The Influence of Beryllium Incorporation into an Al-5wt.%Cu-1wt.%Si Alloy on the Solidification Cooling Rate, Microstructural Length Scale, and Corrosion Resistance
Abstract
1. Introduction
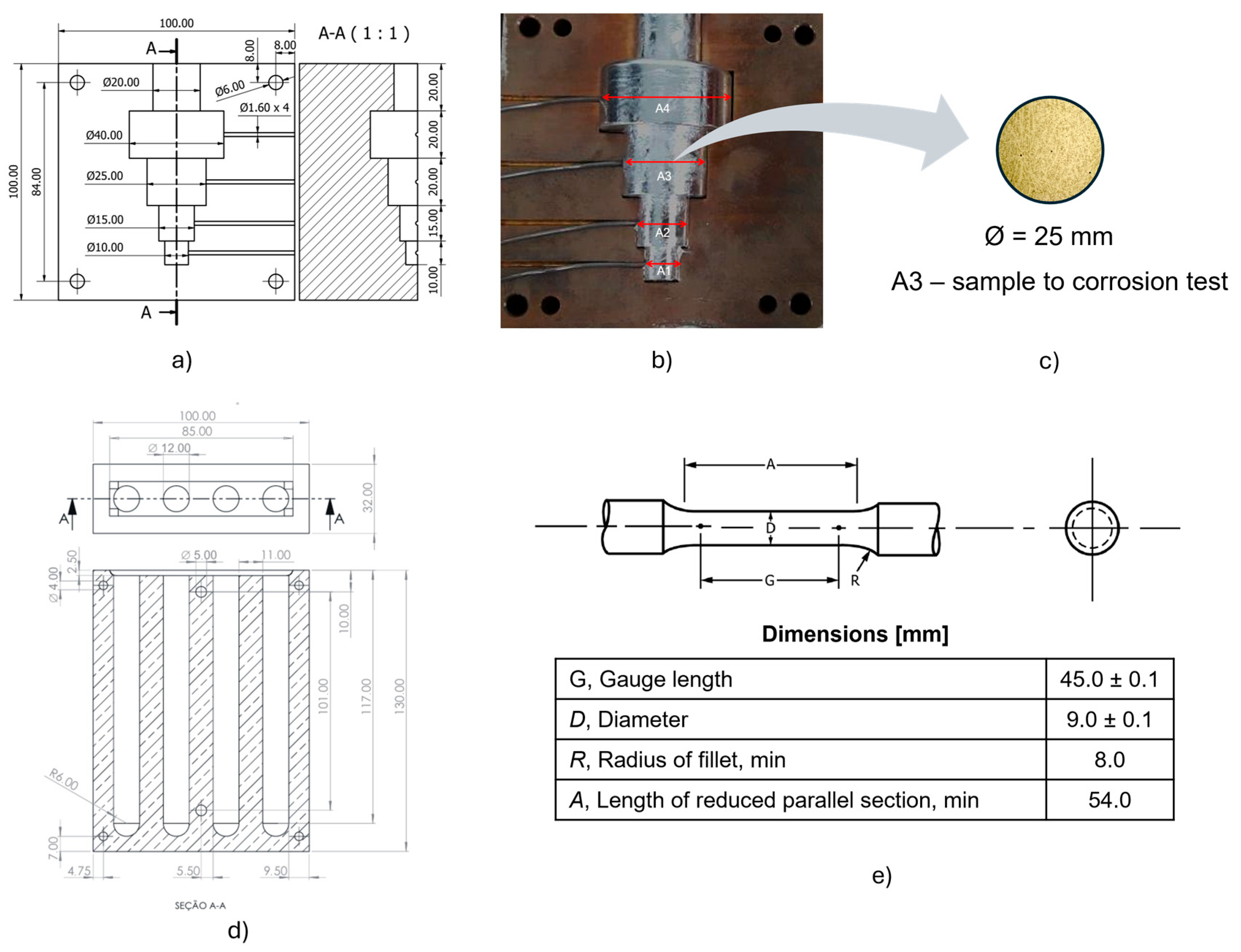
2. Materials and Methods
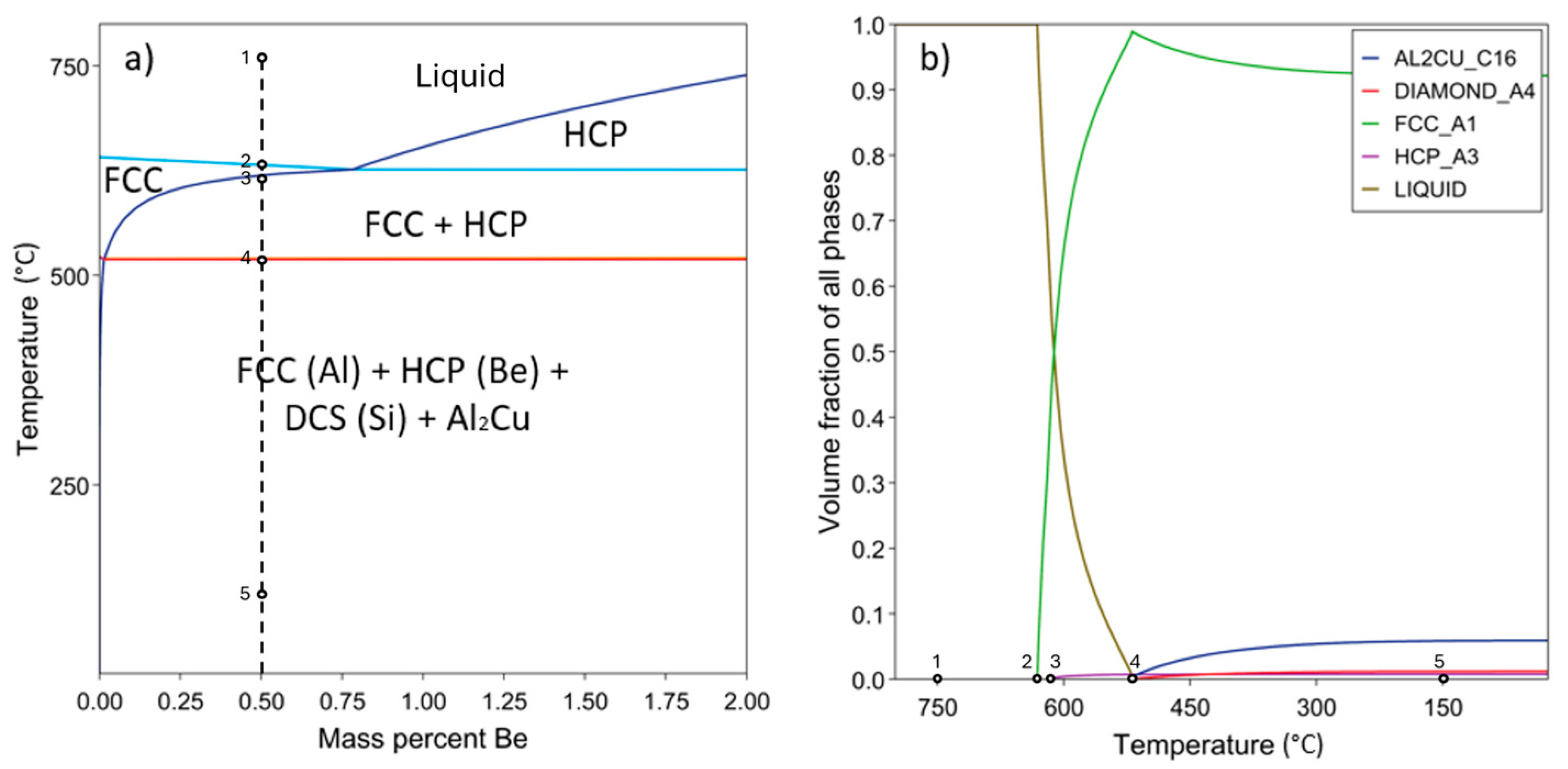
3. Results and Discussion
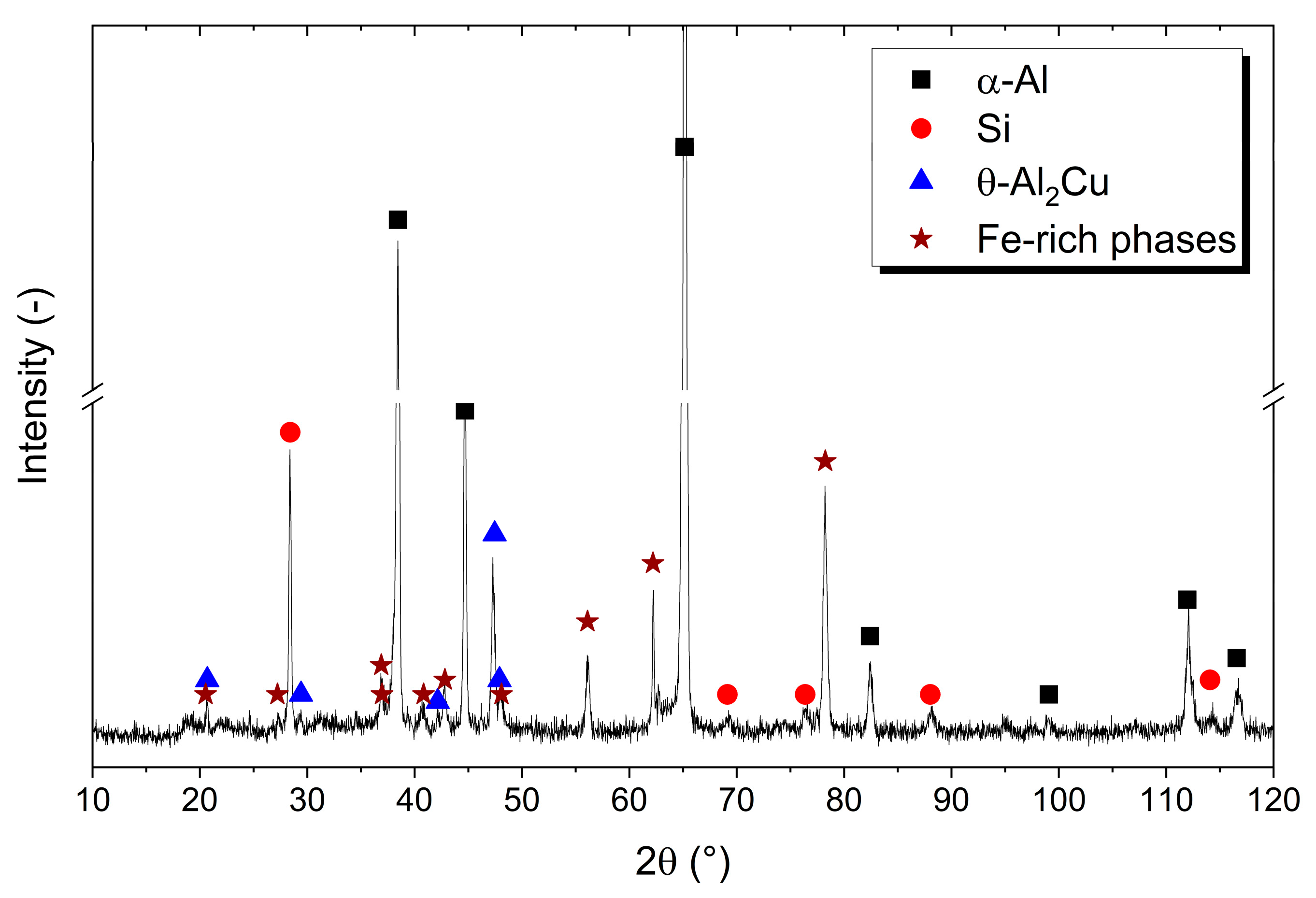
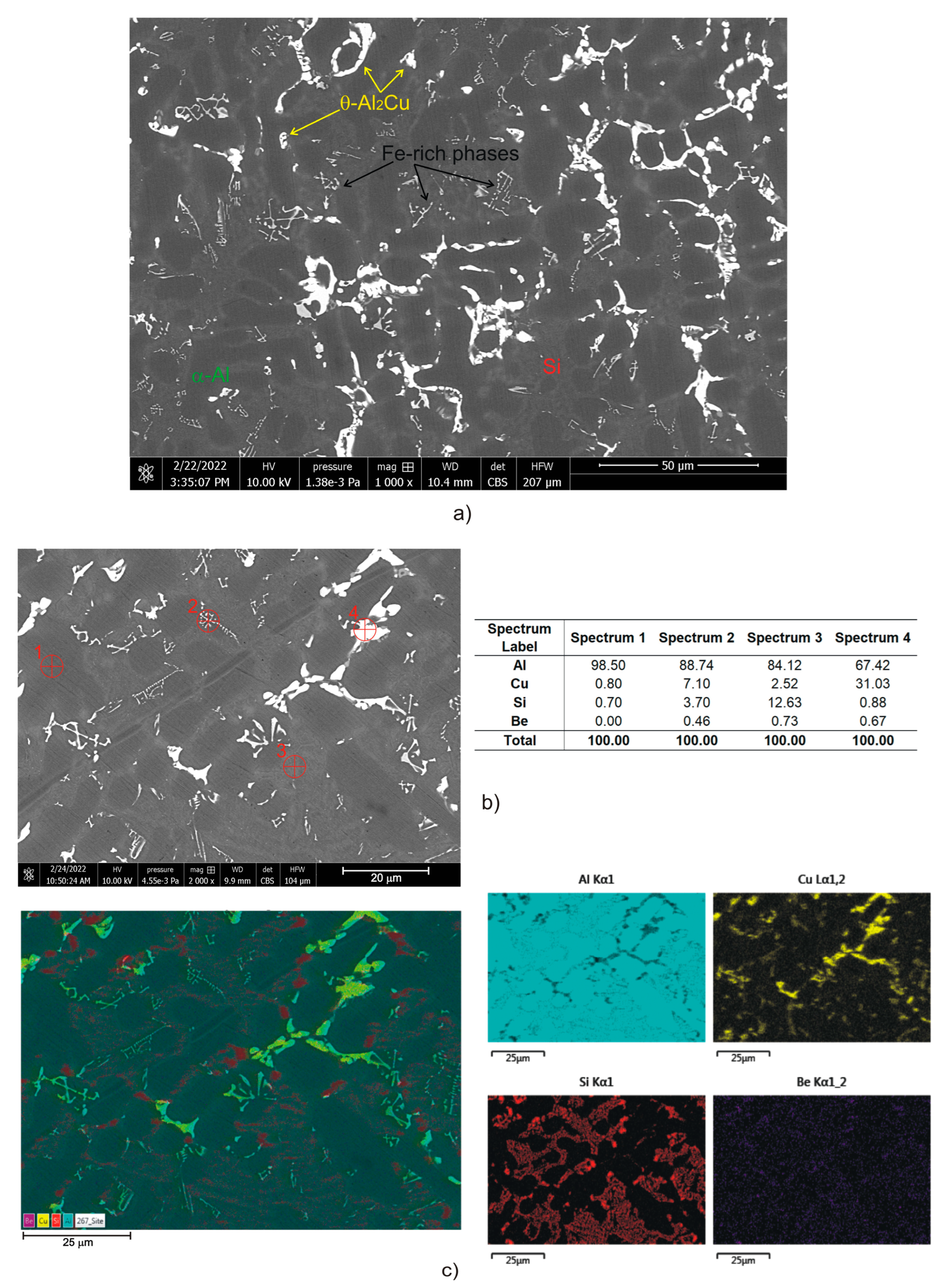
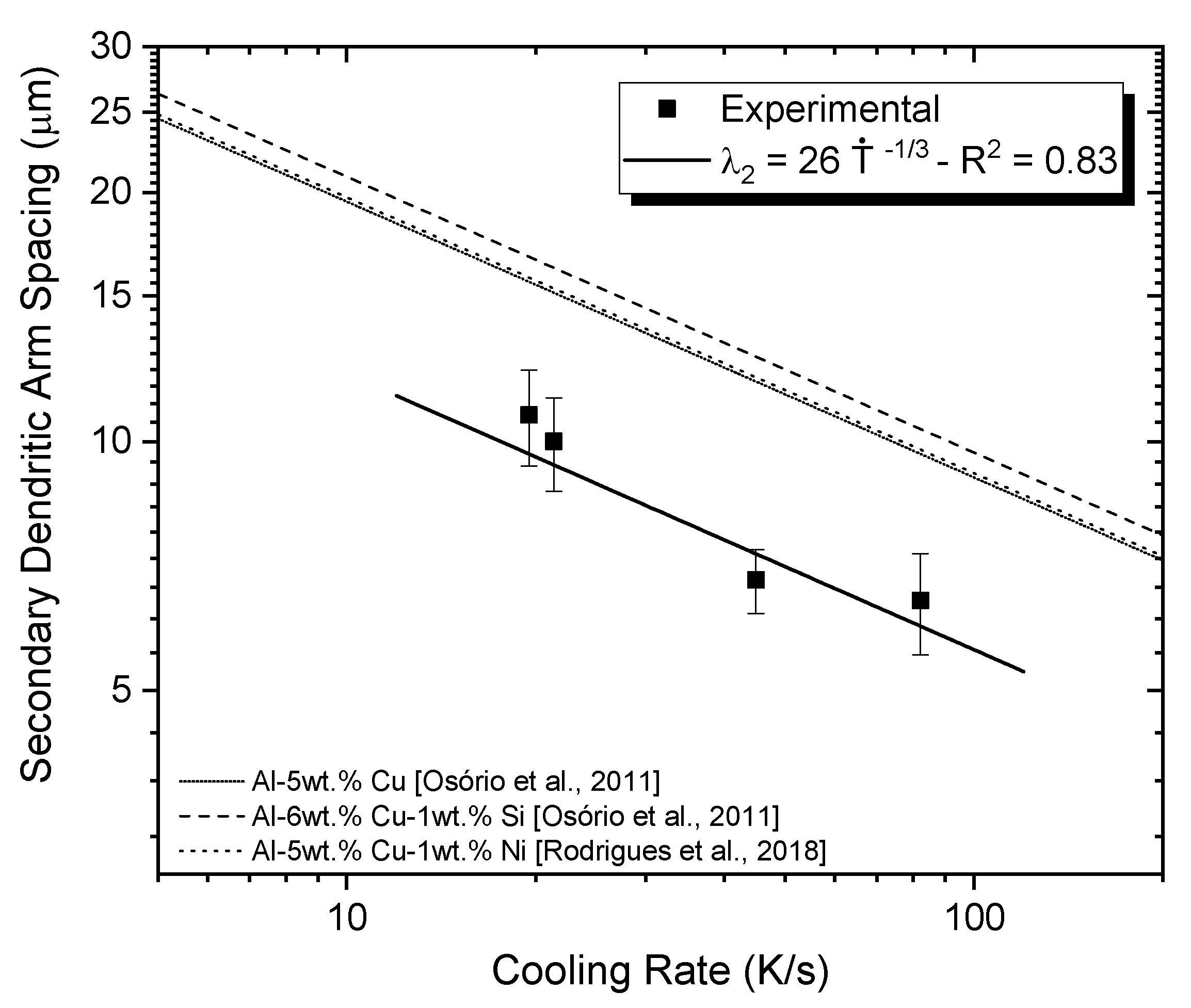
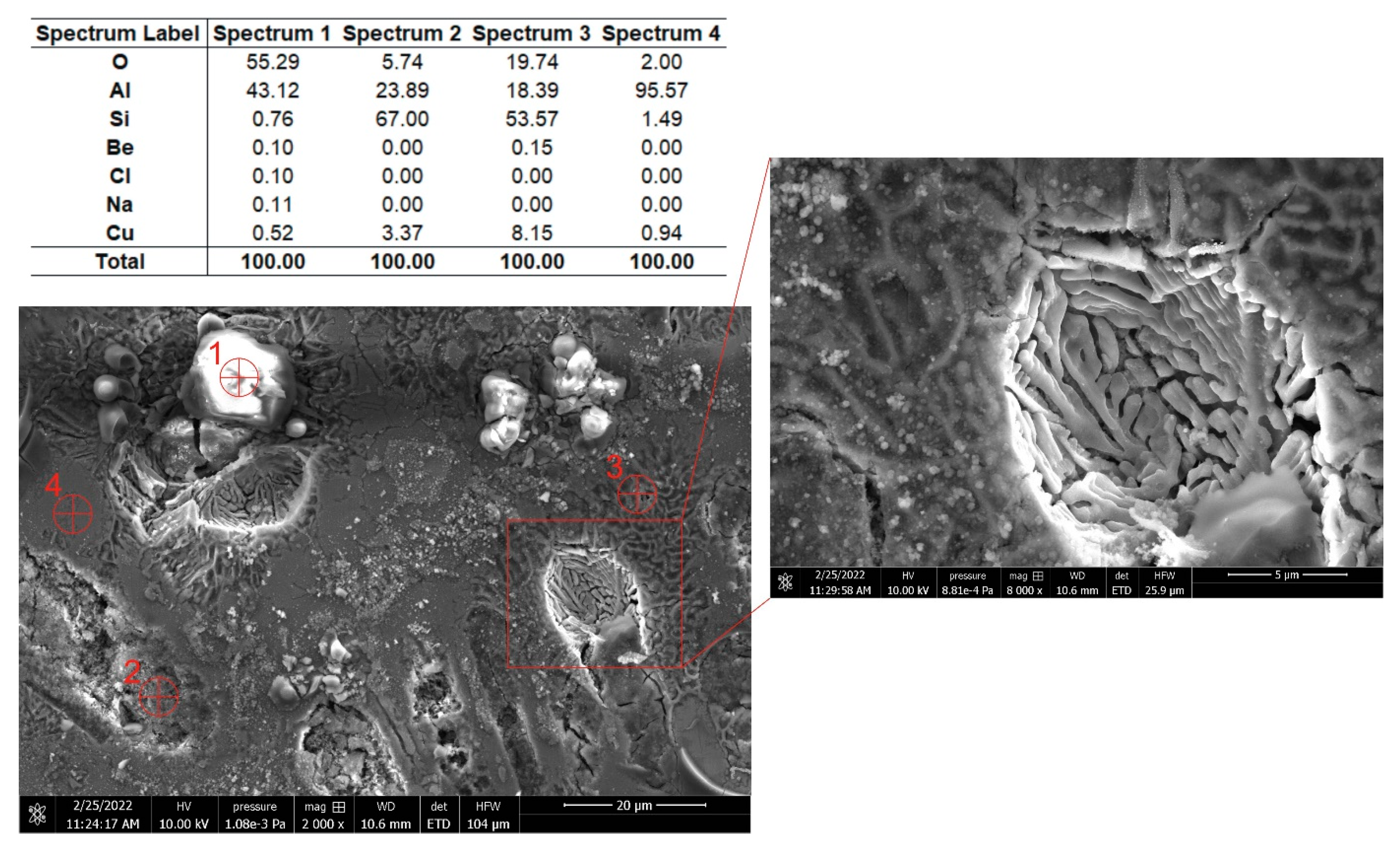
3.1. Microstructural Characterization
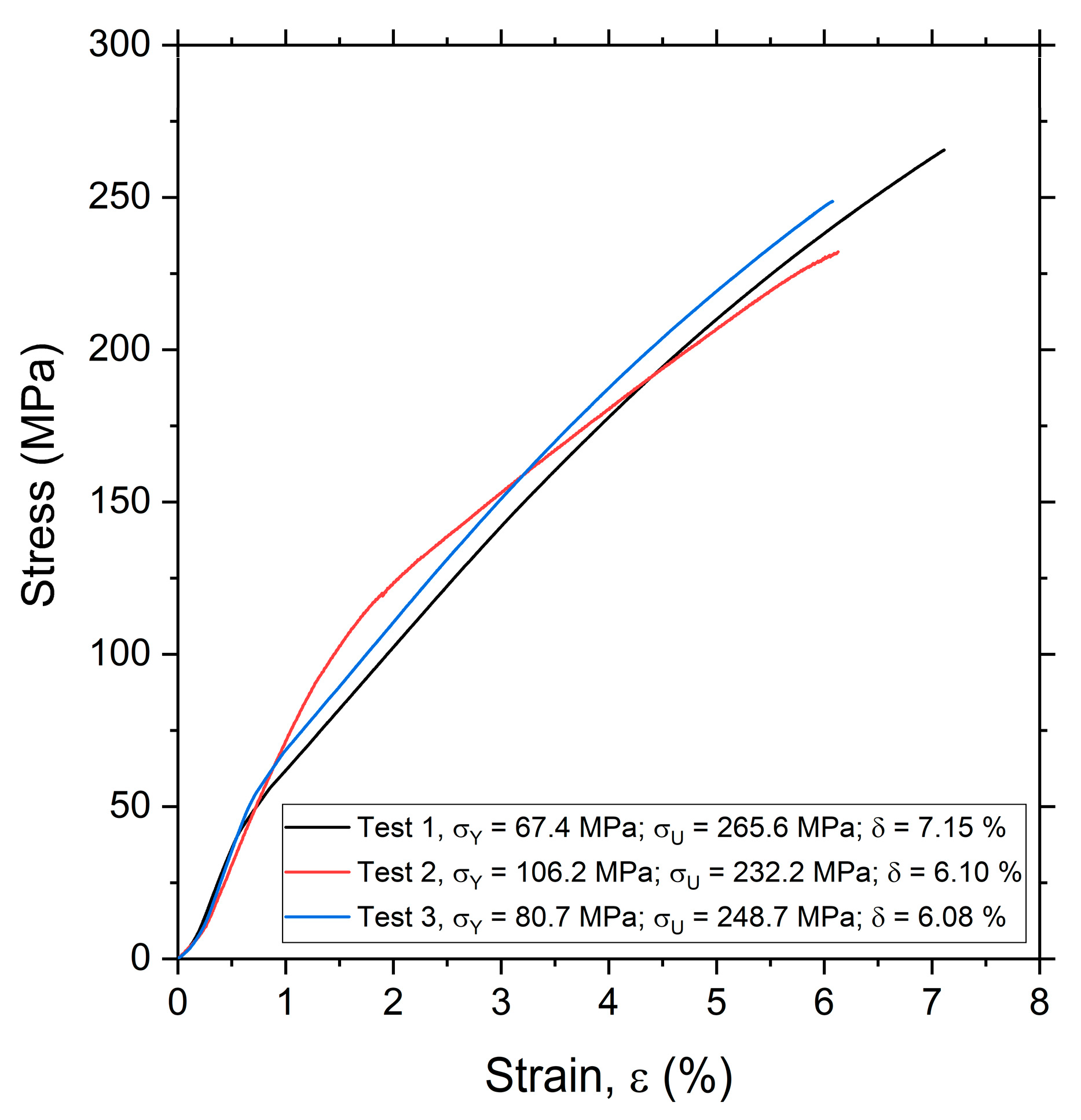
3.2. Tensile Test and Mechanical Behavior
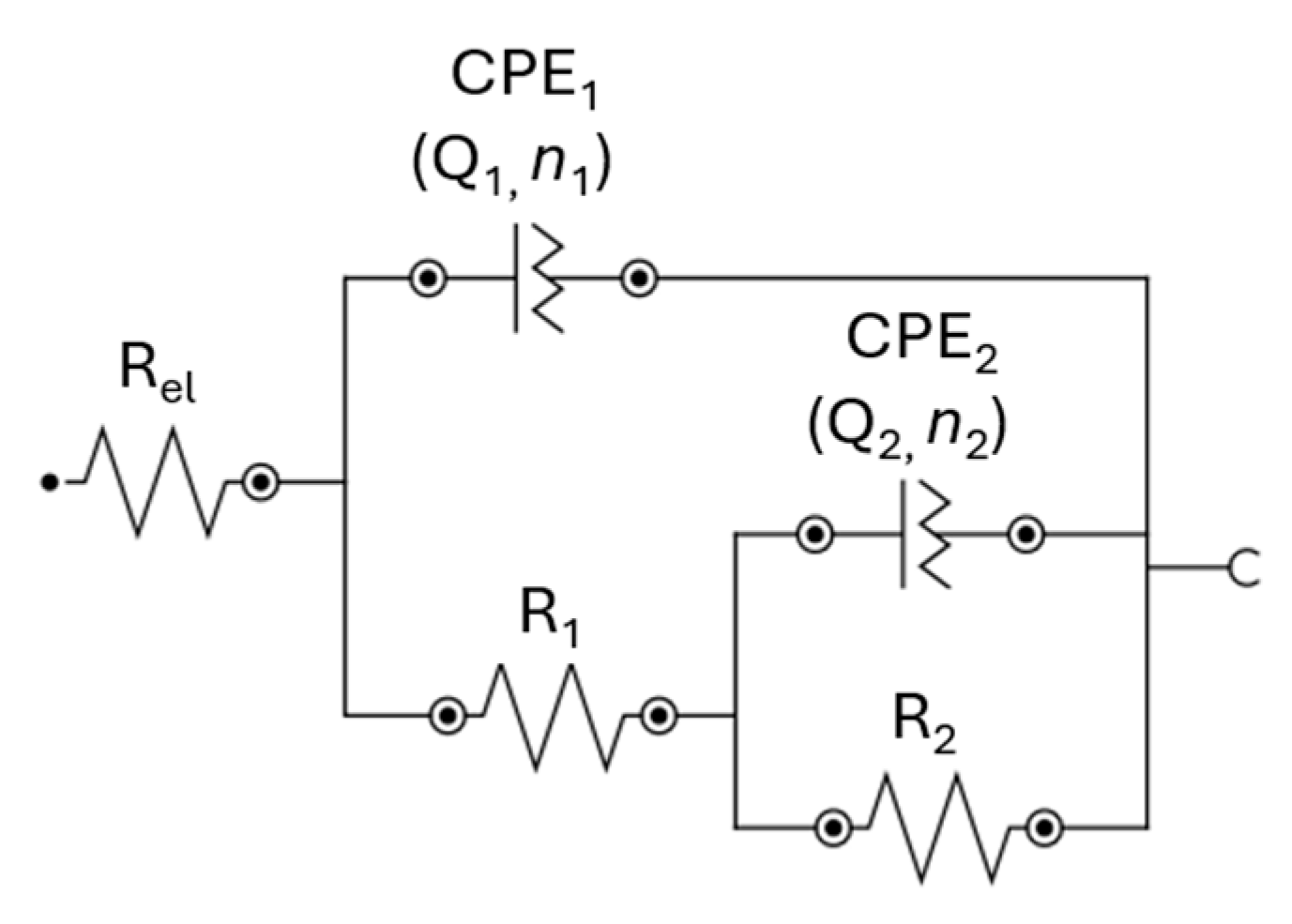
3.3. EIS and Equivalent Circuit Measurements
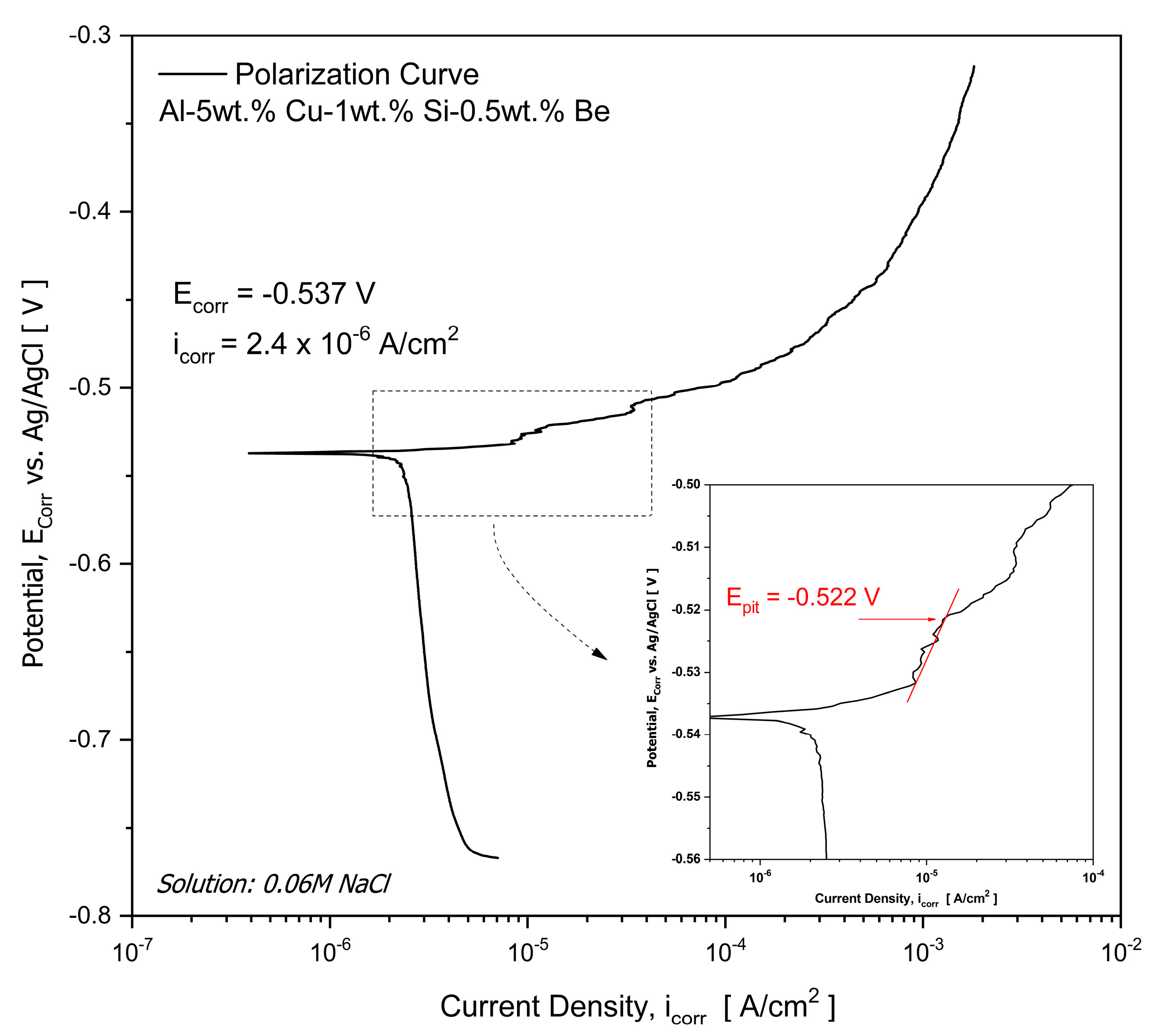
3.4. Potentiodynamic Polarization Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, Z.; Du, Y.; Huang, B.Y.; Liu, Y.; Wang, R.C. A Thermodynamic Description of the Al–Be System: Modeling and Experiment. Calphad 2004, 28, 371–378. [Google Scholar] [CrossRef]
- Molchanova, L.V.; Ilyushin, V.N. Alloying of Aluminum-Beryllium Alloys. Russ. Metall. (Met.) 2013, 2013, 71–73. [Google Scholar] [CrossRef]
- Yie, S.N.; Lee, S.L.; Lin, Y.H.; Lin, J.C. Mechanical Properties of Al-11% Si Casting Alloys Containing Trace Be and Sr. Mater. Trans. JIM 1999, 40, 294–300. [Google Scholar] [CrossRef]
- Wang, G.; Bian, X.; Zhang, J. Effect of Be on the Age-Hardening Characteristics of as-Cast Al-11Si-0.3Mg Alloy. Jinshu Xuebao/Acta Metall. Sin. 2003, 39, 43–46. [Google Scholar]
- Song, G.S.; Fleury, E.; Kim, S.H.; Kim, W.T.; Kim, D.H. Formation and Stability of Quasicrystalline and Hexagonal Approximant Phases in an Al-Mn-Be Alloy. J. Mater. Res. 2002, 17, 1671–1677. [Google Scholar] [CrossRef]
