Research on Corrosion Protection of TETA-Modified Li–Al LDHs for AZ31 Magnesium Alloy in Simulated Seawater
Abstract
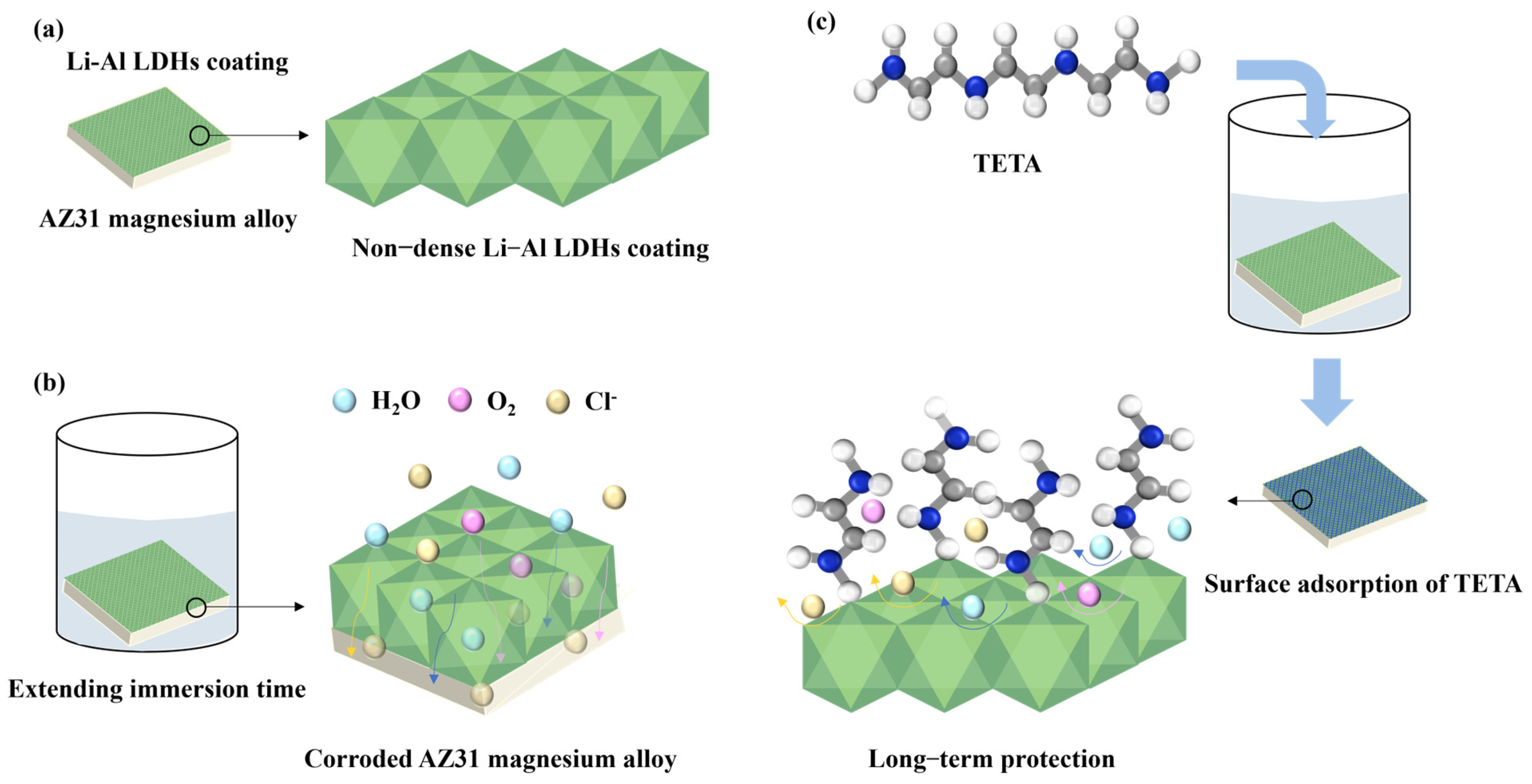
1. Introduction
2. Experimental and Materials
3. Results and Discussion
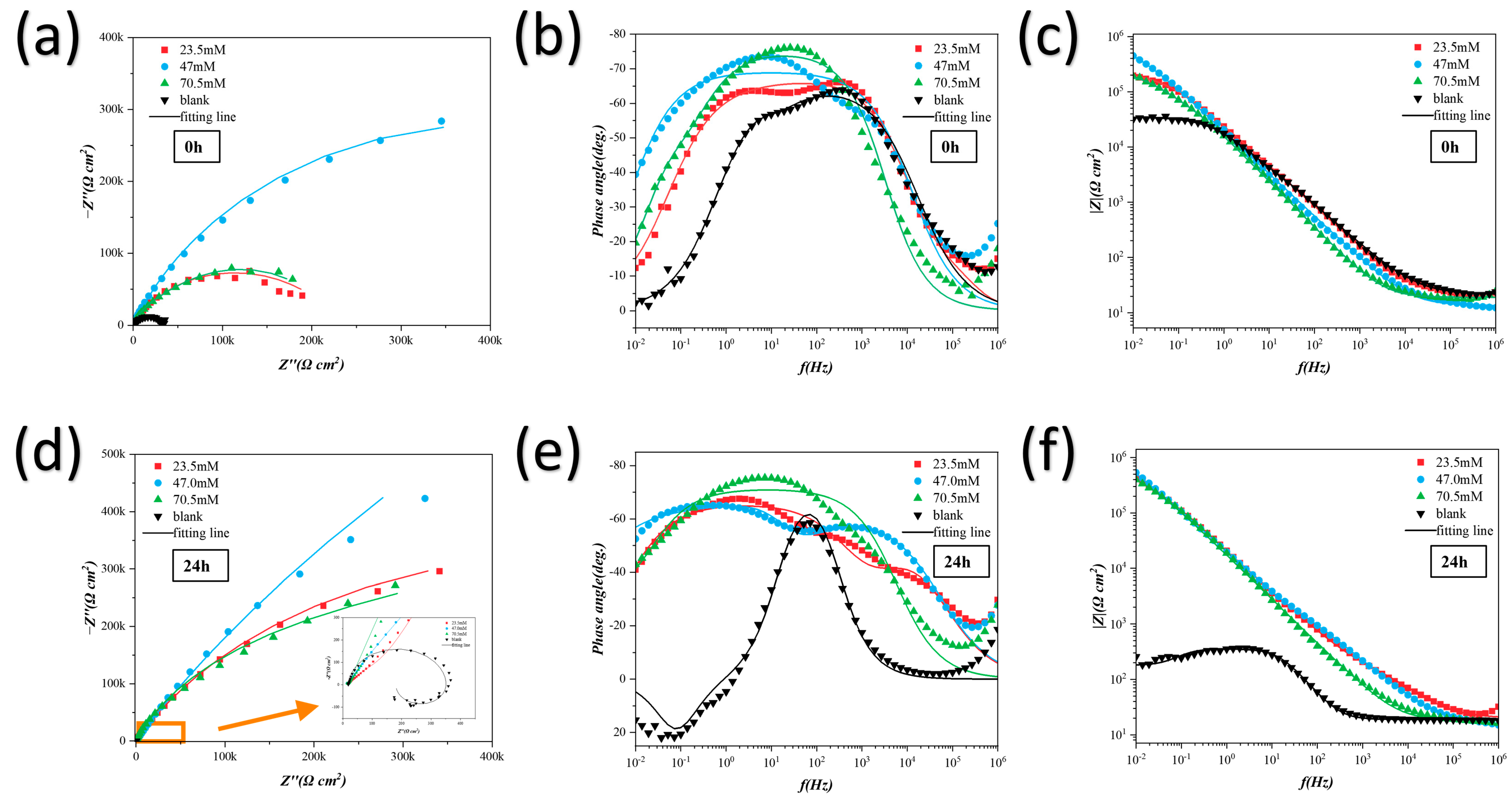
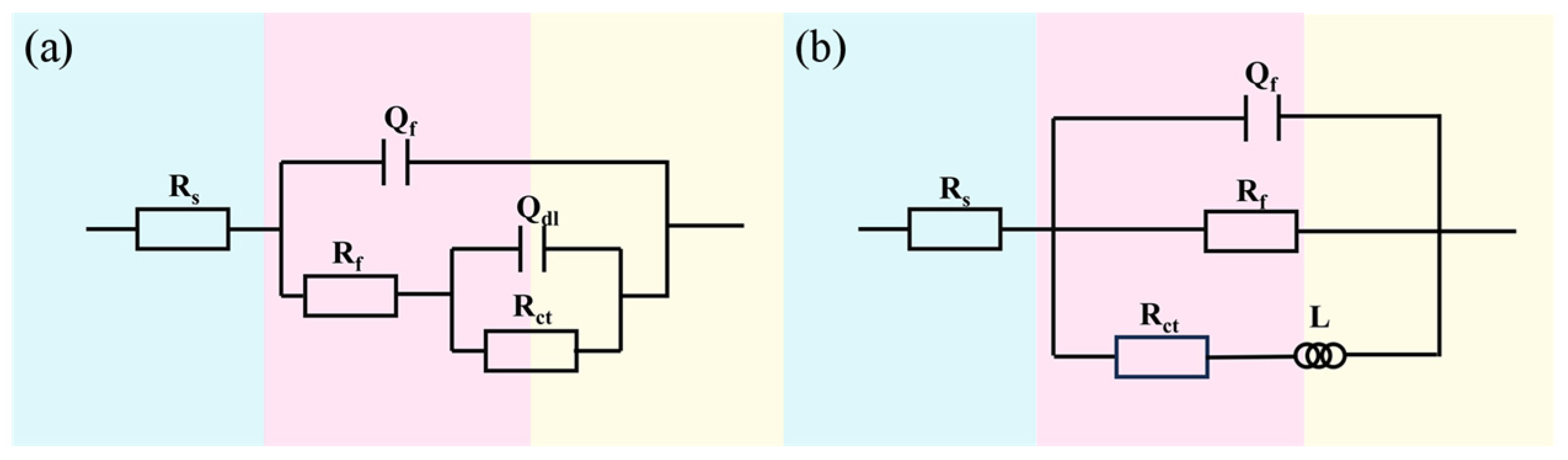
3.1. Electrochemical Analysis
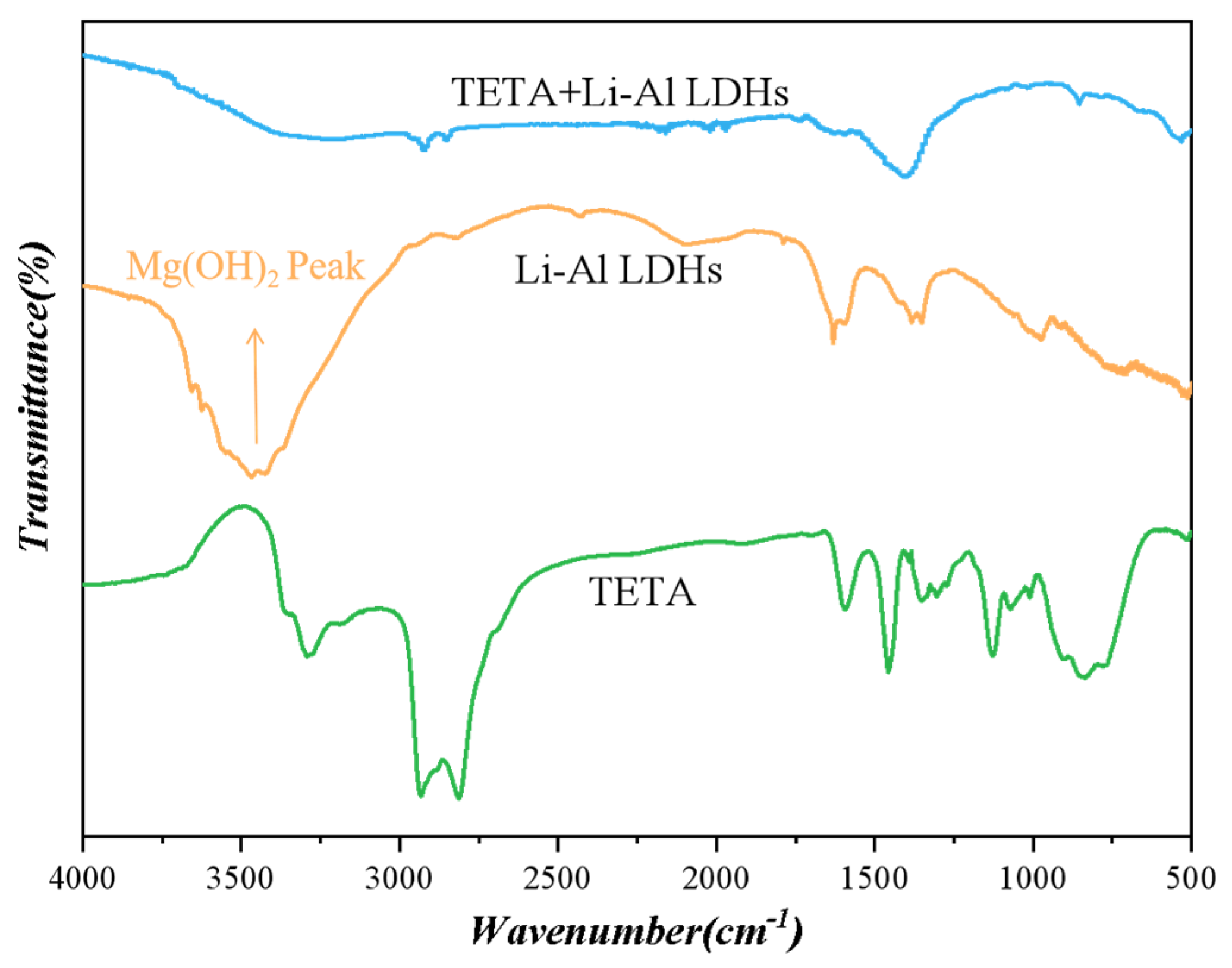
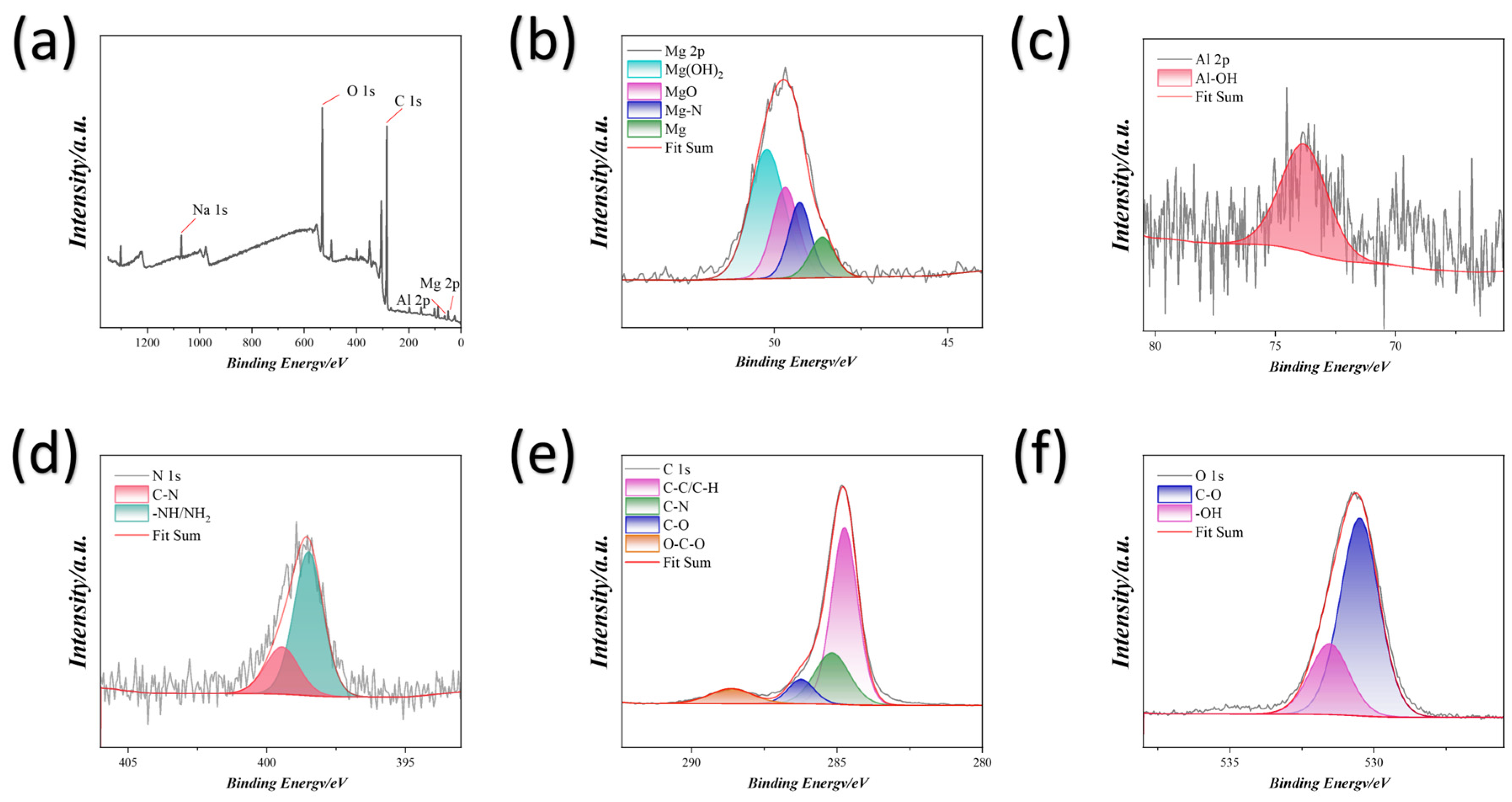
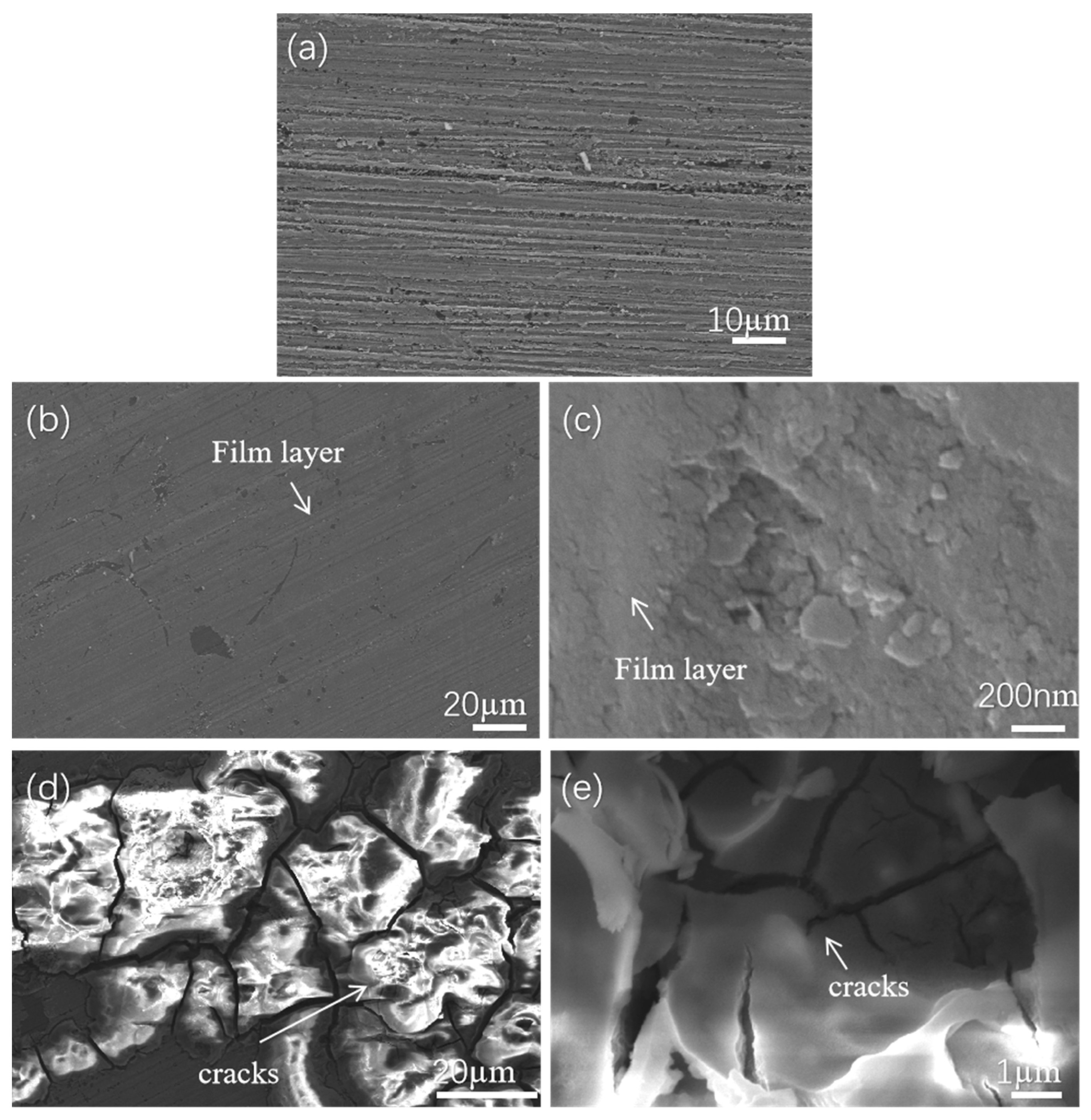
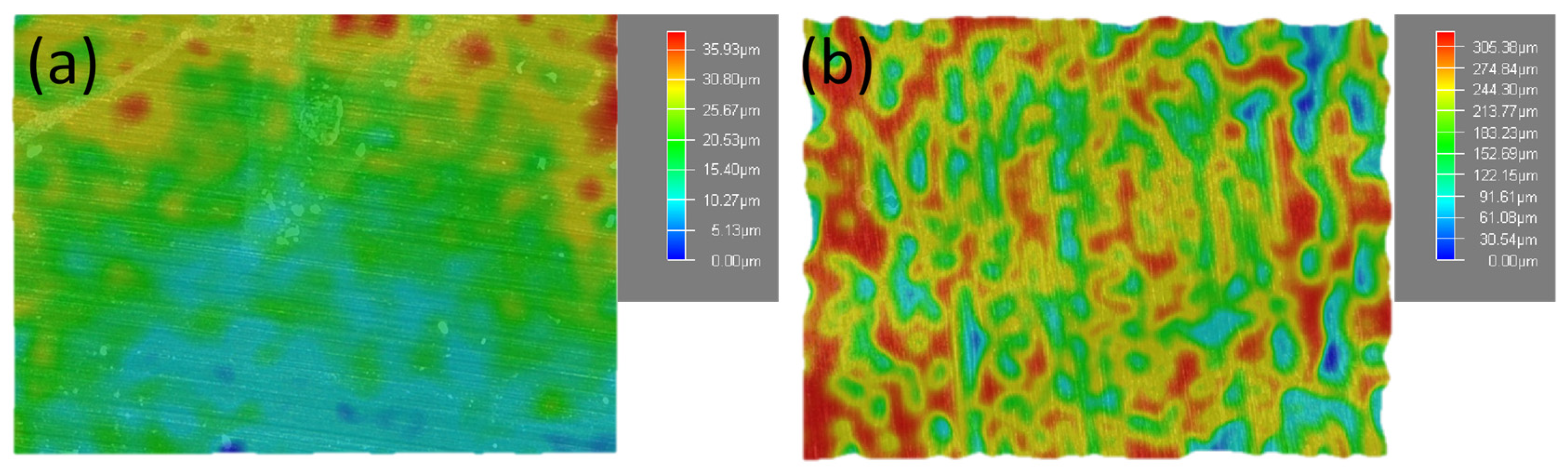
3.2. Analysis of Characterization Test Results
3.3. Discussion and Analysis
4. Conclusions
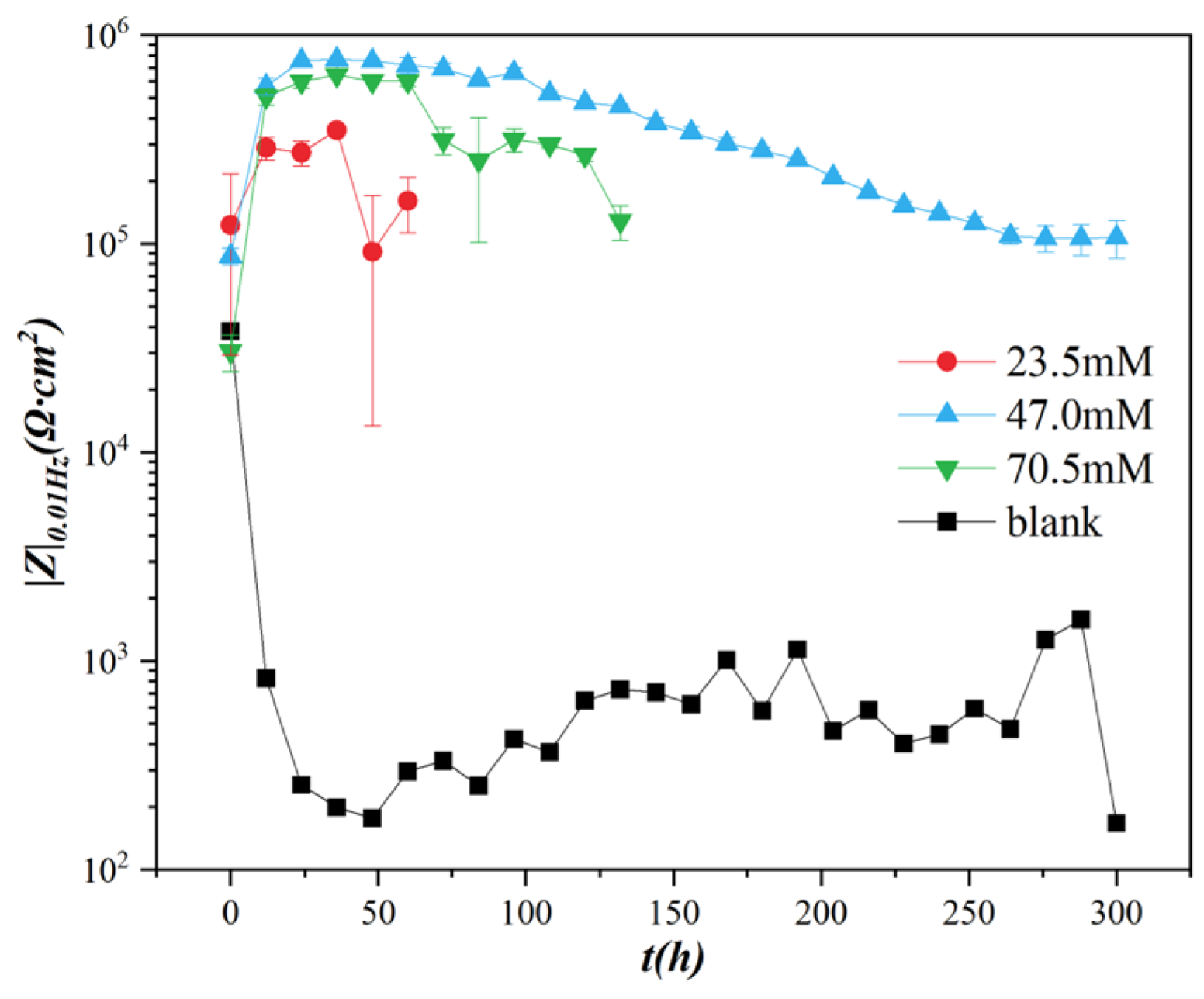
- At the optimal TETA concentration of 47 mM, the maximum |Z|0.01Hz value reached 7.56 × 105 Ω∙cm2 after 24 h of immersion, demonstrating three orders of magnitude of improvement compared to the Li–Al LDHs blank sample without an inhibitor (2.55 × 102 Ω∙cm2). Moreover, after 300 h of immersion, the low-frequency impedance value remained above 105 Ω∙cm2, indicating excellent sustained corrosion protection performance.
- The Li–Al LDHs coating modified with TETA exhibited substantially enhanced long-term corrosion resistance, effectively addressing the limitation of standalone LDHs coatings that only provide short-term corrosion protection.
