Corrosion Resistance Mechanism in WC/FeCrNi Composites: Decoupling the Role of Spherical Versus Angular WC Morphologies
Abstract
1. Introduction
2. Experimental Methodology
2.1. Materials Preparation
2.2. Characterization Techniques
2.3. Electrochemical Tests
3. Results and Discussion
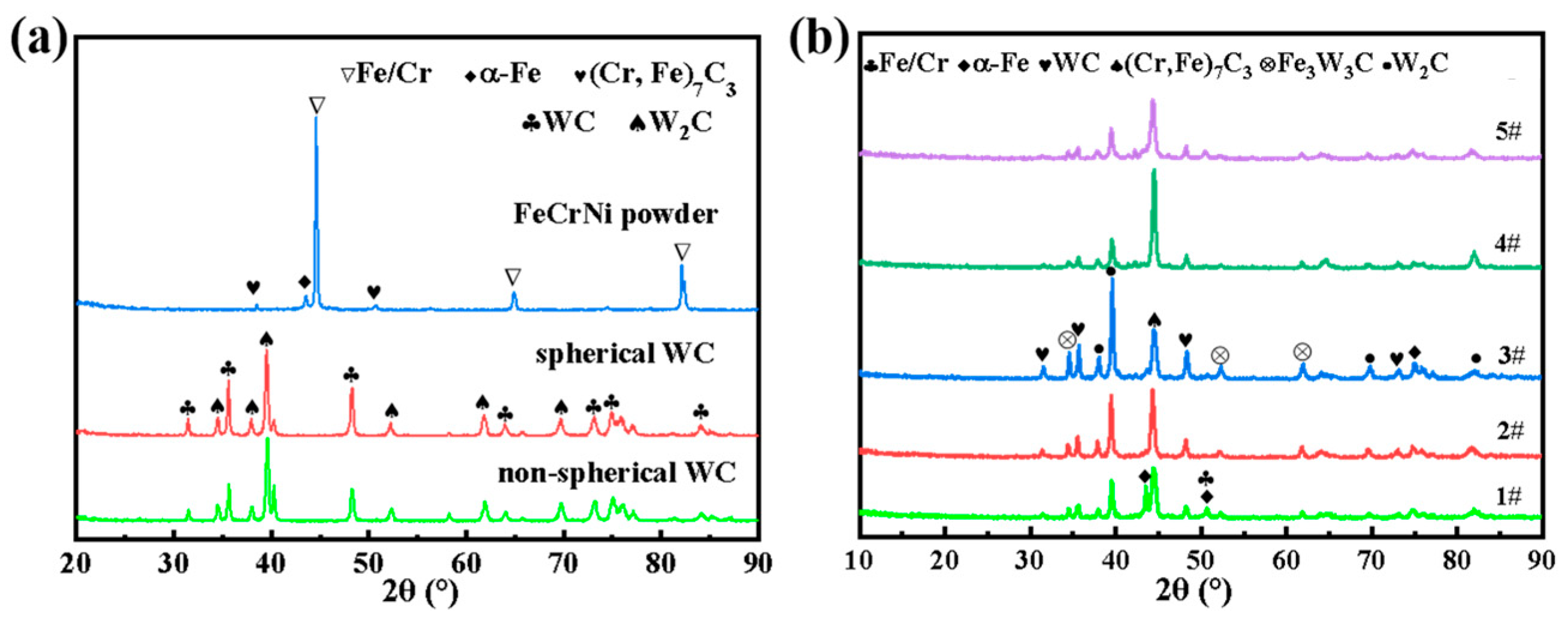
3.1. Phase Analysis of Five Samples
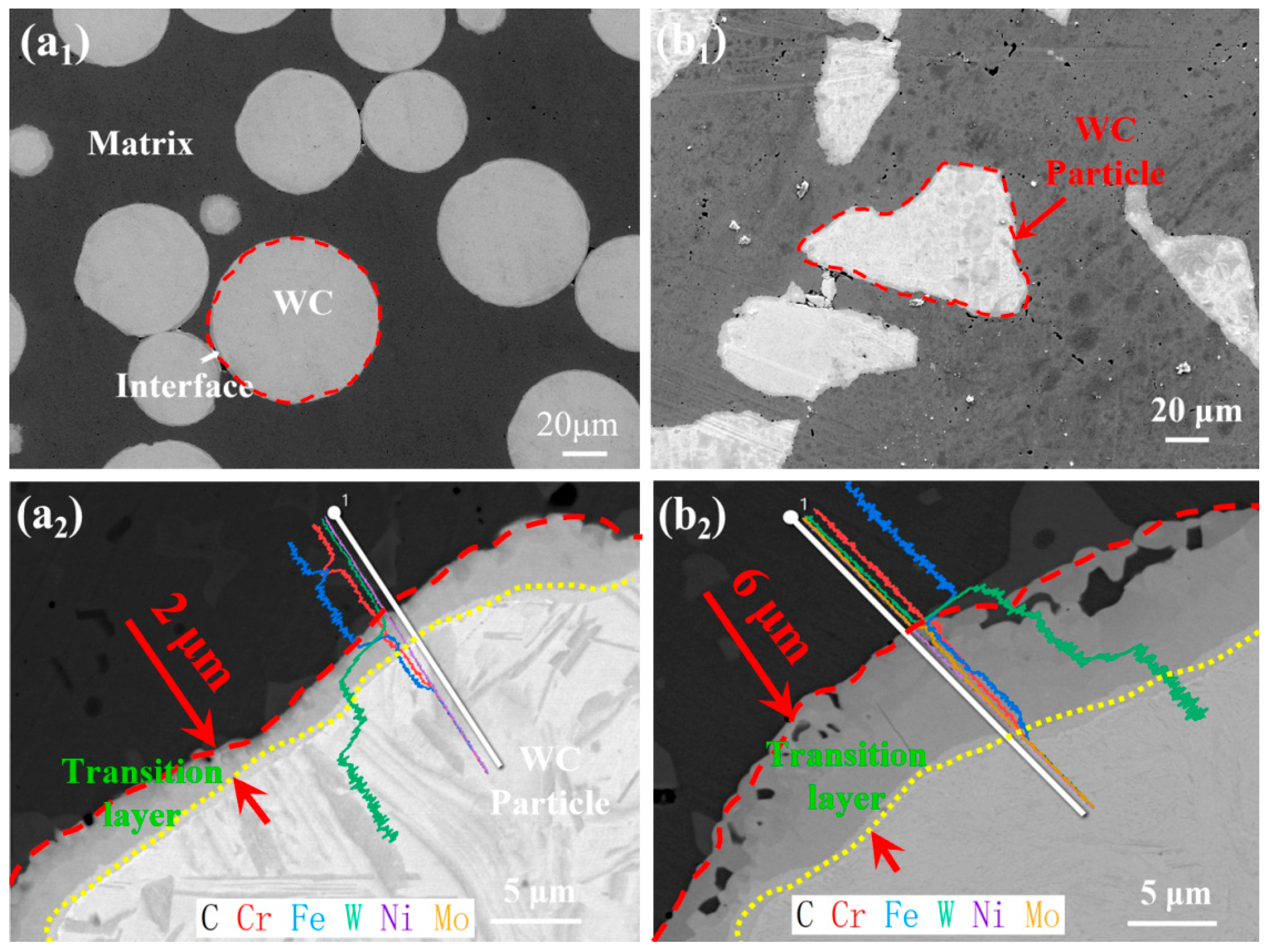
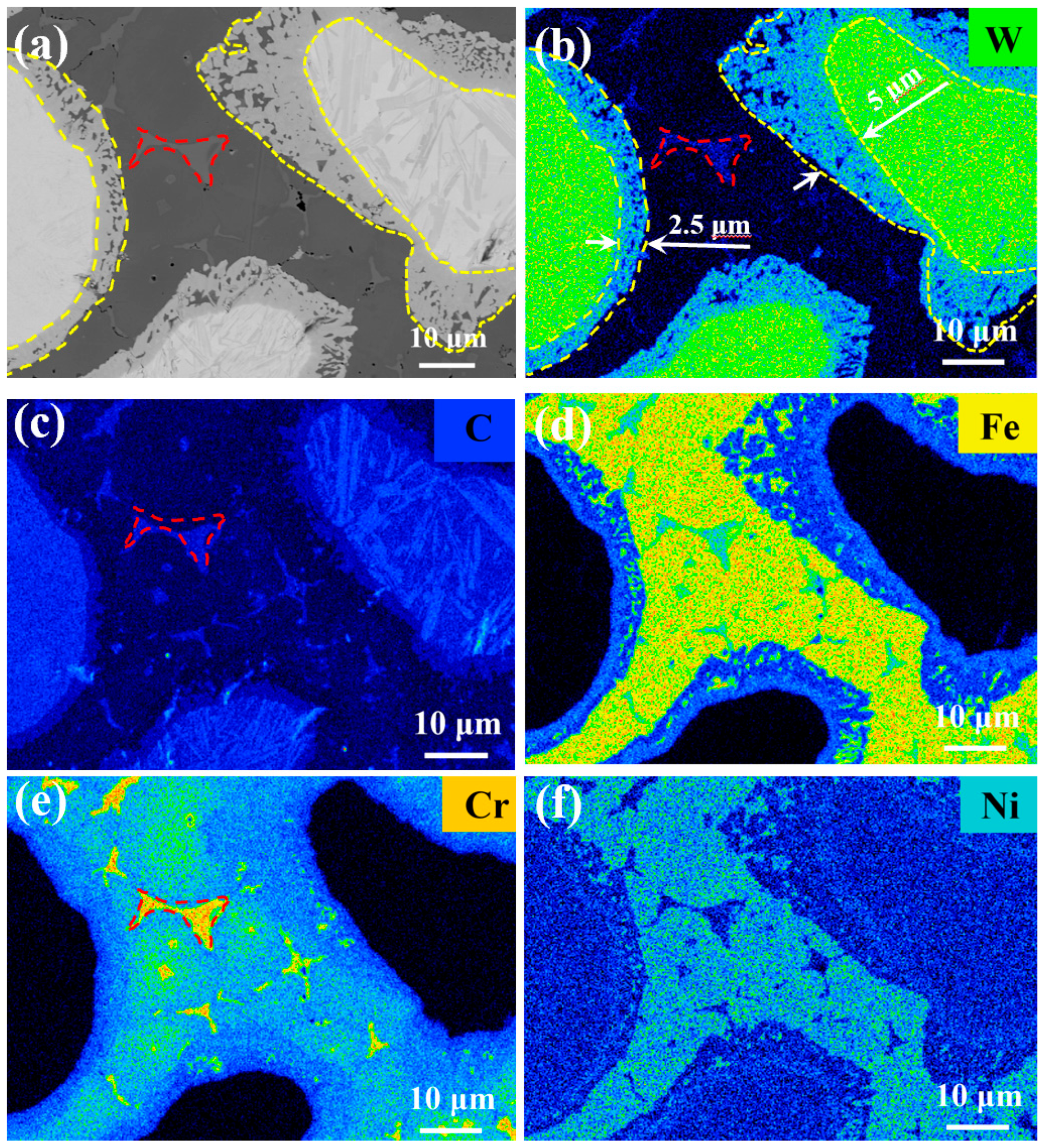
3.2. Microstructure Analysis
3.3. Electrochemical Measurements
3.3.1. The Effect of Adding CTC-A on the Open Circuit Potential of the Alloy
3.3.2. The Effect of Adding CTC-A on the Polarization of the Alloy
3.3.3. The Influence of the Addition of CTC-A on the Electrochemical Impedance of the Alloy
