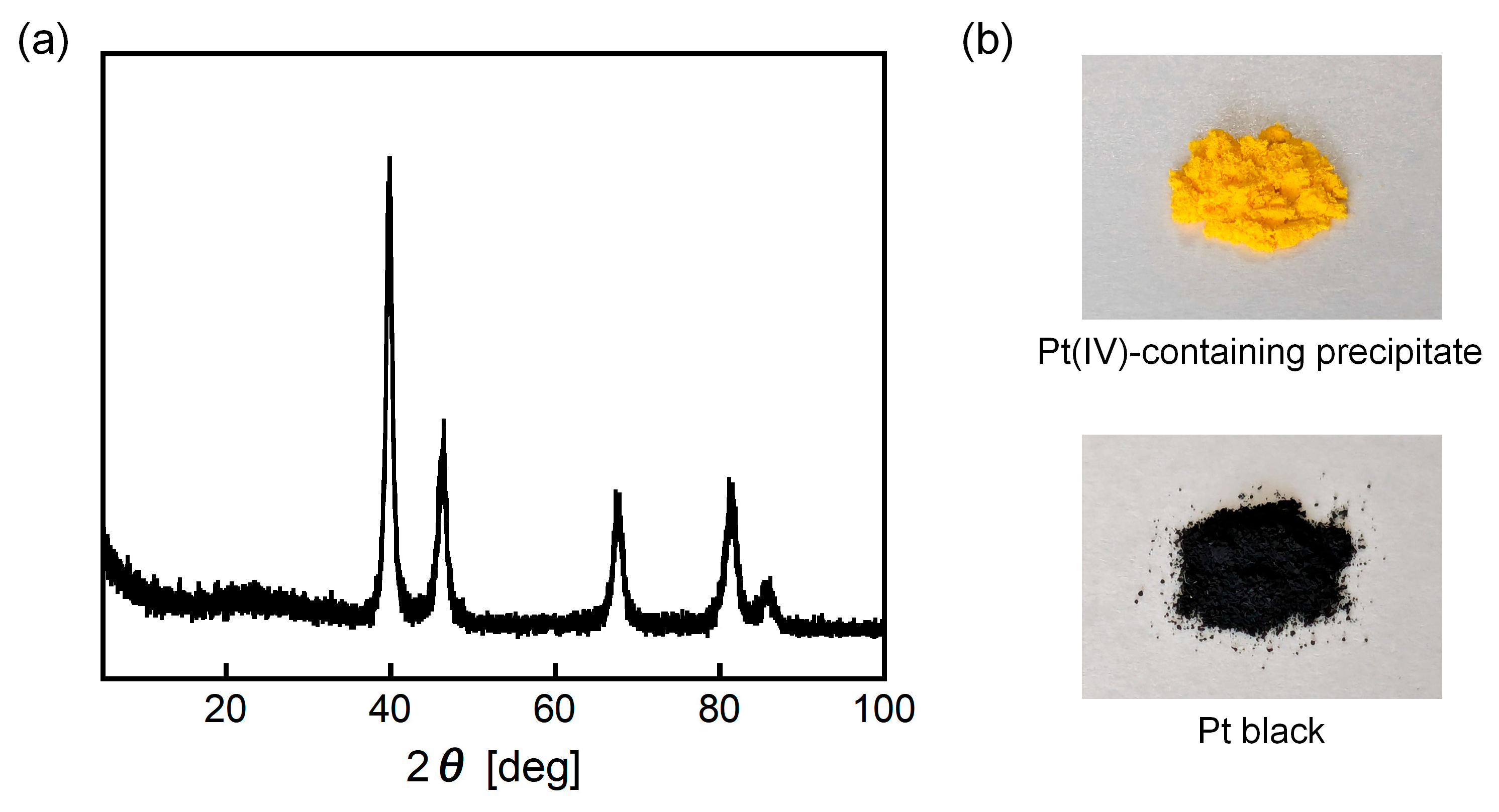
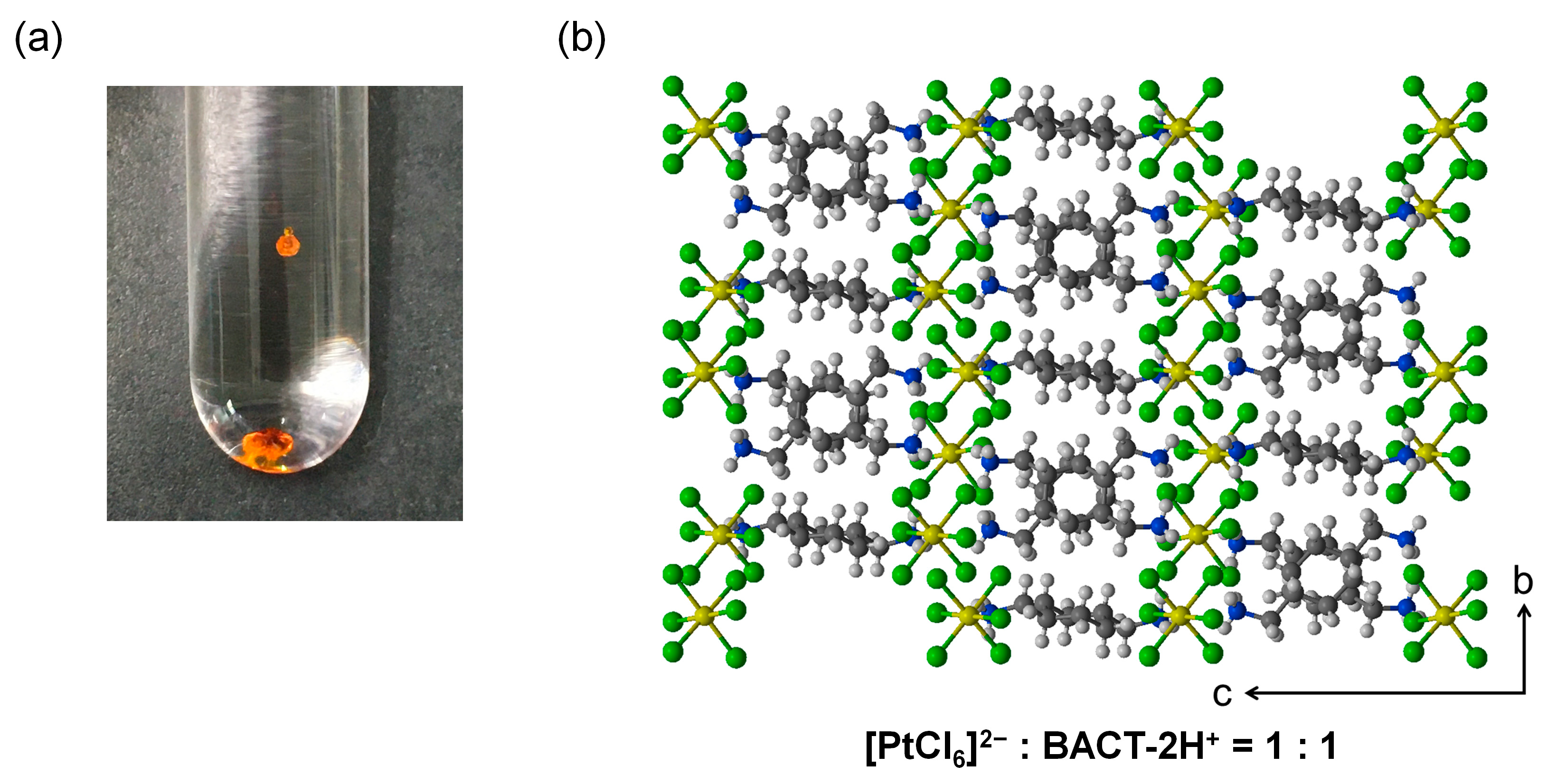
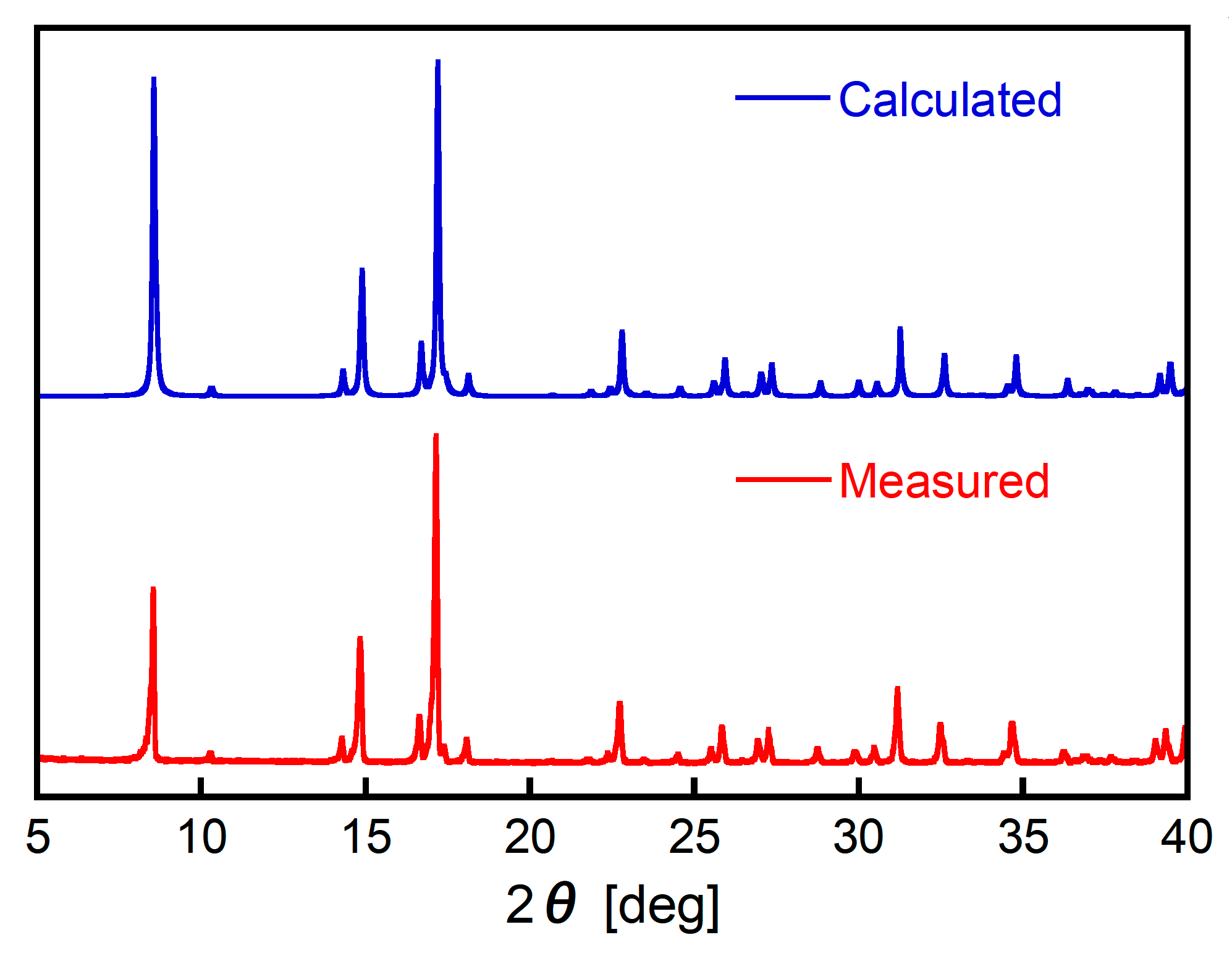
3.6. Characterization of Pt(IV)-Containing Precipitate and Mechanism of Selective Precipitation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seymour, R.J.; O’Farrelly, J. Platinum-group metals. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 1–37. [Google Scholar] [CrossRef]
- Rao, C.R.M.; Reddi, G.S. Platinum group metals (PGM); occurrence, use and recent trends in their determination. TrAC Trends Anal. Chem. 2000, 19, 565–586. [Google Scholar] [CrossRef]
- Hughes, A.E.; Haque, N.; Northey, S.A.; Giddey, S. Platinum group metals: A review of resources, production and usage with a focus on catalysts. Resources 2021, 10, 93. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, J.; Chen, J.; Wu, Y.; Li, B. Recovery of platinum group metals from spent catalysts: A review. Int. J. Miner. Process. 2015, 145, 108–113. [Google Scholar] [CrossRef]
- Kašpar, J.; Fornasiero, P.; Hickey, N. Automotive catalytic converters: Current status and some perspectives. Catal. Today 2003, 77, 419–449. [Google Scholar] [CrossRef]
- Alonso, E.; Field, F.R.; Kirchain, R.E. Platinum availability for future automotive technologies. Environ. Sci. Technol. 2012, 46, 12986–12993. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Ghahreman, A. Platinum group metals recycling from spent automotive catalysts: Metallurgical extraction and recovery technologies. Sep. Purif. Technol. 2023, 311, 123357. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.; Petersen, J. Recovery of Pt, Pd, and Rh from spent automotive catalysts through combined chloride leaching and ion exchange: A review. Hydrometallurgy 2024, 228, 106360. [Google Scholar] [CrossRef]
