Investigation of Surface Oxidation of Cast Austenitic 304 Stainless Steel at High Temperatures
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
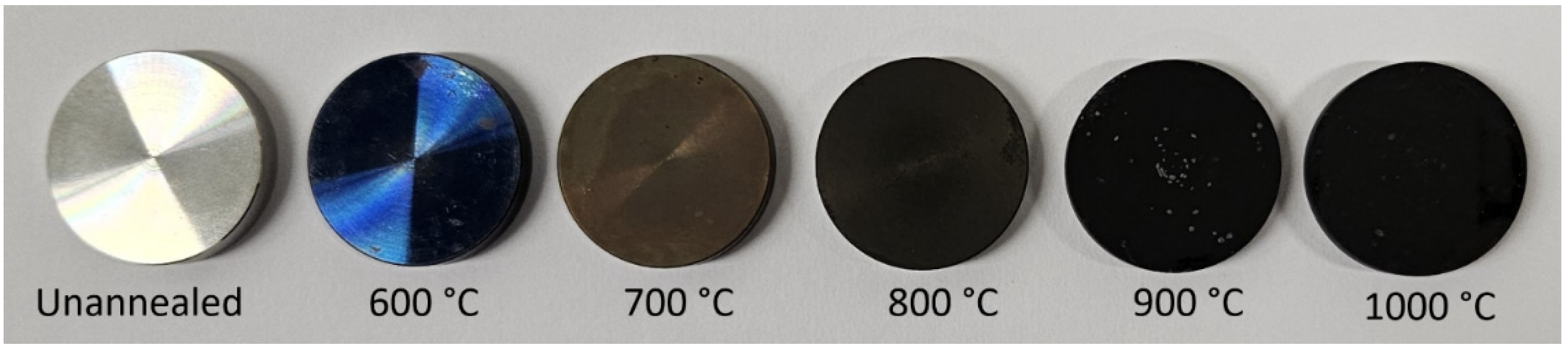
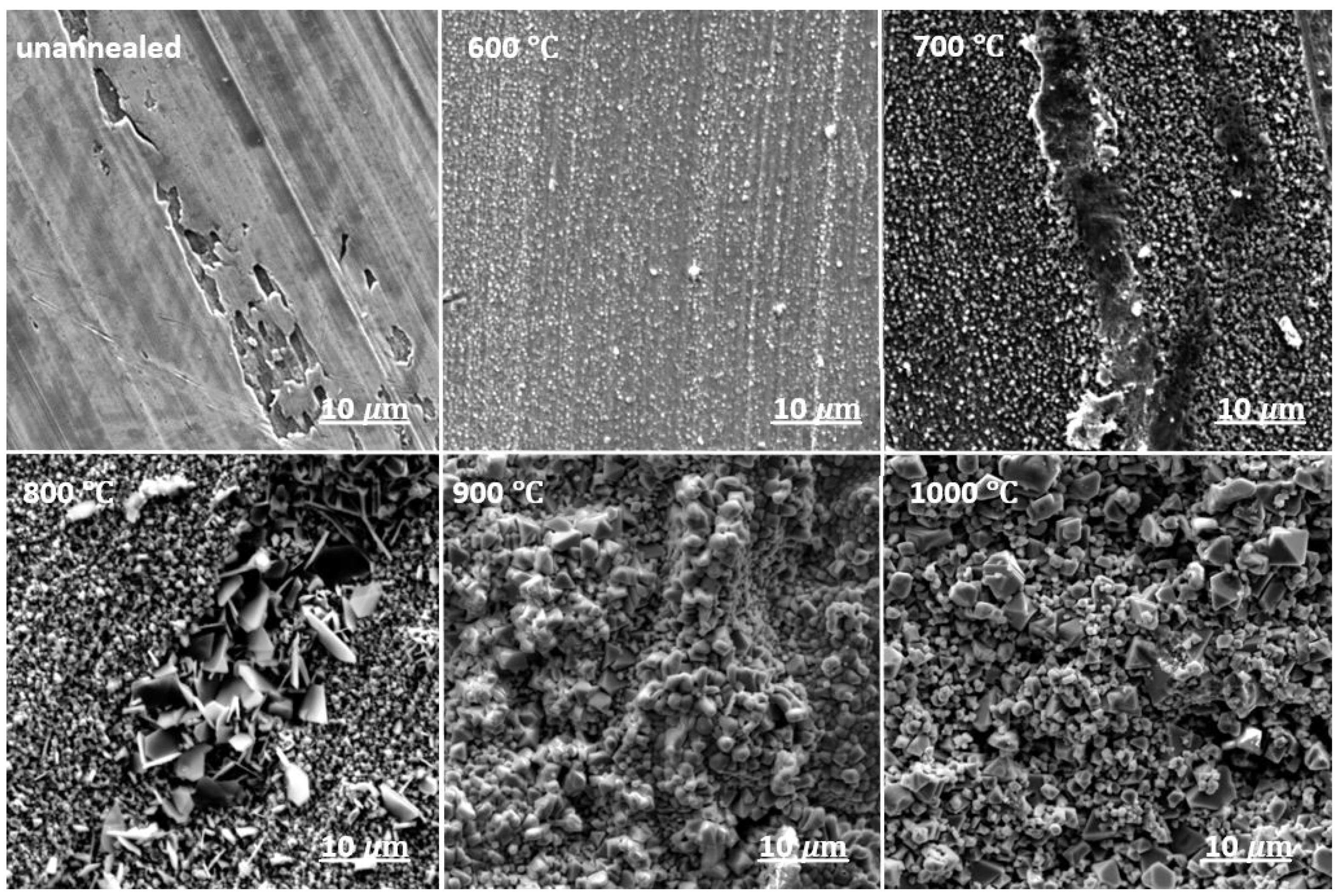
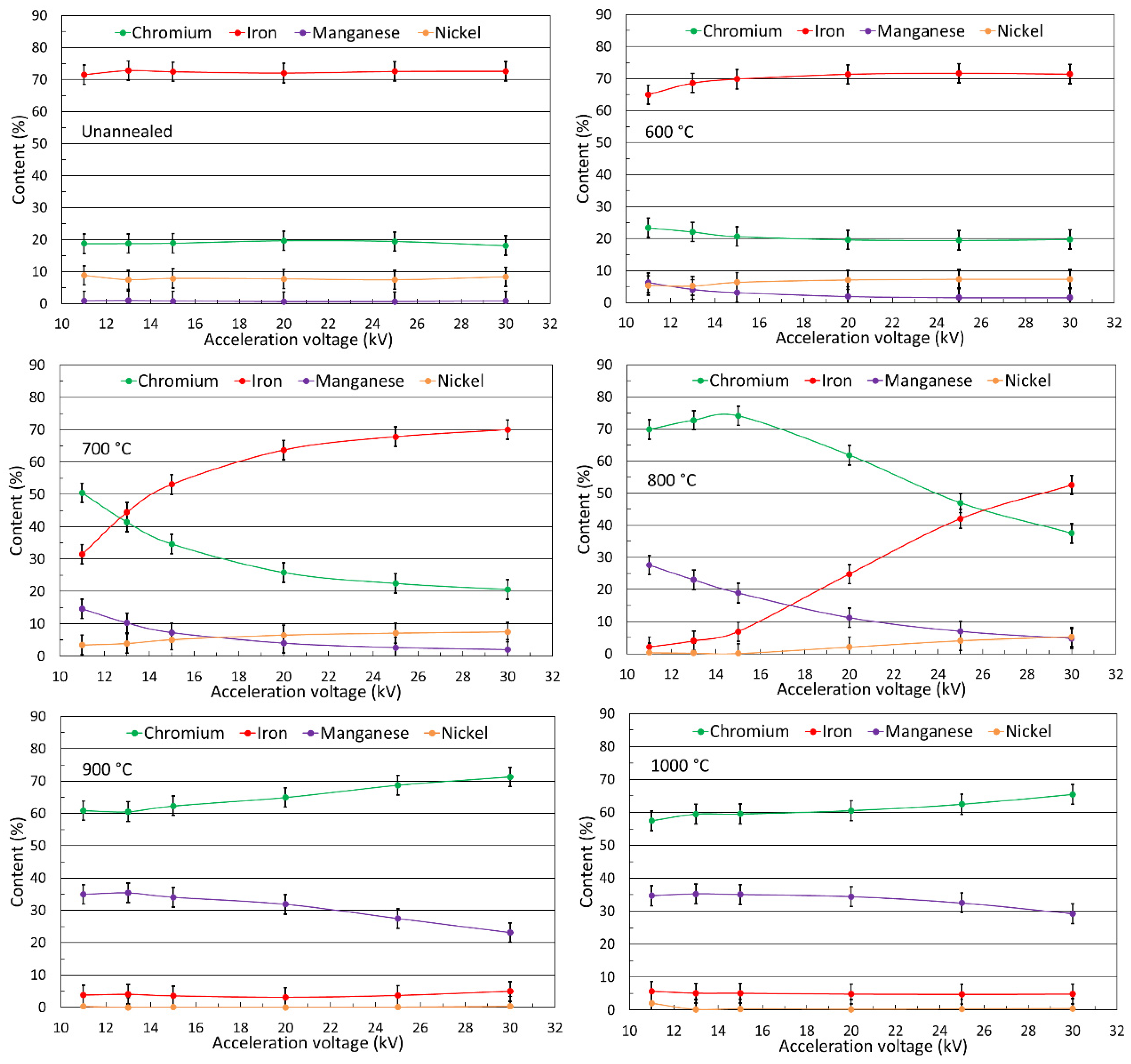
3.1. Oxide Morphology and Element Distribution in the Surface Layer
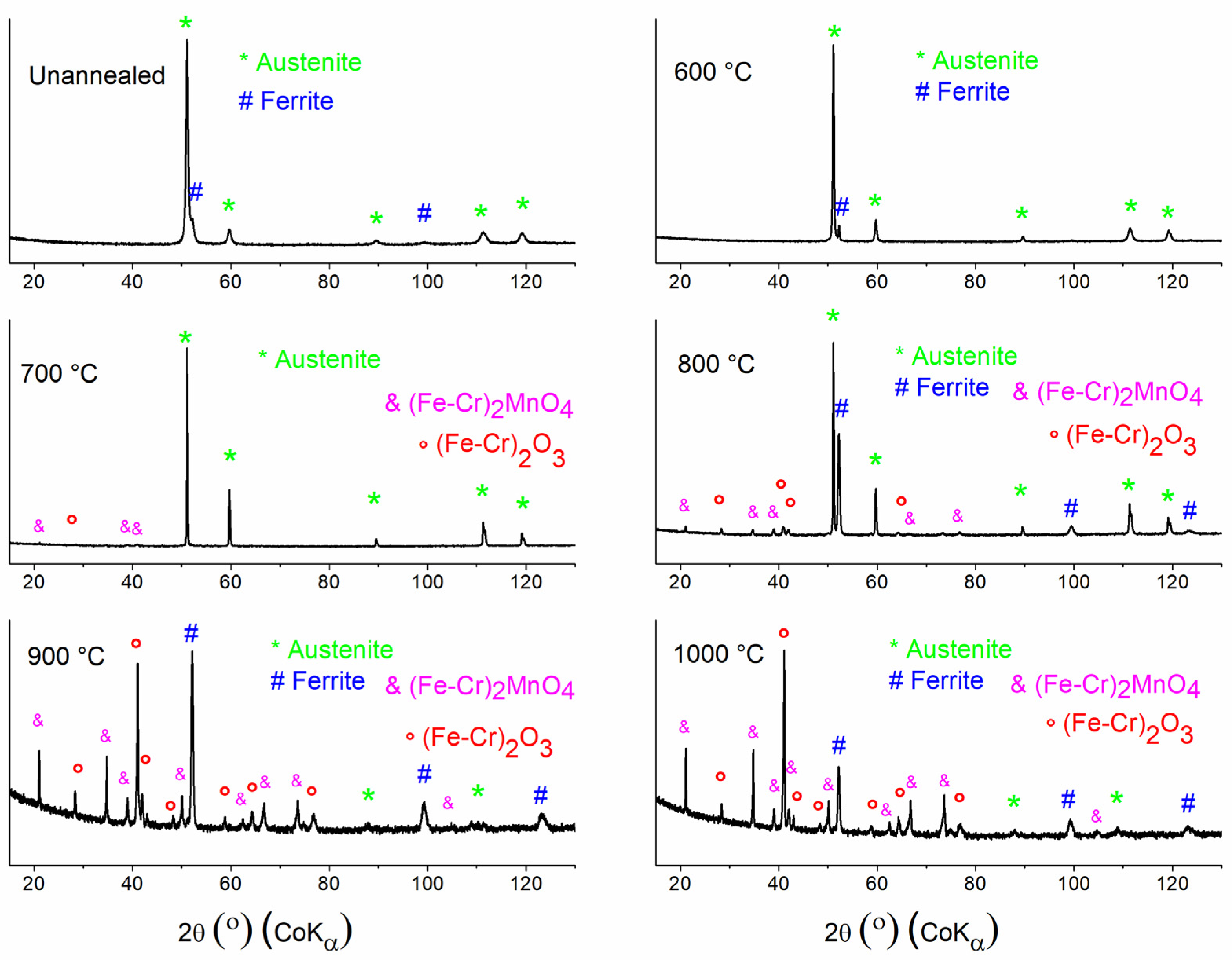
3.2. Surface Phase Transformation Analysis
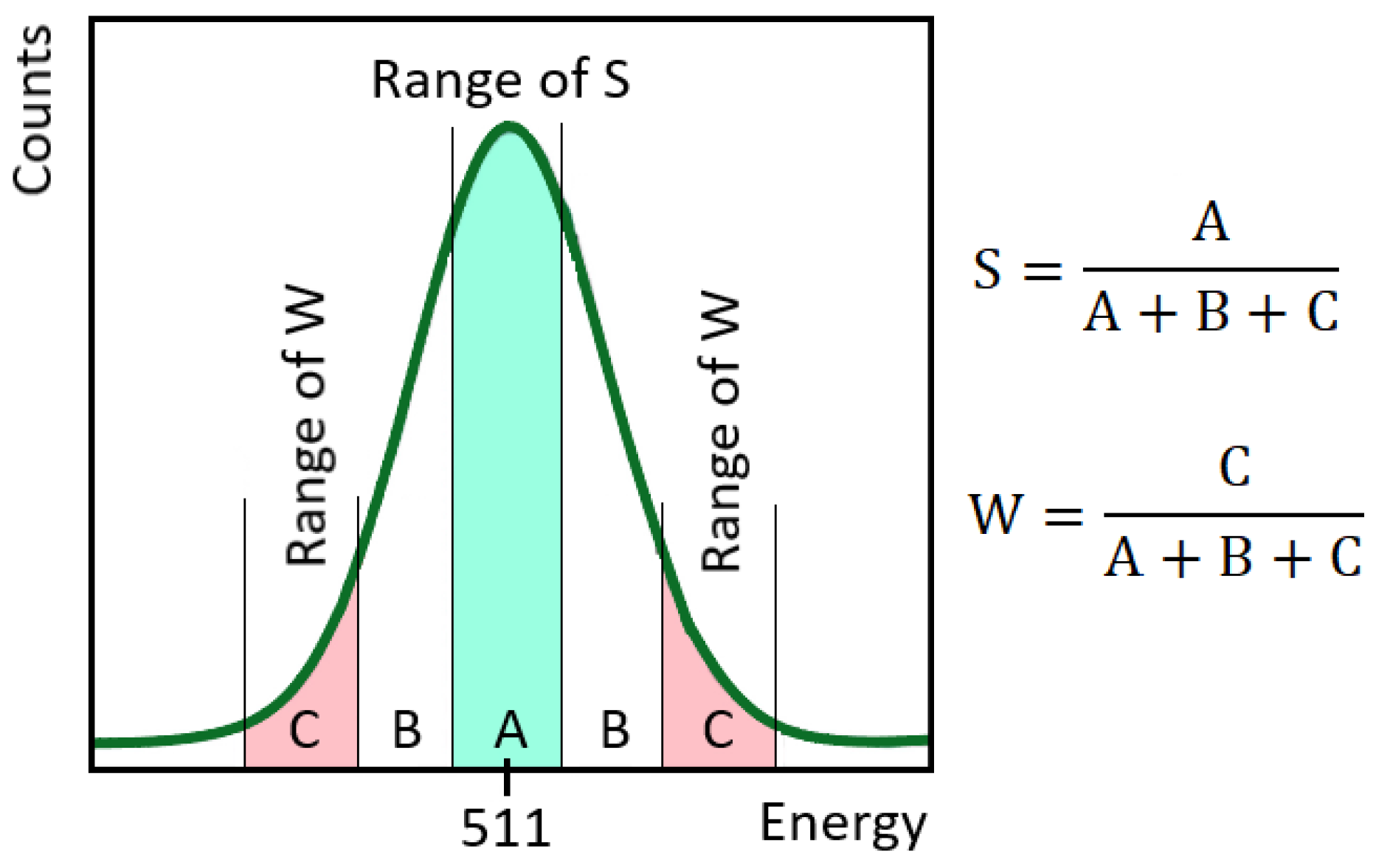
3.3. Application of Mössbauer Spectroscopy for Phase Transformation Analysis
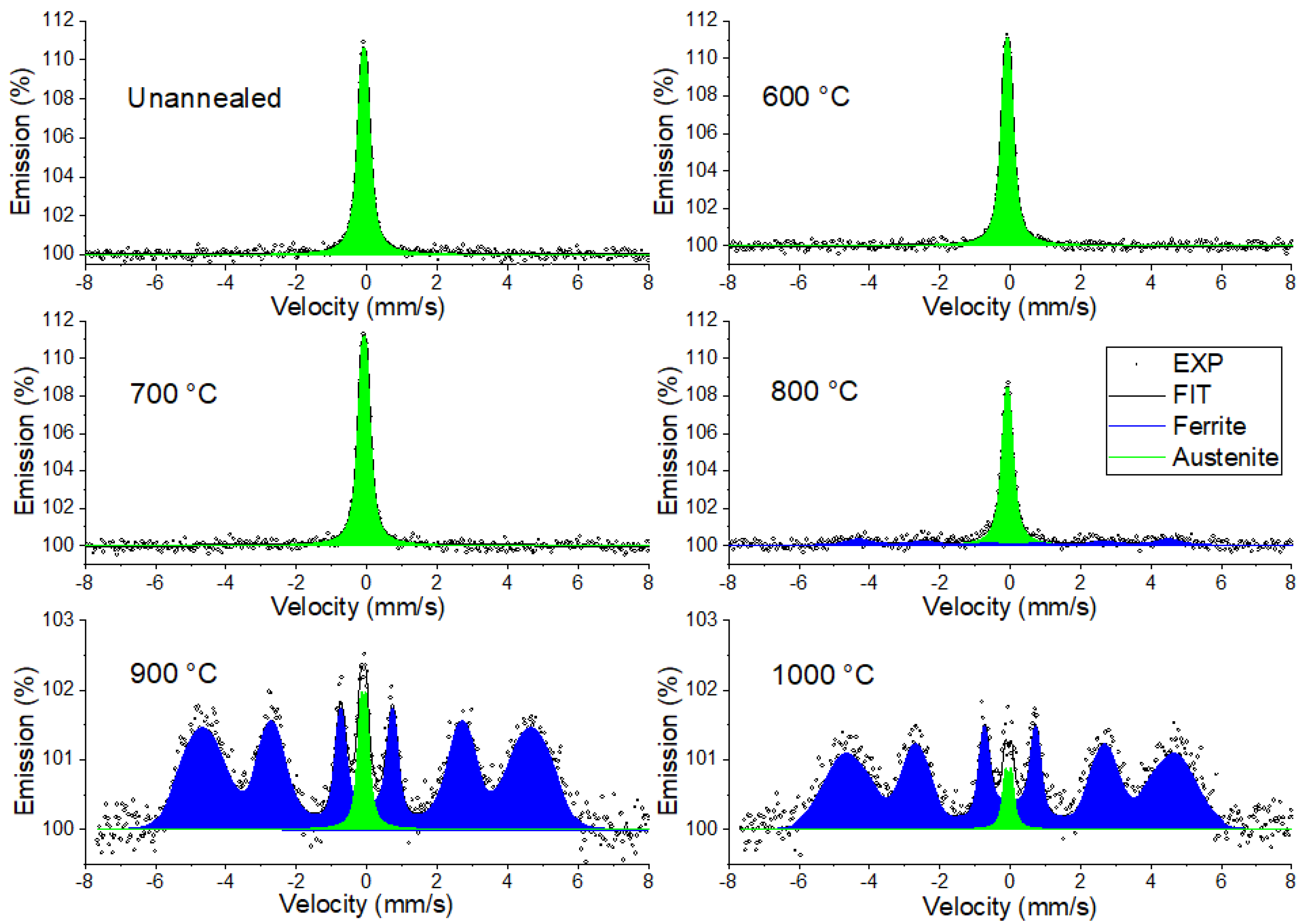
3.3.1. Conversion X-Ray Mössbauer Spectroscopy
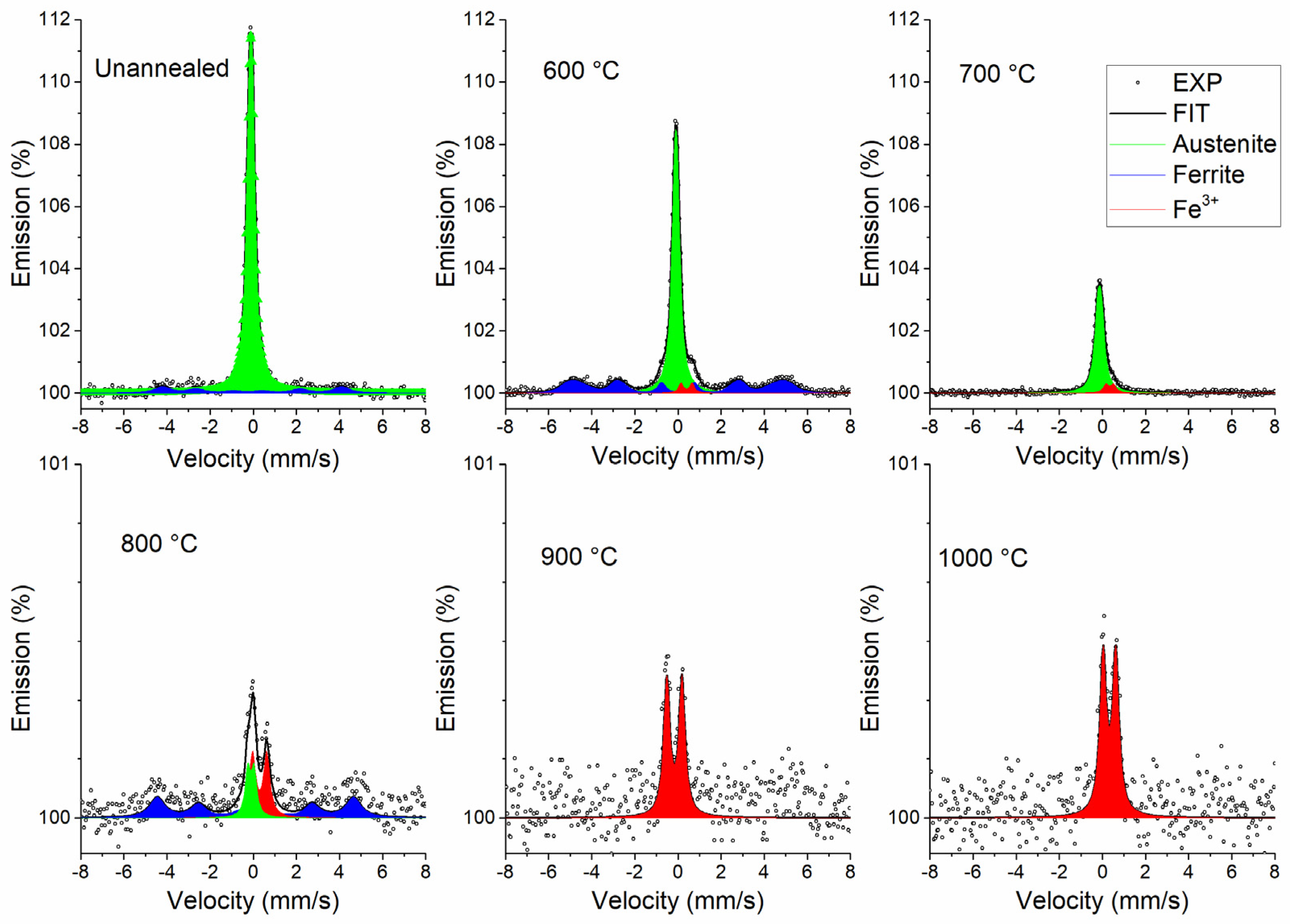
3.3.2. Conversion Electron Mössbauer Spectroscopy
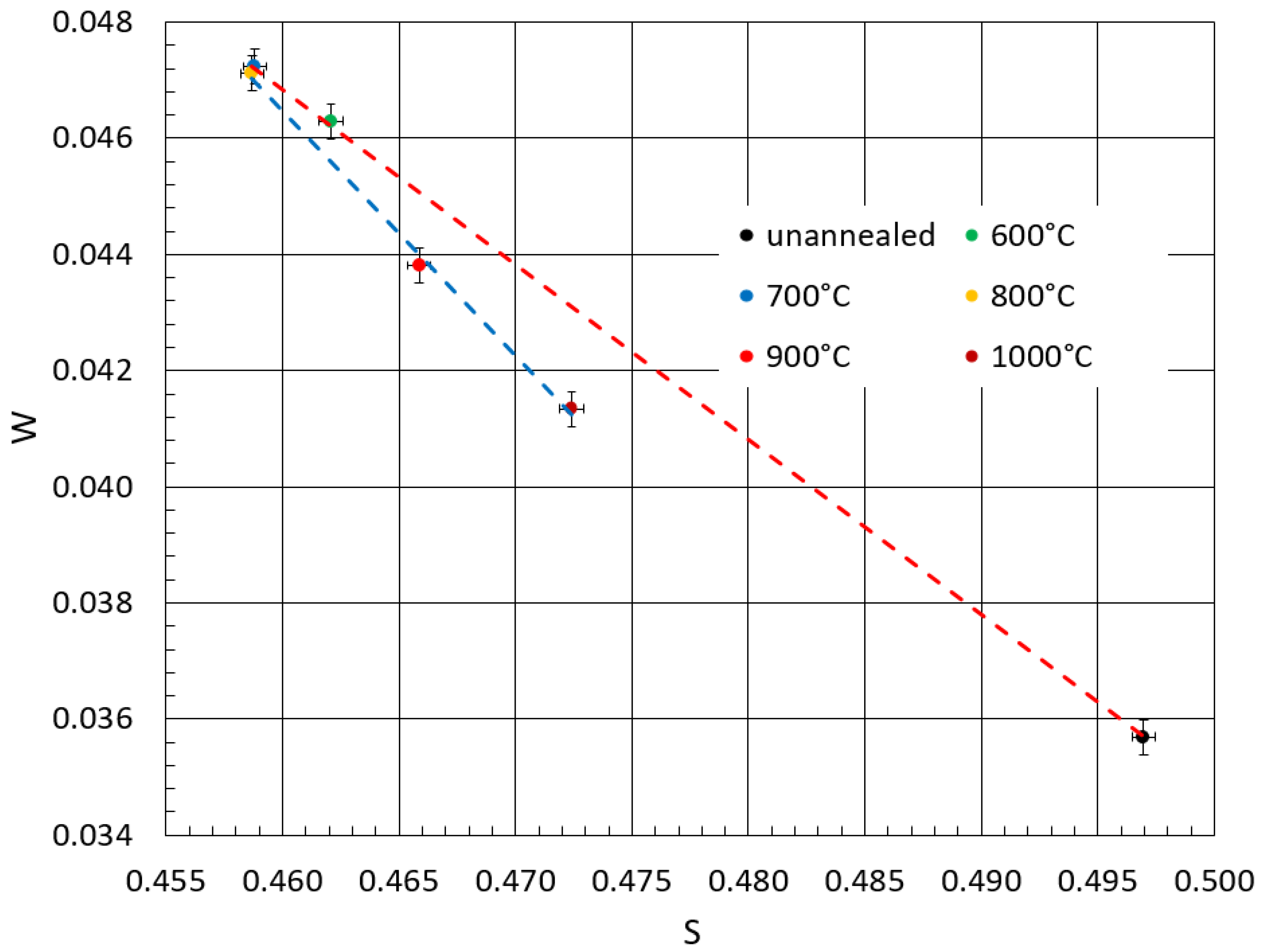
3.4. Positron Annihilation Oxidation Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Podder, A.; Bhanja, A. Application of Stainless Steel in Automobile Industry. Adv. Mater. Res. 2013, 794, 731–740. [Google Scholar] [CrossRef]
- Kangas, P.; Chai, G. Use of advanced austenitic and duplex stainless steels for applications in Oil & Gas and Process industry. Adv. Mater. Res. 2013, 794, 645–669. [Google Scholar] [CrossRef]
- Cetin, M. Effect of boron added corrosion behavior of cast 304 stainless steel. Prot. Met. Phys. Chem. Surf. 2019, 55, 1217–1225. [Google Scholar] [CrossRef]
- Hartwig, A.; Decker, M.; Klein, O.; Karl, H. Stoichiometric titanium dioxide ion implantation in AISI 304 stainless steel for corrosion protection. Nucl. Inst. Met. Phys. Res. Sect. B 2015, 365, 94–99. [Google Scholar] [CrossRef]
- Cetin, M.; Ölmez, E. Corrosion, Wear and Mechanical Properties of Boron Added Cast 304 Stainless Steel. Prot. Met. Phys. Chem. Surf. 2020, 56, 619–627. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Yao, G.; Shi, Y.; Zhang, Y. Effect of annealing treatment on microstructural evolution and deformation behavior of 304L stainless steel made by laser powder bed fusion. Int. J. Plast. 2022, 155, 103335. [Google Scholar] [CrossRef]
- Xu, Q.; Zhu, J.; Zong, Y.; Liu, L.; Zhu, X.; Zhang, F.; Luan, B. Effect of drawing and annealing on the microstructure and mechanical properties of 304 austenitic stainless steel wire. Mater. Res. Express 2021, 8, 126530. [Google Scholar] [CrossRef]