- The adsorption effect of TETA on Li–Al LDHs layers, particularly through rapid adsorption and protection at coating defects, creates effective shielding. This inorganic-organic composite protection design enables defect compensation and forms superior protective barriers through complementary mechanisms.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LDHs | layered double hydroxides |
| TETA | triethylenetetramine |
| ASP | aspartic acid |
| 8HQ | 8-hydroxyquinoline |
| PPA | phenylphosphonic acid |
| EIS | electrochemical impedance spectroscopy |
| FE−SEM | field-emission scanning electron microscopy |
| FT−IR | Fourier transform infrared spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
| SCE | saturated calomel reference electrode |
| OCP | open-circuit potential |
| SEM | scanning electron microscopy |
| CPE | constant phase element |
References
- Li, B.; Zhang, Z.; Liu, T.; Qiu, Z.; Su, Y.; Zhang, J.; Lin, C.; Caporali, S.; Galvanetto, E.; Li, B. Recent Progress in Functionalized Coatings for Corrosion Protection of Magnesium Alloys—A Review. Materials 2022, 15, 3912. [Google Scholar] [CrossRef] [PubMed]
- Cao, H. Low Adhesive and Superhydrophobic LDH Coating for Anti-Corrosion and Self-Cleaning. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129893. [Google Scholar] [CrossRef]
- Cao, J.; Feng, Z.; Liang, H.; Lu, X.; Wang, W. Oriented Self-Assembly of Anisotropic Layered Double Hydroxides (LDHs) with 2D-on-3D Hierarchical Structure. Chem. Eng. J. 2023, 472, 144872. [Google Scholar] [CrossRef]
- Yang, Q.; Tabish, M.; Wang, J.; Zhao, J. Enhanced Corrosion Resistance of Layered Double Hydroxide Films on Mg Alloy: The Key Role of Cationic Surfactant. Materials 2022, 15, 2028. [Google Scholar] [CrossRef]
- Huang, M.; Lu, G.; Pu, J.; Qiang, Y. Superhydrophobic and Smart MgAl-LDH Anti-Corrosion Coating on AZ31 Mg Surface. J. Ind. Eng. Chem. 2021, 103, 154–164. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Wu, L.; Zhang, Y.; Deng, J.; Yao, W.; Wu, J.; Yuan, Y.; Xie, Z.; Atrens, A.; et al. Effect of Solution PH Value on the Corrosion Resistance of Co-Fe LDHs Coating Developed on MAO Treated Magnesium Alloy AZ31. Colloids Surf. A Physicochem. Eng. Asp. 2024, 694, 134220. [Google Scholar] [CrossRef]