3.3.4. The Effect of Adding CTC-A on the Morphology of Corrosion Products of the Alloy
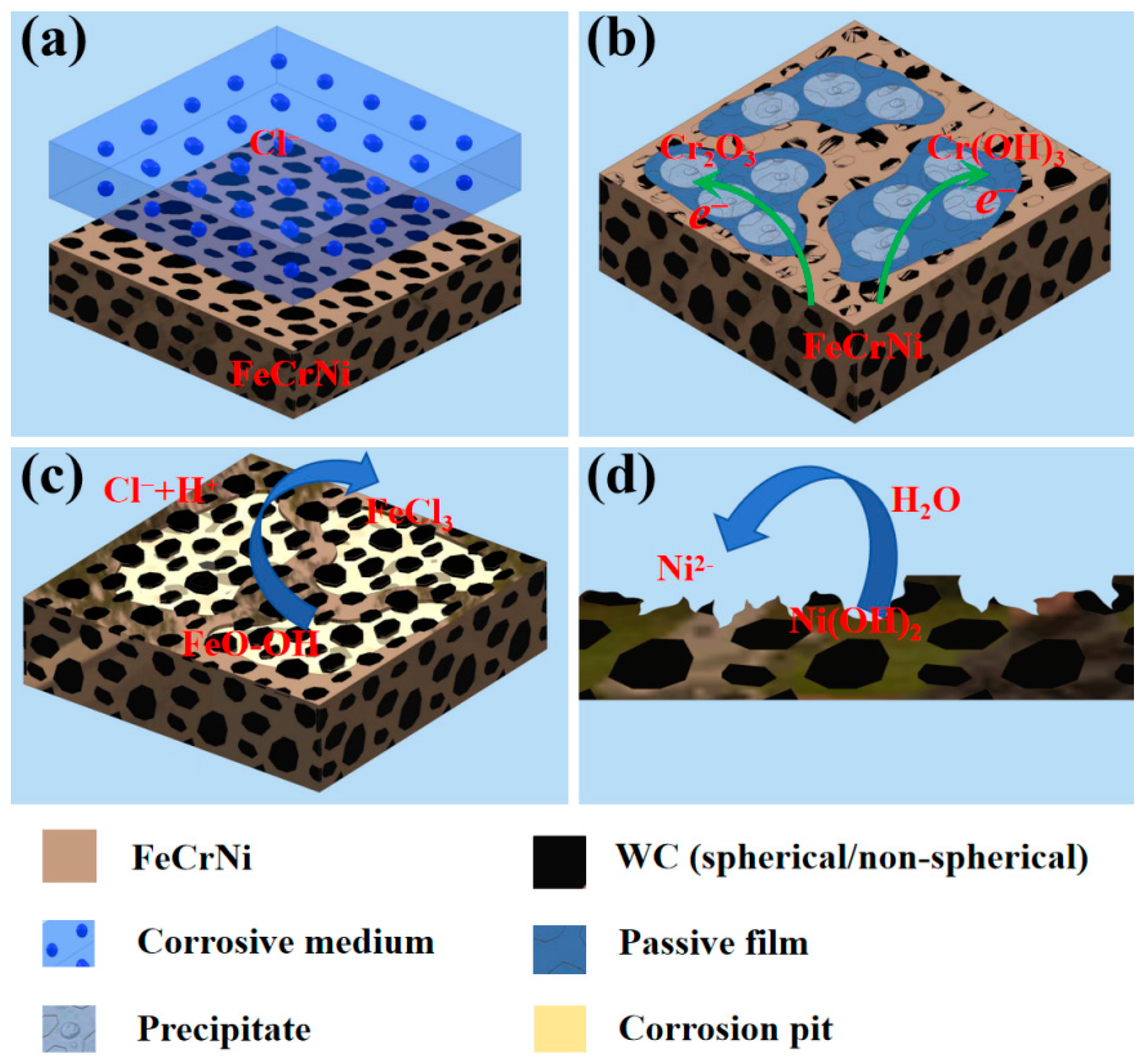
3.3.5. The Effect of the Addition of CTC-A on the Corrosion Mechanism of the Alloy
- (1)
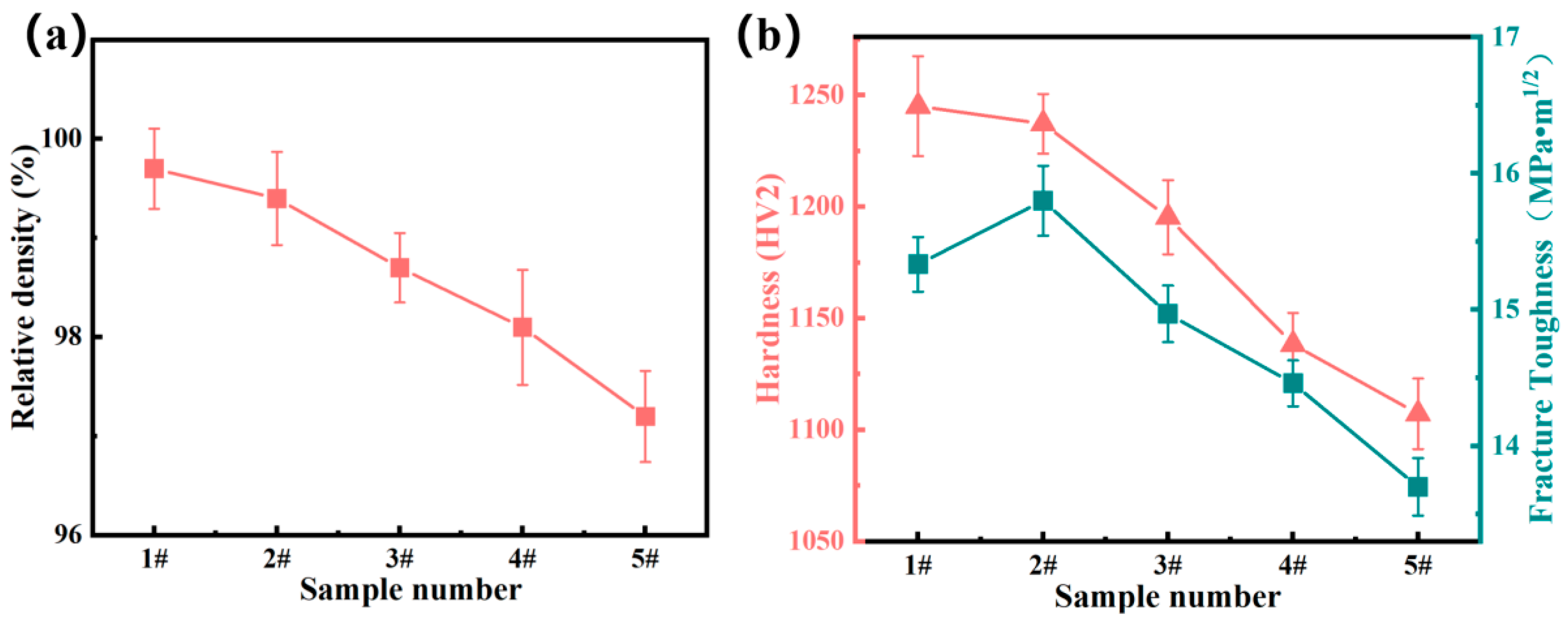
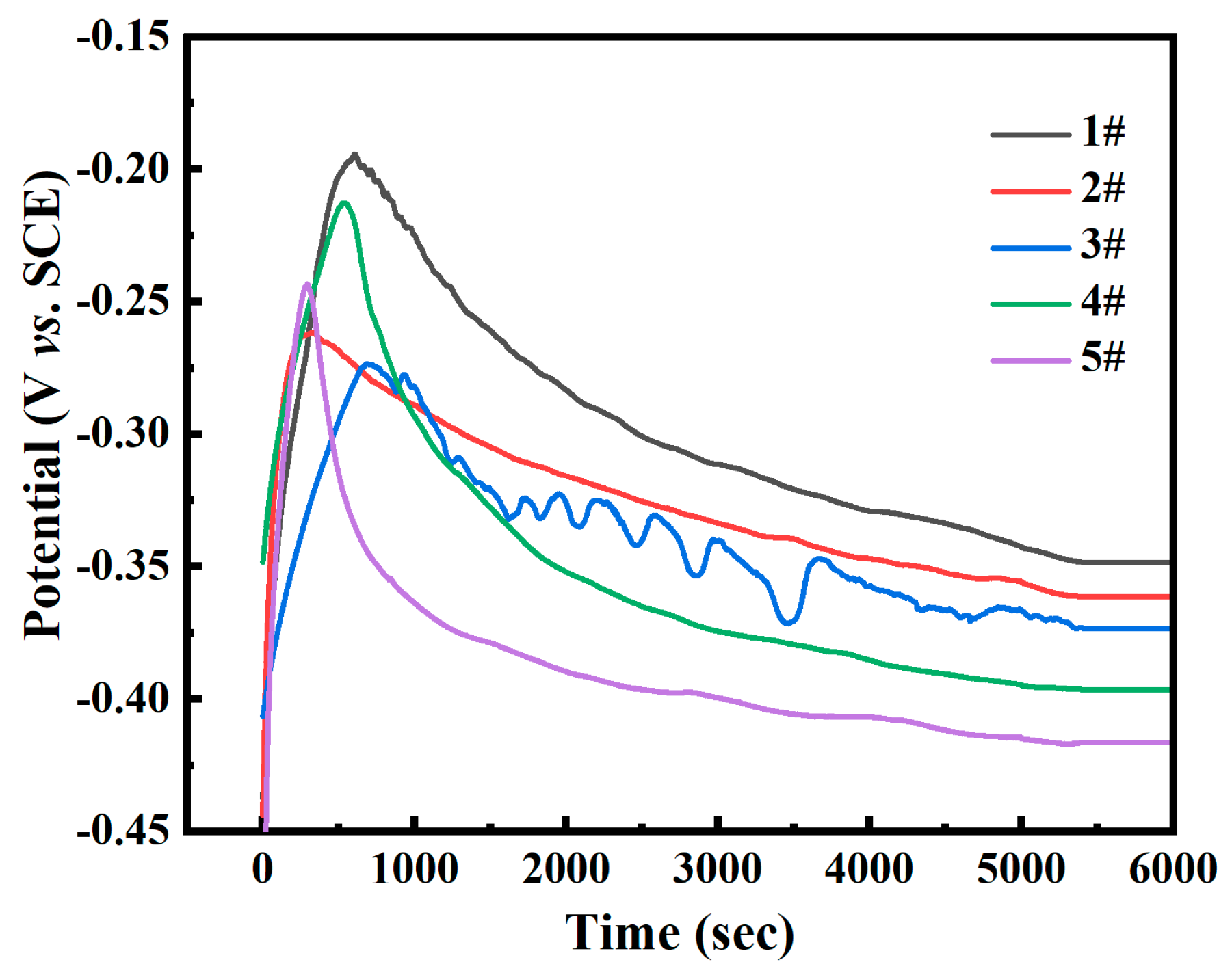
- Passivation film formation stage (<500 s): The open-circuit potential rapidly shifts positively (Figure 8), driven by the preferential oxidation of Cr to Cr3+ in the FeCrNi matrix that forms initial Cr2O3 clusters (Figure 15). A high spherical WC content (sample #1: 40% CTC-S) accelerates dense film formation through its smooth surface and high dispersibility (lowest porosity ~1%, Table 3), as evidenced