- Song, G.S.; Fleury, E.; Lee, S.M.; Kim, W.T.; Kim, D.H. Quasicrystal-Forming Ability of the Icosahedral Phase in Al-Cu-Fe-Be Alloys. Mater. Sci. Eng. A 2003, 346, 42–49. [Google Scholar] [CrossRef]
- Rooy, E.L.; van Linden, J.H.L. Properties and Selection: Nonferrous Alloys and Special-Purpose Materials. In ASM Metals Handbook; ASM International: Materials Park, OH, USA, 1990; Volume 2, pp. 3330–3345. [Google Scholar]
- Zolotorevsky, V.S.; Belov, N.A.; Glazoff, M.V. Casting Aluminum Alloys; Elsevier: Amsterdam, The Netherlands, 2007; Volume 51, ISBN 9788578110796. [Google Scholar]
- Rodrigues, A.V.; Lima, T.S.; Vida, T.A.; Brito, C.; Garcia, A.; Cheung, N. Microstructure and Tensile/Corrosion Properties Relationships of Directionally Solidified Al–Cu–Ni Alloys. Met. Mater. Int. 2018, 24, 1058–1076. [Google Scholar] [CrossRef]
- Brito, C.; Vida, T.; Freitas, E.; Cheung, N.; Spinelli, J.E.; Garcia, A. Cellular/Dendritic Arrays and Intermetallic Phases Affecting Corrosion and Mechanical Resistances of an Al-Mg-Si Alloy. J. Alloys Compd. 2016, 673, 220–230. [Google Scholar] [CrossRef]
- Osório, W.R.; Siqueira, C.A.; Santos, C.A.; Garcia, A. The Correlation between Electrochemical Corrosion Resistance and Mechanical Strength of As-Cast Al-Cu and Al-Si Alloys. Int. J. Electrochem. Sci. 2011, 6, 6275–6289. [Google Scholar] [CrossRef]
- Rodrigues, A.V.; Kakitani, R.; Silva, C.; Giovanetti, L.; Dias, M.; Henein, H.; Garcia, A.; Cheung, N. Influence of Minor Additions of Be on the Eutectic Modification of an Al-33wt.%Cu Alloy Solidified under Transient Conditions. Metals 2023, 13, 94. [Google Scholar] [CrossRef]