- Zeng, R.C.; Liu, Z.G.; Zhang, F.; Li, S.Q.; He, Q.K.; Cui, H.Z.; Han, E.H. Corrosion Resistance of In-Situ Mg-Al Hydrotalcite Conversion Film on AZ31 Magnesium Alloy by One-Step Formation. Trans. Nonferrous Met. Soc. China 2015, 25, 1917–1925. [Google Scholar] [CrossRef]
- Tan, J.K.E.; Balan, P.; Birbilis, N. Advances in LDH Coatings on Mg Alloys for Biomedical Applications: A Corrosion Perspective. Appl. Clay Sci. 2021, 202, 105948. [Google Scholar] [CrossRef]
- Chen, J.; Fang, L.; Wu, F.; Xie, J.; Hu, J.; Jiang, B.; Luo, H. Corrosion Resistance of a Self-Healing Rose-like MgAl-LDH Coating Intercalated with Aspartic Acid on AZ31 Mg Alloy. Prog. Org. Coat. 2019, 136, 105234. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, S.; Liu, Y.; Huangfu, H.; Guo, X.; Zhang, J. Enhancement of Corrosion Resistance of AZ31B Magnesium Alloy by Preparing MgAl-LDHs Coatings Modified with Imidazolium Based Dicationic Ionic Liquids. Surf. Coat. Technol. 2022, 440, 128504. [Google Scholar] [CrossRef]
- Anjum, M.J.; Zhao, J.; Zahedi Asl, V.; Yasin, G.; Wang, W.; Wei, S.; Zhao, Z.; Qamar Khan, W. In-Situ Intercalation of 8-Hydroxyquinoline in Mg-Al LDH Coating to Improve the Corrosion Resistance of AZ31. Corros. Sci. 2019, 157, 1–10. [Google Scholar] [CrossRef]