- Albritton, O.W. A Study of Sensitization in Types 304 and 304L Stainless Steels Using Mössbauer Spectroscopy. Corrosion 1968, 24, 389–392. [Google Scholar] [CrossRef]
- Souza, P.A., Jr.; Macêdo, M.C.S.; Queiroz, R.S.; Klingelhöfer, G. Atmospheric Corrosion Investigation in Industrial, Marine and Rural Environments in South-East Brazil. Hyperfine Interact. 2002, 139, 183–191. [Google Scholar] [CrossRef]
- Carbucicchio, M.; Palombarini, G.; Savorelli, G. Wet corrosion of a cw power laser melted AISI 304 stainless steel. Nucl. Inst. Met. Phys. Res. Sect. B 1993, 76, 170–172. [Google Scholar] [CrossRef]
- Chabica, M.E.; Williamson, D.L.; Wei, R.; Wilbur, P.J. Microstructure and corrosion of nitrogen implanted AISI 304 stainless steel. Surf. Coat. Technol. 1992, 51, 24–29. [Google Scholar] [CrossRef]
- Arshed, M.; Butt, N.M.; Siddique, M.; Anwar-ul-Islam, M. Mössbauer study of corrosion of SS-304 exposed to sea water. J. Radioanal. Nucl. Chem. Lett. 1992, 165, 107–113. [Google Scholar] [CrossRef]
- Kasparova, O.V.; Baldokhin, Y.V. New Concept of the Mechanism of Intergranular Corrosion of Stainless Steels. Prot. Met. 2007, 43, 235–240. [Google Scholar] [CrossRef]
- León-Silva, U.; Nicho, M.E.; González-Rodríguez, J.G. Effect of thermal annealing of poly(3-octylthiophene) films covered stainless steel on corrosion properties. J. Solid State Electrochem. 2010, 14, 1089–1100. [Google Scholar] [CrossRef]
- Etienne, A.; Radiguet, B.; Genevois, C.; Le Breton, J.M.; Valiev, R.; Pareige, P. Thermal stability of ultrafine-grained austenitic stainless steels. Mater. Sci. Eng. A 2010, 527, 5805–5810. [Google Scholar] [CrossRef]
- Chen, A.Y.; Zhang, J.B.; Song, H.W.; Lu, J. Thermal-induced inverse γ/α′ phase transformation in surface nanocrystallization layer of 304 stainless steel. Surf. Coat. Technol. 2007, 201, 7462–7466. [Google Scholar] [CrossRef]
- Merakeb, N.; Messai, A.; Ayesh, A.I. Investigation of phase transformation for ferrite-austenite structure in stainless steel thin films. Thin Solid Film. 2016, 606, 120–126. [Google Scholar] [CrossRef]
- Wu, Y.C.; Zhang, R.; Chen, H.; Li, Y.; Zhang, J.; Zhu, D.M.; Jean, Y.C. Corrosion of iron and stainless steels studied using slow positron beam technique. Rad. Phy. Chem. 2003, 68, 599–603. [Google Scholar] [CrossRef]