- Moran, J.P.; Bovard, F.S.; Chrzan, J.D.; Vandenburgh, P. Corrosion Performance of New Generation Aluminum-Lithium Alloys for Aerospace Applications. In Proceedings of the ICAA13 Pittsburgh: 13th International Conference on Aluminum Alloys, Pittsburgh, PA, USA, 3–7 June 2016; pp. 425–430. [Google Scholar]
- Vargel, C. Corrosion of Aluminium; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 9780080444956. [Google Scholar]
- Vijayarani, K.; Vishnu Devan, M.; Natarajan, R. Effects of Addition of Alkaline Earth Metal-Beryllium on the Dissolution Behaviour of Aluminium-Zinc Alloy. Port. Electrochim. Acta 2017, 35, 91–102. [Google Scholar] [CrossRef]
- Prater, W.L. Pitting Corrosion of Cast Metallurgy Aluminum-Beryllium Alloys in Sulfuric Acid Solutions. Corrosion 2008, 64, 517–531. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Wu, M.; Xiao, D.; Huang, L.; Liu, W. Effects of Beryllium Addition on Microstructure, Mechanical and Corrosion Performance of Al-Mg-Li Alloys. Materials 2023, 16, 6308. [Google Scholar] [CrossRef]
- Camargo, O.A.B.; Padilha, G.S.; Pinto, F.C.; Osório, W.R.; Poloni, E.; Bortolozo, A.D. Synergistic Effects of Minor Be and Zr Contents on Microstructural and Mechanical Properties of A356 Cast Alloy. Int. J. Met. 2024, 19, 1676–1689. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Alkahtani, S.A.; Abuhasel, K.A.; Samuel, F.H. Microstructural Characterization of Beryllium Treated Al-Si Alloys. Adv. Mater. Sci. Eng. 2015, 2015, 673025. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.K.; Singh, V.; Prasad, K.S.; Chakravorty, C.R. On S(Al2CuMg) Precipitation in an AlCuMg Alloy Containing Small Additions of Beryllium. Acta Mater. 1996, 44, 3115–3124. [Google Scholar] [CrossRef]