by the XPS showing 38% Cr3+ enrichment. This enables potential stabilization within ~5000 s (Figure 8) with the most noble Eoc (−0.348 V, Table 3). In contrast, a high angular WC (sample #5: 40% CTC-A) increases the porosity (~3%), while M7C3 carbides consume matrix Cr (XPS-confirmed Cr depletion), delaying stabilization (>5400 s) with the most active Eoc (−0.416 V, Table 3);
- (2)
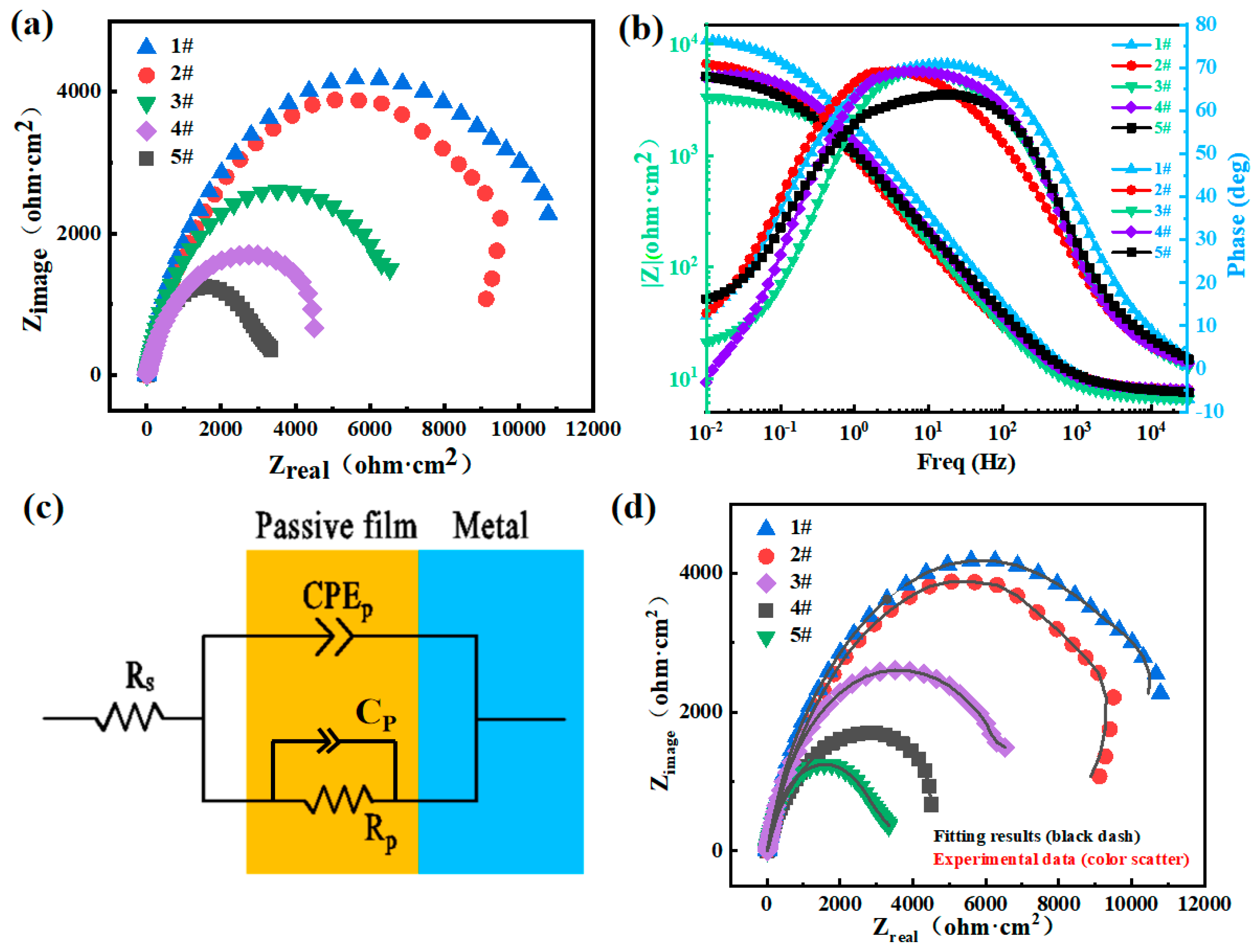
- Passivation film stabilization stage: The Cr2O3 film functions as a charge-transfer barrier (Figure 10c equivalent circuit). sample #1 (40% spherical WC) exhibits superior film continuity (CPEα = 0.912, Table 4), yielding maximum charge-transfer resistance (Rp = 11.22 kΩ·cm2, Table 4) that