- Wu, Y.C.; Chen, C.Q.; Chen, Y.Q.; Hu, Y.; Cheng, J.; Wang, S.J.; Jean, Y.C. Hydrogen- and corrosion-related defect characterization of iron and steels using a slow positron beam. Positron Positronium Chem. 2009, 607, 111–113. [Google Scholar] [CrossRef]
- Yabuuchi, A.; Maekawa, M.; Kawasuso, A. Vacancy defects in a stress-corrosion-cracked Type 304 stainless steel investigated by positron annihilation spectroscopy. J. Nucl. Mat. 2011, 419, 9–14. [Google Scholar] [CrossRef]
- Thaveeprungsriporn, V.; Swatewacharkul, P.; Talerngsuk, K.; Punnachaiya, S.; Thong-Aram, D. Microstructure characterization using compensated Doppler-broadened positron annihilation spectroscopy. Nucl. Inst. Met. Phys. Res. Sect. B 1999, 155, 459–467. [Google Scholar] [CrossRef]
- Horodek, P.; Siemek, K.; Kobets, A.G.; Kulik, M.; Meshkov, I.N. Positron beam and RBS studies of thermally grown oxide films on stainless steel grade 304. Appl. Surf. Sci. 2015, 333, 96–103. [Google Scholar] [CrossRef]
- Maekawa, M.; Yabuuchi, Y.; Kawasuso, A. Evaluation of stainless steel under tensile stress using positron microbeam. J. Phys. Conf. Ser. 2010, 225, 012033. [Google Scholar] [CrossRef]
- Horodek, P.; Dryzek, J.; Kobets, A.; Kulik, M.; Lokhmatov, V.; Meshkov, I.; Orlov, O.; Pavlov, V.; Rudakov, A.; Sidorin, A.; et al. Slow Positron Beam Studies of the Stainless Steel Surface Exposed to Sandblasting. Acta Phys. Pol. A 2014, 125, 714–717. [Google Scholar] [CrossRef]
- Dryzek, E.; Sarnek, M.; Wrobel, M. Reverse transformation of deformation-induced martensite in austenitic stainless steel studied by positron annihilation. J. Mater. Sci. 2014, 49, 8449–8458. [Google Scholar] [CrossRef]
- Zeman, A.; Novotny, R.; Uca, O.; Krsjak, V.; Macak, J.; Debarberis, L. Behavior of cold-worked AISI-304 steel in stress-corrosion cracking process: Microstructural aspects. Appl. Surf. Sci. 2008, 255, 160–163. [Google Scholar] [CrossRef]
- Principi, G. The Mössbauer Effect: A Romantic Scientific Page. Metals 2020, 10, 992. [Google Scholar] [CrossRef]
- Hori, F.; Oshima, R. Positron Annihilation Study of Fatigue in Type 304 Stainless Steel. Mat. Sci. Forum 2001, 363–365, 204–206. [Google Scholar] [CrossRef]