- Froehlich, P.; Lorenz, T.; Martin, G.; Brett, B.; Bertau, M. Valuable metals—Recovery processes, current trends, and recycling strategies. Angew. Chem. Int. Ed. 2017, 56, 2544–2580. [Google Scholar] [CrossRef]
- Pianowska, K.; Kluczka, J.; Benke, G.; Goc, K.; Malarz, J.; Ochmański, M.; Leszczyńska-Sejda, K. Solvent extraction as a method of recovery and separation of platinum group metals. Materials 2023, 16, 4681. [Google Scholar] [CrossRef]
- Zupanc, A.; Install, J.; Jereb, M.; Repo, T. Sustainable and selective modern methods of noble metal recycling. Angew. Chem. Int. Ed. 2023, 62, e202214453. [Google Scholar] [CrossRef]
- Zheng, H.; Ding, Y.; Wen, Q.; Liu, B.; Zhang, S. Separation and purification of platinum group metals from aqueous solution: Recent developments and industrial applications. Resour. Cons. Recycl. 2021, 167, 105417. [Google Scholar] [CrossRef]
- Lee, J.C.; Hong, H.J.; Chung, K.W.; Kim, S. Separation of platinum, palladium and rhodium from aqueous solutions using ion exchange resin: A review. Sep. Purif. Technol. 2020, 246, 116896. [Google Scholar] [CrossRef]
- Ortet, O.; Paiva, A.P. Development of tertiary thioamide derivatives to recover palladium (II) from simulated complex chloride solutions. Hydrometallurgy 2015, 151, 33–41. [Google Scholar] [CrossRef]
- Paiva, A.P.; Martins, M.E.; Ortet, O. Palladium (II) recovery from hydrochloric acid solutions by N,N′-dimethyl-N,N′-dibutylthiodiglycolamide. Metals 2015, 5, 2303–2315. [Google Scholar] [CrossRef]