suppresses the corrosion current (icorr = 0.152 μA·cm−2, Table 3) and Cl− penetration. sample #5 (40% angular WC) shows a degraded film integrity (CPEα = 0.828, Table 4) with minimal Rp (5.15 kΩ·cm2, Table 4), while microgalvanic cells (WC/matrix and M7C3/matrix) accelerate the charge transfer, increasing icorr to 1.120 μA·cm−2 (Table 3);
- (3)
- Passivation film dissolution stage: Under anodic polarization (Figure 9), sample #1’s Cr-rich film demonstrates exceptional dissolution resistance with a wide passive region (−300 to −200 mV) and minimal passive current density (ipass = 2.01 × 10−4 μA·cm−2, Table 3). Conversely, sample #5’s defective film dissolves readily, exhibiting disappearing passive region characteristics and current density surge (0.764 → 1.120 μA·cm−2 at 30 → 40% angular WC, Table 3), which is accompanied by severe surface spalling (Figure 13(c1,c2));
- (4)
- Passivation film breakdown stage: sample #1’s uniform film withstands transpassive dissolution (>−50 mV, Figure 9) with limited pitting (Figure 12(a1–a3)). sample #5’s angular WC triggers catastrophic failure: stress concentration at sharp corners initiates microcrack propagation along interfaces (Figure 13(c2)), while M7C3/matrix galvanic corrosion drives explosive pitting (Figure 13(c2) pits), which manifests as a current surge at low potentials (−0.05 V, Figure 9). This culminates in rapid material degradation through interconnected failure mechanisms.