- Hartley, J.H.; Howell, R.H.; Asoka-Kumar, P.; Sterne, P.A.; Akers, D.; Denison, A. Positron annihilation studies of fatigue in 304 stainless steel. App. Sur. Sci. 1999, 149, 204–206. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, Z.; Wang, J.; Okonkwo, B.O.; Han, E.-H. Effect of MeV Fe Ions Irradiation on the Microstructure and Property of Nuclear Grade 304 Stainless Steel: Characterized by Positron Annihilation Spectroscopy, Transmission Electron Microscope and Nanoindentation. Acta Metall. Sin. (Engl. Lett.) 2021, 34, 1695–1703. [Google Scholar] [CrossRef]
- Wu, Y.C.; Zhang, X.H.; Jean, Y.C.; Suzuki, R.; Ohdaira, T. Positron Annihilation Study of Hyrogen-Induced Defects in AISI 304 Stainless Steel. Mat. Sci. Forum 2004, 445–446, 213–215. [Google Scholar] [CrossRef]
- Horodek, P.; Siemek, K.; Dryzek, J.; Kobets, A.G.; Wróbel, M. Positron Annihilation and Complementary Studies of Stainless Steel Exposed to Sandblasting at Different Angles. Tribol. Lett. 2017, 65, 30. [Google Scholar] [CrossRef]
- Kellai, A.; Kahla, S.; Dehimi, S.; Kaba, L.; Boutaghour, Z. Effect of post weld heat treatment on the microstructure and mechanical properties of a gas-tungsten-arc-welded 304 stainless steel. Int. J. Adv. Manuf. Technol. 2022, 121, 8171–8186. [Google Scholar] [CrossRef]
- Khodadadi, A.; Shamanian, M.; Karimzadeh, M. Microstructure and Mechanical Properties of Dissimilar Friction Stir Spot Welding Between St37 Steel and 304 Stainless Steel. J. Mater. Eng. Perform. 2017, 26, 2847–2858. [Google Scholar] [CrossRef]
- Nam, T.-H.; An, E.; Kim, B.J.; Shin, S.; Ko, W.-S.; Park, N.; Kang, N.; Jeon, J.B. Effect of Post Weld Heat Treatment on the Microstructure and Mechanical Properties of a Submerged-Arc-Welded 304 Stainless Steel. Metals 2018, 8, 26. [Google Scholar] [CrossRef]
- Abioye, T.E.; Omotehinse, I.S.; Oladele, I.O.; Olugbade, T.O.; Ogedengbe, T.I. Effects of post-weld heat treatments on the microstructure, mechanical and corrosion properties of gas metal arc welded 304 stainless steel. World J. Eng. 2020, 17, 87–96. [Google Scholar] [CrossRef]
- Sedláčková, A.; Ivanova, T.; Mashlan, M.; Doláková, H. Phase Changes in the Surface Layer of Stainless Steel Annealed at a Temperature of 550 °C. Materials 2022, 15, 8871. [Google Scholar] [CrossRef]