- Lee, J.Y.; Raju, B.; Kumar, B.N.; Kumar, J.R.; Park, H.K.; Reddy, B.R. Solvent extraction separation and recovery of palladium and platinum from chloride leach liquors of spent automobile catalyst. Sep. Purif. Technol. 2010, 73, 213–218. [Google Scholar] [CrossRef]
- Sun, P.P.; Lee, J.Y.; Lee, M.S. Separation of Pt (IV) and Rh (III) from chloride solution by solvent extraction with amine and neutral extractants. Mater. Trans. 2011, 52, 2071–2076. [Google Scholar] [CrossRef]
- Gupta, B.; Singh, I. Extraction and separation of platinum, palladium and rhodium using Cyanex 923 and their recovery form real samples. Hydrometallurgy 2013, 134–135, 11–18. [Google Scholar] [CrossRef]
- Truong, H.T.; Lee, M.S.; Senanayake, G. Separation of Pt (IV), Rh (III) and Fe (III) in acid chloride leach solutions of glass scraps by solvent extraction with various extractants. Hydrometallurgy 2018, 175, 232–239. [Google Scholar] [CrossRef]
- Rydberg, J.; Cox, M.; Musikas, C.; Choppin, G.R. Solvent Extraction Principles and Practice, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 2004. [Google Scholar]
- Bernardis, F.L.; Grant, R.A.; Sherrington, D.C. A review of methods of separation of the platinum-group metals through their chloro-complexes. React. Funct. Polym. 2005, 65, 205–217. [Google Scholar] [CrossRef]
- Nikoloski, A.N.; Ang, K.L. Review of the application of ion exchange resins for the recovery of platinum-group metals from hydrochloric acid solutions. Miner. Process. Extract. Metall. Rev. 2014, 35, 369–389. [Google Scholar] [CrossRef]
- Ayres, G.H.; Berg, E.W. Separation of palladium from platinum, iridium, and rhodium with dimethylglyoxime. Anal. Chem. 1953, 25, 980–981. [Google Scholar] [CrossRef]