- Wen, T.; Yan, R.; Wang, N.; Li, Y.; Chen, T.; Ma, H. PPA-Containing Layered Double Hydroxide (LDH) Films for Corrosion Protection of a Magnesium Alloy. Surf. Coat. Technol. 2020, 383, 125255. [Google Scholar] [CrossRef]
- Wang, L.; Tu, S.; Huang, K.; Guo, H.; Lei, B.; Yang, Z.; Yong, Q.; Feng, Z.; Liu, X.; Meng, G. Insight into the Corrosion Inhibition Performance of Triethylenetetramine (TETA) for AZ31 Mg Alloy. Colloids Surf. A Physicochem. Eng. Asp. 2025, 710, 136246. [Google Scholar] [CrossRef]
- Wang, L.; Huang, K.; Yang, Z.; Buchheit, R.; Feng, Z. Research on the Corrosion Protection of In-Situ Prepared Li-Al Layered Double Hydroxides on AZ31 Mg Alloy under Standard Atmospheric Pressure and Low Temperature. Appl. Clay Sci. 2024, 261, 107585. [Google Scholar] [CrossRef]
- Dong, Q.; Dai, J.; Qian, K.; Liu, H.; Zhou, X.; Yao, Q.; Lu, M.; Chu, C.; Xue, F.; Bai, J. Dual Self-Healing Inorganic-Organic Hybrid Coating on Biomedical Mg. Corros. Sci. 2022, 200, 110230. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Sabour Rouhaghdam, A. Microstructural, Protective, Inhibitory and Semiconducting Properties of PEO Coatings Containing CeO2 Nanoparticles Formed on AZ31 Mg Alloy. Surf. Coat. Technol. 2018, 352, 561–580. [Google Scholar] [CrossRef]