- Chandra-ambhorn, S.; Saranyachot, P.; Thublaor, T. Higher temperature oxidation behavior of Fe-15.7 wt% Cr-8.5 wt% Mn in oxygen without and with water vapor at 700 °C. Corros. Sci. 2019, 148, 39–47. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, K.; Fang, X.; Xiong, Z.; Wei, L.; Zhu, P.; Li, X. Oxidation behavior of 316L austenitic stainless steel in higher temperature air with long-term exposure. Mater. Res. Express 2020, 7, 066517. [Google Scholar] [CrossRef]
- Pechousek, J.; Mashlan, M. Mössbauer spectrometer as a virtual instrument in the PXI/Compact PCI modular system. Czechoslov. J. Phys. 2005, 55, 853. [Google Scholar] [CrossRef]
- Kholmetskii, A.L.; Misevich, O.V.; Mashlan, M.; Chudakov, V.A.; Anashkevich, A.F.; Gurachevskii, V.L. Air scintillation detector for conversion electrons Mössbauer spectroscopy (CEMS). Nucl. Instrum. Methods Phys. Res. B 1997, 129, 110. [Google Scholar] [CrossRef]
- Klencsár, Z.; Kuzmann, E.; Vértes, A. User-Friendly Program for Multifold Evaluation of Mössbauer Spectra. Hyperfine Interact. 1998, 112, 269. [Google Scholar] [CrossRef]
- Klencsár, Z.; Kuzmann, E.; Vértes, A. User-Friendly software for Mössbauer spectrum analysis. J. Radioanal. Nucl. Chem. 1996, 210, 105. [Google Scholar] [CrossRef]
- Dryzek, J.; Wesseling, C.; Dryzek, E.; Cleff, B. Migration of Vacancies in Stainless Steel Measured by Positron Annihilation. Mater. Lett. 1994, 21, 209–214. [Google Scholar] [CrossRef]
- Selim, F.A. Positron annihilation spectroscopy of defects in nuclear and irradiated materials—A review. Mater. Charact. 2021, 174, 110952. [Google Scholar] [CrossRef]
- Bonny, G.; Konstantinovic, M.I.; Bakaeva, A.; Chao, Y.; Castin, N.; Mergia, K.; Chatzikos, V.; Dellis, S.; Khvan, T.; Bakaev, A.; et al. Trends in vacancy distribution and hardness of high temperature neutron irradiated single crystal tungsten. Acta Mater. 2020, 198, 1–9. [Google Scholar] [CrossRef]
- Han, F.; Lin, G.Y.; Wang, Z.L.; Peng, D.S. Oxidation analysis of 304 Austenitic Stainless Steel Sheet after Intermediate Annealing. Key Eng. Mater. 2011, 474–476, 376–380. [Google Scholar] [CrossRef]