- (1)
- The first mechanistic proof that angular WC’s corner dissolution (Figure 4b) triggers carbon-mediated Cr depletion at interfaces, reducing the passive film stability by 70.7% when exceeding 10 vol% (Rp drop: 11.42 → 3.35 kΩ·cm2, Table 5). This resolves long-standing debates on morphology-corrosion decoupling [5,10];
- (2)
- (3)
4. Conclusions
- (1)
- The open-circuit potential shows a decreasing trend with increases in the angular WC content. When the proportion of angular WC was increased from 0% to 40%, the open-circuit potential gradually decreased from −0.348 V (40% spherical WC for #1 sample) to −0.416 V (40% angular WC for #5 sample). This indicates that spherical WC shows good corrosion resistance by reducing the porosity and decreasing the contact area between the matrix and corrosive liquid;
- (2)
- The kinetic potential polarization test shows that there is a significant WC enhanced phase morphology dependence of the anodic process. When the content of angular WC was increased from 0% to 40%, the corrosion potential gradually decreased from −0.332 V to −0.431 V, and the corrosion current increased from 0.152 μA·cm−2 to 1.120 μA·cm−2, which indicated that the sharp edges of the angular WC might trigger localized pitting corrosion that leads to the deterioration of the passivation film. In turn this reduces the corrosion resistance of the composites;
- (3)
- EIS analysis showed a negative correlation between the passivation film resistance (Rp) and the content of angular WC, with the Rp reaching 11.42 kΩ·cm2 for sample #1 and decreasing to 3.35 kΩ·cm2 for sample #5 (a decrease of 70.7%). XPS confirmed that the spherical WC promotes the enrichment of Cr3+ (up to 38% of Cr2O3 for sample #1), and the proportion of FeO -OH reached 28.5%, leading to a decrease in the self-repairing ability of the passivation film and the deterioration of its material corrosion performance;
- (4)
- Future research guidelines: Based on the limitations identified in this study, two critical research directions are proposed: 1. Long-term corrosion behavior validation in simulated marine environments with synergistic erosion (sand particle concentration: 1–5 g/L) and microbial activity (SRB density: 104–106 cells/mL) to assess real-world durability. 2. High-temperature synchrotron XRD monitoring (20–1300 °C) of carbide transformation kinetics during SPS to establish time–temperature–transformation diagrams with the aim of achieving optimized phase control.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Cao, Y.; Wang, P.; Jin, J.; Deng, L. Evolution of the corrosion layer of Q235 steel in simulated fire-scene smoke. Fire Mater. 2023, 48, 155–165. [Google Scholar] [CrossRef]
- Nie, J.; Wei, L.; Jiang, Y.; Li, Q.; Luo, H. Corrosion mechanism of additively manufactured 316L stainless steel in 3.5 wt.% NaCl solution. Mater. Today Commun. 2021, 26, 145–162. [Google Scholar] [CrossRef]
- ArunKumar, V.; Prakash, N.; Deenadayalan, K. Comparison between the spherical and angular type WC particles reinforced in Nickel-based matrix deposited using Plasma Transferred Arc Welding process. Mater. Today Proc. 2023, 11, 1–10. [Google Scholar] [CrossRef]
- Moreira, A.B.; Ribeiro, L.M.M.; Lacerda, P.; Vieira, M.F. Characterization of iron-matrix composites reinforced by in situ TiC and exsitu WC fabricated by casting. Metals 2021, 11, 862. [Google Scholar] [CrossRef]
- Dai, Z.X.; Jiang, S.L.; Ning, L.K.; Xu, X.D.; Li, S.; Duan, D.L. Effect of friction on corrosion behaviors of AISI 304 and Cr26Mo1 stainless steels in different solutions. J. Iron Steel Res. Int. 2023, 30, 2541–2556. [Google Scholar] [CrossRef]
- Dang, Z.; Qin, G.; Lin, R. A comprehensive study of interfacial microstructure and corrosion behavior of AA2219 to 304SS inertia friction welded joints. Corros. Sci. 2023, 224, 111539. [Google Scholar] [CrossRef]
- Peng, Y.; Li, T.; Long, J.; Li, H.; Lu, B.; Chen, F.; Du, Y. Effect of bimodal WC particle size and binder composition on the morphology of WC grains in WC–Co–Ni3Al cemented carbides. J. Mater. Res. Technol. 2021, 12, 1747–1754. [Google Scholar] [CrossRef]
- Iribecampos, M.; Ulacia, I.; Arana, A.; Larrañaga, J. The combined effect of contact interface size and spin on lubricated traction in rolling-sliding point contacts. Tribol. Int. 2023, 188, 108822. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, K.; Wang, Y. Baffle effect of exposed WC particles in the clearance on slurry erosion behavior of cemented carbide surface. Mater. Today Commun. 2024, 39, 109071. [Google Scholar] [CrossRef]