- Gomes, M.P.; Costa, I.; Pébère, N.; Rossi, J.L.; Tribollet, B.; Vivier, V. On the Corrosion Mechanism of Mg Investigated by Electrochemical Impedance Spectroscopy. Electrochim. Acta 2019, 306, 61–70. [Google Scholar] [CrossRef]
- Thanaa, T.T.; Aadil, M.; Askari, A.; Fattah-alhosseini, A.; Alkaseem, M.; Kaseem, M. Highly Corrosion-Resistant and Photocatalytic Hybrid Coating on AZ31 Mg Alloy via Plasma Electrolytic Oxidation with Organic-Inorganic Integration. J. Magnes. Alloys 2025, 13, 260–282. [Google Scholar] [CrossRef]
- Tran, H.N.; Huang, F.C.; Lee, C.K.; Chao, H.P. Activated Carbon Derived from Spherical Hydrochar Functionalized with Triethylenetetramine: Synthesis, Characterizations, and Adsorption Application. Green Process. Synth. 2017, 6, 565–576. [Google Scholar] [CrossRef]
- Shabanian, M.; Hajibeygi, M.; Raeisi, A. FTIR Characterization of Layered Double Hydroxides and Modified Layered Double Hydroxides. In Layered Double Hydroxide Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 77–101. [Google Scholar]