- Ivanova, T.; Kořenek, M.; Mashlan, M. Using Mössbauer Spectroscopy to Evaluate the Influence of Heat Treatment on the Surface Characteristics of Additive Manufactured 316L Stainless Steel. Materials 2024, 17, 3494. [Google Scholar] [CrossRef]
- Ozturk, B.; Matway, R. Oxidation of Type 304 Stainless Steel under Simulated Annealing Conditions. ISIJ Int. 1997, 37, 169–175. [Google Scholar] [CrossRef]
- Grayeli-Kopri, A.-R.; Savaloni, H.; Habibi, M. Corrosion inhibition of stainless steel type AISI 304 by Mn coating and subsequent annealing with flow of nitrogen at different temperatures. Appl. Surf. Sci. 2013, 276, 269–275. [Google Scholar] [CrossRef]
- Castaing, R. Applications des Sondes Electronique a une Methode D’analyse Ponctuelle Chimique et Cristallographique. Ph.D. Thesis, University of Paris, Paris, France, 1952. [Google Scholar]
- Biehler, J.; Hoche, H.; Oechsner, M. Nitriding behavior and corrosion properties of AISI 304L and 316L austenitic stainless steel with deformation-induced martensite. Surf. Coat. Technol. 2017, 324, 121–128. [Google Scholar] [CrossRef]
- Zhang, X.; Misra, A.; Schulze, R.; Wetteland, C.; Wang, H.; Nastasi, M. Critical factors that determine face-centered cubic to body-centered cubic phase transformation in sputter-deposited austenitic stainless steel films. J. Mater. Res. 2004, 19, 1696–1702. [Google Scholar] [CrossRef]
- Shuro, I.; Umemoto, M.; Todaka, Y.; Yokoyama, S. Phase Transformation and Annealing Behavior of SUS 304 Austenitic Stainless Steel Deformed by High Pressure Torsion. Mater. Sci. Forum 2010, 654–656, 334–337. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Kan, W.; Pan, H. Phase transformations and micro cracks induced by hydrogen in cold-rolled and annealed AISI 304 stainless steels. Int. J. Hydrogen Energy 2012, 37, 8724–8728. [Google Scholar] [CrossRef]
- Riffard, F.; Buscail, H.; Rabaste, F.; Caudron, E.; Cueff, R.; Issartel, C.; Karimi, N.; Perrier, S. Manganese effect on isothermal high temperature oxidation behavior of AISI 304 stainless steel. Mater. Sci. Forum 2008, 595, 1127–1134. [Google Scholar] [CrossRef]
- Lothongkum, G.; Tiyawatwitthaya, C.; Prawetpai, N.; Lothongkum, A.W. XRD investigation of the Cu and Mn effects on the oxide scale of hot rolled AISI 304L stainless steel after annealing and shot-blasting. Mater. Test. 2020, 62, 568–572. [Google Scholar] [CrossRef]