- Tang, P.; Yu, F.; Teng, X.; Peng, L.; Wang, K. Effect of Beryllium Addition and Heat Treatment on the Microstructure and Mechanical Properties of the 15%Mg2Si/Al-8Si Composite. Mater. Charact. 2021, 180, 111416. [Google Scholar] [CrossRef]
- Pawlas, N.; Pałczyński, C.M. Beryllium. In Handbook on the Toxicology of Metals, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 2, pp. 101–119. [Google Scholar] [CrossRef]
- Willis, H.H.; Florig, H.K. Potential Exposures and Risks from Beryllium-Containing Products. Risk Anal. 2002, 22, 1019–1033. [Google Scholar] [CrossRef]
- Speer, W.; Es-Said, O.S. Applications of an Aluminum–Beryllium Composite for Structural Aerospace Components. Eng. Fail. Anal. 2004, 11, 895–902. [Google Scholar] [CrossRef]
- Azarbarmas, M.; Emamy, M.; Rassizadehghani, J.; Alipour, M.; Karamouz, M. The Influence of Beryllium Addition on the Microstructure and Mechanical Properties of Al–15%Mg2Si in-Situ Metal Matrix Composite. Mater. Sci. Eng. A 2011, 528, 8205–8211. [Google Scholar] [CrossRef]
- de Albuquerque, S.; Reinhart, G.; Magalhães, D.C.C.; Spinelli, J.E.; Soltani, H.; Boumechta, W.; Bertelli, F.; Nguyen-Thi, H. Solidification Paths of Al-Cu-Sn Alloys: Comparison of Thermodynamic Analyses and Solidification Experiments Using in Situ X-Radiography. Mater. Today Commun. 2024, 40, 109545. [Google Scholar] [CrossRef]
- Sanchez, J.M.; Galarraga, H.; Marquez, I.; de Cortazar, M.G. High-Throughput CALPHAD-Guided Design and Experimental Study on the Development of a Novel Multicomponent as-Cast Al-Si-Cu-Zn-Fe-Mn-Mg Based Alloy through the Direct Melting of Post-Consumer Scrap. J. Alloys Compd. 2025, 1015, 178888. [Google Scholar] [CrossRef]