- Liu, X.; Sheng, X.; Yao, Q.; Zhao, L.; Xu, Z.; Zhou, Y. Synthesis of a new type of 2-phosphonobutane-1, 2, 4-tricarboxylic-acid-modified terpolymer scale inhibitor and its application in the oil field. Energy Fuels 2021, 35, 6136–6143. [Google Scholar] [CrossRef]
- Gad, S.I.; Attia, M.A.; Hassan, M.A.; El-Shafei, A.G. A random microstructure-based model to study the effect of the shape of reinforcement particles on the damage of elastoplastic particulate metal matrix composites. Ceram. Int. 2021, 47, 3444–3461. [Google Scholar] [CrossRef]
- Mao, L.; Cai, M.; Liu, Q.; Han, L. Effects of spherical WC powders on the erosion behavior of WC-Ni hardfacing used for steel body drill bit. Surf. Coat. Technol. 2021, 409, 126893–126907. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Yin, Z.; Xu, W.; Jiang, L.; Yuan, J. Ultrafine WC-Co cemented carbide end mills prepared by spark plasma sintering and wear mechanism in milling of AISI H13. Int. J. Appl. Ceram. Technol. 2024, 21, 2553–2566. [Google Scholar] [CrossRef]
- Li, W.; Di, R.; Yuan, R.; Song, H.; Lei, J. Microstructure, wear resistance and electrochemical properties of spherical/angular WC reinforced Inconel 625 superalloy by laser melting deposition. J. Manuf. Process. 2022, 74, 413–422. [Google Scholar] [CrossRef]
- An, X.; Zhang, H.; Zhang, D.; Zhu, J.; Wang, Q.; Zhu, T.; Shi, Y.; Cao, X.; Deng, H.; Hu, W.; et al. Mechanisms of hydrogen embrittlement resistances in FCC concentrated solid solution alloys. Corros. Sci. 2024, 229, 111894. [Google Scholar] [CrossRef]
- Lü, Z.; Ding, G.; Liu, M.; Yu, S.; Gao, C. Improved separation performance, anti-fouling property and durability of polyamide-based RO membrane by constructing a polyvinyl alcohol/polyquaternium-10 surface coating layer. Desalination 2023, 564, 116755. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, J.; Liu, G.; Liu, Y.; Chang, S.; Rodrigue, D.; Wang, X. Micronleaf-shape graphene interfaces on wood transverse sections as advanced photothermal evaporators for water purification. J. Mater. Sci. Technol. 2024, 193, 81–89. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Tang, Y.; Huang, X.; Pang, H. Recent advances and challenges of metal–organic framework/graphene-based composites. Compos. Part B Eng. 2022, 230, 109532. [Google Scholar] [CrossRef]
- Weiser, M.; Olszta, M.J.; Engelhard, M.H.; Zhu, Z.; Schreiber, D.K. Visualizing oxygen transport pathways during intergranular oxidation in Ni-Cr. Npj Mater. Degrad. 2023, 7, 70. [Google Scholar] [CrossRef]
- Xie, Y.; Miyamoto, G.; Furuhara, T. High-throughput investigation of Cr-N cluster formation in Fe-35Ni-Cr system during low-temperature nitriding. Acta Mater. 2023, 253, 118921. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Y.; Liu, C.; Zhang, B.; Gao, Y. Enzyme-inspired molecular electrocatalysts for the oxygen reduction reaction. Electrochim. Acta 2024, 479, 143891. [Google Scholar] [CrossRef]
- Cai, X.; Xu, Y.; Liu, M.; Cao, B.; Li, X. Characterization of iron-based surface multilayer-structured tungsten carbide composite layers by EBSD and FIB/TEM. Surf. Coat. Technol. 2020, 403, 126365–126376. [Google Scholar] [CrossRef]
- ASTM E2655-14; Standard Guide for Reporting Uncertainty of Test Results and Use of the Term Measurement Uncertainty in ASTM Test Methods. ASTM International: West Conshohocken, PA, USA, 2024.
- Liu, M.; Liu, B.; Ni, Z.; Du, C.; Li, X. Elucidating the effect of titanium alloying on the pitting corrosion of ferritic stainless steel. J. Mater. Res. Technol. 2024, 28, 1247–1262. [Google Scholar] [CrossRef]
- Yu, R.; Bai, X.; Yu, X.; Zhang, H. WC-reinforced iron matrix composites fabricated by wire arc additive manufacturing combined with gravity-driven powder feeding: Particle transportation and size effects. Rapid Prototyp. J. 2022, 542, 1014–1028. [Google Scholar] [CrossRef]
- Choudhary, S.; Cruz, V.; Pandey, A.; Thomas, S.; Birbilis, N. Element-resolved electrochemical analysis of the passivity of additively manufactured stainless steel 316L. Corros. Sci. 2021, 189, 109576–109585. [Google Scholar] [CrossRef]
- Zhang, Q.; Hao, L.; Chen, W.; Huang, Y.; Xu, Y. Understanding the effect of cathodic reaction on corrosion-enhanced erosion in sand-entraining electrolyte. J. Mater. Sci. Technol. 2025, 209, 103–116. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, X.; He, X.; Liu, C.; Liu, Z.; Zhang, X.; Zhao, M.; Li, X. Mechanism and evaluation method of stress corrosion susceptibility of 904L stainless steel with optimized structure in seawater. Corros. Sci. 2024, 229, 181–192. [Google Scholar] [CrossRef]