- Gordon, L.; Ellefsen, P.R.; Wood, G.; Hileman Jr, O.E. Precipitation of nickel and palladium dimethylglyoximates from homogeneous solution. Talanta 1966, 13, 551–558. [Google Scholar] [CrossRef]
- Ho, H.L.; Wen, J.; Lee, M.S. Development of an efficient process for the separation of Co (II), Mn (II), and Ni (II) from chloride solution by precipitation. Min. Proc. Ext. Met. Rev. 2024, 1–12. [Google Scholar] [CrossRef]
- Nguyen, T.N.H.; Song, S.J.; Lee, M.S. Separation of Au (III) and Pd (II) from the etching solutions of printed circuit boards by chemical reduction and oxidative precipitation. Miner. Process. Extr. Metall. Rev. 2024, 45, 835–845. [Google Scholar] [CrossRef]
- Schreier, G.; Edtmaier, C. Separation of Ir, Pd and Rh from secondary Pt scrap by precipitation and calcination. Hydrometallurgy 2003, 68, 69–75. [Google Scholar] [CrossRef]
- Nguyen, V.N.H.; Song, S.J.; Lee, M.S. Selective precipitation of ammonium hexachloropalladate from leaching solutions of cemented palladium with zinc. J. Min. Metall. Sect. B Metall. 2022, 58, 311–319. [Google Scholar] [CrossRef]
- Sandig-Predzymirska, L.; Barreiros, T.V.; Weigelt, A.; Pitscheider, S.; Pedersen, C.M.; Kallesøe, C.; Thiere, A.; Stelter, M.; Charitos, A. Recovery of platinum and ruthenium from PEM electrodes via hydrometallurgical approach. J. Sustain. Metall. 2025, 11, 145–159. [Google Scholar] [CrossRef]