- Okamoto, T.; Kishitake, K. Dendritic Structure in Unidirectionally Solidified Aluminum, Tin, and Zinc Base Binary Alloys. J. Cryst. Growth 1975, 29, 137–146. [Google Scholar] [CrossRef]
- Soler, G.D.A.; de Faria, A.O.; Borges, J.S.; Vida, T.; Souza, R.C.; Avila, J.A.; Brito, C. Effect of Co Addition on the Microstructure and Mechanical Properties of an Al-2wt.%Ni Alloy. Metals 2024, 14, 1156. [Google Scholar] [CrossRef]
- Vandersluis, E.; Ravindran, C. Comparison of Measurement Methods for Secondary Dendrite Arm Spacing. Metallogr. Microstruct. Anal. 2017, 6, 89–94. [Google Scholar] [CrossRef]
- Silva, C.D.; Soares, T.; Cheung, N.; Garcia, A.; Reis, D.A.P.; Brito, C. Dendritic Spacing and Macrosegregation Affecting Microhardness of an Al-Si-Mg Alloy Solidified Under Unsteady State Conditions. Mater. Res. 2019, 22, e20190390. [Google Scholar] [CrossRef]
- Gündüz, M.; Çadirli, E. Directional Solidification of Aluminium-Copper Alloys. Mater. Sci. Eng. A 2002, 327, 167–185. [Google Scholar] [CrossRef]
- ASTM E8/E8M-22; Standard Test Methods for Tension Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2024. [CrossRef]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Khadke, P.; Tichter, T.; Boettcher, T.; Muench, F.; Ensinger, W.; Roth, C. A Simple and Effective Method for the Accurate Extraction of Kinetic Parameters Using Differential Tafel Plots. Sci. Rep. 2021, 11, 8974. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D.J.; Rappaz, M. Fundamentals of Solidification, 5th Fully Revised ed.; Trans Tech Publications Ltd.: Bäch, Switzerland, 2023. [Google Scholar]