- Copéret, C.; Comas-Vives, A.; Conley, M.P.; Estes, D.P.; Fedorov, A.; Mougel, V.; Nagae, H.; Núnez-Zarur, F.; Zhizhko, P.A. Surface Organometallic and Coordination Chemistry toward Single-Site Heterogeneous Catalysts: Strategies, Methods, Structures, and Activities. Chem. Rev. 2016, 116, 323–421. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Li, R.; Feng, X.; Pang, X.; Rao, J.; Cong, D.; Yin, C.; Zhang, Y. Active Corrosion Protection of Mg−al Layered Double Hydroxide for Magnesium Alloys: A Short Review. Coatings 2021, 11, 1316. [Google Scholar] [CrossRef]
- Shahraki, M.; Dehdab, M.; Elmi, S. Theoretical Studies on the Corrosion Inhibition Performance of Three Amine Derivatives on Carbon Steel: Molecular Dynamics Simulation and Density Functional Theory Approaches. J. Taiwan. Inst. Chem. Eng. 2016, 62, 313–321. [Google Scholar] [CrossRef]
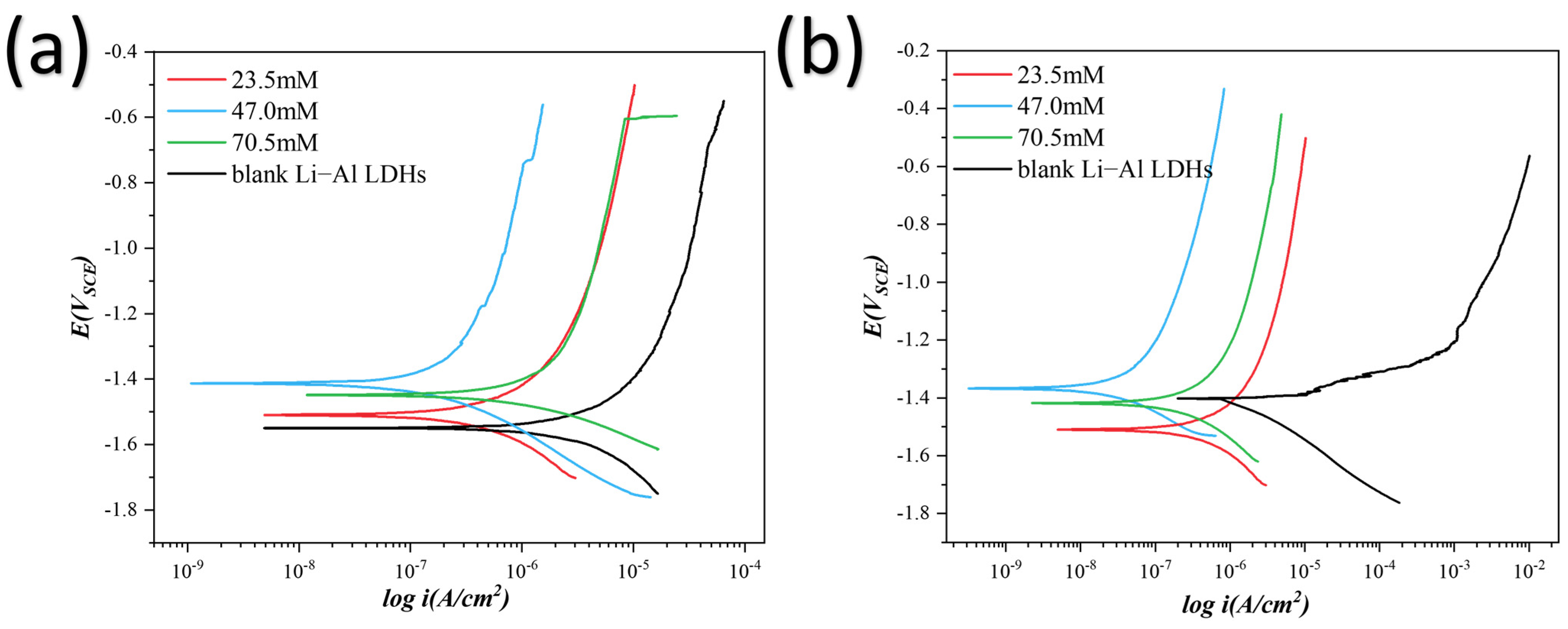
| Time | Concentration mol/L | Ecorr VSCE | icorr A/cm2 |
|---|---|---|---|
| 0 h | 0 | −1.549 | 1.294 × 10−6 |
| 0.0235 | −1.532 ± 0.0254 | 1.294 × 10−6 ± 3.641 × 10−7 | |
| 0.0470 | −1.399 ± 0.0481 | 2.506 × 10−7 ± 1.288 × 10−7 | |
| 0.0705 | −1.425 ± 0.0332 | 4.814 × 10−7 ± 1.518 × 10−7 | |
| 24 h | 0 | −1.402 | 1.812 × 10−6 |
| 0.0235 | −1.506 ± 0.0566 | 3.321 × 10−4 ± 1.518 × 10−4 | |