- Otgonbayar, U.; Sandig-Predzymirska, L.; Thiere, A.; Charitos, A. Solvent extraction of Pt, Ru, and Ir using Cyanex 923 in chloride media to develop a recycling route for spent polymer electrolyte membrane (PEM) electrolyzers. Hydrometallurgy 2024, 226, 106303. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Jones, L.O.; Liu, W.; Zhang, L.; Song, B.; Chen, X.Y.; Stern, C.L.; Schatz, G.C.; Stoddart, J.F. Selective separation of hexachloroplatinate (IV) dianions based on exo-binding with cucurbit[6]uril. Angew. Chem. Int. Ed. 2021, 60, 17587–17594. [Google Scholar] [CrossRef]
- Matsumoto, K.; Sezaki, Y.; Hata, Y.; Jikei, M. Selective recovery of platinum (IV) from HCl solutions using 2-ethylhexylamine as a precipitant. Separations 2021, 8, 40. [Google Scholar] [CrossRef]
- Matsumoto, K.; Hata, Y.; Katagiri, H.; Jikei, M. Highly Selective precipitation of platinum(IV) from HCl solutions using m-phenylenediamine utilizing difference in stability of ionic crystals. Metals 2025, 15, 165. [Google Scholar] [CrossRef]
- Matsumoto, K.; Yamakawa, S.; Sezaki, Y.; Katagiri, H.; Jikei, M. Preferential precipitation and selective separation of Rh(III) from Pd(II) and Pt(IV) using 4-alkylanilines as precipitants. ACS Omega 2019, 4, 1868–1873. [Google Scholar] [CrossRef] [PubMed]
- Harjanto, S.; Cao, Y.; Shibayama, A.; Naitoh, I.; Nanami, T.; Kasahara, K.; Okumura, Y.; Liu, K.; Fujita, T. Leaching of Pt, Pd and Rh from automotive catalyst residue in various chloride based solutions. Mater. Trans. 2006, 47, 129–135. [Google Scholar] [CrossRef]
- CrysAlisPro CCD, CrysAlisPro RED and ABSPACK in CrysAlisPro RED, Oxford Diffraction Ltd.: Abingdon, UK, 2006.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Wakita, K. Yadokari–XG, Software for Crystal Structure Analyses. 2001. Available online: http://chem.s.kanazawa-u.ac.jp/coord/yadokari/ (accessed on 6 July 2025).