- Stadnik, Z.M.; Takeuchi, T.; Tanaka, N.; Mizutani, U. Structural, Mössbauer, and Transport Studies of the Icosahedral Quasicrystals Al55Si7Cu25.5Fe12.5, Al62.5Cu24.5Fe13 and the Crystalline 1/1 Approximant Al55Si7Cu25.5Fe12.5. J. Phys. Condens. Matter 2003, 15, 6365. [Google Scholar] [CrossRef]
- Kurz, W.; Rappaz, M.; Trivedi, R. Progress in Modelling Solidification Microstructures in Metals and Alloys. Part II: Dendrites from 2001 to 2018. Int. Mater. Rev. 2021, 66, 30–76. [Google Scholar] [CrossRef]
- Ganesh, M.R.S.; Reghunath, N.; Levin, M.J.; Prasad, A.; Doondi, S.; Shankar, K.V. Strontium in Al–Si–Mg Alloy: A Review. Met. Mater. Int. 2022, 28, 1–40. [Google Scholar] [CrossRef]
- Fracchia, E.; Gobber, F.S.; Rosso, M. Effect of Alloying Elements on the Sr Modification of Al-Si Cast Alloys. Metals 2021, 11, 342. [Google Scholar] [CrossRef]
- Fortini, A.; Lattanzi, L.; Merlin, M.; Garagnani, G.L. Comprehensive Evaluation of Modification Level Assessment in Sr-Modified Aluminium Alloys. Int. J. Met. 2018, 12, 697–711. [Google Scholar] [CrossRef]
- Osório, W.R.; Moutinho, D.J.; Peixoto, L.C.; Ferreira, I.L.; Garcia, A. Macrosegregation and Microstructure Dendritic Array Affecting the Electrochemical Behaviour of Ternary Al-Cu-Si Alloys. Electrochim. Acta 2011, 56, 8412–8421. [Google Scholar] [CrossRef]
- Rodrigues, A.V.; Lima, T.S.; Vida, T.A.; Brito, C.; Garcia, A.; Cheung, N. Microstructure Features and Mechanical/Electrochemical Behavior of Directionally Solidified Al−6wt.%Cu−5wt.%Ni Alloy. Trans. Nonferrous Met. Soc. China 2021, 31, 1529–1549. [Google Scholar] [CrossRef]