| 0.047 | −1.349 ± 0.0246 | 1.254 × 10−8 ± 3.335 × 10−9 | |
| 0.0705 | −1.457 ± 0.0551 | 2.637 × 10−7 ± 3.451 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, S.; Wang, L.; Wang, S.; Chen, H.; Huang, Q.; Hou, N.; Feng, Z.; Meng, G. Research on Corrosion Protection of TETA-Modified Li–Al LDHs for AZ31 Magnesium Alloy in Simulated Seawater. Metals 2025, 15, 724. https://doi.org/10.3390/met15070724
Tu S, Wang L, Wang S, Chen H, Huang Q, Hou N, Feng Z, Meng G. Research on Corrosion Protection of TETA-Modified Li–Al LDHs for AZ31 Magnesium Alloy in Simulated Seawater. Metals. 2025; 15(7):724. https://doi.org/10.3390/met15070724
Chicago/Turabian StyleTu, Sifan, Liyan Wang, Sixu Wang, Haoran Chen, Qian Huang, Ning Hou, Zhiyuan Feng, and Guozhe Meng. 2025. "Research on Corrosion Protection of TETA-Modified Li–Al LDHs for AZ31 Magnesium Alloy in Simulated Seawater" Metals 15, no. 7: 724. https://doi.org/10.3390/met15070724
APA StyleTu, S., Wang, L., Wang, S., Chen, H., Huang, Q., Hou, N., Feng, Z., & Meng, G. (2025). Research on Corrosion Protection of TETA-Modified Li–Al LDHs for AZ31 Magnesium Alloy in Simulated Seawater. Metals, 15(7), 724. https://doi.org/10.3390/met15070724