- Cook, D.C. Strain induced martensite formation in stainless steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 1987, 18, 201–210. [Google Scholar] [CrossRef]
- Kanaya, K.; Okayama, S. Penetration and energy-loss theory of electrons in solid targets. J. Phys. D Appl. 1972, 5, 43–58. [Google Scholar] [CrossRef]
- Procházka, I. Positron annihilation spectroscopy. Mater. Struct. 2001, 8, 55–60. [Google Scholar]
- Dryzek, E.; Sarnek, M. Reverse Transformation of Deformation Induced Martensite in Austenitic Stainless Steel. In Proceedings of the 41st Polish Seminar on Positron Annihilation, Lublin, Poland, 9–13 September 2013; Volume 125, pp. 710–713. [Google Scholar] [CrossRef]
- Ovchinnikov, V.V. Mössbauer Analysis of the Atomic and Magnetic Structure of Alloys; Cambridge International Science Publishing: Cambridge, UK, 2006; p. 248. [Google Scholar]
- Gütlich, P.; Bill, E.; Trautwein, A.X. Mössbauer Spectroscopy and Transition Metal Chemistry, Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2011; p. 568. [Google Scholar]
- Kuzmann, E.; Nagy, D.; Vértes, A. Critical Review of Analytical Applications of Mössbauer Spectroscopy Illustrated by Mineralogical and Geological Examples (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 801–858. [Google Scholar] [CrossRef]
- Puska, M.J.; Nieminen, R.M. Theory of positrons in solids and on solid surface. Rev. Mod. Phys. 1994, 66, 841–896. [Google Scholar] [CrossRef]
- Schultz, P.J.; Lynn, K.G. Interactions of positron beams with surface, thin films and interfaces. Rev. Mod. Phys. 1994, 60, 701–779. [Google Scholar] [CrossRef]

| Steel | Element Concentration, wt % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | C | Si | Mn | P | S | Cr | Ni | N | |
| 304 | Bal. | 0.07 | 1.0 | 2.0 | 0.045 | ≤0.01 | 17.50–19.50 | 8.00–10.50 | 0.10 |
| Annealing Temperature | Phase | δ [mm/s] | Δ [mm/s] | B [T] | W [mm/s] | A [%] |
|---|---|---|---|---|---|---|
| Unannealed | austenite | −0.08 ± 0.01 | 0.15 ± 0.01 | - | 0.28 ± 0.01 | 100 |
| 600 °C | austenite | −0.08 ± 0.01 | 0.14 ± 0.01 | - | 0.27 ± 0.01 | 100 |
| 700 °C | austenite | −0.08 ± 0.01 | 0.15 ± 0.01 | - | 0.27 ± 0.01 | 100 |
| 800 °C | austenite | −0.08 ± 0.01 | 0.14 ± 0.01 | - | 0.28 ± 0.01 | 70 ± 2 |