- Guo, H.; Hu, W.; Yang, K.; Yu, C. Effects of Si and Fe Additions on Mechanical Properties of As-Cast Al–5Cu Alloys. Mater. Sci. Technol. 2022, 38, 352–362. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Y.; Li, Y.; Wang, H.; Wang, Z.; Xu, G.; Li, J.; Tang, H.; Yu, F.; Qian, X. Segregation-Induced Abnormal Recrystallization Behavior, Texture Evolution, and Effects on Mechanical Properties in Twin-Roll Casting 2060 Al-Cu-Li Alloys. J. Alloys Compd. 2024, 991, 174472. [Google Scholar] [CrossRef]
- Cai, Q.; Mendis, C.L.; Chang, I.T.H.; Fan, Z. Microstructure and Mechanical Properties of New Die-Cast Quaternary Al-Cu-Si-Mg Alloys. Mater. Sci. Eng. A 2021, 800, 140357. [Google Scholar] [CrossRef]
- Arthanari, S.; Jang, J.C.; Shin, K.S. Corrosion Studies of High Pressure Die-Cast Al-Si-Ni and Al-Si-Ni-Cu Alloys. J. Alloys Compd. 2018, 749, 146–154. [Google Scholar] [CrossRef]
- Semiletov, A.M.; Chirkunov, A.A.; Kuznetsov, Y.I. Protection of D16 Alloy against Corrosion in Neutral Aqueous Solutions and in an Aggressive Atmosphere Using Organic Inhibitors. Prot. Met. Phys. Chem. Surf. 2020, 56, 1285–1292. [Google Scholar] [CrossRef]
- Pulido-González, N.; Torres, B.; Zheludkevich, M.L.; Rams, J. High Power Diode Laser (HPDL) Surface Treatments to Improve the Mechanical Properties and the Corrosion Behaviour of Mg-Zn-Ca Alloys for Biodegradable Implants. Surf. Coat. Technol. 2020, 402, 126314. [Google Scholar] [CrossRef]
- Chen, Z.; Han, P.; He, B.; Sun, F.; Bai, X.; Wang, X.; Guo, T.; Wang, X. Electrochemical Impedance Spectroscopy (EIS) of NaCl-Saturated Sandy Soil at Sub-Zero Temperatures. Int. J. Electrochem. Sci. 2021, 16, 210914. [Google Scholar] [CrossRef]
- ASTM DESIGNATION G102; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. In Annual Book of ASTM Standars; ASTM International: West Conshohocken, PA, USA, 2012; Volume 66.