- Kabuto, C.; Akine, S.; Nemoto, T.; Kwon, E. Release of software (Yadokari–XG 2009) for crystal structure analyses. J. Cryst. Soc. Jpn. 2009, 51, 218–224. [Google Scholar] [CrossRef]
- Shah, M.A. Growth of uniform nanoparticles of platinum by an economical approach at relatively low temperature. Sci. Iran. 2012, 19, 964–966. [Google Scholar] [CrossRef]


| Metals | Initial Precipitate | Reprecipitated Solid |
|---|---|---|
| Concentration [mg/L] | Concentration [mg/L] | |
| Pt | 970 | 931 |
| Pd | 5 | 0.6 |
| Rh | 33 | 0.7 |
| Metals | Catalyst Leachate | Initial Precipitate | Purified Precipitate | ||
|---|---|---|---|---|---|
| Concentration | Concentration | Recovery | Concentration | Recovery | |
| [mg/L] | [mg/L] | [%] | [mg/L] | [%] | |
| Pt | 1680 | 1666.6 | 99.2 | 1606.1 | 95.6 |
| Pd | 979 | 146.5 | 15.0 | 5.4 | 0.6 |
| Rh | 256 | 36.7 | 14.3 | <0.1 | <0.1 |
| Ce | 70,997 | 0.1 | <0.1 | <0.1 | <0.1 |
| Al | 1563 | 6.5 | 0.4 | 4.7 | 0.3 |
| Fe | 2351 | 44.9 | 1.9 | <0.1 | <0.1 |
| Zr | 328 | 0.2 | <0.1 | 0.2 | <0.1 |
| La | 1237 | 0.7 | <0.1 | 0.2 | <0.1 |
| Mg | 36,625 | 13.4 | <0.1 | <0.1 | <0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, K.; Sakamoto, R.; Sakuta, Y.; Aoki, R.; Katagiri, H.; Jikei, M. Highly Selective Recovery of Pt(IV) from HCl Solutions by Precipitation Using 1,4-Bis(aminomethyl)cyclohexane as a Precipitating Agent. Metals 2025, 15, 778. https://doi.org/10.3390/met15070778
Matsumoto K, Sakamoto R, Sakuta Y, Aoki R, Katagiri H, Jikei M. Highly Selective Recovery of Pt(IV) from HCl Solutions by Precipitation Using 1,4-Bis(aminomethyl)cyclohexane as a Precipitating Agent. Metals. 2025; 15(7):778. https://doi.org/10.3390/met15070778
Chicago/Turabian StyleMatsumoto, Kazuya, Ryu Sakamoto, Yoshiya Sakuta, Ryota Aoki, Hiroshi Katagiri, and Mitsutoshi Jikei. 2025. "Highly Selective Recovery of Pt(IV) from HCl Solutions by Precipitation Using 1,4-Bis(aminomethyl)cyclohexane as a Precipitating Agent" Metals 15, no. 7: 778. https://doi.org/10.3390/met15070778
APA StyleMatsumoto, K., Sakamoto, R., Sakuta, Y., Aoki, R., Katagiri, H., & Jikei, M. (2025). Highly Selective Recovery of Pt(IV) from HCl Solutions by Precipitation Using 1,4-Bis(aminomethyl)cyclohexane as a Precipitating Agent. Metals, 15(7), 778. https://doi.org/10.3390/met15070778