| ferrite | 0.09 ± 0.05 | - | 27.4 ± 0.5 | 0.91 ± 0.14 | 30 ± 2 | |
| 900 °C | austenite | −0.09 ± 0.01 | 0.16 ± 0.01 | - | 0.22 ± 0.03 | 9 ± 2 |
| ferrite | 0.01 ± 0.01 | - | 27.7 * | 0.25 ± 0.02 | 91 ± 2 | |
| 1000 °C | austenite | −0.05 ± 0.03 | 0.17 ± 0.01 | - | 0.19 ± 0.03 | 5 ± 2 |
| ferrite | -0.01 ± 0.01 | - | 26.8 * | 0.22 ± 0.02 | 95 ± 2 |
| Annealing Temperature | Phase | δ [mm/s] | Δ [mm/s] | B [T] | W [mm/s] | A [%] |
|---|---|---|---|---|---|---|
| Unannealed | austenite | −0.11 ± 0.01 | 0.17 ± 0.01 | - | 0.32 ± 0.01 | 86 ± 2 |
| ferrite | −0.08 ± 0.06 | - | 25.9 ± 0.5 | 0.98 ± 0.15 | 14 ± 2 | |
| 600 °C | austenite | −0.10 ± 0.01 | 0.15 ± 0.01 | - | 0.34 ± 0.01 | 61 ± 2 |
| ferrite | 0.01 ± 0.02 | - | 30.1 ± 0.1 | 0.97 ± 0.04 | 35 ± 2 | |
| Fe3+ oxide | 0.35 * | 0.58 * | - | 0.37 * | 4 ± 2 | |
| 700 °C | austenite | −0.11 ± 0.01 | 0.19 ± 0.01 | - | 0.37 ± 0.01 | 92 ± 2 |
| Fe3+ oxide | 0.35 * | 0.58 * | - | 0.37 * | 8 ± 2 | |
| 800 °C | austenite | −0.08 ± 0.02 | 0.22 ± 0.02 | - | 0.29 ± 0.05 | 19 ± 2 |
| ferrite | 0.12 ± 0.05 | - | 28.2 ± 0.4 | 0.86 ± 0.14 | 49 ± 2 | |
| Fe3+ oxide | 0.37 ± 0.02 | 0.56 ± 0.03 | - | 0.35 ± 0.04 | 32 ± 2 | |
| 900 °C | Fe3+ oxide | 0.36 ± 0.01 | 0.63 ± 0.02 | - | 0.30 ± 0.03 | 100 |
| 1000 °C | Fe3+ oxide | 0.35 ± 0.01 | 0.58 ± 0.02 | - | 0.37 ± 0.02 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, T.; Kořenek, M.; Mashlan, M.; Fryšák, M. Investigation of Surface Oxidation of Cast Austenitic 304 Stainless Steel at High Temperatures. Metals 2025, 15, 748. https://doi.org/10.3390/met15070748
Ivanova T, Kořenek M, Mashlan M, Fryšák M. Investigation of Surface Oxidation of Cast Austenitic 304 Stainless Steel at High Temperatures. Metals. 2025; 15(7):748. https://doi.org/10.3390/met15070748
Chicago/Turabian StyleIvanova, Tatiana, Michal Kořenek, Miroslav Mashlan, and Martin Fryšák. 2025. "Investigation of Surface Oxidation of Cast Austenitic 304 Stainless Steel at High Temperatures" Metals 15, no. 7: 748. https://doi.org/10.3390/met15070748
APA StyleIvanova, T., Kořenek, M., Mashlan, M., & Fryšák, M. (2025). Investigation of Surface Oxidation of Cast Austenitic 304 Stainless Steel at High Temperatures. Metals, 15(7), 748. https://doi.org/10.3390/met15070748