- Li, C.; Li, X.; Zhang, Y.; Wen, K.; Yan, L.; Li, Y.; Li, Y.; Yu, M.; Gao, G.; Yan, H.; et al. Effect of Cu/Li Ratio on Mechanical Properties and Corrosion Behavior of Sc-Containing Al-Cu-Li Alloys. Materials 2025, 18, 2254. [Google Scholar] [CrossRef]
- Geng, J.; Li, Y.; Liu, G.; Xiao, H.; Hong, T.; Huang, J.; Wang, M.; Chen, D.; Wang, H. Segregation of S Precipitates at T/Al Interfaces and Relevant Effects on Localized Corrosion Mechanisms in Al-Cu-Mg Alloy. Mater. Charact. 2020, 168, 110571. [Google Scholar] [CrossRef]


| Alloy Elements | Mg | Al | Be | Si | Cu | Ni | Fe | Mn |
|---|---|---|---|---|---|---|---|---|
| Al | 0.01 | Balance | - | 0.13 | 0.01 | - | 0.23 | - |
| Cu | - | - | - | - | Balance | 0.008 | - | 0.08 |
| Si | - | 0.11 | - | Balance | - | 0.01 | 0.32 | - |
| Cu-Be | - | 0.029 | 9.83 | 0.0097 | Balance | 0.01 | 0.053 | - |
| Sample (Diameter [mm]) | Cooling Rate () [K/s] |
|---|---|
| A1 (10 mm) | 82.2 |
| A2 (15 mm) | 45.0 |
| A3 (25 mm) | 21.4 |
| A4 (40 mm) | 19.5 |
| Alloy | [K/s] | [μm] | σY [MPa] | σU [MPa] | δ [%] |
|---|---|---|---|---|---|
| Al-5Cu [11] | 9.8 | 24.3 ± 2.3 | 112 ± 13.0 | 225 ± 21.0 | - |
| Al-5Cu-1Ni [9] | 12.6 | 10.5 ± 2.2 | - | 190 ± 8.0 | 25 ± 2.4 |
| Al-5Cu-2.5Si-1.1Mg [46] | - | - | 219 ± 3.1 | 344.7 ± 6.5 | 7.72 ± 1.4 |
| 2060-T6 Al-Cu-Li [45] | - | - | ~348 | −378 | 10.2 |
| A356—T6 [18] | 1.5 | 35 ± 6.0 | - | 243 ± 17 | 11.0 ± 1.4 |
| Al-5Cu-1Si-0.5Be [this work] | 21.4 | 10.0 ± 2.8 | 84.8 ± 14.3 | 248.8 ± 11.2 | 6.4 ± 0.5 |
| Parameter | Value | Estimated Error (%) |
|---|---|---|
| Rel (Ω∙cm2) | 85.48 | 0.42 |
| Q1 (S∙sn/cm2) | 12.20 | 1.90 |
| R1 (kΩ∙cm2) | 5.94 | 0.73 |
| n1 | 0.92 | - |
| Q2 (S∙sn/cm2) | 167.52 | 1.57 |
| R2 (kΩ∙cm2) | 12.65 | 1.59 |
| n2 | 0.99 | - |
| χ2 | 0.9 × 10−3 |
| Alloy | [K/s] | [μm] | [] | [] | [] |
|---|---|---|---|---|---|
| Al-5Cu [11] | 9.8 | 24.3 ± 2.3 | 4.15 | −0.678 | 46.9 * |
| Al-5Cu-1Ni [9] | 12.6 | 10.5 ± 2.2 | 0.15 | −0.525 | 1.7 * |
| Al-6Cu-1Si [42] | 13.5 | 10.2 ± 1.2 | 4.36 | −0.620 | 47.2 * |
| Al-Cu-Li [52] | - | - | 0.31 | −0.642 | - |
| Al-5Cu-1Si-0.5Be [this work] | 21.4 | 10.0 ± 2.8 | 2.40 | −0.537 | 28.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.R.; Araújo, M.P.; Vida, T.; Conde, F.F.; Cheung, N.; Garcia, A.; Brito, C. The Influence of Beryllium Incorporation into an Al-5wt.%Cu-1wt.%Si Alloy on the Solidification Cooling Rate, Microstructural Length Scale, and Corrosion Resistance. Metals 2025, 15, 736. https://doi.org/10.3390/met15070736
Santos JR, Araújo MP, Vida T, Conde FF, Cheung N, Garcia A, Brito C. The Influence of Beryllium Incorporation into an Al-5wt.%Cu-1wt.%Si Alloy on the Solidification Cooling Rate, Microstructural Length Scale, and Corrosion Resistance. Metals. 2025; 15(7):736. https://doi.org/10.3390/met15070736
Chicago/Turabian StyleSantos, Joyce Ranay, Milena Poletto Araújo, Talita Vida, Fabio Faria Conde, Noé Cheung, Amauri Garcia, and Crystopher Brito. 2025. "The Influence of Beryllium Incorporation into an Al-5wt.%Cu-1wt.%Si Alloy on the Solidification Cooling Rate, Microstructural Length Scale, and Corrosion Resistance" Metals 15, no. 7: 736. https://doi.org/10.3390/met15070736
APA StyleSantos, J. R., Araújo, M. P., Vida, T., Conde, F. F., Cheung, N., Garcia, A., & Brito, C. (2025). The Influence of Beryllium Incorporation into an Al-5wt.%Cu-1wt.%Si Alloy on the Solidification Cooling Rate, Microstructural Length Scale, and Corrosion Resistance. Metals, 15(7), 736. https://doi.org/10.3390/met15070736