- Ye, Y.; Guo, Z.; Zhou, Z.; Zhang, B.; Wang, Q.; Shen, B. Improved corrosion and corrosion-wear properties of Fe-based high-entropy amorphous coatings by modulating heat input of HVAF. Corros. Sci. 2024, 232, 577–587. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, W.; Li, T.; Zhang, M.; Liu, B.; Liu, Y.; Wang, L.; Hu, S. Effect of WC content on microstructures and mechanical properties of FeCoCrNi high-entropy alloy/WC composite coatings by plasma cladding. Surf. Coat. Technol. 2020, 385, 125326–125336. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Q.; Zhou, Q.; Gao, S.; Wang, B.; Wang, X.; Huang, Y. Flow accelerated corrosion and erosion−corrosion behavior of marine carbon steel in natural seawater. Mater. Degrad. 2021, 5, 784–793. [Google Scholar] [CrossRef]
- Li, N.; Wang, B.; Liu, T.; Liu, C.; Che, Z.; Chen, T.; Li, Q.; Li, Z.; Wang, L.; Cheng, X.; et al. Revealing the coupling of multiple corrosion behaviors in the corrosion process of titanium-steel composites in marine environment. Corros. Sci. 2024, 233, 784–793. [Google Scholar] [CrossRef]
- Ko, G.; Kim, W.; Kwon, K.; Lee, T.-K. The corrosion of stainless steel made by additive manufacturing: A review. Metals 2021, 11, 516. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Bai, X.; Zhao, W.; Zhang, P.; Rao, W.-F. Laser cladding highly corrosion-resistant nano/submicron ultrafine-grained Fe-based composite layers. Surf. Coat. Technol. 2021, 424, 424–433. [Google Scholar] [CrossRef]








| Element | C | Fe | Cr | Ni | Si | Mo | B | Mn |
|---|---|---|---|---|---|---|---|---|
| Content | 0.48 | 69.42 | 17.13 | 3.20 | 2.79 | 0.69 | 5.86 | 0.42 |
| Sample Number | CTC-S (Spherical WC) | CTC-A (Angular WC) | Fe-Based Alloy |
|---|---|---|---|
| #1 | 40 | 0 | 60 |
| #2 | 30 | 10 | 60 |
| #3 | 20 | 20 | 60 |
| #4 | 10 | 30 | 60 |
| #5 | 0 | 40 | 60 |
| Sample | Eoc | βa | βc | jcorr | Ecorr | jpass |
|---|---|---|---|---|---|---|
| Number | (V vs. SCE) | mV/Decade | mV/Decade | (μA·cm−2) | (V vs. SCE) | (×10−4 μA·cm−2) |
| #1 | −0.348 | 82.1 | 120.8 | 0.152 | −0.332 | 2.01 |
| #2 | −0.361 | 79.4 | 135.4 | 0.213 | −0.351 | 1.34 |
| #3 | −0.373 | 75.2 | 115.3 | 0.423 | −0.375 | |
| #4 | −0.396 | 73.9 | 128.6 | 0.764 | −0.394 | |
| #5 | −0.416 | 68.5 | 110.7 | 1.120 | −0.431 |
| Sample | Rs | Rp | Cp | CPEp Parameter | Chi-Squared | SSQ | |
|---|---|---|---|---|---|---|---|
| Number | (Ω·cm2) | (103Ω·cm2) | (10−3 mF·cm−2) | Y0 (10−3 Ω−1·sα·cm−2) | α | (10−3) | (10−3) |
| #1 | 3.868 | 11.221 | 1.215 | 1.913 | 0.912 | 1.2 | 1.8 |
| #2 | 3.676 | 10.883 | 1.818 | 1.744 | 0.880 | 0.9 | 1.7 |
| #3 | 4.264 | 8.107 | 2.282 | 1.468 | 0.858 | 0.6 | 1.9 |
| #4 | 4.613 | 6.332 | 2.671 | 1.245 | 0.835 | 1.1 | 1.5 |
| #5 | 5.091 | 5.148 | 2.745 | 1.016 | 0.828 | 0.4 | 1.2 |
| Element | C | O | Cl | Cr | Fe | Ni | W |
|---|---|---|---|---|---|---|---|
| 1 | 10.81 | 43.41 | 5.63 | 8.87 | 27.81 | 1.99 | 1.48 |
| 2 | 11.61 | 44.2 | 6.87 | 8.56 | 26.48 | 0.35 | 1.93 |
| 3 | 13.66 | 50.63 | 0.12 | 12.81 | 19.12 | 0.54 | 3.11 |
| 4 | 46.44 | 15.03 | / | / | 3.16 | / | 35.37 |
| Elemental | C | O | Cl | Cr | Fe | Ni | W |
|---|---|---|---|---|---|---|---|
| 1 | 12.23 | 44.98 | 6.87 | 7.89 | 23.23 | 1.64 | 2.16 |
| 2 | 12.86 | 48.75 | 4.86 | 7.61 | 21.81 | 1.48 | 2.63 |
| 3 | 11.61 | 52.23 | 4.39 | 7.1 | 20.06 | 1.85 | 2.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Wang, R.; Tian, X.; Liu, Y. Corrosion Resistance Mechanism in WC/FeCrNi Composites: Decoupling the Role of Spherical Versus Angular WC Morphologies. Metals 2025, 15, 777. https://doi.org/10.3390/met15070777
Zeng X, Wang R, Tian X, Liu Y. Corrosion Resistance Mechanism in WC/FeCrNi Composites: Decoupling the Role of Spherical Versus Angular WC Morphologies. Metals. 2025; 15(7):777. https://doi.org/10.3390/met15070777
Chicago/Turabian StyleZeng, Xiaoyi, Renquan Wang, Xin Tian, and Ying Liu. 2025. "Corrosion Resistance Mechanism in WC/FeCrNi Composites: Decoupling the Role of Spherical Versus Angular WC Morphologies" Metals 15, no. 7: 777. https://doi.org/10.3390/met15070777
APA StyleZeng, X., Wang, R., Tian, X., & Liu, Y. (2025). Corrosion Resistance Mechanism in WC/FeCrNi Composites: Decoupling the Role of Spherical Versus Angular WC Morphologies. Metals, 15(7), 777. https://doi.org/10.3390/met15070777






